Molecular Genetic Mechanisms in Age-Related Macular Degeneration
Abstract
:1. Introduction
2. Immune Dysregulation and the Complement System
2.1. Complement Factor H (CFH)
2.2. Complement Factor I (CFI)
2.3. Complement Component 3 (C3)
2.4. Complement Component 5 (C5)
2.5. Complement Component 9 (C9)
2.6. Complement Component 2 (C2) and Complement Factor B (CFB)
2.7. Complement Factor D (CFD)
3. Extracellular Matrix (ECM) Remodeling
3.1. Tissue Inhibitor of Metalloproteinases (TIMPs)
3.2. Matrix Metalloproteinases (MMPs)
3.3. Other Extracellular Matrix Components
4. Lipid Metabolism
4.1. Apolipoprotein E (ApoE)
4.2. Hepatic Lipase (LIPC)
4.3. Cholesteryl Ester Transfer Protein (CETP)
4.4. ATP-Binding Cassette Transporter A1 (ABCA1)
4.5. Lipoprotein Lipase (LPL)
5. Angiogenesis
5.1. Vascular Endothelial Growth Factor (VEGF)
5.2. Fibulin 5
6. Oxidative Stress Response and Photoreceptor Survival
7. Genes Implicated in Multiple Pathways
Age-Related Maculopathy Susceptibility 2 (ARMS2) and High-Temperature Requirement Factor A Serine Peptidase 1 (HTRA1)
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wong, W.L.; Su, X.; Li, X.; Cheung, C.M.; Klein, R.; Cheng, C.Y.; Wong, T.Y. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet Glob. Health 2014, 2, e106–e116. [Google Scholar] [CrossRef] [Green Version]
- Brown, M.M.; Brown, G.C.; Sharma, S.; Kistler, J.; Brown, H. Utility values associated with blindness in an adult population. Br. J. Ophthalmol. 2001, 85, 327–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernandez Trillo, A.; Dickinson, C.M. The impact of visual and nonvisual factors on quality of life and adaptation in adults with visual impairment. Investig. Ophthalmol. Vis. Sci. 2012, 53, 4234–4241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, R.A.; Brody, B.L.; Thomas, R.G.; Kaplan, R.M.; Brown, S.I. The psychosocial impact of macular degeneration. Arch. Ophthalmol. 1998, 116, 514–520. [Google Scholar] [CrossRef]
- Spooner, K.L.; Mhlanga, C.T.; Hong, T.H.; Broadhead, G.K.; Chang, A.A. The burden of neovascular age-related macular degeneration: A patient’s perspective. Clin. Ophthalmol. 2018, 12, 2483–2491. [Google Scholar] [CrossRef] [Green Version]
- Brown, M.M.; Brown, G.C.; Stein, J.D.; Roth, Z.; Campanella, J.; Beauchamp, G.R. Age-related macular degeneration: Economic burden and value-based medicine analysis. Can. J. Ophthalmol. 2005, 40, 277–287. [Google Scholar] [CrossRef]
- Rein, D.B.; Wittenborn, J.S.; Zhang, X.; Honeycutt, A.A.; Lesesne, S.B.; Saaddine, J.; Vision Health Cost-Effectiveness Study, G. Forecasting age-related macular degeneration through the year 2050: The potential impact of new treatments. Arch. Ophthalmol. 2009, 127, 533–540. [Google Scholar] [CrossRef] [Green Version]
- Sparrow, J.R. Bisretinoids of RPE lipofuscin: Trigger for complement activation in age-related macular degeneration. Adv. Exp. Med. Biol. 2010, 703, 63–74. [Google Scholar] [CrossRef]
- Strauss, O. The retinal pigment epithelium in visual function. Physiol. Rev. 2005, 85, 845–881. [Google Scholar] [CrossRef] [Green Version]
- Beatty, S.; Koh, H.; Phil, M.; Henson, D.; Boulton, M. The role of oxidative stress in the pathogenesis of age-related macular degeneration. Surv. Ophthalmol. 2000, 45, 115–134. [Google Scholar] [CrossRef] [Green Version]
- Van der Vaart, H.; Postma, D.S.; Timens, W.; ten Hacken, N.H. Acute effects of cigarette smoke on inflammation and oxidative stress: A review. Thorax 2004, 59, 713–721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thornton, J.; Edwards, R.; Mitchell, P.; Harrison, R.A.; Buchan, I.; Kelly, S.P. Smoking and age-related macular degeneration: A review of association. Eye 2005, 19, 935–944. [Google Scholar] [CrossRef] [PubMed]
- Smith, W.; Assink, J.; Klein, R.; Mitchell, P.; Klaver, C.C.; Klein, B.E.; Hofman, A.; Jensen, S.; Wang, J.J.; de Jong, P.T. Risk factors for age-related macular degeneration: Pooled findings from three continents. Ophthalmology 2001, 108, 697–704. [Google Scholar] [CrossRef]
- Age-Related Eye Disease Study Research Group. Risk factors associated with age-related macular degeneration. A case-control study in the age-related eye disease study: Age-Related Eye Disease Study Report Number 3. Ophthalmology 2000, 107, 2224–2232. [Google Scholar] [CrossRef]
- Friedman, E. A hemodynamic model of the pathogenesis of age-related macular degeneration. Am. J. Ophthalmol. 1997, 124, 677–682. [Google Scholar] [CrossRef]
- Curcio, C.A. Complementing apolipoprotein secretion by cultured retinal pigment epithelium. Proc. Natl. Acad. Sci. USA 2011, 108, 18569–18570. [Google Scholar] [CrossRef] [Green Version]
- Fleckenstein, M.; Keenan, T.D.L.; Guymer, R.H.; Chakravarthy, U.; Schmitz-Valckenberg, S.; Klaver, C.C.; Wong, W.T.; Chew, E.Y. Age-related macular degeneration. Nat. Rev. Dis. Primers 2021, 7, 31. [Google Scholar] [CrossRef]
- Spaide, R.F.; Jaffe, G.J.; Sarraf, D.; Freund, K.B.; Sadda, S.R.; Staurenghi, G.; Waheed, N.K.; Chakravarthy, U.; Rosenfeld, P.J.; Holz, F.G.; et al. Consensus Nomenclature for Reporting Neovascular Age-Related Macular Degeneration Data: Consensus on Neovascular Age-Related Macular Degeneration Nomenclature Study Group. Ophthalmology 2020, 127, 616–636. [Google Scholar] [CrossRef]
- Palejwala, N.V.; Jia, Y.; Gao, S.S.; Liu, L.; Flaxel, C.J.; Hwang, T.S.; Lauer, A.K.; Wilson, D.J.; Huang, D.; Bailey, S.T. Detection of Nonexudative Choroidal Neovascularization in Age-Related Macular Degeneration with Optical Coherence Tomography Angiography. Retina 2015, 35, 2204–2211. [Google Scholar] [CrossRef] [Green Version]
- Choudhury, F.; Varma, R.; McKean-Cowdin, R.; Klein, R.; Azen, S.P.; Los Angeles Latino Eye Study, G. Risk factors for four-year incidence and progression of age-related macular degeneration: The los angeles latino eye study. Am. J. Ophthalmol. 2011, 152, 385–395. [Google Scholar] [CrossRef] [Green Version]
- Seddon, J.M.; Willett, W.C.; Speizer, F.E.; Hankinson, S.E. A prospective study of cigarette smoking and age-related macular degeneration in women. JAMA 1996, 276, 1141–1146. [Google Scholar] [CrossRef] [PubMed]
- Christen, W.G.; Glynn, R.J.; Manson, J.E.; Ajani, U.A.; Buring, J.E. A prospective study of cigarette smoking and risk of age-related macular degeneration in men. JAMA 1996, 276, 1147–1151. [Google Scholar] [CrossRef]
- Chakravarthy, U.; Augood, C.; Bentham, G.C.; de Jong, P.T.; Rahu, M.; Seland, J.; Soubrane, G.; Tomazzoli, L.; Topouzis, F.; Vingerling, J.R.; et al. Cigarette smoking and age-related macular degeneration in the EUREYE Study. Ophthalmology 2007, 114, 1157–1163. [Google Scholar] [CrossRef] [PubMed]
- Seddon, J.M.; Rosner, B.; Sperduto, R.D.; Yannuzzi, L.; Haller, J.A.; Blair, N.P.; Willett, W. Dietary fat and risk for advanced age-related macular degeneration. Arch. Ophthalmol. 2001, 119, 1191–1199. [Google Scholar] [CrossRef] [Green Version]
- Van Leeuwen, R.; Boekhoorn, S.; Vingerling, J.R.; Witteman, J.C.; Klaver, C.C.; Hofman, A.; de Jong, P.T. Dietary intake of antioxidants and risk of age-related macular degeneration. JAMA 2005, 294, 3101–3107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hyman, L.G.; Lilienfeld, A.M.; Ferris, F.L., 3rd; Fine, S.L. Senile macular degeneration: A case-control study. Am. J. Epidemiol. 1983, 118, 213–227. [Google Scholar] [CrossRef]
- Klein, R.; Klein, B.E.; Cruickshanks, K.J. The prevalence of age-related maculopathy by geographic region and ethnicity. Prog. Retin. Eye Res. 1999, 18, 371–389. [Google Scholar] [CrossRef]
- Klein, R.; Klein, B.E.; Knudtson, M.D.; Wong, T.Y.; Cotch, M.F.; Liu, K.; Burke, G.; Saad, M.F.; Jacobs, D.R., Jr. Prevalence of age-related macular degeneration in 4 racial/ethnic groups in the multi-ethnic study of atherosclerosis. Ophthalmology 2006, 113, 373–380. [Google Scholar] [CrossRef]
- Klein, M.L.; Mauldin, W.M.; Stoumbos, V.D. Heredity and age-related macular degeneration. Observations in monozygotic twins. Arch. Ophthalmol. 1994, 112, 932–937. [Google Scholar] [CrossRef]
- Meyers, S.M.; Greene, T.; Gutman, F.A. A twin study of age-related macular degeneration. Am. J. Ophthalmol. 1995, 120, 757–766. [Google Scholar] [CrossRef] [Green Version]
- Seddon, J.M.; Ajani, U.A.; Mitchell, B.D. Familial aggregation of age-related maculopathy. Am. J. Ophthalmol. 1997, 123, 199–206. [Google Scholar] [CrossRef]
- Klein, B.E.; Klein, R.; Lee, K.E.; Moore, E.L.; Danforth, L. Risk of incident age-related eye diseases in people with an affected sibling: The Beaver Dam Eye Study. Am. J. Epidemiol. 2001, 154, 207–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hammond, C.J.; Webster, A.R.; Snieder, H.; Bird, A.C.; Gilbert, C.E.; Spector, T.D. Genetic influence on early age-related maculopathy: A twin study. Ophthalmology 2002, 109, 730–736. [Google Scholar] [CrossRef]
- Bojanowski, C.M.; Tuo, J.; Chew, E.Y.; Csaky, K.G.; Chan, C.C. Analysis of Hemicentin-1, hOgg1, and E-selectin single nucleotide polymorphisms in age-related macular degeneration. Trans. Am. Ophthalmol. Soc. 2005, 103, 37–44; discussion 44–35. [Google Scholar]
- Maguire, M.G.; Ying, G.S.; Jaffe, G.J.; Toth, C.A.; Daniel, E.; Grunwald, J.; Martin, D.F.; Hagstrom, S.A.; Group, C.R. Single-Nucleotide Polymorphisms Associated With Age-Related Macular Degeneration and Lesion Phenotypes in the Comparison of Age-Related Macular Degeneration Treatments Trials. JAMA Ophthalmol. 2016, 134, 674–681. [Google Scholar] [CrossRef] [Green Version]
- Popp, N.A.; Agron, E.; Hageman, G.S.; Tuo, J.; Chew, E.Y.; Chan, C.C. No Sex Differences in the Frequencies of Common Single Nucleotide Polymorphisms Associated with Age-Related Macular Degeneration. Curr. Eye Res. 2017, 42, 470–475. [Google Scholar] [CrossRef]
- Lin, L.Y.; Zhou, Q.; Hagstrom, S.; Maguire, M.G.; Daniel, E.; Grunwald, J.E.; Martin, D.F.; Ying, G.S.; Group, C.R. Association of Single-Nucleotide Polymorphisms in Age-Related Macular Degeneration With Pseudodrusen: Secondary Analysis of Data From the Comparison of AMD Treatments Trials. JAMA Ophthalmol. 2018, 136, 682–688. [Google Scholar] [CrossRef]
- Richardson, A.J.; Islam, F.M.; Guymer, R.H.; Cain, M.; Baird, P.N. A tag-single nucleotide polymorphisms approach to the vascular endothelial growth factor-A gene in age-related macular degeneration. Mol. Vis. 2007, 13, 2148–2152. [Google Scholar]
- Zhou, T.Q.; Guan, H.J.; Hu, J.Y. Genome-wide analysis of single nucleotide polymorphisms in patients with atrophic age-related macular degeneration in oldest old Han Chinese. Genet. Mol. Res. 2015, 14, 17432–17438. [Google Scholar] [CrossRef]
- Shin, H.T.; Yoon, B.W.; Seo, J.H. Comparison of risk allele frequencies of single nucleotide polymorphisms associated with age-related macular degeneration in different ethnic groups. BMC Ophthalmol. 2021, 21, 97. [Google Scholar] [CrossRef]
- Klaver, C.C.; Kliffen, M.; van Duijn, C.M.; Hofman, A.; Cruts, M.; Grobbee, D.E.; van Broeckhoven, C.; de Jong, P.T. Genetic association of apolipoprotein E with age-related macular degeneration. Am. J. Hum. Genet. 1998, 63, 200–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raychaudhuri, S.; Iartchouk, O.; Chin, K.; Tan, P.L.; Tai, A.K.; Ripke, S.; Gowrisankar, S.; Vemuri, S.; Montgomery, K.; Yu, Y.; et al. A rare penetrant mutation in CFH confers high risk of age-related macular degeneration. Nat. Genet. 2011, 43, 1232–1236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Helgason, H.; Sulem, P.; Duvvari, M.R.; Luo, H.; Thorleifsson, G.; Stefansson, H.; Jonsdottir, I.; Masson, G.; Gudbjartsson, D.F.; Walters, G.B.; et al. A rare nonsynonymous sequence variant in C3 is associated with high risk of age-related macular degeneration. Nat. Genet. 2013, 45, 1371–1374. [Google Scholar] [CrossRef] [PubMed]
- Seddon, J.M.; Yu, Y.; Miller, E.C.; Reynolds, R.; Tan, P.L.; Gowrisankar, S.; Goldstein, J.I.; Triebwasser, M.; Anderson, H.E.; Zerbib, J.; et al. Rare variants in CFI, C3 and C9 are associated with high risk of advanced age-related macular degeneration. Nat. Genet. 2013, 45, 1366–1370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhan, X.; Larson, D.E.; Wang, C.; Koboldt, D.C.; Sergeev, Y.V.; Fulton, R.S.; Fulton, L.L.; Fronick, C.C.; Branham, K.E.; Bragg-Gresham, J.; et al. Identification of a rare coding variant in complement 3 associated with age-related macular degeneration. Nat. Genet. 2013, 45, 1375–1379. [Google Scholar] [CrossRef]
- Chen, W.; Stambolian, D.; Edwards, A.O.; Branham, K.E.; Othman, M.; Jakobsdottir, J.; Tosakulwong, N.; Pericak-Vance, M.A.; Campochiaro, P.A.; Klein, M.L.; et al. Genetic variants near TIMP3 and high-density lipoprotein-associated loci influence susceptibility to age-related macular degeneration. Proc. Natl. Acad. Sci. USA 2010, 107, 7401–7406. [Google Scholar] [CrossRef] [Green Version]
- Neale, B.M.; Fagerness, J.; Reynolds, R.; Sobrin, L.; Parker, M.; Raychaudhuri, S.; Tan, P.L.; Oh, E.C.; Merriam, J.E.; Souied, E.; et al. Genome-wide association study of advanced age-related macular degeneration identifies a role of the hepatic lipase gene (LIPC). Proc. Natl. Acad. Sci. USA 2010, 107, 7395–7400. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Bhangale, T.R.; Fagerness, J.; Ripke, S.; Thorleifsson, G.; Tan, P.L.; Souied, E.H.; Richardson, A.J.; Merriam, J.E.; Buitendijk, G.H.; et al. Common variants near FRK/COL10A1 and VEGFA are associated with advanced age-related macular degeneration. Hum. Mol. Genet. 2011, 20, 3699–3709. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Reynolds, R.; Fagerness, J.; Rosner, B.; Daly, M.J.; Seddon, J.M. Association of variants in the LIPC and ABCA1 genes with intermediate and large drusen and advanced age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2011, 52, 4663–4670. [Google Scholar] [CrossRef] [Green Version]
- Fritsche, L.G.; Chen, W.; Schu, M.; Yaspan, B.L.; Yu, Y.; Thorleifsson, G.; Zack, D.J.; Arakawa, S.; Cipriani, V.; Ripke, S.; et al. Seven new loci associated with age-related macular degeneration. Nat. Genet. 2013, 45, 433–439. [Google Scholar] [CrossRef]
- Fritsche, L.G.; Igl, W.; Bailey, J.N.; Grassmann, F.; Sengupta, S.; Bragg-Gresham, J.L.; Burdon, K.P.; Hebbring, S.J.; Wen, C.; Gorski, M.; et al. A large genome-wide association study of age-related macular degeneration highlights contributions of rare and common variants. Nat. Genet. 2016, 48, 134–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, X.; Gharahkhani, P.; Mitchell, P.; Liew, G.; Hewitt, A.W.; MacGregor, S. Genome-wide meta-analysis identifies novel loci associated with age-related macular degeneration. J. Hum. Genet. 2020, 65, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Black, J.R.; Clark, S.J. Age-related macular degeneration: Genome-wide association studies to translation. Genet. Med. 2016, 18, 283–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fritsche, L.G.; Fariss, R.N.; Stambolian, D.; Abecasis, G.R.; Curcio, C.A.; Swaroop, A. Age-related macular degeneration: Genetics and biology coming together. Annu. Rev. Genom. Hum. Genet. 2014, 15, 151–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamashiro, K.; Hosoda, Y.; Miyake, M.; Ooto, S.; Tsujikawa, A. Characteristics of Pachychoroid Diseases and Age-Related Macular Degeneration: Multimodal Imaging and Genetic Backgrounds. J. Clin. Med. 2020, 9, 2034. [Google Scholar] [CrossRef]
- Al-Khersan, H.; Hussain, R.M.; Ciulla, T.A.; Dugel, P.U. Innovative therapies for neovascular age-related macular degeneration. Expert Opin. Pharmacother. 2019, 20, 1879–1891. [Google Scholar] [CrossRef]
- Guimaraes, T.A.C.; Georgiou, M.; Bainbridge, J.W.B.; Michaelides, M. Gene therapy for neovascular age-related macular degeneration: Rationale, clinical trials and future directions. Br. J. Ophthalmol. 2021, 105, 151–157. [Google Scholar] [CrossRef] [Green Version]
- Anderson, D.H.; Radeke, M.J.; Gallo, N.B.; Chapin, E.A.; Johnson, P.T.; Curletti, C.R.; Hancox, L.S.; Hu, J.; Ebright, J.N.; Malek, G.; et al. The pivotal role of the complement system in aging and age-related macular degeneration: Hypothesis re-visited. Prog. Retin. Eye Res. 2010, 29, 95–112. [Google Scholar] [CrossRef] [Green Version]
- Hageman, G.S.; Luthert, P.J.; Victor Chong, N.H.; Johnson, L.V.; Anderson, D.H.; Mullins, R.F. An integrated hypothesis that considers drusen as biomarkers of immune-mediated processes at the RPE-Bruch’s membrane interface in aging and age-related macular degeneration. Prog. Retin. Eye Res. 2001, 20, 705–732. [Google Scholar] [CrossRef]
- Colijn, J.M.; Meester-Smoor, M.; Verzijden, T.; de Breuk, A.; Silva, R.; Merle, B.M.J.; Cougnard-Gregoire, A.; Hoyng, C.B.; Fauser, S.; Coolen, A.; et al. Genetic Risk, Lifestyle, and Age-Related Macular Degeneration in Europe: The EYE-RISK Consortium. Ophthalmology 2021, 128, 1039–1049. [Google Scholar] [CrossRef]
- Janeway, C.A., Jr. How the immune system protects the host from infection. Microbes Infect. 2001, 3, 1167–1171. [Google Scholar] [CrossRef]
- Kilgore, K.S.; Imlay, M.M.; Szaflarski, J.P.; Silverstein, F.S.; Malani, A.N.; Evans, V.M.; Warren, J.S. Neutrophils and reactive oxygen intermediates mediate glucan-induced pulmonary granuloma formation through the local induction of monocyte chemoattractant protein-1. Lab. Investig. 1997, 76, 191–201. [Google Scholar] [PubMed]
- Triantafilou, K.; Hughes, T.R.; Triantafilou, M.; Morgan, B.P. The complement membrane attack complex triggers intracellular Ca2+ fluxes leading to NLRP3 inflammasome activation. J. Cell Sci. 2013, 126, 2903–2913. [Google Scholar] [CrossRef] [Green Version]
- Vlaicu, S.I.; Tegla, C.A.; Cudrici, C.D.; Danoff, J.; Madani, H.; Sugarman, A.; Niculescu, F.; Mircea, P.A.; Rus, V.; Rus, H. Role of C5b-9 complement complex and response gene to complement-32 (RGC-32) in cancer. Immunol. Res. 2013, 56, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Danobeitia, J.S.; Djamali, A.; Fernandez, L.A. The role of complement in the pathogenesis of renal ischemia-reperfusion injury and fibrosis. Fibrogenesis Tissue Repair 2014, 7, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edwards, A.O.; Ritter, R., III; Abel, K.J.; Manning, A.; Panhuysen, C.; Farrer, L.A. Complement factor H polymorphism and age-related macular degeneration. Science 2005, 308, 421–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hageman, G.S.; Anderson, D.H.; Johnson, L.V.; Hancox, L.S.; Taiber, A.J.; Hardisty, L.I.; Hageman, J.L.; Stockman, H.A.; Borchardt, J.D.; Gehrs, K.M.; et al. A common haplotype in the complement regulatory gene factor H (HF1/CFH) predisposes individuals to age-related macular degeneration. Proc. Natl. Acad. Sci. USA 2005, 102, 7227–7232. [Google Scholar] [CrossRef] [Green Version]
- Haines, J.L.; Hauser, M.A.; Schmidt, S.; Scott, W.K.; Olson, L.M.; Gallins, P.; Spencer, K.L.; Kwan, S.Y.; Noureddine, M.; Gilbert, J.R.; et al. Complement factor H variant increases the risk of age-related macular degeneration. Science 2005, 308, 419–421. [Google Scholar] [CrossRef] [Green Version]
- Klein, R.J.; Zeiss, C.; Chew, E.Y.; Tsai, J.Y.; Sackler, R.S.; Haynes, C.; Henning, A.K.; SanGiovanni, J.P.; Mane, S.M.; Mayne, S.T.; et al. Complement factor H polymorphism in age-related macular degeneration. Science 2005, 308, 385–389. [Google Scholar] [CrossRef]
- Zareparsi, S.; Branham, K.E.; Li, M.; Shah, S.; Klein, R.J.; Ott, J.; Hoh, J.; Abecasis, G.R.; Swaroop, A. Strong association of the Y402H variant in complement factor H at 1q32 with susceptibility to age-related macular degeneration. Am. J. Hum. Genet. 2005, 77, 149–153. [Google Scholar] [CrossRef] [Green Version]
- Conley, Y.P.; Thalamuthu, A.; Jakobsdottir, J.; Weeks, D.E.; Mah, T.; Ferrell, R.E.; Gorin, M.B. Candidate gene analysis suggests a role for fatty acid biosynthesis and regulation of the complement system in the etiology of age-related maculopathy. Hum. Mol. Genet. 2005, 14, 1991–2002. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.Y.; Yamashiro, K.; Chen, L.J.; Ahn, J.; Huang, L.; Huang, L.; Cheung, C.M.; Miyake, M.; Cackett, P.D.; Yeo, I.Y.; et al. New loci and coding variants confer risk for age-related macular degeneration in East Asians. Nat. Commun. 2015, 6, 6063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyake, M.; Saito, M.; Yamashiro, K.; Sekiryu, T.; Yoshimura, N. Complement factor H R1210C among Japanese patients with age-related macular degeneration. Jpn J. Ophthalmol. 2015, 59, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P.T.; Betts, K.E.; Radeke, M.J.; Hageman, G.S.; Anderson, D.H.; Johnson, L.V. Individuals homozygous for the age-related macular degeneration risk-conferring variant of complement factor H have elevated levels of CRP in the choroid. Proc. Natl. Acad. Sci. USA 2006, 103, 17456–17461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laine, M.; Jarva, H.; Seitsonen, S.; Haapasalo, K.; Lehtinen, M.J.; Lindeman, N.; Anderson, D.H.; Johnson, P.T.; Jarvela, I.; Jokiranta, T.S.; et al. Y402H polymorphism of complement factor H affects binding affinity to C-reactive protein. J. Immunol. 2007, 178, 3831–3836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Triebwasser, M.P.; Roberson, E.D.; Yu, Y.; Schramm, E.C.; Wagner, E.K.; Raychaudhuri, S.; Seddon, J.M.; Atkinson, J.P. Rare Variants in the Functional Domains of Complement Factor H Are Associated With Age-Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 2015, 56, 6873–6878. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez de Cordoba, S.; Esparza-Gordillo, J.; Goicoechea de Jorge, E.; Lopez-Trascasa, M.; Sanchez-Corral, P. The human complement factor H: Functional roles, genetic variations and disease associations. Mol. Immunol. 2004, 41, 355–367. [Google Scholar] [CrossRef]
- DiScipio, R.G. Formation and structure of the C5b-7 complex of the lytic pathway of complement. J. Biol. Chem. 1992, 267, 17087–17094. [Google Scholar] [CrossRef]
- Sharma, A.K.; Pangburn, M.K. Identification of three physically and functionally distinct binding sites for C3b in human complement factor H by deletion mutagenesis. Proc. Natl. Acad. Sci. USA 1996, 93, 10996–11001. [Google Scholar] [CrossRef] [Green Version]
- Skerka, C.; Lauer, N.; Weinberger, A.A.; Keilhauer, C.N.; Suhnel, J.; Smith, R.; Schlotzer-Schrehardt, U.; Fritsche, L.; Heinen, S.; Hartmann, A.; et al. Defective complement control of factor H (Y402H) and FHL-1 in age-related macular degeneration. Mol. Immunol. 2007, 44, 3398–3406. [Google Scholar] [CrossRef]
- Makou, E.; Herbert, A.P.; Barlow, P.N. Functional anatomy of complement factor H. Biochemistry 2013, 52, 3949–3962. [Google Scholar] [CrossRef] [PubMed]
- Molins, B.; Fuentes-Prior, P.; Adan, A.; Anton, R.; Arostegui, J.I.; Yague, J.; Dick, A.D. Complement factor H binding of monomeric C-reactive protein downregulates proinflammatory activity and is impaired with at risk polymorphic CFH variants. Sci. Rep. 2016, 6, 22889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calippe, B.; Augustin, S.; Beguier, F.; Charles-Messance, H.; Poupel, L.; Conart, J.B.; Hu, S.J.; Lavalette, S.; Fauvet, A.; Rayes, J.; et al. Complement Factor H Inhibits CD47-Mediated Resolution of Inflammation. Immunity 2017, 46, 261–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mulfaul, K.; Mullin, N.K.; Giacalone, J.C.; Voigt, A.P.; DeVore, M.R.; Stone, E.M.; Tucker, B.A.; Mullins, R.F. Local factor H production by human choroidal endothelial cells mitigates complement deposition: Implications for macular degeneration. J. Pathol. 2022, 257, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Clark, S.J.; Perveen, R.; Hakobyan, S.; Morgan, B.P.; Sim, R.B.; Bishop, P.N.; Day, A.J. Impaired binding of the age-related macular degeneration-associated complement factor H 402H allotype to Bruch’s membrane in human retina. J. Biol. Chem. 2010, 285, 30192–30202. [Google Scholar] [CrossRef] [Green Version]
- Borras, C.; Canonica, J.; Jorieux, S.; Abache, T.; El Sanharawi, M.; Klein, C.; Delaunay, K.; Jonet, L.; Salvodelli, M.; Naud, M.C.; et al. CFH exerts anti-oxidant effects on retinal pigment epithelial cells independently from protecting against membrane attack complex. Sci. Rep. 2019, 9, 13873. [Google Scholar] [CrossRef] [Green Version]
- Armento, A.; Honisch, S.; Panagiotakopoulou, V.; Sonntag, I.; Jacob, A.; Bolz, S.; Kilger, E.; Deleidi, M.; Clark, S.; Ueffing, M. Loss of Complement Factor H impairs antioxidant capacity and energy metabolism of human RPE cells. Sci. Rep. 2020, 10, 10320. [Google Scholar] [CrossRef]
- Weismann, D.; Hartvigsen, K.; Lauer, N.; Bennett, K.L.; Scholl, H.P.; Charbel Issa, P.; Cano, M.; Brandstatter, H.; Tsimikas, S.; Skerka, C.; et al. Complement factor H binds malondialdehyde epitopes and protects from oxidative stress. Nature 2011, 478, 76–81. [Google Scholar] [CrossRef] [Green Version]
- Landowski, M.; Kelly, U.; Klingeborn, M.; Groelle, M.; Ding, J.D.; Grigsby, D.; Bowes Rickman, C. Human complement factor H Y402H polymorphism causes an age-related macular degeneration phenotype and lipoprotein dysregulation in mice. Proc. Natl. Acad. Sci. USA 2019, 116, 3703–3711. [Google Scholar] [CrossRef] [Green Version]
- Geerlings, M.J.; Kremlitzka, M.; Bakker, B.; Nilsson, S.C.; Saksens, N.T.; Lechanteur, Y.T.; Pauper, M.; Corominas, J.; Fauser, S.; Hoyng, C.B.; et al. The Functional Effect of Rare Variants in Complement Genes on C3b Degradation in Patients With Age-Related Macular Degeneration. JAMA Ophthalmol. 2017, 135, 39–46. [Google Scholar] [CrossRef]
- Maller, J.; George, S.; Purcell, S.; Fagerness, J.; Altshuler, D.; Daly, M.J.; Seddon, J.M. Common variation in three genes, including a noncoding variant in CFH, strongly influences risk of age-related macular degeneration. Nat. Genet. 2006, 38, 1055–1059. [Google Scholar] [CrossRef] [PubMed]
- Maugeri, A.; Barchitta, M.; Mazzone, M.G.; Giuliano, F.; Agodi, A. Complement System and Age-Related Macular Degeneration: Implications of Gene-Environment Interaction for Preventive and Personalized Medicine. Biomed. Res. Int. 2018, 2018, 7532507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajendran, A.; Dhoble, P.; Sundaresan, P.; Saravanan, V.; Vashist, P.; Nitsch, D.; Smeeth, L.; Chakravarthy, U.; Ravindran, R.D.; Fletcher, A.E. Genetic risk factors for late age-related macular degeneration in India. Br. J. Ophthalmol. 2018, 102, 1213–1217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Connolly, E.; Rhatigan, M.; O’Halloran, A.M.; Muldrew, K.A.; Chakravarthy, U.; Cahill, M.; Kenny, R.A.; Doyle, S.L. Prevalence of age-related macular degeneration associated genetic risk factors and 4-year progression data in the Irish population. Br. J. Ophthalmol. 2018, 102, 1691–1695. [Google Scholar] [CrossRef]
- Supanji, S.; Romdhoniyyah, D.F.; Sasongko, M.B.; Agni, A.N.; Wardhana, F.S.; Widayanti, T.W.; Prayogo, M.E.; Perdamaian, A.B.I.; Dianratri, A.; Kawaichi, M.; et al. Associations of ARMS2 and CFH Gene Polymorphisms with Neovascular Age-Related Macular Degeneration. Clin. Ophthalmol. 2021, 15, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Matuskova, V.; Zeman, T.; Ewerlingova, L.; Hlinomazova, Z.; Soucek, J.; Vlkova, E.; Goswami, N.; Balcar, V.J.; Sery, O. An association of neovascular age-related macular degeneration with polymorphisms of CFH, ARMS2, HTRA1 and C3 genes in Czech population. Acta Ophthalmol. 2020, 98, e691–e699. [Google Scholar] [CrossRef] [PubMed]
- Gili, P.; Lloreda Martin, L.; Martin-Rodrigo, J.C.; Kim-Yeon, N.; Modamio-Gardeta, L.; Fernandez-Garcia, J.L.; Rebolledo-Poves, A.B.; Gomez-Blazquez, E.; Pazos-Rodriguez, R.; Perez-Fernandez, E.; et al. Gene polymorphisms associated with an increased risk of exudative age-related macular degeneration in a Spanish population. Eur. J. Ophthalmol. 2022, 32, 651–657. [Google Scholar] [CrossRef]
- Gourgouli, K.; Gourgouli, I.; Tsaousis, G.; Spai, S.; Niskopoulou, M.; Efthimiopoulos, S.; Lamnissou, K. Investigation of genetic base in the treatment of age-related macular degeneration. Int. Ophthalmol. 2020, 40, 985–997. [Google Scholar] [CrossRef]
- Neto, J.M.; Viturino, M.G.; Ananina, G.; Bajano, F.F.; Costa, S.; Roque, A.B.; Borges, G.F.; Franchi, R.; Rim, P.H.; Medina, F.M.; et al. Association of genetic variants rs641153 (CFB), rs2230199 (C3), and rs1410996 (CFH) with age-related macular degeneration in a Brazilian population. Exp. Biol. Med. 2021, 246, 2290–2296. [Google Scholar] [CrossRef]
- Thakkinstian, A.; Bowe, S.; McEvoy, M.; Smith, W.; Attia, J. Association between apolipoprotein E polymorphisms and age-related macular degeneration: A HuGE review and meta-analysis. Am. J. Epidemiol. 2006, 164, 813–822. [Google Scholar] [CrossRef]
- Lores-Motta, L.; Paun, C.C.; Corominas, J.; Pauper, M.; Geerlings, M.J.; Altay, L.; Schick, T.; Daha, M.R.; Fauser, S.; Hoyng, C.B.; et al. Genome-Wide Association Study Reveals Variants in CFH and CFHR4 Associated with Systemic Complement Activation: Implications in Age-Related Macular Degeneration. Ophthalmology 2018, 125, 1064–1074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Zou, M.; Chen, A.; Liu, Z.; Young, C.A.; Wang, S.B.; Zheng, D.; Jin, G. Genetic associations of anti-vascular endothelial growth factor therapy response in age-related macular degeneration: A systematic review and meta-analysis. Acta Ophthalmol. 2022, 100, e669–e680. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Triebwasser, M.P.; Wong, E.K.; Schramm, E.C.; Thomas, B.; Reynolds, R.; Mardis, E.R.; Atkinson, J.P.; Daly, M.; Raychaudhuri, S.; et al. Whole-exome sequencing identifies rare, functional CFH variants in families with macular degeneration. Hum. Mol. Genet. 2014, 23, 5283–5293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Association Between Complement Factor C2/C3/CFB/CFH Polymorphisms and Age-Related Macular Degeneration: A Meta-Analysis. Genet. Test. Mol. Biomark. 2018, 22, 526–540. [CrossRef] [PubMed]
- Supanji, S.; Perdamaian, A.B.I.; Anindita, D.A.; Widayanti, T.W.; Wardhana, F.S.; Sasongko, M.B.; Prayogo, M.E.; Agni, A.N.; Oka, C. rs3753394 Complement Factor H (CFH) Gene Polymorphism in Patients with Age-Related Macular Degeneration (AMD) in Indonesian Population. BIO Web Conf. 2021, 41, 06001. [Google Scholar] [CrossRef]
- Duvvari, M.R.; Saksens, N.T.; van de Ven, J.P.; de Jong-Hesse, Y.; Schick, T.; Nillesen, W.M.; Fauser, S.; Hoefsloot, L.H.; Hoyng, C.B.; de Jong, E.K.; et al. Analysis of rare variants in the CFH gene in patients with the cuticular drusen subtype of age-related macular degeneration. Mol. Vis. 2015, 21, 285–292. [Google Scholar] [PubMed]
- Ferrara, D.; Seddon, J.M. Phenotypic Characterization of Complement Factor H R1210C Rare Genetic Variant in Age-Related Macular Degeneration. JAMA Ophthalmol. 2015, 133, 785–791. [Google Scholar] [CrossRef] [Green Version]
- Kersten, E.; Paun, C.C.; Schellevis, R.L.; Hoyng, C.B.; Delcourt, C.; Lengyel, I.; Peto, T.; Ueffing, M.; Klaver, C.C.W.; Dammeier, S.; et al. Systemic and ocular fluid compounds as potential biomarkers in age-related macular degeneration. Surv. Ophthalmol. 2018, 63, 9–39. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Wagner, E.K.; Souied, E.H.; Seitsonen, S.; Immonen, I.J.; Happola, P.; Raychaudhuri, S.; Daly, M.J.; Seddon, J.M. Protective coding variants in CFH and PELI3 and a variant near CTRB1 are associated with age-related macular degenerationdagger. Hum. Mol. Genet. 2016, 25, 5276–5285. [Google Scholar] [CrossRef] [Green Version]
- De Breuk, A.; Lechanteur, Y.T.E.; Heesterbeek, T.J.; Fauser, S.; Klaver, C.C.W.; Hoyng, C.B.; den Hollander, A.I. Genetic Risk in Families with Age-Related Macular Degeneration. Ophthalmol. Sci. 2021, 1, 100087. [Google Scholar] [CrossRef]
- Klein, M.L.; Francis, P.J.; Rosner, B.; Reynolds, R.; Hamon, S.C.; Schultz, D.W.; Ott, J.; Seddon, J.M. CFH and LOC387715/ARMS2 genotypes and treatment with antioxidants and zinc for age-related macular degeneration. Ophthalmology 2008, 115, 1019–1025. [Google Scholar] [CrossRef] [PubMed]
- Awh, C.C.; Hawken, S.; Zanke, B.W. Treatment response to antioxidants and zinc based on CFH and ARMS2 genetic risk allele number in the Age-Related Eye Disease Study. Ophthalmology 2015, 122, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Seddon, J.M.; Silver, R.E.; Rosner, B. Response to AREDS supplements according to genetic factors: Survival analysis approach using the eye as the unit of analysis. Br. J. Ophthalmol. 2016, 100, 1731–1737. [Google Scholar] [CrossRef] [PubMed]
- Seddon, J.M.; Reynolds, R.; Rosner, B. Peripheral retinal drusen and reticular pigment: Association with CFHY402H and CFHrs1410996 genotypes in family and twin studies. Investig. Ophthalmol. Vis. Sci. 2009, 50, 586–591. [Google Scholar] [CrossRef] [Green Version]
- Geerlings, M.J.; Kersten, E.; Groenewoud, J.M.M.; Fritsche, L.G.; Hoyng, C.B.; de Jong, E.K.; den Hollander, A.I. Geographic distribution of rare variants associated with age-related macular degeneration. Mol. Vis. 2018, 24, 75–82. [Google Scholar]
- Fagerness, J.A.; Maller, J.B.; Neale, B.M.; Reynolds, R.C.; Daly, M.J.; Seddon, J.M. Variation near complement factor I is associated with risk of advanced AMD. Eur. J. Hum. Genet. 2009, 17, 100–104. [Google Scholar] [CrossRef]
- Van de Ven, J.P.; Nilsson, S.C.; Tan, P.L.; Buitendijk, G.H.; Ristau, T.; Mohlin, F.C.; Nabuurs, S.B.; Schoenmaker-Koller, F.E.; Smailhodzic, D.; Campochiaro, P.A.; et al. A functional variant in the CFI gene confers a high risk of age-related macular degeneration. Nat. Genet. 2013, 45, 813–817. [Google Scholar] [CrossRef]
- Hallam, T.M.; Marchbank, K.J.; Harris, C.L.; Osmond, C.; Shuttleworth, V.G.; Griffiths, H.; Cree, A.J.; Kavanagh, D.; Lotery, A.J. Rare Genetic Variants in Complement Factor I Lead to Low FI Plasma Levels Resulting in Increased Risk of Age-Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 2020, 61, 18. [Google Scholar] [CrossRef]
- Java, A.; Baciu, P.; Widjajahakim, R.; Sung, Y.J.; Yang, J.; Kavanagh, D.; Atkinson, J.; Seddon, J. Functional Analysis of Rare Genetic Variants in Complement Factor I (CFI) using a Serum-Based Assay in Advanced Age-related Macular Degeneration. Transl. Vis. Sci. Technol. 2020, 9, 37. [Google Scholar] [CrossRef]
- De Jong, S.; Volokhina, E.B.; de Breuk, A.; Nilsson, S.C.; de Jong, E.K.; van der Kar, N.; Bakker, B.; Hoyng, C.B.; van den Heuvel, L.P.; Blom, A.M.; et al. Effect of rare coding variants in the CFI gene on Factor I expression levels. Hum. Mol. Genet. 2020, 29, 2313–2324. [Google Scholar] [CrossRef]
- Geerlings, M.J.; de Jong, E.K.; den Hollander, A.I. The complement system in age-related macular degeneration: A review of rare genetic variants and implications for personalized treatment. Mol. Immunol. 2017, 84, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Ellis, S.; Buchberger, A.; Holder, J.; Orhan, e.; Hughes, J. GT005, a gene therapy for the treatment of dry age-related macular degeneration (AMD). Investig. Ophthalmol. Vis. Sci. 2020, 61, 2295. [Google Scholar]
- Maller, J.B.; Fagerness, J.A.; Reynolds, R.C.; Neale, B.M.; Daly, M.J.; Seddon, J.M. Variation in complement factor 3 is associated with risk of age-related macular degeneration. Nat. Genet. 2007, 39, 1200–1201. [Google Scholar] [CrossRef]
- Yates, J.R.; Sepp, T.; Matharu, B.K.; Khan, J.C.; Thurlby, D.A.; Shahid, H.; Clayton, D.G.; Hayward, C.; Morgan, J.; Wright, A.F.; et al. Complement C3 variant and the risk of age-related macular degeneration. N. Engl. J. Med. 2007, 357, 553–561. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Li, S.; Hu, S.; Yu, J.; Xiang, Y. Association between genetic variation of complement C3 and the susceptibility to advanced age-related macular degeneration: A meta-analysis. BMC Ophthalmol. 2018, 18, 274. [Google Scholar] [CrossRef] [PubMed]
- Heurich, M.; Martinez-Barricarte, R.; Francis, N.J.; Roberts, D.L.; Rodriguez de Cordoba, S.; Morgan, B.P.; Harris, C.L. Common polymorphisms in C3, factor B, and factor H collaborate to determine systemic complement activity and disease risk. Proc. Natl. Acad. Sci. USA 2011, 108, 8761–8766. [Google Scholar] [CrossRef] [Green Version]
- Duvvari, M.R.; Paun, C.C.; Buitendijk, G.H.; Saksens, N.T.; Volokhina, E.B.; Ristau, T.; Schoenmaker-Koller, F.E.; van de Ven, J.P.; Groenewoud, J.M.; van den Heuvel, L.P.; et al. Analysis of rare variants in the C3 gene in patients with age-related macular degeneration. PLoS ONE 2014, 9, e94165. [Google Scholar] [CrossRef] [Green Version]
- Pei, X.T.; Li, X.X.; Bao, Y.Z.; Yu, W.Z.; Yan, Z.; Qi, H.J.; Qian, T.; Xiao, H.X. Association of c3 gene polymorphisms with neovascular age-related macular degeneration in a chinese population. Curr. Eye Res. 2009, 34, 615–622. [Google Scholar] [CrossRef]
- Liao, D.S.; Grossi, F.V.; El Mehdi, D.; Gerber, M.R.; Brown, D.M.; Heier, J.S.; Wykoff, C.C.; Singerman, L.J.; Abraham, P.; Grassmann, F.; et al. Complement C3 Inhibitor Pegcetacoplan for Geographic Atrophy Secondary to Age-Related Macular Degeneration: A Randomized Phase 2 Trial. Ophthalmology 2020, 127, 186–195. [Google Scholar] [CrossRef] [Green Version]
- Anderson, D.H.; Mullins, R.F.; Hageman, G.S.; Johnson, L.V. A role for local inflammation in the formation of drusen in the aging eye. Am. J. Ophthalmol. 2002, 134, 411–431. [Google Scholar] [CrossRef]
- Johnson, L.V.; Ozaki, S.; Staples, M.K.; Erickson, P.A.; Anderson, D.H. A potential role for immune complex pathogenesis in drusen formation. Exp. Eye Res. 2000, 70, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Scholl, H.P.; Charbel Issa, P.; Walier, M.; Janzer, S.; Pollok-Kopp, B.; Borncke, F.; Fritsche, L.G.; Chong, N.V.; Fimmers, R.; Wienker, T.; et al. Systemic complement activation in age-related macular degeneration. PLoS ONE 2008, 3, e2593. [Google Scholar] [CrossRef]
- Nozaki, M.; Raisler, B.J.; Sakurai, E.; Sarma, J.V.; Barnum, S.R.; Lambris, J.D.; Chen, Y.; Zhang, K.; Ambati, B.K.; Baffi, J.Z.; et al. Drusen complement components C3a and C5a promote choroidal neovascularization. Proc. Natl. Acad. Sci. USA 2006, 103, 2328–2333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baas, D.C.; Ho, L.; Ennis, S.; Merriam, J.E.; Tanck, M.W.; Uitterlinden, A.G.; de Jong, P.T.; Cree, A.J.; Griffiths, H.L.; Rivadeneira, F.; et al. The complement component 5 gene and age-related macular degeneration. Ophthalmology 2010, 117, 500–511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, K.; Ma, L.; Lai, T.Y.Y.; Brelen, M.E.; Tam, P.O.S.; Tham, C.C.; Pang, C.P.; Chen, L.J. Evaluation of the association of C5 with neovascular age-related macular degeneration and polypoidal choroidal vasculopathy. Eye Vis. 2019, 6, 34. [Google Scholar] [CrossRef]
- Jaffe, G.J.; Westby, K.; Csaky, K.G.; Mones, J.; Pearlman, J.A.; Patel, S.S.; Joondeph, B.C.; Randolph, J.; Masonson, H.; Rezaei, K.A. C5 Inhibitor Avacincaptad Pegol for Geographic Atrophy Due to Age-Related Macular Degeneration: A Randomized Pivotal Phase 2/3 Trial. Ophthalmology 2021, 128, 576–586. [Google Scholar] [CrossRef]
- Saksens, N.T.; Geerlings, M.J.; Bakker, B.; Schick, T.; Daha, M.R.; Fauser, S.; Boon, C.J.; de Jong, E.K.; Hoyng, C.B.; den Hollander, A.I. Rare Genetic Variants Associated With Development of Age-Related Macular Degeneration. JAMA Ophthalmol. 2016, 134, 287–293. [Google Scholar] [CrossRef]
- Ratnapriya, R.; Acar, I.E.; Geerlings, M.J.; Branham, K.; Kwong, A.; Saksens, N.T.M.; Pauper, M.; Corominas, J.; Kwicklis, M.; Zipprer, D.; et al. Family-based exome sequencing identifies rare coding variants in age-related macular degeneration. Hum Mol. Genet. 2020, 29, 2022–2034. [Google Scholar] [CrossRef]
- McMahon, O.; Hallam, T.M.; Patel, S.; Harris, C.L.; Menny, A.; Zelek, W.M.; Widjajahakim, R.; Java, A.; Cox, T.E.; Tzoumas, N.; et al. The rare C9 P167S risk variant for age-related macular degeneration increases polymerization of the terminal component of the complement cascade. Hum. Mol. Genet. 2021, 30, 1188–1199. [Google Scholar] [CrossRef]
- Kremlitzka, M.; Geerlings, M.J.; de Jong, S.; Bakker, B.; Nilsson, S.C.; Fauser, S.; Hoyng, C.B.; de Jong, E.K.; den Hollander, A.I.; Blom, A.M. Functional analyses of rare genetic variants in complement component C9 identified in patients with age-related macular degeneration. Hum. Mol. Genet. 2018, 27, 2678–2688. [Google Scholar] [CrossRef]
- Horiuchi, T.; Nishizaka, H.; Kojima, T.; Sawabe, T.; Niho, Y.; Schneider, P.M.; Inaba, S.; Sakai, K.; Hayashi, K.; Hashimura, C.; et al. A non-sense mutation at Arg95 is predominant in complement 9 deficiency in Japanese. J. Immunol. 1998, 160, 1509–1513. [Google Scholar] [PubMed]
- Nishiguchi, K.M.; Yasuma, T.R.; Tomida, D.; Nakamura, M.; Ishikawa, K.; Kikuchi, M.; Ohmi, Y.; Niwa, T.; Hamajima, N.; Furukawa, K.; et al. C9-R95X polymorphism in patients with neovascular age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2012, 53, 508–512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gold, B.; Merriam, J.E.; Zernant, J.; Hancox, L.S.; Taiber, A.J.; Gehrs, K.; Cramer, K.; Neel, J.; Bergeron, J.; Barile, G.R.; et al. Variation in factor B (BF) and complement component 2 (C2) genes is associated with age-related macular degeneration. Nat. Genet. 2006, 38, 458–462. [Google Scholar] [CrossRef] [Green Version]
- Mantel, I.; Ambresin, A.; Moetteli, L.; Droz, I.; Roduit, R.; Munier, F.L.; Schorderet, D.F. Complement factor B polymorphism and the phenotype of early age-related macular degeneration. Ophthalmic. Genet. 2014, 35, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Spencer, K.L.; Hauser, M.A.; Olson, L.M.; Schmidt, S.; Scott, W.K.; Gallins, P.; Agarwal, A.; Postel, E.A.; Pericak-Vance, M.A.; Haines, J.L. Protective effect of complement factor B and complement component 2 variants in age-related macular degeneration. Hum. Mol. Genet. 2007, 16, 1986–1992. [Google Scholar] [CrossRef]
- Sun, C.; Zhao, M.; Li, X. CFB/C2 gene polymorphisms and risk of age-related macular degeneration: A systematic review and meta-analysis. Curr. Eye Res. 2012, 37, 259–271. [Google Scholar] [CrossRef]
- Thakkinstian, A.; McEvoy, M.; Chakravarthy, U.; Chakrabarti, S.; McKay, G.J.; Ryu, E.; Silvestri, G.; Kaur, I.; Francis, P.; Iwata, T.; et al. The association between complement component 2/complement factor B polymorphisms and age-related macular degeneration: A HuGE review and meta-analysis. Am. J. Epidemiol. 2012, 176, 361–372. [Google Scholar] [CrossRef]
- Gehrs, K.M.; Jackson, J.R.; Brown, E.N.; Allikmets, R.; Hageman, G.S. Complement, age-related macular degeneration and a vision of the future. Arch. Ophthalmol. 2010, 128, 349–358. [Google Scholar] [CrossRef] [Green Version]
- Lokki, M.L.; Koskimies, S.A. Allelic differences in hemolytic activity and protein concentration of BF molecules are found in association with particular HLA haplotypes. Immunogenetics 1991, 34, 242–246. [Google Scholar] [CrossRef]
- Yoneyama, S.; Sakurada, Y.; Kikushima, W.; Sugiyama, A.; Matsubara, M.; Fukuda, Y.; Tanabe, N.; Parikh, R.; Mabuchi, F.; Kashiwagi, K.; et al. Genetic factors associated with response to as-needed aflibercept therapy for typical neovascular age-related macular degeneration and polypoidal choroidal vasculopathy. Sci. Rep. 2020, 10, 7188. [Google Scholar] [CrossRef]
- Nakata, I.; Yamashiro, K.; Yamada, R.; Gotoh, N.; Nakanishi, H.; Hayashi, H.; Akagi-Kurashige, Y.; Tsujikawa, A.; Otani, A.; Saito, M.; et al. Significance of C2/CFB variants in age-related macular degeneration and polypoidal choroidal vasculopathy in a Japanese population. Investig. Ophthalmol. Vis. Sci. 2012, 53, 794–798. [Google Scholar] [CrossRef] [Green Version]
- Forneris, F.; Ricklin, D.; Wu, J.; Tzekou, A.; Wallace, R.S.; Lambris, J.D.; Gros, P. Structures of C3b in complex with factors B and D give insight into complement convertase formation. Science 2010, 330, 1816–1820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stanton, C.M.; Yates, J.R.; den Hollander, A.I.; Seddon, J.M.; Swaroop, A.; Stambolian, D.; Fauser, S.; Hoyng, C.; Yu, Y.; Atsuhiro, K.; et al. Complement factor D in age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2011, 52, 8828–8834. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Chen, Y.; Tong, Z.; Zhou, X.; Zhao, C.; Wang, K.; Hughes, G.; Kasuga, D.; Bedell, M.; Lee, C.; et al. Lack of association of CFD polymorphisms with advanced age-related macular degeneration. Mol. Vis. 2010, 16, 2273–2278. [Google Scholar]
- Holz, F.G.; Sadda, S.R.; Busbee, B.; Chew, E.Y.; Mitchell, P.; Tufail, A.; Brittain, C.; Ferrara, D.; Gray, S.; Honigberg, L.; et al. Efficacy and Safety of Lampalizumab for Geographic Atrophy Due to Age-Related Macular Degeneration: Chroma and Spectri Phase 3 Randomized Clinical Trials. JAMA Ophthalmol. 2018, 136, 666–677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hogan, M.J. Ultrastructure of the choroid. Its role in the pathogenesis of chorioretinal disease. Trans. Pac. Coast Otoophthalmol. Soc. Annu. Meet 1961, 42, 61–87. [Google Scholar] [PubMed]
- Booij, J.C.; Baas, D.C.; Beisekeeva, J.; Gorgels, T.G.; Bergen, A.A. The dynamic nature of Bruch’s membrane. Prog. Retin. Eye Res. 2010, 29, 1–18. [Google Scholar] [CrossRef]
- Massova, I.; Kotra, L.P.; Fridman, R.; Mobashery, S. Matrix metalloproteinases: Structures, evolution, and diversification. FASEB J. 1998, 12, 1075–1095. [Google Scholar] [CrossRef] [Green Version]
- Hussain, A.A.; Lee, Y.; Zhang, J.J.; Marshall, J. Disturbed matrix metalloproteinase activity of Bruch’s membrane in age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2011, 52, 4459–4466. [Google Scholar] [CrossRef]
- Garcia-Onrubia, L.; Valentin-Bravo, F.J.; Coco-Martin, R.M.; Gonzalez-Sarmiento, R.; Pastor, J.C.; Usategui-Martin, R.; Pastor-Idoate, S. Matrix Metalloproteinases in Age-Related Macular Degeneration (AMD). Int. J. Mol. Sci. 2020, 21, 5934. [Google Scholar] [CrossRef]
- Guo, L.; Hussain, A.A.; Limb, G.A.; Marshall, J. Age-dependent variation in metalloproteinase activity of isolated human Bruch’s membrane and choroid. Investig. Ophthalmol. Vis. Sci. 1999, 40, 2676–2682. [Google Scholar]
- Kamei, M.; Hollyfield, J.G. TIMP-3 in Bruch’s membrane: Changes during aging and in age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 1999, 40, 2367–2375. [Google Scholar]
- Krogh Nielsen, M.; Subhi, Y.; Rue Molbech, C.; Nilsson, L.L.; Nissen, M.H.; Sorensen, T.L. Imbalances in tissue inhibitors of metalloproteinases differentiate choroidal neovascularization from geographic atrophy. Acta Ophthalmol. 2019, 97, 84–90. [Google Scholar] [CrossRef] [Green Version]
- Abecasis, G.R.; Yashar, B.M.; Zhao, Y.; Ghiasvand, N.M.; Zareparsi, S.; Branham, K.E.; Reddick, A.C.; Trager, E.H.; Yoshida, S.; Bahling, J.; et al. Age-related macular degeneration: A high-resolution genome scan for susceptibility loci in a population enriched for late-stage disease. Am. J. Hum. Genet. 2004, 74, 482–494. [Google Scholar] [CrossRef] [Green Version]
- Ardeljan, D.; Chan, C.C. Aging is not a disease: Distinguishing age-related macular degeneration from aging. Prog. Retin. Eye Res. 2013, 37, 68–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Della, N.G.; Campochiaro, P.A.; Zack, D.J. Localization of TIMP-3 mRNA expression to the retinal pigment epithelium. Investig. Ophthalmol. Vis. Sci. 1996, 37, 1921–1924. [Google Scholar]
- Yu, W.H.; Yu, S.; Meng, Q.; Brew, K.; Woessner, J.F., Jr. TIMP-3 binds to sulfated glycosaminoglycans of the extracellular matrix. J. Biol. Chem. 2000, 275, 31226–31232. [Google Scholar] [CrossRef] [Green Version]
- Fariss, R.N.; Apte, S.S.; Olsen, B.R.; Iwata, K.; Milam, A.H. Tissue inhibitor of metalloproteinases-3 is a component of Bruch’s membrane of the eye. Am. J. Pathol. 1997, 150, 323–328. [Google Scholar]
- Arpino, V.; Brock, M.; Gill, S.E. The role of TIMPs in regulation of extracellular matrix proteolysis. Matrix Biol. 2015, 44–46, 247–254. [Google Scholar] [CrossRef]
- Bailey, T.A.; Alexander, R.A.; Dubovy, S.R.; Luthert, P.J.; Chong, N.H. Measurement of TIMP-3 expression and Bruch’s membrane thickness in human macula. Exp. Eye Res. 2001, 73, 851–858. [Google Scholar] [CrossRef]
- Qi, J.H.; Ebrahem, Q.; Moore, N.; Murphy, G.; Claesson-Welsh, L.; Bond, M.; Baker, A.; Anand-Apte, B. A novel function for tissue inhibitor of metalloproteinases-3 (TIMP3): Inhibition of angiogenesis by blockage of VEGF binding to VEGF receptor-2. Nat. Med. 2003, 9, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Weber, B.H.; Vogt, G.; Wolz, W.; Ives, E.J.; Ewing, C.C. Sorsby’s fundus dystrophy is genetically linked to chromosome 22q13-qter. Nat. Genet. 1994, 7, 158–161. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Cheng, T.; Qu, Y. TIMP-3 suppression induces choroidal neovascularization by moderating the polarization of macrophages in age-related macular degeneration. Mol. Immunol. 2019, 106, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Kauer, W.K.; Stein, H.J. Emerging concepts of bile reflux in the constellation of gastroesophageal reflux disease. J. Gastrointest. Surg. 2010, 14, S9–S16. [Google Scholar] [CrossRef]
- Cascella, R.; Strafella, C.; Longo, G.; Ragazzo, M.; Manzo, L.; De Felici, C.; Errichiello, V.; Caputo, V.; Viola, F.; Eandi, C.M.; et al. Uncovering genetic and non-genetic biomarkers specific for exudative age-related macular degeneration: Significant association of twelve variants. Oncotarget 2018, 9, 7812–7821. [Google Scholar] [CrossRef] [Green Version]
- Fauser, S.; Smailhodzic, D.; Caramoy, A.; van de Ven, J.P.; Kirchhof, B.; Hoyng, C.B.; Klevering, B.J.; Liakopoulos, S.; den Hollander, A.I. Evaluation of serum lipid concentrations and genetic variants at high-density lipoprotein metabolism loci and TIMP3 in age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2011, 52, 5525–5528. [Google Scholar] [CrossRef] [Green Version]
- Tuo, J.; Grob, S.; Zhang, K.; Chan, C.C. Genetics of immunological and inflammatory components in age-related macular degeneration. Ocul. Immunol. Inflamm. 2012, 20, 27–36. [Google Scholar] [CrossRef] [Green Version]
- Zeng, R.; Wen, F.; Zhang, X.; Zuo, C.; Li, M.; Chen, H.; Wu, K. An rs9621532 variant near the TIMP3 gene is not associated with neovascular age-related macular degeneration and polypoidal choroidal vasculopathy in a Chinese Han population. Ophthalmic Genet. 2012, 33, 139–143. [Google Scholar] [CrossRef]
- Liutkeviciene, R.; Vilkeviciute, A.; Gedvilaite, G.; Kaikaryte, K.; Kriauciuniene, L. Haplotypes of HTRA1 rs1120638, TIMP3 rs9621532, VEGFA rs833068, CFI rs10033900, ERCC6 rs3793784, and KCTD10 rs56209061 Gene Polymorphisms in Age-Related Macular Degeneration. Dis. Markers 2019, 2019, 9602949. [Google Scholar] [CrossRef] [Green Version]
- Ardeljan, D.; Meyerle, C.B.; Agron, E.; Wang, J.J.; Mitchell, P.; Chew, E.Y.; Zhao, J.; Maminishkis, A.; Chan, C.C.; Tuo, J. Influence of TIMP3/SYN3 polymorphisms on the phenotypic presentation of age-related macular degeneration. Eur. J. Hum. Genet. 2013, 21, 1152–1157. [Google Scholar] [CrossRef]
- Ortak, H.; Demir, S.; Ates, O.; Benli, I.; Sogut, E.; Sahin, M. The role of MMP2 (-1306C>T) and TIMP2 (-418 G>C) promoter variants in age-related macular degeneration. Ophthalmic Genet 2013, 34, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Oszajca, K.; Szemraj, M.; Szemraj, J.; Jurowski, P. Association analysis of genetic polymorphisms and expression levels of selected genes involved in extracellular matrix turnover and angiogenesis with the risk of age-related macular degeneration. Ophthalmic Genet. 2018, 39, 684–698. [Google Scholar] [CrossRef] [PubMed]
- Liutkeviciene, R.; Lesauskaite, V.; Sinkunaite-Marsalkiene, G.; Simonyte, S.; Zemaitiene, R.; Kriauciuniene, L.; Zaliuniene, D. MMP-2 Rs24386 (C-->T) gene polymorphism and the phenotype of age-related macular degeneration. Int. J. Ophthalmol. 2017, 10, 1349–1353. [Google Scholar] [CrossRef] [PubMed]
- Liutkeviciene, R.; Lesauskaite, V.; Sinkunaite-Marsalkiene, G.; Zaliuniene, D.; Zaliaduonyte-Peksiene, D.; Mizariene, V.; Gustiene, O.; Jasinskas, V.; Jariene, G.; Tamosiunas, A. The Role of Matrix Metalloproteinases Polymorphisms in Age-Related Macular Degeneration. Ophthalmic Genet. 2015, 36, 149–155. [Google Scholar] [CrossRef]
- Liutkeviciene, R.; Vilkeviciute, A.; Borisovaite, D.; Miniauskiene, G. Association of exudative age-related macular degeneration with matrix metalloproteinases-2 (-1306 C/T) rs243865 gene polymorphism. Indian J. Ophthalmol. 2018, 66, 551–557. [Google Scholar] [CrossRef]
- Seitzman, R.L.; Mahajan, V.B.; Mangione, C.; Cauley, J.A.; Ensrud, K.E.; Stone, K.L.; Cummings, S.R.; Hochberg, M.C.; Hillier, T.A.; Sinsheimer, J.S.; et al. Estrogen receptor α and matrix metalloproteinase 2 polymorphisms and age-related maculopathy in older women. Am. J. Epidemiol. 2008, 167, 1217–1225. [Google Scholar] [CrossRef] [Green Version]
- Usategui-Martin, R.; Pastor-Idoate, S.; Chamorro, A.J.; Fernandez, I.; Fernandez-Bueno, I.; Marcos-Martin, M.; Gonzalez-Sarmiento, R.; Carlos Pastor, J. Meta-analysis of the rs243865 MMP-2 polymorphism and age-related macular degeneration risk. PLoS ONE 2019, 14, e0213624. [Google Scholar] [CrossRef] [Green Version]
- Cheng, J.; Hao, X.; Zhang, Z. Risk of macular degeneration affected by polymorphisms in Matrix metalloproteinase-2: A case-control study in Chinese Han population. Medicine 2017, 96, e8190. [Google Scholar] [CrossRef]
- Yan, Q.; Ding, Y.; Liu, Y.; Sun, T.; Fritsche, L.G.; Clemons, T.; Ratnapriya, R.; Klein, M.L.; Cook, R.J.; Liu, Y.; et al. Genome-wide analysis of disease progression in age-related macular degeneration. Hum. Mol. Genet. 2018, 27, 929–940. [Google Scholar] [CrossRef] [Green Version]
- Sohn, E.H.; Han, I.C.; Roos, B.R.; Faga, B.; Luse, M.A.; Binkley, E.M.; Boldt, H.C.; Folk, J.C.; Russell, S.R.; Mullins, R.F.; et al. Genetic Association between MMP9 and Choroidal Neovascularization in Age-Related Macular Degeneration. Ophthalmol. Sci. 2021, 1, 100002. [Google Scholar] [CrossRef]
- Fiotti, N.; Pedio, M.; Battaglia Parodi, M.; Altamura, N.; Uxa, L.; Guarnieri, G.; Giansante, C.; Ravalico, G. MMP-9 microsatellite polymorphism and susceptibility to exudative form of age-related macular degeneration. Genet. Med. 2005, 7, 272–277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Budiene, B.; Liutkeviciene, R.; Gustiene, O.; Ugenskiene, R.; Laukaitiene, D.; Savukaityte, A.; Vilkeviciute, A.; Steponaviciute, R.; Rocyte, A.; Zaliuniene, D. The association of matrix metalloproteinases polymorphisms and interleukins in advanced age-related macular degeneration. Ophthalmic Genet. 2018, 39, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Liutkeviciene, R.; Zaliaduonyte-Peksiene, D.; Zaliuniene, D.; Gustiene, O.; Jasinskas, V.; Lesauskaite, V.; Tamosiunas, A.; Zaliunas, R. Does matrix metalloproteinase-3 polymorphism play a role in age-related macular degeneration in patients with myocardial infarction? Medicina 2012, 48, 404–409. [Google Scholar] [CrossRef] [PubMed]
- Tamura, Y.; Konomi, H.; Sawada, H.; Takashima, S.; Nakajima, A. Tissue distribution of type VIII collagen in human adult and fetal eyes. Investig. Ophthalmol. Vis. Sci. 1991, 32, 2636–2644. [Google Scholar]
- Corominas, J.; Colijn, J.M.; Geerlings, M.J.; Pauper, M.; Bakker, B.; Amin, N.; Lores Motta, L.; Kersten, E.; Garanto, A.; Verlouw, J.A.M.; et al. Whole-Exome Sequencing in Age-Related Macular Degeneration Identifies Rare Variants in COL8A1, a Component of Bruch’s Membrane. Ophthalmology 2018, 125, 1433–1443. [Google Scholar] [CrossRef] [Green Version]
- Den Hollander, A.I.; Hoyng, C.B.; Boon, C.J.F. Complement Factor H Gene Mutations: Implications for Genetic Testing and Precision Medicine in Macular Degeneration. Ophthalmology 2019, 126, 1422–1423. [Google Scholar] [CrossRef] [Green Version]
- Meyer, K.J.; Davis, L.K.; Schindler, E.I.; Beck, J.S.; Rudd, D.S.; Grundstad, A.J.; Scheetz, T.E.; Braun, T.A.; Fingert, J.H.; Alward, W.L.; et al. Genome-wide analysis of copy number variants in age-related macular degeneration. Hum. Genet. 2011, 129, 91–100. [Google Scholar] [CrossRef] [Green Version]
- Marmorstein, A.D.; Marmorstein, L.Y.; Sakaguchi, H.; Hollyfield, J.G. Spectral profiling of autofluorescence associated with lipofuscin, Bruch’s Membrane, and sub-RPE deposits in normal and AMD eyes. Investig. Ophthalmol. Vis. Sci. 2002, 43, 2435–2441. [Google Scholar]
- Van Leeuwen, E.M.; Emri, E.; Merle, B.M.J.; Colijn, J.M.; Kersten, E.; Cougnard-Gregoire, A.; Dammeier, S.; Meester-Smoor, M.; Pool, F.M.; de Jong, E.K.; et al. A new perspective on lipid research in age-related macular degeneration. Prog. Retin. Eye Res. 2018, 67, 56–86. [Google Scholar] [CrossRef]
- Curcio, C.A. Antecedents of Soft Drusen, the Specific Deposits of Age-Related Macular Degeneration, in the Biology of Human Macula. Investig. Ophthalmol. Vis. Sci. 2018, 59, AMD182–AMD194. [Google Scholar] [CrossRef] [Green Version]
- Curcio, C.A. Photoreceptor topography in ageing and age-related maculopathy. Eye 2001, 15, 376–383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Curcio, C.A.; Millican, C.L.; Bailey, T.; Kruth, H.S. Accumulation of cholesterol with age in human Bruch’s membrane. Investig. Ophthalmol. Vis. Sci. 2001, 42, 265–274. [Google Scholar]
- Curcio, C.A. Soft Drusen in Age-Related Macular Degeneration: Biology and Targeting Via the Oil Spill Strategies. Investig. Ophthalmol. Vis. Sci. 2018, 59, AMD160–AMD181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crabb, J.W.; Miyagi, M.; Gu, X.; Shadrach, K.; West, K.A.; Sakaguchi, H.; Kamei, M.; Hasan, A.; Yan, L.; Rayborn, M.E.; et al. Drusen proteome analysis: An approach to the etiology of age-related macular degeneration. Proc. Natl. Acad. Sci. USA 2002, 99, 14682–14687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Curcio, C.A.; Johnson, M.; Huang, J.D.; Rudolf, M. Aging, age-related macular degeneration, and the response-to-retention of apolipoprotein B-containing lipoproteins. Prog. Retin. Eye Res. 2009, 28, 393–422. [Google Scholar] [CrossRef] [Green Version]
- Mullins, R.F.; Russell, S.R.; Anderson, D.H.; Hageman, G.S. Drusen associated with aging and age-related macular degeneration contain proteins common to extracellular deposits associated with atherosclerosis, elastosis, amyloidosis, and dense deposit disease. FASEB J. 2000, 14, 835–846. [Google Scholar] [CrossRef]
- Zweifel, S.A.; Spaide, R.F.; Curcio, C.A.; Malek, G.; Imamura, Y. Reticular pseudodrusen are subretinal drusenoid deposits. Ophthalmology 2010, 117, 303–312.e301. [Google Scholar] [CrossRef]
- Zweifel, S.A.; Imamura, Y.; Spaide, T.C.; Fujiwara, T.; Spaide, R.F. Prevalence and significance of subretinal drusenoid deposits (reticular pseudodrusen) in age-related macular degeneration. Ophthalmology 2010, 117, 1775–1781. [Google Scholar] [CrossRef]
- Ross, R. Atherosclerosis—an inflammatory disease. N. Engl. J. Med. 1999, 340, 115–126. [Google Scholar] [CrossRef]
- Tall, A.R.; Yvan-Charvet, L. Cholesterol, inflammation and innate immunity. Nat. Rev. Immunol. 2015, 15, 104–116. [Google Scholar] [CrossRef] [Green Version]
- Lusis, A.J. Atherosclerosis. Nature 2000, 407, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Murphy, A.J.; Woollard, K.J.; Hoang, A.; Mukhamedova, N.; Stirzaker, R.A.; McCormick, S.P.; Remaley, A.T.; Sviridov, D.; Chin-Dusting, J. High-density lipoprotein reduces the human monocyte inflammatory response. Arter. Thromb. Vasc. Biol. 2008, 28, 2071–2077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Connor, S.L.; Johnson, E.J.; Klein, M.L.; Hughes, S.; Connor, W.E. Effect of dietary lutein and zeaxanthin on plasma carotenoids and their transport in lipoproteins in age-related macular degeneration. Am. J. Clin. Nutr. 2007, 85, 762–769. [Google Scholar] [CrossRef]
- Mahley, R.W.; Innerarity, T.L.; Rall, S.C., Jr.; Weisgraber, K.H. Plasma lipoproteins: Apolipoprotein structure and function. J. Lipid Res. 1984, 25, 1277–1294. [Google Scholar] [CrossRef]
- Mahley, R.W. Apolipoprotein E: Cholesterol transport protein with expanding role in cell biology. Science 1988, 240, 622–630. [Google Scholar] [CrossRef] [PubMed]
- Zannis, V.I.; Just, P.W.; Breslow, J.L. Human apolipoprotein E isoprotein subclasses are genetically determined. Am. J. Hum. Genet. 1981, 33, 11–24. [Google Scholar] [PubMed]
- Farrer, L.A.; Cupples, L.A.; Haines, J.L.; Hyman, B.; Kukull, W.A.; Mayeux, R.; Myers, R.H.; Pericak-Vance, M.A.; Risch, N.; van Duijn, C.M. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA 1997, 278, 1349–1356. [Google Scholar] [CrossRef]
- Zannis, V.I.; Kardassis, D.; Zanni, E.E. Genetic mutations affecting human lipoproteins, their receptors, and their enzymes. Adv. Hum. Genet. 1993, 21, 145–319. [Google Scholar] [CrossRef]
- Souied, E.H.; Benlian, P.; Amouyel, P.; Feingold, J.; Lagarde, J.P.; Munnich, A.; Kaplan, J.; Coscas, G.; Soubrane, G. The epsilon4 allele of the apolipoprotein E gene as a potential protective factor for exudative age-related macular degeneration. Am. J. Ophthalmol. 1998, 125, 353–359. [Google Scholar] [CrossRef]
- Schmidt, S.; Klaver, C.; Saunders, A.; Postel, E.; De La Paz, M.; Agarwal, A.; Small, K.; Udar, N.; Ong, J.; Chalukya, M.; et al. A pooled case-control study of the apolipoprotein E (APOE) gene in age-related maculopathy. Ophthalmic Genet. 2002, 23, 209–223. [Google Scholar] [CrossRef]
- Zareparsi, S.; Reddick, A.C.; Branham, K.E.; Moore, K.B.; Jessup, L.; Thoms, S.; Smith-Wheelock, M.; Yashar, B.M.; Swaroop, A. Association of apolipoprotein E alleles with susceptibility to age-related macular degeneration in a large cohort from a single center. Investig. Ophthalmol. Vis. Sci. 2004, 45, 1306–1310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McKay, G.J.; Patterson, C.C.; Chakravarthy, U.; Dasari, S.; Klaver, C.C.; Vingerling, J.R.; Ho, L.; de Jong, P.T.; Fletcher, A.E.; Young, I.S.; et al. Evidence of association of APOE with age-related macular degeneration: A pooled analysis of 15 studies. Hum. Mutat. 2011, 32, 1407–1416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, L.; Hoffmann, T.J.; Melles, R.B.; Sakoda, L.C.; Kvale, M.N.; Banda, Y.; Schaefer, C.; Risch, N.; Jorgenson, E. Differences in the Genetic Susceptibility to Age-Related Macular Degeneration Clinical Subtypes. Investig. Ophthalmol. Vis. Sci. 2015, 56, 4290–4299. [Google Scholar] [CrossRef] [Green Version]
- Xiying, M.; Wenbo, W.; Wangyi, F.; Qinghuai, L. Association of Apolipoprotein E Polymorphisms with Age-related Macular Degeneration Subtypes: An Updated Systematic Review and Meta-analysis. Arch. Med. Res. 2017, 48, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Viturino, M.G.; Neto, J.M.; Bajano, F.F.; Costa, S.M.; Roque, A.B.; Borges, G.F.; Ananina, G.; Rim, P.H.; Medina, F.M.; Costa, F.F.; et al. Evaluation of APOE polymorphisms and the risk for age-related macular degeneration in a Southeastern Brazilian population. Exp. Biol. Med. 2021, 246, 1148–1155. [Google Scholar] [CrossRef] [PubMed]
- Liutkeviciene, R.; Vilkeviciute, A.; Smalinskiene, A.; Tamosiunas, A.; Petkeviciene, J.; Zaliuniene, D.; Lesauskaite, V. The role of apolipoprotein E (rs7412 and rs429358) in age-related macular degeneration. Ophthalmic Genet. 2018, 39, 457–462. [Google Scholar] [CrossRef]
- Baird, P.N.; Richardson, A.J.; Robman, L.D.; Dimitrov, P.N.; Tikellis, G.; McCarty, C.A.; Guymer, R.H. Apolipoprotein (APOE) gene is associated with progression of age-related macular degeneration (AMD). Hum. Mutat. 2006, 27, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Adams, M.K.; Simpson, J.A.; Richardson, A.J.; English, D.R.; Aung, K.Z.; Makeyeva, G.A.; Guymer, R.H.; Giles, G.G.; Hopper, J.; Robman, L.D.; et al. Apolipoprotein E gene associations in age-related macular degeneration: The Melbourne Collaborative Cohort Study. Am. J. Epidemiol. 2012, 175, 511–518. [Google Scholar] [CrossRef] [Green Version]
- Holliday, E.G.; Smith, A.V.; Cornes, B.K.; Buitendijk, G.H.; Jensen, R.A.; Sim, X.; Aspelund, T.; Aung, T.; Baird, P.N.; Boerwinkle, E.; et al. Insights into the genetic architecture of early stage age-related macular degeneration: A genome-wide association study meta-analysis. PLoS ONE 2013, 8, e53830. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Song, R.; Ai, Y.; Zhu, J.; He, J.; Dang, M.; Li, H. APOE2 promotes the development and progression of subretinal neovascularization in age-related macular degeneration via MAPKs signaling pathway. Saudi J. Biol. Sci. 2020, 27, 2770–2777. [Google Scholar] [CrossRef]
- Guerra, R.; Wang, J.; Grundy, S.M.; Cohen, J.C. A hepatic lipase (LIPC) allele associated with high plasma concentrations of high density lipoprotein cholesterol. Proc. Natl. Acad. Sci. USA 1997, 94, 4532–4537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chavali, V.R.M.; Haider, N.; Bell, B.A.; Song, Y.; Dunaief, J.L. Hepatic Lipase C knockout mouse has diminished ERGs and Bruch’s lipid deposits. Investig. Ophthalmol. Vis. Sci. 2019, 60, 1343. [Google Scholar]
- Reynolds, R.; Rosner, B.; Seddon, J.M. Serum lipid biomarkers and hepatic lipase gene associations with age-related macular degeneration. Ophthalmology 2010, 117, 1989–1995. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.F.; Han, Y.; Zhang, R.; Qin, L.; Wang, M.X.; Ma, L. CETP/LPL/LIPC gene polymorphisms and susceptibility to age-related macular degeneration. Sci. Rep. 2015, 5, 15711. [Google Scholar] [CrossRef]
- Liutkeviciene, R.; Vilkeviciute, A.; Kriauciuniene, L.; Deltuva, V.P. SIRT1 rs12778366, FGFR2 rs2981582, STAT3 rs744166, LIPC rs10468017, rs493258 and LPL rs12678919 genotypes and haplotype evaluation in patients with age-related macular degeneration. Gene 2019, 686, 8–15. [Google Scholar] [CrossRef]
- Lee, J.; Zeng, J.; Hughes, G.; Chen, Y.; Grob, S.; Zhao, L.; Lee, C.; Krupa, M.; Quach, J.; Luo, J.; et al. Association of LIPC and advanced age-related macular degeneration. Eye 2013, 27, 265–270; quiz 271. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Vega, B.; García, M.; Olivares, L.; Álvarez, L.; González-Fernández, A.; Artime, E.; Fernández-Vega Cueto, A.; Cobo, T.; Coca-Prados, M.; Vega, J.A.; et al. The association study of lipid metabolism gene polymorphisms with AMD identifies a protective role for APOE-E2 allele in the wet form in a Northern Spanish population. Acta Ophthalmol. 2020, 98, e282–e291. [Google Scholar] [CrossRef] [PubMed]
- Kathiresan, S.; Willer, C.J.; Peloso, G.M.; Demissie, S.; Musunuru, K.; Schadt, E.E.; Kaplan, L.; Bennett, D.; Li, Y.; Tanaka, T.; et al. Common variants at 30 loci contribute to polygenic dyslipidemia. Nat. Genet. 2009, 41, 56–65. [Google Scholar] [CrossRef] [Green Version]
- Sharma, K.; Battu, P.; Singh, R.; Sharma, S.K.; Anand, A. Modulated anti-VEGF therapy under the influence of lipid metabolizing proteins in Age related macular degeneration: A pilot study. Sci. Rep. 2022, 12, 714. [Google Scholar] [CrossRef]
- Barter, P.; Kastelein, J.; Nunn, A.; Hobbs, R.; Future Forum Editorial, B. High density lipoproteins (HDLs) and atherosclerosis; the unanswered questions. Atherosclerosis 2003, 168, 195–211. [Google Scholar] [CrossRef]
- Tserentsoodol, N.; Sztein, J.; Campos, M.; Gordiyenko, N.V.; Fariss, R.N.; Lee, J.W.; Fliesler, S.J.; Rodriguez, I.R. Uptake of cholesterol by the retina occurs primarily via a low density lipoprotein receptor-mediated process. Mol. Vis. 2006, 12, 1306–1318. [Google Scholar]
- Van Leeuwen, R.; Klaver, C.C.; Vingerling, J.R.; Hofman, A.; van Duijn, C.M.; Stricker, B.H.; de Jong, P.T. Cholesterol and age-related macular degeneration: Is there a link? Am. J. Ophthalmol. 2004, 137, 750–752. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, S. ABCA1 and biogenesis of HDL. J. Atheroscler. Thromb. 2006, 13, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Storti, F.; Klee, K.; Todorova, V.; Steiner, R.; Othman, A.; van der Velde-Visser, S.; Samardzija, M.; Meneau, I.; Barben, M.; Karademir, D.; et al. Impaired ABCA1/ABCG1-mediated lipid efflux in the mouse retinal pigment epithelium (RPE) leads to retinal degeneration. eLife 2019, 8, e45100. [Google Scholar] [CrossRef] [PubMed]
- Peters, F.; Ebner, L.J.A.; Atac, D.; Maggi, J.; Berger, W.; den Hollander, A.I.; Grimm, C. Regulation of ABCA1 by AMD-Associated Genetic Variants and Hypoxia in iPSC-RPE. Int. J. Mol. Sci. 2022, 23, 3194. [Google Scholar] [CrossRef]
- Pikuleva, I.A.; Curcio, C.A. Cholesterol in the retina: The best is yet to come. Prog. Retin. Eye Res. 2014, 41, 64–89. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Wang, M.; Han, Y.; Zhang, R.; Ma, L. ABCA1 rs1883025 polymorphism and risk of age-related macular degeneration. Graefes Arch. Clin. Exp. Ophthalmol. 2016, 254, 323–332. [Google Scholar] [CrossRef]
- Mead, J.R.; Irvine, S.A.; Ramji, D.P. Lipoprotein lipase: Structure, function, regulation, and role in disease. J. Mol. Med. 2002, 80, 753–769. [Google Scholar] [CrossRef]
- Peter, I.; Huggins, G.S.; Ordovas, J.M.; Haan, M.; Seddon, J.M. Evaluation of new and established age-related macular degeneration susceptibility genes in the Women’s Health Initiative Sight Exam (WHI-SE) Study. Am. J. Ophthalmol. 2011, 152, 1005–1013.e1001. [Google Scholar] [CrossRef] [Green Version]
- Tian, J.; Yu, W.; Qin, X.; Fang, K.; Chen, Q.; Hou, J.; Li, J.; Chen, D.; Hu, Y.; Li, X. Association of genetic polymorphisms and age-related macular degeneration in Chinese population. Investig. Ophthalmol. Vis. Sci. 2012, 53, 4262–4269. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Li, M.; Wen, F.; Zuo, C.; Chen, H.; Wu, K.; Zeng, R. Different impact of high-density lipoprotein-related genetic variants on polypoidal choroidal vasculopathy and neovascular age-related macular degeneration in a Chinese Han population. Exp. Eye Res. 2013, 108, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Merle, B.M.; Maubaret, C.; Korobelnik, J.F.; Delyfer, M.N.; Rougier, M.B.; Lambert, J.C.; Amouyel, P.; Malet, F.; Le Goff, M.; Dartigues, J.F.; et al. Association of HDL-related loci with age-related macular degeneration and plasma lutein and zeaxanthin: The Alienor study. PLoS ONE 2013, 8, e79848. [Google Scholar] [CrossRef]
- Grossniklaus, H.E.; Green, W.R. Choroidal neovascularization. Am. J. Ophthalmol. 2004, 137, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, H.J.; Leibole, M.A.; Tezel, T.; Ferguson, T.A. Fas ligand (CD95 ligand) controls angiogenesis beneath the retina. Nat. Med. 1999, 5, 292–297. [Google Scholar] [CrossRef]
- Kim, I.; Ryan, A.M.; Rohan, R.; Amano, S.; Agular, S.; Miller, J.W.; Adamis, A.P. Constitutive expression of VEGF, VEGFR-1, and VEGFR-2 in normal eyes. Investig. Ophthalmol. Vis. Sci. 1999, 40, 2115–2121. [Google Scholar]
- Ferrara, N. VEGF and Intraocular Neovascularization: From Discovery to Therapy. Transl. Vis. Sci. Technol. 2016, 5, 10. [Google Scholar] [CrossRef] [Green Version]
- Dawson, D.W.; Volpert, O.V.; Gillis, P.; Crawford, S.E.; Xu, H.; Benedict, W.; Bouck, N.P. Pigment epithelium-derived factor: A potent inhibitor of angiogenesis. Science 1999, 285, 245–248. [Google Scholar] [CrossRef]
- Zarbin, M.A. Current concepts in the pathogenesis of age-related macular degeneration. Arch. Ophthalmol. 2004, 122, 598–614. [Google Scholar] [CrossRef] [Green Version]
- Ferrara, N.; Adamis, A.P. Ten years of anti-vascular endothelial growth factor therapy. Nat. Rev. Drug Discov. 2016, 15, 385–403. [Google Scholar] [CrossRef] [Green Version]
- Apte, R.S.; Chen, D.S.; Ferrara, N. VEGF in Signaling and Disease: Beyond Discovery and Development. Cell 2019, 176, 1248–1264. [Google Scholar] [CrossRef] [Green Version]
- Miller, J.W.; Le Couter, J.; Strauss, E.C.; Ferrara, N. Vascular endothelial growth factor a in intraocular vascular disease. Ophthalmology 2013, 120, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Fang, A.M.; Lee, A.Y.; Kulkarni, M.; Osborn, M.P.; Brantley, M.A., Jr. Polymorphisms in the VEGFA and VEGFR-2 genes and neovascular age-related macular degeneration. Mol. Vis. 2009, 15, 2710–2719. [Google Scholar] [PubMed]
- Vilkeviciute, A.; Cebatoriene, D.; Kriauciuniene, L.; Liutkeviciene, R. VEGFA Haplotype and VEGF-A and VEGF-R2 Protein Associations with Exudative Age-Related Macular Degeneration. Cells 2022, 11, 996. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Mo, M.; Liu, G.Y.; Gong, Y.M.; Yu, K.D.; Xu, G.Z. Interaction of two functional genetic variants LOXL1 rs1048661 and VEGFA rs3025039 on the risk of age-related macular degeneration in Chinese women. Ann. Transl. Med. 2020, 8, 818. [Google Scholar] [CrossRef] [PubMed]
- Abedi, F.; Wickremasinghe, S.; Richardson, A.J.; Islam, A.F.; Guymer, R.H.; Baird, P.N. Genetic influences on the outcome of anti-vascular endothelial growth factor treatment in neovascular age-related macular degeneration. Ophthalmology 2013, 120, 1641–1648. [Google Scholar] [CrossRef]
- Hagstrom, S.A.; Ying, G.S.; Pauer, G.J.; Sturgill-Short, G.M.; Huang, J.; Maguire, M.G.; Martin, D.F.; Comparison of Age-Related Macular Degeneration Treatments Trials (CATT) Research Group. VEGFA and VEGFR2 gene polymorphisms and response to anti-vascular endothelial growth factor therapy: Comparison of age-related macular degeneration treatments trials (CATT). JAMA Ophthalmol. 2014, 132, 521–527. [Google Scholar] [CrossRef] [Green Version]
- Finger, R.P.; Wickremasinghe, S.S.; Baird, P.N.; Guymer, R.H. Predictors of anti-VEGF treatment response in neovascular age-related macular degeneration. Surv. Ophthalmol. 2014, 59, 1–18. [Google Scholar] [CrossRef]
- Kucukevcilioglu, M.; Patel, C.B.; Stone, E.M.; Russell, S.R. Clinically detectable drusen domains in fibulin-5-associated age-related macular degeneration (AMD): Drusen subdomains in fibulin-5 AMD. Int. Ophthalmol. 2016, 36, 569–575. [Google Scholar] [CrossRef]
- Mullins, R.F.; Olvera, M.A.; Clark, A.F.; Stone, E.M. Fibulin-5 distribution in human eyes: Relevance to age-related macular degeneration. Exp. Eye Res. 2007, 84, 378–380. [Google Scholar] [CrossRef] [Green Version]
- Albig, A.R.; Schiemann, W.P. Fibulin-5 antagonizes vascular endothelial growth factor (VEGF) signaling and angiogenic sprouting by endothelial cells. DNA Cell Biol. 2004, 23, 367–379. [Google Scholar] [CrossRef]
- Lotery, A.J.; Baas, D.; Ridley, C.; Jones, R.P.; Klaver, C.C.; Stone, E.; Nakamura, T.; Luff, A.; Griffiths, H.; Wang, T.; et al. Reduced secretion of fibulin 5 in age-related macular degeneration and cutis laxa. Hum. Mutat. 2006, 27, 568–574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stone, E.M.; Braun, T.A.; Russell, S.R.; Kuehn, M.H.; Lotery, A.J.; Moore, P.A.; Eastman, C.G.; Casavant, T.L.; Sheffield, V.C. Missense variations in the fibulin 5 gene and age-related macular degeneration. N. Engl. J. Med. 2004, 351, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Auer-Grumbach, M.; Weger, M.; Fink-Puches, R.; Papic, L.; Frohlich, E.; Auer-Grumbach, P.; El Shabrawi-Caelen, L.; Schabhuttl, M.; Windpassinger, C.; Senderek, J.; et al. Fibulin-5 mutations link inherited neuropathies, age-related macular degeneration and hyperelastic skin. Brain 2011, 134, 1839–1852. [Google Scholar] [CrossRef] [PubMed]
- Bhutto, I.; Lutty, G. Understanding age-related macular degeneration (AMD): Relationships between the photoreceptor/retinal pigment epithelium/Bruch’s membrane/choriocapillaris complex. Mol. Asp. Med. 2012, 33, 295–317. [Google Scholar] [CrossRef] [Green Version]
- Winkler, B.S.; Kapousta-Bruneau, N.; Arnold, M.J.; Green, D.G. Effects of inhibiting glutamine synthetase and blocking glutamate uptake on b-wave generation in the isolated rat retina. Vis. Neurosci. 1999, 16, 345–353. [Google Scholar] [CrossRef] [Green Version]
- Bonilla, B.; Hengel, S.R.; Grundy, M.K.; Bernstein, K.A. RAD51 Gene Family Structure and Function. Annu. Rev. Genet. 2020, 54, 25–46. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, D.; Zhang, J.; Zhang, M.; Lu, F.; Qiu, G.; Zhao, L.; Nguyen, D.H.; Luo, H.; Cao, G.; et al. RAD51 gene is associated with advanced age-related macular degeneration in Chinese population. Clin. Biochem. 2013, 46, 1689–1693. [Google Scholar] [CrossRef]
- Chu, X.K.; Meyerle, C.B.; Liang, X.; Chew, E.Y.; Chan, C.C.; Tuo, J. In-depth analyses unveil the association and possible functional involvement of novel RAD51B polymorphisms in age-related macular degeneration. Age 2014, 36, 9627. [Google Scholar] [CrossRef] [Green Version]
- Battu, P.; Sharma, K.; Rain, M.; Singh, R.; Anand, A. Serum Levels of ARMS2, COL8A1, RAD51B, and VEGF and their Correlations in Age-related Macular Degeneration. Curr. Neurovasc. Res. 2021, 18, 181–188. [Google Scholar] [CrossRef]
- Sartorius, U.; Schmitz, I.; Krammer, P.H. Molecular mechanisms of death-receptor-mediated apoptosis. Chembiochem 2001, 2, 20–29. [Google Scholar] [CrossRef]
- Mori, K.; Ishikawa, K.; Fukuda, Y.; Ji, R.; Wada, I.; Kubo, Y.; Akiyama, M.; Notomi, S.; Murakami, Y.; Nakao, S.; et al. TNFRSF10A downregulation induces retinal pigment epithelium degeneration during the pathogenesis of age-related macular degeneration and central serous chorioretinopathy. Hum. Mol. Genet. 2022, ddac020. [Google Scholar] [CrossRef] [PubMed]
- Arakawa, S.; Takahashi, A.; Ashikawa, K.; Hosono, N.; Aoi, T.; Yasuda, M.; Oshima, Y.; Yoshida, S.; Enaida, H.; Tsuchihashi, T.; et al. Genome-wide association study identifies two susceptibility loci for exudative age-related macular degeneration in the Japanese population. Nat. Genet. 2011, 43, 1001–1004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Y.; Li, S.; Li, H.; Yang, F.; Bai, Y.; Zhao, M.; Guo, J.; Zhao, M.; Zhou, P.; Khor, C.C.; et al. TNFRSF10A-LOC389641 rs13278062 but not REST-C4orf14-POLR2B-IGFBP7 rs1713985 was found associated with age-related macular degeneration in a Chinese population. Investig. Ophthalmol. Vis. Sci. 2013, 54, 8199–8203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tuo, J.; Ning, B.; Bojanowski, C.M.; Lin, Z.N.; Ross, R.J.; Reed, G.F.; Shen, D.; Jiao, X.; Zhou, M.; Chew, E.Y.; et al. Synergic effect of polymorphisms in ERCC6 5’ flanking region and complement factor H on age-related macular degeneration predisposition. Proc. Natl. Acad. Sci. USA 2006, 103, 9256–9261. [Google Scholar] [CrossRef] [Green Version]
- Troelstra, C.; van Gool, A.; de Wit, J.; Vermeulen, W.; Bootsma, D.; Hoeijmakers, J.H. ERCC6, a member of a subfamily of putative helicases, is involved in Cockayne’s syndrome and preferential repair of active genes. Cell 1992, 71, 939–953. [Google Scholar] [CrossRef] [Green Version]
- Baas, D.C.; Despriet, D.D.; Gorgels, T.G.; Bergeron-Sawitzke, J.; Uitterlinden, A.G.; Hofman, A.; van Duijn, C.M.; Merriam, J.E.; Smith, R.T.; Barile, G.R.; et al. The ERCC6 gene and age-related macular degeneration. PLoS ONE 2010, 5, e13786. [Google Scholar] [CrossRef] [Green Version]
- Blasiak, J.; Synowiec, E.; Salminen, A.; Kaarniranta, K. Genetic variability in DNA repair proteins in age-related macular degeneration. Int. J. Mol. Sci. 2012, 13, 13378–13397. [Google Scholar] [CrossRef]
- Liu, M.M.; Agron, E.; Chew, E.; Meyerle, C.; Ferris, F.L., 3rd; Chan, C.C.; Tuo, J. Copy number variations in candidate genes in neovascular age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2011, 52, 3129–3135. [Google Scholar] [CrossRef]
- Weeks, D.E.; Conley, Y.P.; Tsai, H.J.; Mah, T.S.; Rosenfeld, P.J.; Paul, T.O.; Eller, A.W.; Morse, L.S.; Dailey, J.P.; Ferrell, R.E.; et al. Age-related maculopathy: An expanded genome-wide scan with evidence of susceptibility loci within the 1q31 and 17q25 regions. Am. J. Ophthalmol. 2001, 132, 682–692. [Google Scholar] [CrossRef]
- Weeks, D.E.; Conley, Y.P.; Tsai, H.J.; Mah, T.S.; Schmidt, S.; Postel, E.A.; Agarwal, A.; Haines, J.L.; Pericak-Vance, M.A.; Rosenfeld, P.J.; et al. Age-related maculopathy: A genomewide scan with continued evidence of susceptibility loci within the 1q31, 10q26, and 17q25 regions. Am. J. Hum. Genet. 2004, 75, 174–189. [Google Scholar] [CrossRef] [Green Version]
- Iyengar, S.K.; Song, D.; Klein, B.E.; Klein, R.; Schick, J.H.; Humphrey, J.; Millard, C.; Liptak, R.; Russo, K.; Jun, G.; et al. Dissection of genomewide-scan data in extended families reveals a major locus and oligogenic susceptibility for age-related macular degeneration. Am. J. Hum. Genet. 2004, 74, 20–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kenealy, S.J.; Schmidt, S.; Agarwal, A.; Postel, E.A.; De La Paz, M.A.; Pericak-Vance, M.A.; Haines, J.L. Linkage analysis for age-related macular degeneration supports a gene on chromosome 10q26. Mol. Vis. 2004, 10, 57–61. [Google Scholar] [CrossRef]
- Fisher, S.A.; Abecasis, G.R.; Yashar, B.M.; Zareparsi, S.; Swaroop, A.; Iyengar, S.K.; Klein, B.E.; Klein, R.; Lee, K.E.; Majewski, J.; et al. Meta-analysis of genome scans of age-related macular degeneration. Hum. Mol. Genet. 2005, 14, 2257–2264. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Camp, N.J.; Sun, H.; Tong, Z.; Gibbs, D.; Cameron, D.J.; Chen, H.; Zhao, Y.; Pearson, E.; Li, X.; et al. A variant of the HTRA1 gene increases susceptibility to age-related macular degeneration. Science 2006, 314, 992–993. [Google Scholar] [CrossRef] [PubMed]
- Marmorstein, A.D.; Marmorstein, L.Y. The challenge of modeling macular degeneration in mice. Trends Genet. 2007, 23, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.; Myers, C.E.; Meuer, S.M.; Gangnon, R.E.; Sivakumaran, T.A.; Iyengar, S.K.; Lee, K.E.; Klein, B.E. Risk alleles in CFH and ARMS2 and the long-term natural history of age-related macular degeneration: The Beaver Dam Eye Study. JAMA Ophthalmol. 2013, 131, 383–392. [Google Scholar] [CrossRef] [Green Version]
- Wyatt, M.K.; Tsai, J.Y.; Mishra, S.; Campos, M.; Jaworski, C.; Fariss, R.N.; Bernstein, S.L.; Wistow, G. Interaction of complement factor h and fibulin3 in age-related macular degeneration. PLoS ONE 2013, 8, e68088. [Google Scholar] [CrossRef]
- Jakobsdottir, J.; Conley, Y.P.; Weeks, D.E.; Mah, T.S.; Ferrell, R.E.; Gorin, M.B. Susceptibility genes for age-related maculopathy on chromosome 10q26. Am. J. Hum. Genet. 2005, 77, 389–407. [Google Scholar] [CrossRef] [Green Version]
- Rivera, A.; Fisher, S.A.; Fritsche, L.G.; Keilhauer, C.N.; Lichtner, P.; Meitinger, T.; Weber, B.H. Hypothetical LOC387715 is a second major susceptibility gene for age-related macular degeneration, contributing independently of complement factor H to disease risk. Hum. Mol. Genet. 2005, 14, 3227–3236. [Google Scholar] [CrossRef] [Green Version]
- Dewan, A.; Liu, M.; Hartman, S.; Zhang, S.S.; Liu, D.T.; Zhao, C.; Tam, P.O.; Chan, W.M.; Lam, D.S.; Snyder, M.; et al. HTRA1 promoter polymorphism in wet age-related macular degeneration. Science 2006, 314, 989–992. [Google Scholar] [CrossRef]
- Conley, Y.P.; Jakobsdottir, J.; Mah, T.; Weeks, D.E.; Klein, R.; Kuller, L.; Ferrell, R.E.; Gorin, M.B. CFH, ELOVL4, PLEKHA1 and LOC387715 genes and susceptibility to age-related maculopathy: AREDS and CHS cohorts and meta-analyses. Hum. Mol. Genet. 2006, 15, 3206–3218. [Google Scholar] [CrossRef] [PubMed]
- Kanda, A.; Chen, W.; Othman, M.; Branham, K.E.; Brooks, M.; Khanna, R.; He, S.; Lyons, R.; Abecasis, G.R.; Swaroop, A. A variant of mitochondrial protein LOC387715/ARMS2, not HTRA1, is strongly associated with age-related macular degeneration. Proc. Natl. Acad. Sci. USA 2007, 104, 16227–16232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Francis, P.J.; Klein, M.L. Update on the role of genetics in the onset of age-related macular degeneration. Clin. Ophthalmol. 2011, 5, 1127–1133. [Google Scholar] [CrossRef] [Green Version]
- Hautamaki, A.; Seitsonen, S.; Holopainen, J.M.; Moilanen, J.A.; Kivioja, J.; Onkamo, P.; Jarvela, I.; Immonen, I. The genetic variant rs4073 AT of the Interleukin-8 promoter region is associated with the earlier onset of exudative age-related macular degeneration. Acta Ophthalmol. 2015, 93, 726–733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woo, S.J.; Ahn, J.; Morrison, M.A.; Ahn, S.Y.; Lee, J.; Kim, K.W.; DeAngelis, M.M.; Park, K.H. Analysis of Genetic and Environmental Risk Factors and Their Interactions in Korean Patients with Age-Related Macular Degeneration. PLoS ONE 2015, 10, e0132771. [Google Scholar] [CrossRef] [Green Version]
- Lu, Z.G.; May, A.; Dinh, B.; Lin, V.; Su, F.; Tran, C.; Adivikolanu, H.; Ehlen, R.; Che, B.; Wang, Z.H.; et al. The interplay of oxidative stress and ARMS2-HTRA1 genetic risk in neovascular AMD. Vessel. Plus 2021, 5, 4. [Google Scholar] [CrossRef]
- Smailhodzic, D.; Klaver, C.C.; Klevering, B.J.; Boon, C.J.; Groenewoud, J.M.; Kirchhof, B.; Daha, M.R.; den Hollander, A.I.; Hoyng, C.B. Risk alleles in CFH and ARMS2 are independently associated with systemic complement activation in age-related macular degeneration. Ophthalmology 2012, 119, 339–346. [Google Scholar] [CrossRef]
- Fritsche, L.G.; Loenhardt, T.; Janssen, A.; Fisher, S.A.; Rivera, A.; Keilhauer, C.N.; Weber, B.H. Age-related macular degeneration is associated with an unstable ARMS2 (LOC387715) mRNA. Nat. Genet. 2008, 40, 892–896. [Google Scholar] [CrossRef]
- Wang, G.; Spencer, K.L.; Court, B.L.; Olson, L.M.; Scott, W.K.; Haines, J.L.; Pericak-Vance, M.A. Localization of age-related macular degeneration-associated ARMS2 in cytosol, not mitochondria. Investig. Ophthalmol. Vis. Sci. 2009, 50, 3084–3090. [Google Scholar] [CrossRef]
- Kortvely, E.; Hauck, S.M.; Duetsch, G.; Gloeckner, C.J.; Kremmer, E.; Alge-Priglinger, C.S.; Deeg, C.A.; Ueffing, M. ARMS2 is a constituent of the extracellular matrix providing a link between familial and sporadic age-related macular degenerations. Investig. Ophthalmol. Vis. Sci. 2010, 51, 79–88. [Google Scholar] [CrossRef] [Green Version]
- Micklisch, S.; Lin, Y.; Jacob, S.; Karlstetter, M.; Dannhausen, K.; Dasari, P.; von der Heide, M.; Dahse, H.M.; Schmolz, L.; Grassmann, F.; et al. Age-related macular degeneration associated polymorphism rs10490924 in ARMS2 results in deficiency of a complement activator. J. Neuroinflam. 2017, 14, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Canfield, A.E.; Hadfield, K.D.; Rock, C.F.; Wylie, E.C.; Wilkinson, F.L. HtrA1: A novel regulator of physiological and pathological matrix mineralization? Biochem. Soc. Trans. 2007, 35, 669–671. [Google Scholar] [CrossRef] [PubMed]
- Beguier, F.; Housset, M.; Roubeix, C.; Augustin, S.; Zagar, Y.; Nous, C.; Mathis, T.; Eandi, C.; Benchaboune, M.; Drame-Maigne, A.; et al. The 10q26 Risk Haplotype of Age-Related Macular Degeneration Aggravates Subretinal Inflammation by Impairing Monocyte Elimination. Immunity 2020, 53, 429–441.e428. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.L.; Seager, N.A.; Gardiner, J.D.; Pappas, C.M.; Cronin, M.C.; Amat di San Filippo, C.; Anstadt, R.A.; Liu, J.; Toso, M.A.; Nichols, L.; et al. Chromosome 10q26-driven age-related macular degeneration is associated with reduced levels of HTRA1 in human retinal pigment epithelium. Proc. Natl. Acad. Sci. USA 2021, 118, e2103617118. [Google Scholar] [CrossRef] [PubMed]
- Ross, R.J.; Verma, V.; Rosenberg, K.I.; Chan, C.C.; Tuo, J. Genetic markers and biomarkers for age-related macular degeneration. Expert Rev. Ophthalmol. 2007, 2, 443–457. [Google Scholar] [CrossRef] [Green Version]
- Bergeron-Sawitzke, J.; Gold, B.; Olsh, A.; Schlotterbeck, S.; Lemon, K.; Visvanathan, K.; Allikmets, R.; Dean, M. Multilocus analysis of age-related macular degeneration. Eur. J. Hum. Genet. 2009, 17, 1190–1199. [Google Scholar] [CrossRef]
- Mohamad, N.A.; Ramachandran, V.; Mohd Isa, H.; Chan, Y.M.; Ngah, N.F.; Ching, S.M.; Hoo, F.K.; Wan Sulaiman, W.A.; Inche Mat, L.N.; Mohamed, M.H. Association of HTRA1 and ARMS2 gene polymorphisms with response to intravitreal ranibizumab among neovascular age-related macular degenerative subjects. Hum. Genom. 2019, 13, 13. [Google Scholar] [CrossRef]
- Shijo, T.; Sakurada, Y.; Fukuda, Y.; Yoneyama, S.; Sugiyama, A.; Matsubara, M.; Kikushima, W.; Tanabe, N.; Parikh, R.; Kashiwagi, K. Association of CRP levels with ARMS2 and CFH variants in age-related macular degeneration. Int. Ophthalmol. 2020, 40, 2735–2742. [Google Scholar] [CrossRef]
- Kitchens, J.W.; Kassem, N.; Wood, W.; Stone, T.W.; Isernhagen, R.; Wood, E.; Hancock, B.A.; Radovich, M.; Waymire, J.; Li, L.; et al. A pharmacogenetics study to predict outcome in patients receiving anti-VEGF therapy in age related macular degeneration. Clin. Ophthalmol. 2013, 7, 1987–1993. [Google Scholar] [CrossRef] [Green Version]
- Nakai, S.; Matsumiya, W.; Miki, A.; Honda, S.; Nakamura, M. Association of an age-related maculopathy susceptibility 2 gene variant with the 12-month outcomes of intravitreal aflibercept combined with photodynamic therapy for polypoidal choroidal vasculopathy. Jpn J. Ophthalmol. 2019, 63, 389–395. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Z.; Hu, S.; Qi, J. Meta-Analysis of the Pharmacogenetics of ARMS2 A69S Polymorphism and the Response to Advanced Age-Related Macular Degeneration. Ophthalmic Res. 2021, 64, 192–204. [Google Scholar] [CrossRef]
- Fang, K.; Gao, P.; Tian, J.; Qin, X.; Yu, W.; Li, J.; Chen, Q.; Huang, L.; Chen, D.; Hu, Y.; et al. Joint Effect of CFH and ARMS2/HTRA1 Polymorphisms on Neovascular Age-Related Macular Degeneration in Chinese Population. J. Ophthalmol. 2015, 2015, 821918. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, R.; Yasuda, M.; Song, S.J.; Chen, S.J.; Jonas, J.B.; Wang, J.J.; Mitchell, P.; Wong, T.Y. The prevalence of age-related macular degeneration in Asians: A systematic review and meta-analysis. Ophthalmology 2010, 117, 921–927. [Google Scholar] [CrossRef] [PubMed]
- Yasuma, T.; Nakamura, M.; Kikuchi, M.; Ishikawa, K.; Nishihara, H.; Kaneko, H.; Nishiguchi, K.M.; Terasaki, H. Association of c.*372_815del443ins54 Polymorphism in the ARMS2 (LOC387715) Gene With Neovascular Age-Related Macular Degeneration (AMD) and Polypoidal Choroidal Vasculopathy (PCV) in a Japanese Population. Investig. Ophthalmol. Vis. Sci. 2009, 50, 3436. [Google Scholar]
- Akagi-Kurashige, Y.; Yamashiro, K.; Gotoh, N.; Miyake, M.; Morooka, S.; Yoshikawa, M.; Nakata, I.; Kumagai, K.; Tsujikawa, A.; Yamada, R.; et al. MMP20 and ARMS2/HTRA1 Are Associated with Neovascular Lesion Size in Age-Related Macular Degeneration. Ophthalmology 2015, 122, 2295–2302.e2292. [Google Scholar] [CrossRef]
- Yang, Z.; Tong, Z.; Chen, Y.; Zeng, J.; Lu, F.; Sun, X.; Zhao, C.; Wang, K.; Davey, L.; Chen, H.; et al. Genetic and functional dissection of HTRA1 and LOC387715 in age-related macular degeneration. PLoS Genet. 2010, 6, e1000836. [Google Scholar] [CrossRef]
- Andreoli, M.T.; Morrison, M.A.; Kim, B.J.; Chen, L.; Adams, S.M.; Miller, J.W.; DeAngelis, M.M.; Kim, I.K. Comprehensive analysis of complement factor H and LOC387715/ARMS2/HTRA1 variants with respect to phenotype in advanced age-related macular degeneration. Am. J. Ophthalmol. 2009, 148, 869–874. [Google Scholar] [CrossRef] [Green Version]
- Deangelis, M.M.; Ji, F.; Adams, S.; Morrison, M.A.; Harring, A.J.; Sweeney, M.O.; Capone, A., Jr.; Miller, J.W.; Dryja, T.P.; Ott, J.; et al. Alleles in the HtrA serine peptidase 1 gene alter the risk of neovascular age-related macular degeneration. Ophthalmology 2008, 115, 1209–1215.e1207. [Google Scholar] [CrossRef] [Green Version]
- Tam, P.O.; Ng, T.K.; Liu, D.T.; Chan, W.M.; Chiang, S.W.; Chen, L.J.; DeWan, A.; Hoh, J.; Lam, D.S.; Pang, C.P. HTRA1 variants in exudative age-related macular degeneration and interactions with smoking and CFH. Investig. Ophthalmol. Vis. Sci. 2008, 49, 2357–2365. [Google Scholar] [CrossRef] [Green Version]
- Gerhardt, M.J.; Marsh, J.A.; Morrison, M.; Kazlauskas, A.; Khadka, A.; Rosenkranz, S.; DeAngelis, M.M.; Saint-Geniez, M.; Jacobo, S.M.P. ER stress-induced aggresome trafficking of HtrA1 protects against proteotoxicity. J. Mol. Cell Biol. 2017, 9, 516–532. [Google Scholar] [CrossRef]
- Jacobo, S.M.; Deangelis, M.M.; Kim, I.K.; Kazlauskas, A. Age-related macular degeneration-associated silent polymorphisms in HtrA1 impair its ability to antagonize insulin-like growth factor 1. Mol. Cell Biol. 2013, 33, 1976–1990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanda, H.; Nakano, Y.; Terasawa, Y.; Morimoto, T.; Fujikado, T. The relationship between retinal damage and current intensity in a pre-clinical suprachoroidal-transretinal stimulation model using a laser-formed microporous electrode. J. Neural. Eng. 2017, 14, 056013. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Dean, D.C.; Kaplan, H.J. Age-related macular degeneration. Discov. Med. 2010, 9, 13–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, G.; Dubovy, S.R.; Kovach, J.L.; Schwartz, S.G.; Agarwal, A.; Scott, W.K.; Haines, J.L.; Pericak-Vance, M.A. Variants at chromosome 10q26 locus and the expression of HTRA1 in the retina. Exp. Eye Res. 2013, 112, 102–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friedrich, U.; Myers, C.A.; Fritsche, L.G.; Milenkovich, A.; Wolf, A.; Corbo, J.C.; Weber, B.H.F. Risk- and non-risk-associated variants at the 10q26 AMD locus influence ARMS2 mRNA expression but exclude pathogenic effects due to protein deficiency. Hum. Mol. Genet. 2011, 20, 1387–1399. [Google Scholar] [CrossRef]
- Ajana, S.; Cougnard-Gregoire, A.; Colijn, J.M.; Merle, B.M.J.; Verzijden, T.; de Jong, P.; Hofman, A.; Vingerling, J.R.; Hejblum, B.P.; Korobelnik, J.F.; et al. Predicting Progression to Advanced Age-Related Macular Degeneration from Clinical, Genetic, and Lifestyle Factors Using Machine Learning. Ophthalmology 2021, 128, 587–597. [Google Scholar] [CrossRef]
- Csaky, K.G.; Schachat, A.P.; Kaiser, P.K.; Small, K.W.; Heier, J.S. The Use of Genetic Testing in the Management of Patients With Age-Related Macular Degeneration:American Society of Retina Specialists Genetics Task Force Special Report. J. Vitr. Dis. 2017, 1, 75–78. [Google Scholar] [CrossRef] [Green Version]
- Cabral de Guimaraes, T.A.; Daich Varela, M.; Georgiou, M.; Michaelides, M. Treatments for dry age-related macular degeneration: Therapeutic avenues, clinical trials and future directions. Br. J. Ophthalmol. 2022, 106, 297–304. [Google Scholar] [CrossRef]
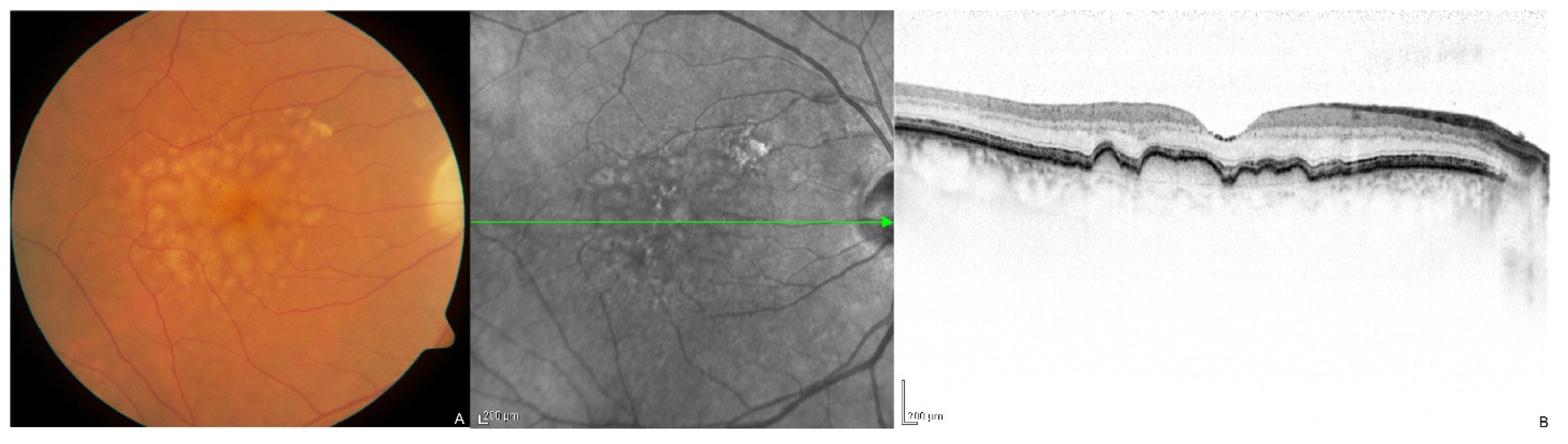
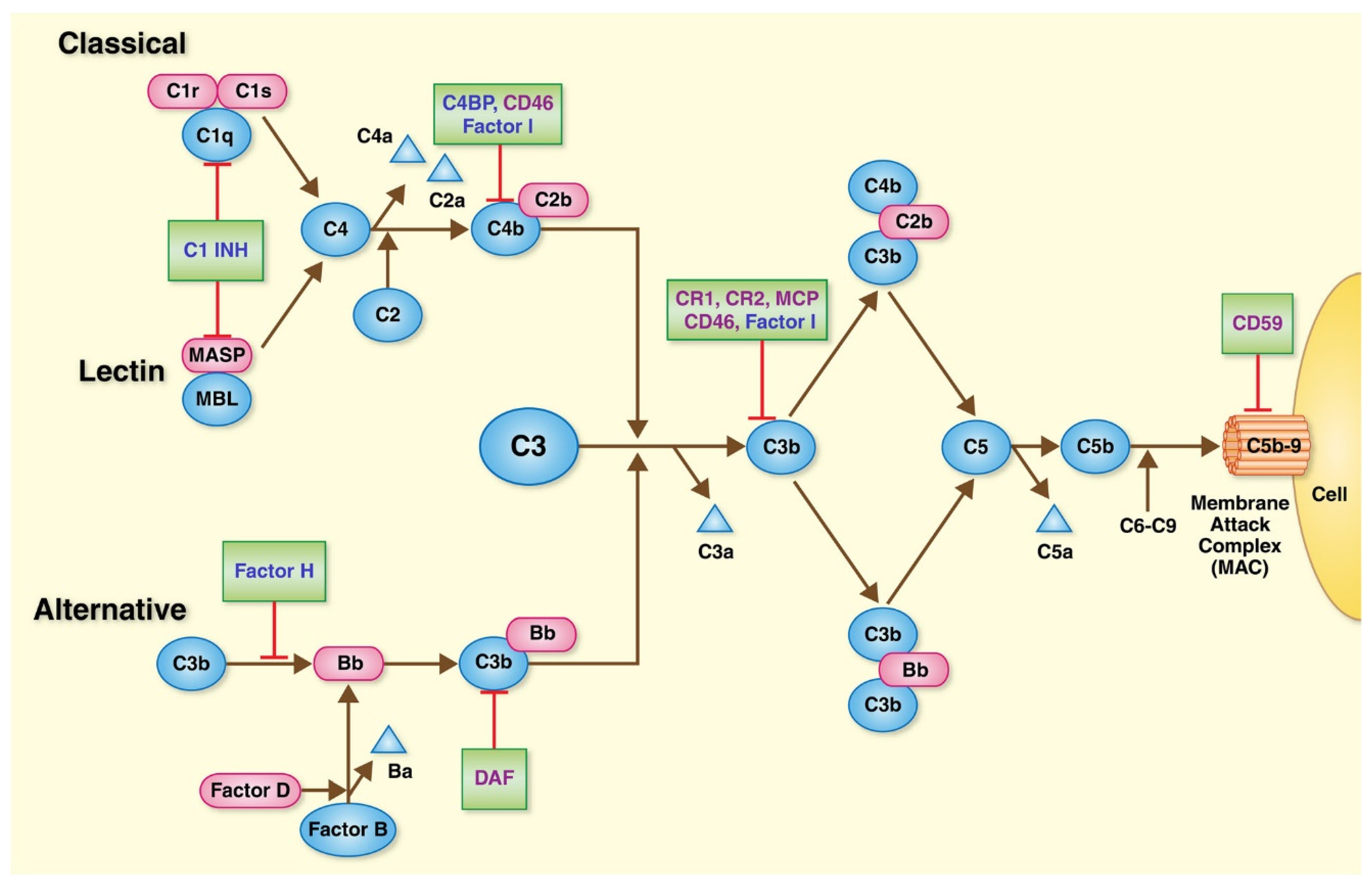

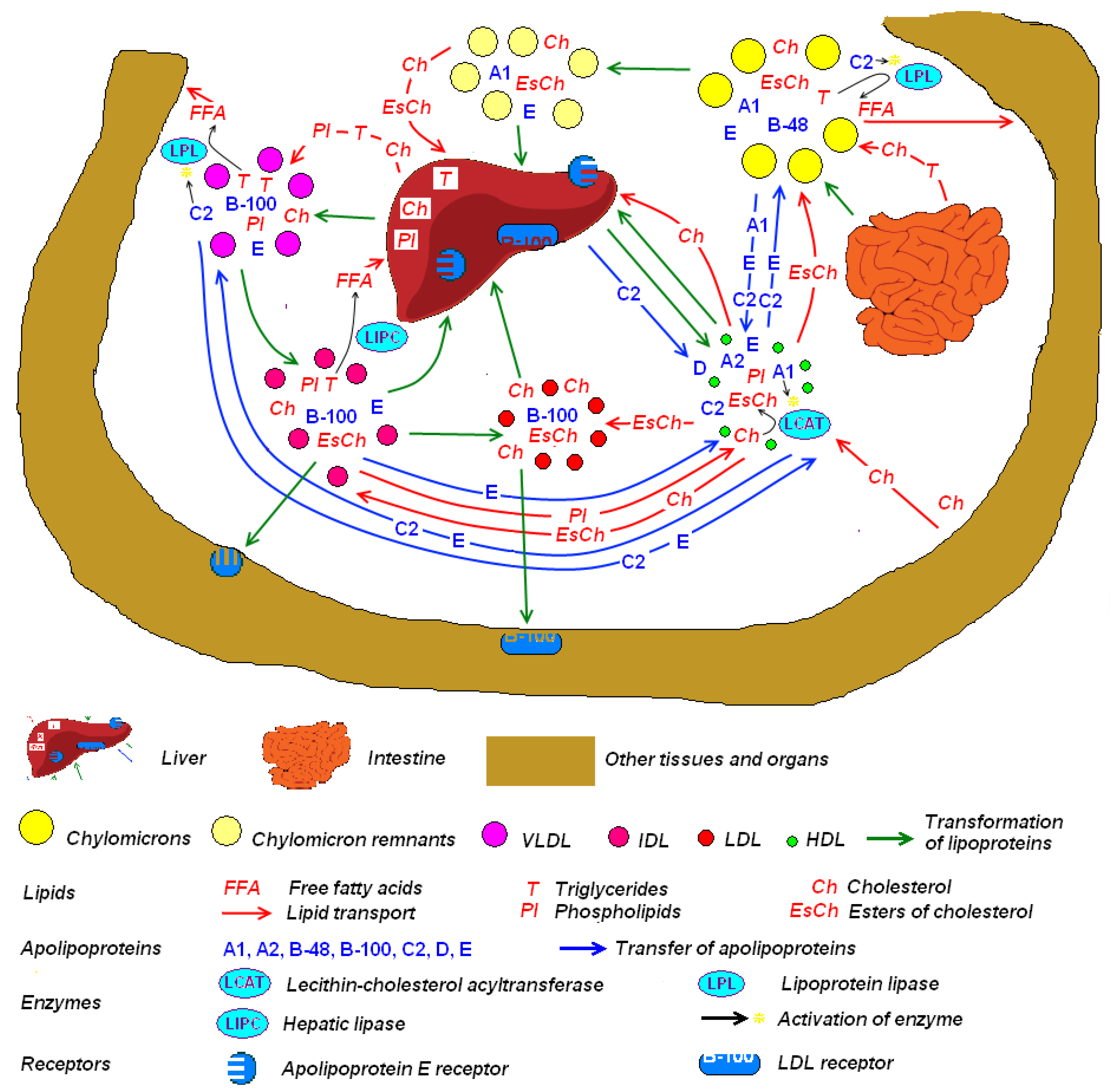
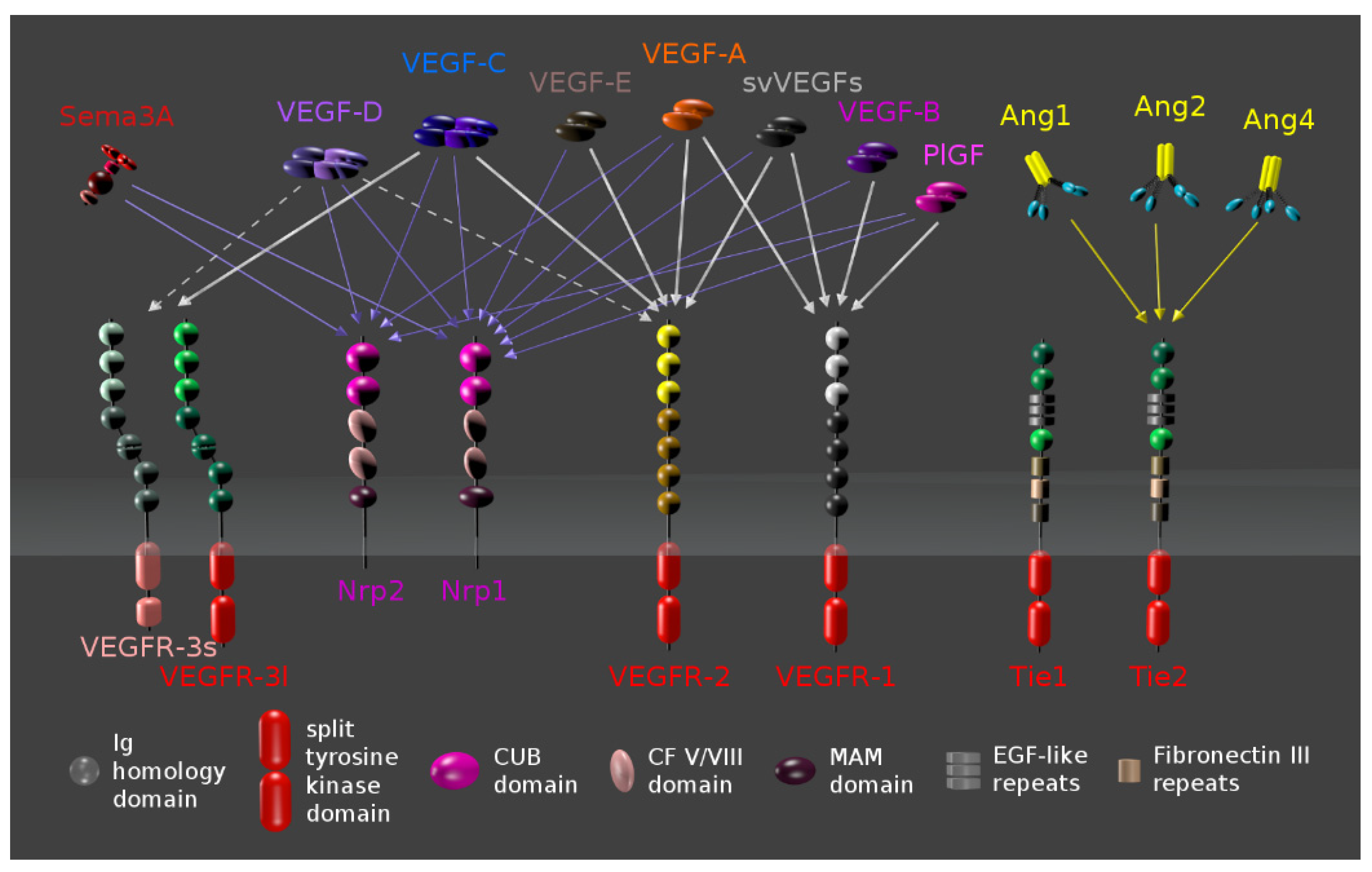
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shughoury, A.; Sevgi, D.D.; Ciulla, T.A. Molecular Genetic Mechanisms in Age-Related Macular Degeneration. Genes 2022, 13, 1233. https://doi.org/10.3390/genes13071233
Shughoury A, Sevgi DD, Ciulla TA. Molecular Genetic Mechanisms in Age-Related Macular Degeneration. Genes. 2022; 13(7):1233. https://doi.org/10.3390/genes13071233
Chicago/Turabian StyleShughoury, Aumer, Duriye Damla Sevgi, and Thomas A. Ciulla. 2022. "Molecular Genetic Mechanisms in Age-Related Macular Degeneration" Genes 13, no. 7: 1233. https://doi.org/10.3390/genes13071233
APA StyleShughoury, A., Sevgi, D. D., & Ciulla, T. A. (2022). Molecular Genetic Mechanisms in Age-Related Macular Degeneration. Genes, 13(7), 1233. https://doi.org/10.3390/genes13071233





