Leukocyte Telomere Length as a Molecular Biomarker of Coronary Heart Disease
Abstract
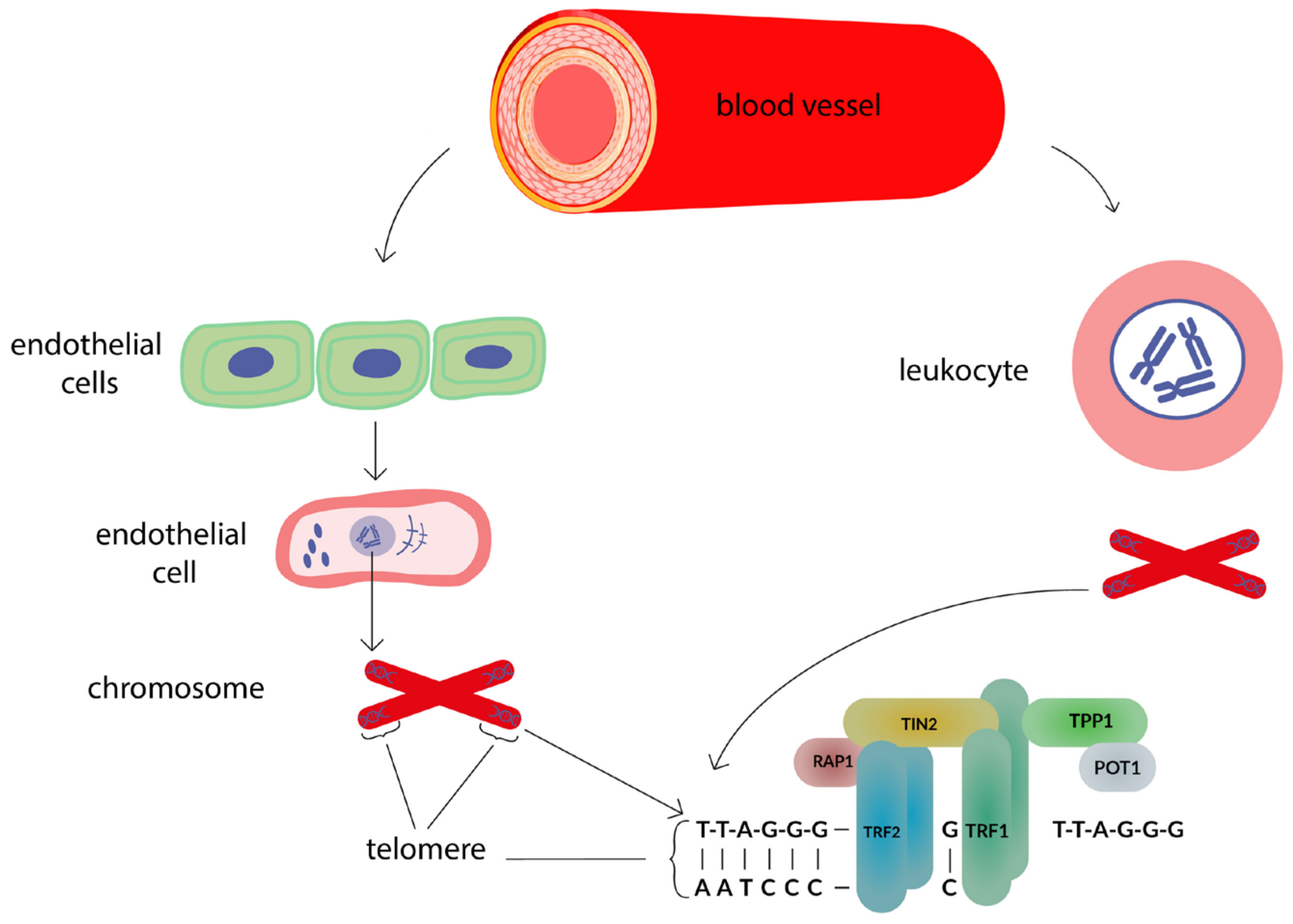
:1. Introduction
2. Materials and Methods
3. Results
3.1. Leukocyte Telomere Length in Patients with Stable Coronary Heart Disease
3.2. Leukocyte Telomere Length in Patients with Acute Myocardial Infarction
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Timmis, A.; Townsend, N.; Gale, C.P.; Torbica, A.; Lettino, M.; Petersen, S.E.; Mossialos, E.A.; Maggioni, A.P.; Kazakiewicz, D.; May, H.T.; et al. European Society of Cardiology: Cardiovascular Disease Statistics 2019. Eur. Heart J. 2020, 41, 12–85. [Google Scholar] [CrossRef] [PubMed]
- Shalnova, S.A.; Drapkina, O.M. The trends of cardiovascular and cancer mortality in Russian men and women from 2000 to 2016 years. Ration. Pharmacother. Cardiol. 2019, 15, 77–83. [Google Scholar] [CrossRef]
- Ageeva, L.I.; Alexandrova, G.A.; Golubev, N.A.; Kirillova, G.N.; Ogryzko, E.V.; Oskov, Y.I.; Nam, P.D.; Kharkov, T.L.; Chumarina, V.Z. Healthcare in Russia. 2021: Stat.sat./Rosstat.—M., 2021, 171 p. Available online: https://ghdx.healthdata.org/organizations/federal-state-statistics-service-russia (accessed on 1 June 2022).
- Kolber, M.R.; Scrimshaw, C. Family history of cardiovascular disease. Can. Fam Physician 2014, 60, 1016. [Google Scholar] [PubMed]
- Hamczyk, M.R.; Nevado, R.M.; Barettino, A.; Fuster, V.; Andres, V. Biological versus chronological aging: JACC focus seminar. J. Am. Coll. Cardiol. 2020, 75, 919–930. [Google Scholar] [CrossRef]
- Nilsson, P.M. Early vascular aging (EVA): Consequences and prevention. Vasc. Health Risk Manag. 2008, 4, 547–552. [Google Scholar] [CrossRef] [Green Version]
- Thijssen, D.H.J.; Bruno, R.M.; Mil, A.C.C.M.; Holder, S.M.; Faita, F.; Greyling, A.; Zock, P.L.; Taddei, S.; Deanfield, J.E.; Luscher, T.; et al. Expert consensus and evidence-based recommendations for the assessment of flow mediated dilation in humans. Eur. Heart J. 2019, 40, 2534–2547. [Google Scholar] [CrossRef]
- Bauer, M.; Caviezel, S.; Teynor, A.; Erbel, R.; Mahabadi, A.A.; Schmidt-Trucksass, A. Carotid intima-media thickness as a bio-marker of subclinical atherosclerosis. Swiss Med. Wkly. 2012, 142, w13705. [Google Scholar] [CrossRef]
- Grillo, A.; Lonati, A.M.; Guida, V.; Parati, G. Cardio-ankle vascular stiffness index (CAVI) and 24-h blood pressure profiles. Eur. Heart J. Suppl. 2017, 19 (Suppl. B), 17–23. [Google Scholar] [CrossRef]
- Townsend, R.R.; Wilkinson, I.B.; Schiffrin, E.L.; Townsend, R.R.; Wilkinson, I.B.; Schiffrin, E.L.; Avolio, A.P.; Chirinos, J.A.; Cockcroft, J.R.; Heffernan, K.S.; et al. Recommendations for improving and standardizing vascular research on arterial stiffness: A scientific statement from the American Heart Association. Hypertension 2015, 66, 698–722. [Google Scholar] [CrossRef] [Green Version]
- Lanzer, P.; Boehm, M.; Sorribas, V.; Thiriet, M.; Janzen, J.; Zeller, T.; Hilaire, C.S.; Shanahan, C. Medial vascular calcification revisited: Review and perspectives. Eur. Heart J. 2014, 35, 1515–1525. [Google Scholar] [CrossRef]
- De Meyer, T.; Nawrot, T.; Bekaert, S.; De Buyzere, M.L.; Rietzschel, E.R.; Andres, V. Telomere length as cardiovascular aging biomarker: JACC review topic of the week. J. Am. Coll. Cardiol. 2018, 72, 805–813. [Google Scholar] [CrossRef] [PubMed]
- Burko, N.V.; Avdeeva, I.V.; Oleynikov, V.E.; Boytsov, S.A. The concept of early vascular aging. Ration. Pharmacother. Cardiol. 2019, 15, 742–749. [Google Scholar] [CrossRef] [Green Version]
- Marco, L.D.; Bellasi, A.; Raggi, P. Cardiovascular biomarkers in chronic kidney disease: State of current research and clinical applicability. Dis. Markers 2015, 586569. [Google Scholar] [CrossRef] [Green Version]
- Gregoli, K.D.; George, S.J.; Jackson, C.L.; Newby, A.C.; Johnson, J.L. Differential effects of tissue inhibitor of metalloproteinase (TIMP)-1 and TIMP-2 on atherosclerosis and monocyte/macrophage invasion. Cardiovasc. Res. 2016, 109, 318–330. [Google Scholar] [CrossRef]
- Freitas, I.A.; Lima, N.A.; Silva, G.B.; Castro, R.L.; Patel, P.; Vasconcelos Lima, C.C.; Costa Lino, D.O. Novel biomarkers in the prognosis of patients with atherosclerotic coronary artery disease. Port. J. Cardiol. 2020, 39, 667–672. [Google Scholar] [CrossRef]
- Wainstein, M.V.; Mossmann, M.; Araujo, G.N.; Gonçalves, S.C.; Gravina, G.L.; Sangalli, M.; Veadrigo, F.; Matte, R.; Reich, R.; Costa, F.G.; et al. Elevated serum interleukin-6 is predictive of coronary artery disease in intermediate risk over-weight patients referred for coronary angiography. Diabetol. Metab. Syndr. 2017, 9, 67. [Google Scholar] [CrossRef]
- Velde, A.R.; Lexis, C.P.H.; Meijers, W.C.; Horst, I.C.; Lipsic, E.; Dokter, M.M.; Veldhuisen, D.J.; Harst, P.; Boer, R.A. Galectin-3 and sST2 in prediction of left ventricular ejection fraction after myocardial infarction. Clin. Chim. Acta 2016, 452, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhao, J.Q.; Jiao, Y.R.; Ren, J.; Zhou, Y.H.; Li, L.; Yao, H.C. Predictive value of leukocyte telomere length for the severity of coronary artery disease. Pers. Med. 2020, 17, 175–183. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, W.; Jiao, Y.R.; Ren, J.; Gao, L.; Li, Y.; Hu, P.; Ren, T.Y.; Han, Q.F.; Chen, C.; et al. Leukocyte telomere length: A potential biomarker for the prognosis of coronary artery disease. Biomark. Med. 2020, 14, 933–941. [Google Scholar] [CrossRef]
- Xu, X.; Hu, H.; Lin, Y.; Huang, F.; Ji, H.; Li, Y.; Lin, S.; Chen, X.; Duan, S. Differences in leukocyte telomere length between coronary heart disease and normal population: A Multipopulation Meta-Analysis. BioMed Res. Int. 2019, 5046867. [Google Scholar] [CrossRef]
- Ma, L.N.; Li, Y.; Wang, J.Y. Telomeres, and essential hypertension. Clin. Biochem. 2015, 48, 1195–1199. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Wang, L.; Dai, M.; Wei, F.; Xu, D. Shorter leukocyte telomere length coupled with lower expression of telomerase genes in patients with essential hypertension. Int. J. Med. Sci. 2020, 17, 2180–2186. [Google Scholar] [CrossRef]
- Allende, M.; Molina, E.; González-Porras, J.R.; Toledo, E.; Lecumberri, R.; Hermida, J. Short leukocyte telomere length is associated with cardioembolic stroke risk in patients with atrial fibrillation. Stroke 2016, 47, 863–865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Gao, Y.; Zhao, L.; Hu, R.; Yang, X.; Liu, Y. Shortened leukocyte telomere length as a potential biomarker for predicting the progression of atrial fibrillation from paroxysm to persistence in the short-term. Medicine 2021, 100, e26020. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T. Vascular senescense and endothelial function—Can we apply it to atrial fibrillation? Circ. J. 2019, 83, 1439–1440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nikulina, S.I.; Shishkova, K.I.; Shulman, V.A.; Chernova, A.A.; Maksimov, V.N. Peripheral blood leukocyte telomere length as a possible prognostic marker for the development of atrial fibrillation. CardioSomatics 2020, 11, 50–54. [Google Scholar] [CrossRef]
- Chatterjee, S.; Gonzalo-Calvo, D.; Derda, A.A.; Schimmel, K.; Sonnenschein, K.; Bavendiek, U.; Bauersachs, J.; Bar, C.; Thum, T. Leukocyte telomere length correlates with hypertrophic cardiomyopathy severity. Sci. Rep. 2018, 8, 11227. [Google Scholar] [CrossRef]
- Wang, Y.; Jiao, F.; Zheng, H.; Kong, Q.; Li, R.; Zhang, X.; Yan, L.; Hao, Y.; Wu, Y. Gender difference in associations between telomere length and risk factors in patients with stroke. Front. Aging Neurosci. 2021, 13, 719538. [Google Scholar] [CrossRef]
- Yetim, E.; Topcuoglu, M.A.; Kutlay, N.Y.; Tukun, A.; Oguz, K.K.; Arsava, E.M. The association between telomere length and ischemic stroke risk and phenotype. Sci. Rep. 2021, 11, 10967. [Google Scholar] [CrossRef]
- Cao, W.; Zheng, D.; Zhang, J.; Wang, A.; Liu, D.; Zhang, J.; Singh, M.; Maranga, I.E.; Cao, M.; Wu, L.; et al. Association between telomere length in peripheral blood leukocytes and risk of ischemic stroke in a Han Chinese population: A linear and non-linear Mendelian randomization analysis. J. Transl. Med. 2020, 18, 385. [Google Scholar] [CrossRef]
- Tian, Y.J.; Wang, S.; Jiao, F.J.; Kong, Q.; Liu, C.; Wu, Y. Telomere length: A potential biomarker for the risk and prognosis of stroke. Front. Neurol. 2019, 10, 624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Feng, C.; Li, L.; Yang, S.; Chen, Y.; Hui, R.; Zhang, M.; Zhang, W. The association of telomere attrition with first-onset stroke in Southern Chinese: A case-control study and meta-analysis. Sci. Rep. 2018, 8, 2290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, J.; Kanchi, M.M.; Rawtaer, I.; Feng, L.; Kumar, A.P.; Kua, E.H.; Mahendran, R. The functional and structural connectomes of telomere length and their association with cognition in mild cognitive impairment. Cortex 2020, 132, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Wang, H.; Xiong, Y.; Chen, C.; Duan, K.; Jia, J.; Ma, F. Vitamin D supplementation improves cognitive function through reducing oxidative stress regulated by telomere length in older adults with mild cognitive impairment: A 12-month randomized controlled trial. J. Alzheimers Dis. 2020, 78, 1509–1518. [Google Scholar] [CrossRef]
- Hinterberger, M.; Fischer, P.; Huber, K.; Krugluger, W.; Zehetmayer, S. Leukocyte telomere length is linked to vascular risk factors not to Alzheimer’s disease in the VITA study. J. Neural Transm. 2017, 124, 809–819. [Google Scholar] [CrossRef]
- Blackburn, E.H.; Appel, E.S.; Link, J. Human telomere biology: A contributory and interactive factor in aging, disease risks, and protection. Science 2015, 350, 1193–1198. [Google Scholar] [CrossRef] [Green Version]
- Yeh, J.K.; Wang, C.Y. Telomeres and telomerase in cardiovascular diseases. Genes 2016, 7, 58. [Google Scholar] [CrossRef] [Green Version]
- Herrmann, M.; Pusceddu, I.; Marz, W.; Herrmann, W. Telomere biology and age-related diseases. Clin. Chem. Labor-Atory Med. 2018, 56, 1210–1222. [Google Scholar] [CrossRef]
- Pusceddu, I.; Farrell, C.J.L.; Di Pierro, A.M.; Jani, E.; Herrmann, W.; Herrmann, M. The role of telomeres and vitamin D in cellular aging and age-related diseases. Clin. Chem. Lab. Med. 2015, 53, 1661–1678. [Google Scholar] [CrossRef] [Green Version]
- Dorajoo, R.; Chang, X.; Gurung, R.L.; Li, Z.; Wang, L.; Wang, R.; Beckman, K.B.; Adams-Haduch, J.; M, Y.; Liu, S.; et al. Loci for human leukocyte telomere length in the Singaporean Chinese population and trans-ethnic genetic studies. Nat. Commun. 2019, 10, 2491. [Google Scholar] [CrossRef] [Green Version]
- Turner, K.J.; Vasu, V.; Darren, K.; Griffin, D.K. Telomere biology and human phenotype. Cells 2019, 8, 73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salakhov, R.R.; Ponasenko, A.V. Telomere length and cardiovascular diseases. Complex. Issues Cardiovasc. Dis. 2018, 7, 101–107. [Google Scholar] [CrossRef]
- McClintock, B. The stability of broken ends of chromosomes in zea mays. Genetics 1941, 26, 234–282. [Google Scholar] [CrossRef] [PubMed]
- Moyzis, R.K.; Buckingham, J.M.; Cram, L.S.; Dani, M.; Deaven, L.L.; Jones, M.D.; Meyne, J.; Ratliff, R.L.; Wu, J.R. A highly con-served repetitive DNA sequence, (TTAGGG)n, present at the telomeres of human chromosomes. Proc. Natl. Acad. Sci. USA 1988, 85, 6622–6666. [Google Scholar] [CrossRef] [Green Version]
- Armanios, M. Telomeres and age-related disease: How telomere biology informs clinical paradigms. J. Clin. Investig. 2013, 123, 996–1002. [Google Scholar] [CrossRef] [Green Version]
- Brown, L.L.; Zhang, Y.S.; Mitchell, C.; Ailshire, J. Does telomere length indicate biological, physical, and cognitive health among older adults? Evidence from the Health and Retirement Study. J. Gerontol. 2018, 73, 1626–1632. [Google Scholar] [CrossRef] [Green Version]
- Hayflick, L. The limited in vitro lifetime of human diploid cell strains. Exp. Cell Res. 1965, 37, 614–636. [Google Scholar] [CrossRef]
- Samani, N.J.; Boultby, R.; Butler, R.; Thompson, J.R.; Goodall, A.H. Telomere shortening in atherosclerosis. Lancet 2001, 358, 472–473. [Google Scholar] [CrossRef]
- Rubtsova, M.; Dontsova, O. Human telomerase RNA: Telomerase component or more? Biomolecules 2020, 10, 873. [Google Scholar] [CrossRef]
- Arai, Y.; Martin-Ruiz, C.M.; Takayama, M.; Abe, Y.; Takebayashi, T.; Koyasu, S.; Suematsu, M.; Hirose, N.; von Zglinicki, T. Inflammation, but not telomere length, predicts successful ageing at extreme old age: A longitudinal study of semi-supercentenarians. EBioMedicine 2015, 2, 1549–1558. [Google Scholar] [CrossRef] [Green Version]
- Allsopp, R.C.; Morin, G.B.; DePinho, R.; Harley, C.B.; Weissman, I.L. Telomerase is required to slow telomere shortening and extend replicative lifespan of HSCs during serial transplantation. Blood 2003, 102, 517–520. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharyya, J.; Mihara, K.; Bhattacharjee, D.; Mukherjee, M. Telomere length as a potential biomarker of coronary artery disease. Indian J. Med. Res. 2017, 145, 730–737. [Google Scholar] [CrossRef] [PubMed]
- Cawthon, R.M. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002, 30, e47. [Google Scholar] [CrossRef] [PubMed]
- Willeit, P.; Willeit, J.; Brandstätter, A.; Ehrlenbach, S.; Mayr, A.; Gasperi, A.; Weger, S.; Oberhollenzer, F.; Reindl, M.; Kronenberg, F.; et al. Cellular aging reflected by leukocyte telomere length predicts advanced atherosclerosis and cardiovascular disease risk. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 1649–1656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hassler, E.; Almer, G.; Reishofer, G.; Marsche, G.; Mangge, H.; Deutschmann, H.; Herrmann, M.; Leber, S.; Gunzer, F.; Renner, W. Sex-specific association of serum antioxidative capacity and leukocyte telomere length. Antioxidants 2021, 10, 1908. [Google Scholar] [CrossRef]
- Dlouha, D.; Pitha, J.; Mesanyova, J.; Mrazkova, J.; Fellnerova, A.; Stanek, V.; Lanska, V.; Hubacek, J.A. Genetic variants within telomere-associated genes, leukocyte telomere length and the risk of acute coronary syndrome in Czech women. Clin. Chim. Acta 2016, 454, 62–65. [Google Scholar] [CrossRef]
- Tian, R.; Zhang, L.N.; Zhang, T.T.; Pang, H.Y.; Chen, L.F.; Shen, Z.J.; Liu, Z.; Fang, Q.; Zhang, S.Y. Association between oxidative stress and peripheral leukocyte telomere length in patients with premature coronary artery disease. Med. Sci. Monit. 2017, 23, 4382–4390. [Google Scholar] [CrossRef] [Green Version]
- Pejenaute, A.; Cortes, A.; Marques, J.; Montero, L.; Beloqui, O.; Fortuno, A.; Martí, A.; Orbe, J.; Zalba, G. NADPH oxidase overactivity underlies telomere shortening in human atherosclerosis. Int. J. Mol. Sci. 2020, 21, 1434. [Google Scholar] [CrossRef] [Green Version]
- Gupta, M.D.; Miglani, M.; Bansal, A.; Jain, V.; Arora, S.; Kumar, S.; Virani, S.S.; Kalra, A.; Yadav, R.; Pasha, Q.; et al. Telomere length in young patients with acute myocardial infarction without conventional risk factors: A pilot study from a South Asian population. Indian Heart J. 2020, 72, 619–622. [Google Scholar] [CrossRef]
- Starnino, L.; Dupuis, G.; Busque, L.; Bourgoin, V.; Dube, M.P.; Busseuil, D.; D’Antono, B. The associations of hostility and defensiveness with telomere length are influenced by sex and health status. Biol. Sex. Differ. 2021, 12, 2. [Google Scholar] [CrossRef]
- Mazidi, M.; Shekoohi, N.; Katsiki, N.; Rakowski, M.; Mikhailidis, D.P.; Banach, M. Serum anti-inflammatory and inflammatory markers have no causal impact on telomere length: A Mendelian randomization study. Arch. Med. Sci. 2021, 17, 739–751. [Google Scholar] [CrossRef] [PubMed]
- Hammadah, M.; Mheid, I.A.; Wilmot, K.; Ramadan, R.; Abdelhadi, N.; Alkhoder, A.; Obideen, M.; Pimple, P.M.; Levantsevych, O.; Kelli, H.M.; et al. Telomere shortening, regenerative capacity, and cardiovascular outcomes. Circ. Res. 2017, 120, 1130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yakhontov, D.A.; Ostanina, J.O.; Pakharukova, M.Y.; Mordvinov, V.A. Clinical sings and symptoms of polyvascular disease in coronary artery disease patients of different age groups. Complex. Issues Cardiovasc. Dis. 2017, 6, 36–43. [Google Scholar] [CrossRef] [Green Version]
- Yakhontov, D.A.; Ostanina, J.O. Early vascular aging syndrome in young and middle age patients with hypertension and coronary artery disease. Med. Alph. 2018, 1, 33–36. [Google Scholar]
- Wilson, R.W.; Herbert, K.E.; Mistry, Y.; Stevens, S.E.; Patel, H.R.; Hastings, R.A.; Thompson, M.M.; Williams, B. Blood leukocyte telomere DNA content predicts vascular telomere DNA content in humans with and without vascular disease. Eur. Heart J. 2008, 29, 2689–2694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.Q.; Lo, K.; Feng, Y.Q.; Zhang, B. The association of mean telomere length with all-cause, cerebrovascular and cardiovascular mortality. Biosci. Rep. 2019, 39, BSR20192306. [Google Scholar] [CrossRef] [Green Version]
- Clinical Recommendations. Stable Coronary Heart Disease. Russian Society of Cardiology 2020.—Text: Electronic. Available online: https://scardio.ru/content/Guidelines/2020/Clinic_rekom_IBS.pdf (accessed on 20 November 2021).
- Russo, A.; Palumbo, L.; Fornengo, C. Telomere length variation in juvenile acute myocardial infarction. PLoS ONE 2012, 7, e49206. [Google Scholar] [CrossRef] [PubMed]
- Margaritis, M.; Sanna, F.; Lazaros, G.; Akoumianakis, I.; Patel, S.; Antonopoulos, A.S.; Duke, C.; Herdman, L.; Psarros, C.; Oikonomou, E.K.; et al. Predictive value of telomere length on outcome following acute myocardial infarction: Evidence for contrasting effects of vascular vs. blood oxidative stress. Eur. Heart J. 2017, 38, 3094–3104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, D.; Martin-Ruiz, C.; Saretzki, G.; Neely, D.; Qiu, W.; Kunadian, V. The association of telomere length and telomerase activity with adverse outcomes in older patients with non-ST-elevation acute coronary syndrome. PLoS ONE 2020, 15, e0227616. [Google Scholar] [CrossRef] [PubMed]
- Maximov, V.N.; Malyutina, S.K.; Orlov, P.S.; Ivanoschuk, D.E.; Voropaeva, E.N.; Bobak, M.; Voevoda, M.I. Length telomere leukocytes as aging markers and risk factors for age-related disease in humans. Adv. Gerontol. 2016, 29, 702–708. [Google Scholar] [CrossRef]
- Strajesko, I.D.; Tkacheva, I.N.; Akasheva, D.U.; Dudinskaya, E.V.; Agaltsov, M.V.; Kruglikova, A.S.; Brailova, N.V.; Pykhtina, V.S.; Plokhova, E.V.; Ozerova, I.N.; et al. Relation of cardiovascular risk factors and leukocyte telomere length. Cardiovasc. Ther. Prev. 2016, 15, 52–57. [Google Scholar] [CrossRef] [Green Version]
- Doroshchuk, N.A.; Tikhase, A.K.; Lankin, V.Z.; Konovalova, G.G.; Mednikova, T.K.; Postnov, A.Y.; Kukharchuk, V.V. The influence of oxidative stress on the length of telomeric repeats in chromosomes o white blood cells in patients with coronary artery disease. Cardiol. Bull. 2017, 12, 32–37. [Google Scholar]
- Wang, H.; Naghavi, M.; Allen, C.; Barber, R.M.; Bhutta, Z.B.; Carter, A.; Casey, D.C.; Charlson, F.J.; Chen, A.Z.; Coates, M.M.; et al. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: A systematic analysis for the Global Burden of Disease Study 2015. The Lancet. 2016, 388, 1459–1544. [Google Scholar] [CrossRef] [Green Version]
- Oganov, R.G.; Simanenkov, V.I.; Bakulin, I.G.; Bakulina, N.V.; Barbarash, O.L.; Boytsov, S.A.; Boldueva, S.A.; Garganeeva, N.P.; Doshchitsin, V.L.; Karateev, A.E.; et al. Comorbidities in clinical practice. Algorithms for diagnostics and treatment. Cardiovasc. Ther. Prevention. 2019, 18, 5–66. [Google Scholar] [CrossRef]
- Paluch, W.; Semczuk, K.; Rys, A.; Szymanski, F.M.; Filipiak, K.J. Anti-hypertensive treatment efficacy in patients with arterial hypertension and coronary artery disease or coronary equivalent. Arter. Hypertens. 2017, 21, 93–98. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.Y.; Zhang, J.; Jin, F.; Zhou, H. Efficacy of felodipine and enalapril in the treatment of essential hypertension with coronary artery disease and the effect on levels of salusin-β, apelin, and PON1 gene expression in patients. Cell. Mol. Biol. 2021, 67, 174–180. [Google Scholar] [CrossRef]
- Sarkar, G.; Gaikwad, V.B.; Sharma, A.; Halder, S.K.; Kumar, D.A.; Anand, J.; Agrawal, S.; Kumbhar, A.; Kinholkar, B.; Mathur, R.; et al. Fixed-dose combination of metoprolol, telmisartan, and chlorthalidone for essential hypertension in adults with stable coronary artery disease: Phase III Study. Adv. Ther. 2022, 39, 923–942. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, D.; Zeng, N.; Guo, H.; Li, H.; Shen, S. Trends of antihypertensive agents in patients with hypertension and coronary artery disease in a tertiary hospital of China. Int. J. Clin. Pharmacol. Ther. 2020, 42, 482–488. [Google Scholar] [CrossRef]
- Guo, Q.; Lu, X.; Gao, Y.; Zhang, J.; Yan, B.; Su, D.; Song, A.; Zhao, X.; Wang, G. Cluster analysis: A new approach for identification of underlying risk factors for coronary artery disease in essential hypertensive patients. Sci. Rep. 2017, 7, 43965. [Google Scholar] [CrossRef] [Green Version]
- Steensig, K.; Olesen, K.K.W.; Thim, T.; Nielsen, J.C.; Jensen, S.E.; Jensen, L.O.; Kristensen, S.D.; Botker, H.E.; Lip, G.Y.H.; Maeng, M. CAD is an independent risk factor for stroke among patients with atrial fibrillation. J. Am. Coll. Cardiol. 2018, 72, 2540–2542. [Google Scholar] [CrossRef]
- Michniewicz, E.; Mlodawska, E.; Lopatowska, P.; Tomaszuk-Kazberuk, A.; Malyszko, J. Patients with atrial fibrillation and coronary artery disease—Double trouble. Adv. Med. Sci. 2018, 63, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Gladding, P.A.; Legget, M.; Fatkin, D.; Larsen, P.; Doughty, R. Polygenic risk scores in coronary artery disease and atrial fibrillation. Heart Lung Circ. 2020, 29, 634–640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alkindi, F.A.; Rafie, I.M. Anticoagulation in patients with atrial fibrillation and coronary artery disease. Heart Views 2020, 21, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Nortamo, S.; Kentta, T.V.; Ukkola, O.; Huikuri, H.V.; Perkiomaki, J.S. Supraventricular premature beats and risk of new-onset atrial fibrillation in coronary artery disease. J. Cardiovasc. Electrophysiol. 2017, 28, 1269–1274. [Google Scholar] [CrossRef]
- Zheng, Y.J.; He, J.Q. Common differentially expressed genes and pathways correlating both coronary artery disease and atrial fibrillation. Excli J. 2021, 20, 126–141. [Google Scholar] [CrossRef] [PubMed]
- Pastori, D.; Pignatelli, P.; Sciacqua, A.; Perticone, M.; Violi, F.; Lip, G.Y.H. Relationship of peripheral and coronary artery disease to cardiovascular events in patients with atrial fibrillation. Int. J. Cardiol. 2018, 255, 69–73. [Google Scholar] [CrossRef]
- Inohara, T.; Shrader, P.; Pieper, K.; Blanco, R.G.; Allen, L.A.; Fonarow, G.C.; Gersh, B.J.; Go, A.S.; Ezekowitz, M.D.; Kowey, P.R.; et al. Treatment of atrial fibrillation with concomitant coronary or peripheral artery disease: Results from the outcomes registry for better informed treatment of atrial fibrillation II. Am. Heart J. 2019, 213, 81–90. [Google Scholar] [CrossRef]
- Lamblin, N.; Ninni, S.; Tricot, O.; Meurice, T.; Lemesle, G.; Bauters, C. Secondary prevention and outcomes in outpatients with coronary artery disease, atrial fibrillation or heart failure: A focus on disease overlap. Open Heart 2020, 7, e001165. [Google Scholar] [CrossRef] [Green Version]
- Wakili, R.; Riesinger, L.; Fender, A.C.; Dobrev, D. Double Jeopardy: Will the new trials tell us how to manage patients with atrial fibrillation and coronary artery disease? IJC Heart Vasc. 2019, 23, 100369. [Google Scholar] [CrossRef]
- Suridjan, I.; Herrmann, N.; Adibfar, A.; Saleem, M.; Andreazza, A.; Oh, P.I.; Lanctot, K.L. Lipid peroxidation markers in coronary artery disease patients with possible vascular mild cognitive impairment. J. Alzheimer’s Dis. 2017, 58, 885–896. [Google Scholar] [CrossRef] [Green Version]
- Xia, C.; Vonder, M.; Sidorenkov, G.; Oudkerk, M.; de Groot, J.C.; Harst, P.; Bock, G.H.; De Deyn, P.P.; Vliegenthart, R. The relationship of coronary artery calcium and clinical coronary artery disease with cognitive function: A systematic review and meta-analysis. J. Atheroscler. Thromb. 2020, 27, 934–958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saleem, M.; Herrmann, N.; Dinoff, A.; Mazereeuw, G.; Oh, P.I.; Goldstein, B.I.; Kiss, A.; Shammi, P.; Lanctot, K.L. Association between endothelial function and cognitive performance in patients with coronary artery disease during cardiac rehabilitation. Psychosom. Med. 2019, 81, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Tarasova, I.V.; Trubnikova, O.A.; Barbarash, O.L. EEG and clinical factors associated with mild cognitive impairment in coronary artery disease patients. Dement. Geriatr. Cogn. Disord. 2018, 46, 275–284. [Google Scholar] [CrossRef] [PubMed]


| Authors [References] | Study Characteristics | Group Characteristics | Participants (n) | Age, in Years (M ± SE or Me [P25; P75]) | Sex (Male/Female, %) | Method | Telomere Length | |
|---|---|---|---|---|---|---|---|---|
| Absolute, bp | Relative (T/S Ratio), CU | |||||||
| Williet et al., 2010 [55] | Prospective, population-based study | Austrians without CAD | 712 | 61.8 ± 10.8 | 47.6/52.4 | Real- time quantitative PCR | N/A | 1.52 ± 0.81 |
| Dlouha et al., 2016 [57] | Observational, cross-sectional case-control study | Czechs without CAD | 642 | 50 ± 2.7 | 0/100 | Real-time quantitative PCR | N/A | 0.93 ± 0.38 |
| Tian et al., 2017 [58] | Observational, cross-sectional study | Chinese without CAD | 128 | 48.5 ± 7.33 | 57.8/42.2 | Real- time quantitative PCR | N/A | 1.1 ± 0.57 |
| Pejenaute et al., 2020 [59] | Observational, cross-sectional study | Spaniards without CAD | 389 | 54 ± 1 | 80/20 | Real- time quantitative PCR | 8591 ± 84 | N/A |
| Gupta et al., 2020 [60] | Observational, cross-sectional study | Indians without CVDs | 77 | 34.38 ± 5.86 | 75/25 | Real- time quantitative PCR | N/A | 0.792 |
| Starnino et al., 2021 [61] | Observational, cross-sectional study | Canadians without CVDs | 25 | 55.68 ± 0.19 | 56/44 | Real- time quantitative PCR | N/A | 0.94 ± 0.15 |
| Mazidi et al., 2021 * [62] | Mendelian randomized trial | British without CAD | 20 | 22.3 ± 1.8 | 100/0 | Real-time quantitative PCR | 12 420 ± 80 | N/A |
| Mazidi et al., 2021 * [62] | Mendelian randomized trial | British without CAD | 20 | 62.75 ± 2.1 | 100/0 | Real-time quantitative PCR | 6 380 ± 60 | N/A |
| Hassler et al., 2021 ** [56] | Observational, cross-sectional study | Austrians without CVDs | 90 | 40.77 ± 11.62 | 100/0 | Real- time quantitative PCR | N/A | 0.7 ± 0.28 |
| Hassler et al., 2021 ** [56] | Observational, cross-sectional study | Austrians without CVDs | 90 | 44.71 ± 10.96 | 0/100 | Real- time quantitative PCR | N/A | 0.69 ± 0.31 |
| Authors [References] | Study Characteristics | Group Characteristics | Participants (n) | Age, in Years (M ± SE or Me [P25; P75]) | Sex (Male/Female, %) | Method | Telomere Length | |
|---|---|---|---|---|---|---|---|---|
| Absolute, bp | Relative (T/S Ratio), CU | |||||||
| Williet et al., 2010 [55] | Prospective, population-based study | Austrians with a stable CAD | 88 | 70 ± 10.5 | 63.6/26.4 | Real- time quantitative PCR | N/A | 1.13 ± 0.52 |
| Yakhontov et al., 2017 * [64] | Observational, cross-sectional study | Russians with stable CAD I-III FC | 59 | 52 [46.5; 55] | 100/0 | Real- time quantitative PCR | N/A | 0.84 [0.2; 1.9] |
| Yakhontov et al., 2017 * [64] | Observational, cross-sectional study | Russians with stable CAD I-III FC | 47 | 64 [62; 67] | 100/0 | Real- time quantitative PCR | N/A | 0.3 [0.09; 1.2] |
| Hammadah et al., 2017 [63] | Observational, cross-sectional study | Canadians with stable CAD | 566 | 63 ± 9,0 | 63.6/26.4 | Real- time quantitative PCR | N/A | 0.82 ± 0.14 |
| Tian et al., 2017 [58] | Observational, cross-sectional study | Chinese with premature CAD | 128 | 48.6 ± 7.26 | 57.8/42.2 | Real- time quantitative PCR | N/A | 0.88 ± 0.86 |
| Yakhontov et al., 2018 [65] | Observational, cross-sectional study | Russians with essential hypertension and stable CAD I-III FC | 43 | 52 [46.5; 55.0] | 100/0 | Real- time quantitative PCR | N/A | 0.7 [0.12; 0.92] |
| Pejenaute et al., 2020 [59] | Observational, cross-sectional study | Spaniards with coronary atherosclerosis | 116 | 61 ± 1 | 88/12 | Real- time quantitative PCR | 8315 ± 98 | N/A |
| Starnino et al., 2021 [61] | Observational, cross-sectional study | Canadians with stable CAD | 598 | 66.13 ± 6.25 | 80.6/19.4 | Real- time quantitative PCR | N/A | 0.83 ± 0.18 |
| Authors [References] | Study Characteristics | Group Characteristics | Participants (n) | Age, in Years (M ± SE or Me [P25; P75]) | Sex (Male/Female, %) | Method | Telomere Length | |
|---|---|---|---|---|---|---|---|---|
| Absolute, bp | Relative (T/S Ratio), CU | |||||||
| Russo A. et al., 2012 [69] | Observational, open, cross-sectional, longitudinal study. | Italians with AMI | 199 | 40.1 ± 5 | 89.4/10.6 | Real- time quantitative PCR | N/A | 0.77 ± 0.2 |
| Dlouha, D. et al., 2016 [57] | Observational, cross-sectional case-control study | Czechs with AMI | 505 | 61 ± 9.7 | 0/100 | Real- time quantitative PCR | N/A | 0.86 ± 0.32 |
| Margaritis, M. et al., 2017 [70] | Observational, open, cross-sectional, longitudinal study | British with AMI | 290 | 63 ± 12.7 | 85.2/14.8 | Real- time quantitative PCR | N/A | 1.08 [0.41—2.66] * |
| Gupta M.D. et al., 2020 [60] | Observatio-nal, open, cross-sectional study | Indians with AMI | 77 | 35.33 ± 6.22 | 84.4/15.6 | Real- time quantitative PCR | N/A | 0.115 |
| Chan D. et al., 2020 [71] | Prospective, observation, cohort, longitudinal study. | British with AMI | 135 | 81 ± 4 | 64/36 | Real- time quantitative PCR | N/A | 0.47 ± 0.25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zimnitskaya, O.V.; Petrova, M.M.; Lareva, N.V.; Cherniaeva, M.S.; Al-Zamil, M.; Ivanova, A.E.; Shnayder, N.A. Leukocyte Telomere Length as a Molecular Biomarker of Coronary Heart Disease. Genes 2022, 13, 1234. https://doi.org/10.3390/genes13071234
Zimnitskaya OV, Petrova MM, Lareva NV, Cherniaeva MS, Al-Zamil M, Ivanova AE, Shnayder NA. Leukocyte Telomere Length as a Molecular Biomarker of Coronary Heart Disease. Genes. 2022; 13(7):1234. https://doi.org/10.3390/genes13071234
Chicago/Turabian StyleZimnitskaya, Olga V., Marina M. Petrova, Natalia V. Lareva, Marina S. Cherniaeva, Mustafa Al-Zamil, Anastasia E. Ivanova, and Natalia A. Shnayder. 2022. "Leukocyte Telomere Length as a Molecular Biomarker of Coronary Heart Disease" Genes 13, no. 7: 1234. https://doi.org/10.3390/genes13071234
APA StyleZimnitskaya, O. V., Petrova, M. M., Lareva, N. V., Cherniaeva, M. S., Al-Zamil, M., Ivanova, A. E., & Shnayder, N. A. (2022). Leukocyte Telomere Length as a Molecular Biomarker of Coronary Heart Disease. Genes, 13(7), 1234. https://doi.org/10.3390/genes13071234








