A Preparative Method for the Isolation of Calponin from Molluscan Catch Muscle
Abstract
1. Introduction
2. Results
2.1. Rigorization and Washing of Mussel Muscles
2.2. Mussel Calponin Fraction
2.3. Calponin Chromatography
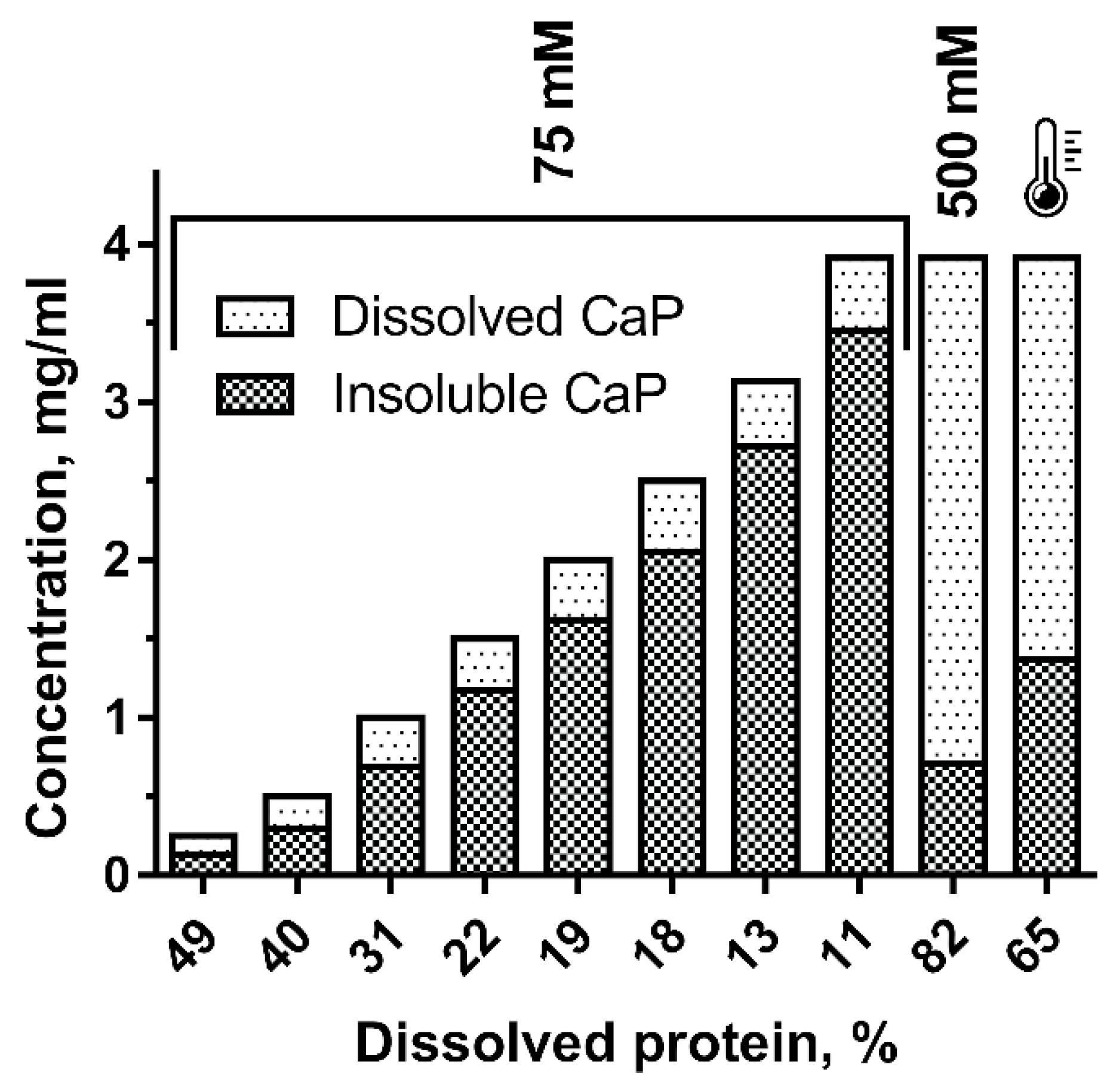
2.4. Calponin Solubility
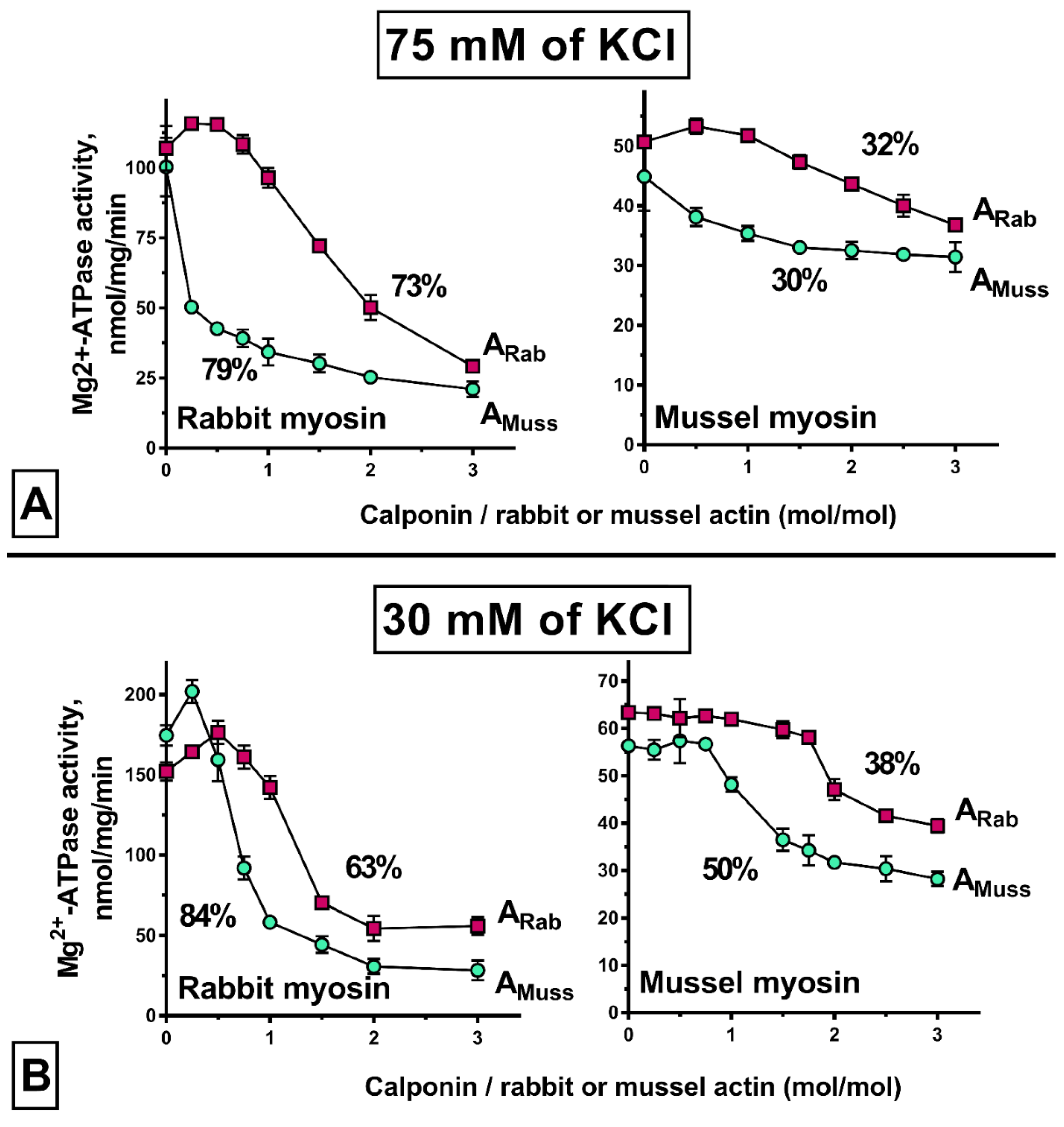
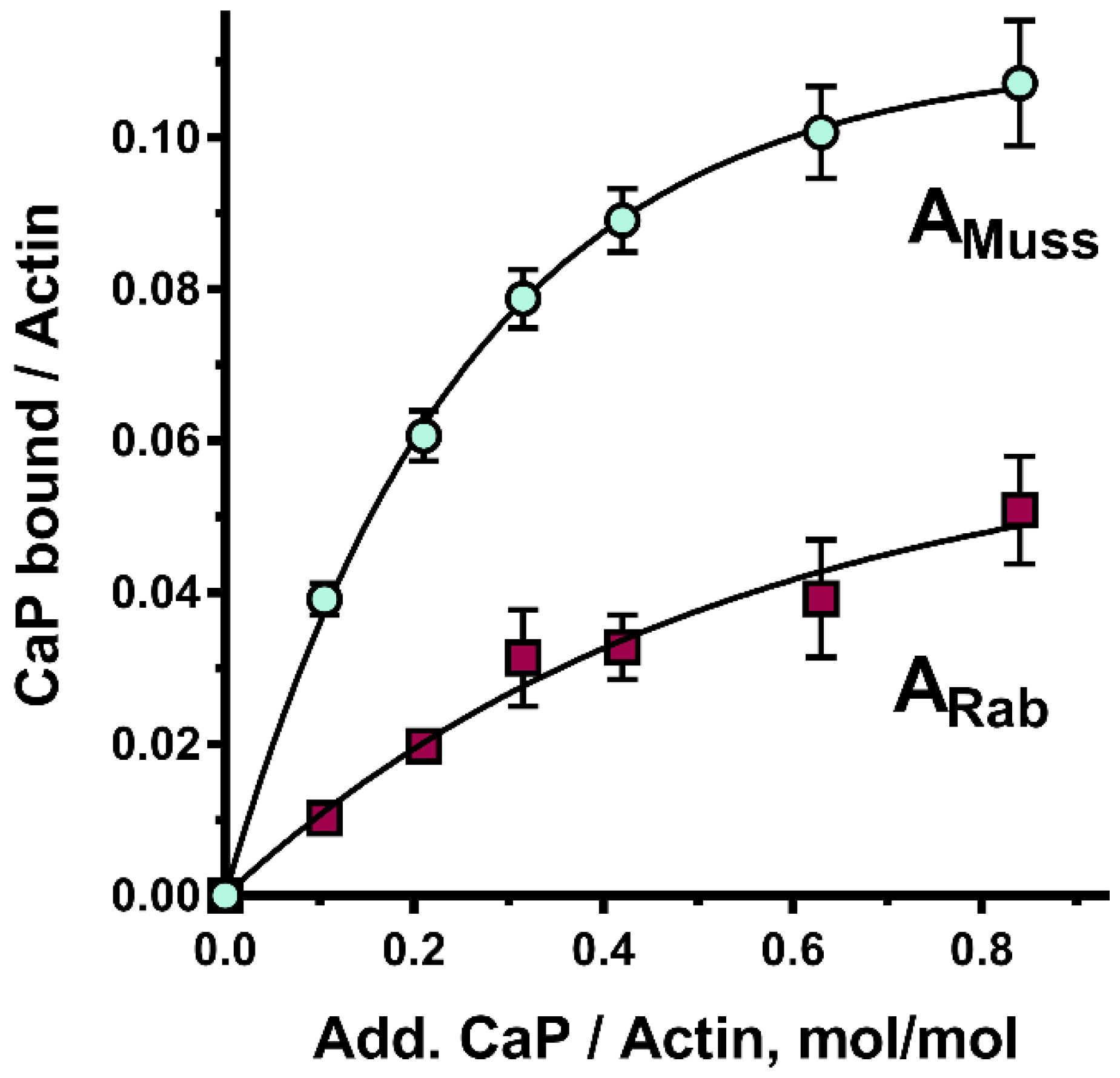
2.5. Properties of Mussel Calponin
3. Discussion
4. Materials and Methods
4.1. Protein Isolation
4.2. Mg2+-ATPase Assay
4.3. Protein Binding
4.4. Calponin Solubility
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rozenblum, G.T.; Gimona, M. Calponins: Adaptable modular regulators of the actin cytoskeleton. Int. J. Biochem. Cell Biol. 2008, 40, 1990–1995. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Hiwada, K.; Kokubu, T. Isolation and characterization of a 34000-dalton calmodulin- and F-actin-binding protein from chicken gizzard smooth muscle. Biochem. Biophys. Res. Commun. 1986, 141, 20–26. [Google Scholar] [CrossRef]
- Winder, S.J.; Allen, B.G.; Fraser, E.D.; Kang, H.M.; Kargacin, G.J.; Walsh, M.P. Calponin phosphorylation in vitro and in intact muscle. Biochem. J. 1993, 296, 827–836. [Google Scholar] [CrossRef]
- Winder, S.J.; Walsh, M.P. Smooth muscle calponin. Inhibition of actomyosin MgATPase and regulation by phosphorylation. J. Biol. Chem. 1990, 265, 10148–10155. [Google Scholar] [CrossRef]
- Draeger, A.; Gimona, M.; Stuckert, A.; Celis, J.E.; Small, J.V. Calponin Developmental isoforms and a low molecular weight variant. FEBS Lett. 1991, 291, 24–28. [Google Scholar] [CrossRef]
- Hossain, M.M.; Hwang, D.Y.; Huang, Q.Q.; Sasaki, Y.; Jin, J.P. Developmentally regulated expression of calponin isoforms and the effect of h2-calponin on cell proliferation. Am. J. Physiol. Cell Physiol. 2003, 284, C156–C167. [Google Scholar] [CrossRef]
- Dillon, P.; Aksoy, M.; Driska, S.; Murphy, R. Myosin phosphorylation and the cross-bridge cycle in arterial smooth muscle. Science 1981, 211, 495–497. [Google Scholar] [CrossRef]
- Marston, S.B. What is latch? New ideas about tonic contraction in smooth muscle. J. Muscle Res. Cell Motil. 1989, 10, 97–100. [Google Scholar] [CrossRef]
- Murphy, R.A.; Rembold, C.M. The latch-bridge hypothesis of smooth muscle contraction. Can. J. Physiol. Pharmacol. 2005, 83, 857–864. [Google Scholar] [CrossRef]
- Funabara, D.; Osakabe, Y.; Kanoh, S. Calponin Isoform Expression in the Japanese Pearl Oyster, Pinctada fucata. Am. J. Mol. Biol. 2019, 9, 154–172. [Google Scholar] [CrossRef][Green Version]
- Szymanski, P.T.; Program, P. Calponin (CaP) as a latch-bridge protein—a new concept in regulation of contractility in smooth muscles. J. Muscle Res. Cell Motil. 2004, 25, 7–19. [Google Scholar] [CrossRef]
- Funabara, D.; Nakaya, M.; Watabe, S. Isolation and characterization of a novel 45 kDa calponin-like protein from anterior byssus retractor muscle of the mussel Mytilus galloprovincialis. Fish. Sci. 2001, 67, 511–517. [Google Scholar] [CrossRef]
- Dobrzhanskaya, A.V.; Vyatchin, I.G.; Lazarev, S.S.; Matusovsky, O.S.; Shelud’ko, N.S. Molluscan smooth catch muscle contains calponin but not caldesmon. J. Muscle Res. Cell Motil. 2013, 34, 23–33. [Google Scholar] [CrossRef]
- Sirenko, V.V.; Simonyan, A.O.; Dobrzhanskaya, A.V.; Shelud’ko, N.S.; Borovikov, Y.S. Modulation of conformations of myosin subfragment-1 (S-1) and inhibition of S-1 ATPase by mussel calponin. Cell Tissue Biol. 2015, 9, 64–70. [Google Scholar] [CrossRef]
- Sirenko, V.V.; Dobrzhanskaya, A.V.; Shelud’ko, N.S.; Borovikov, Y.S. Calponin-like protein from mussel smooth muscle is a competitive inhibitor of actomyosin ATPase. Biochemistry (Moscow) 2016, 81, 28–33. [Google Scholar] [CrossRef]
- Sirenko, V.V.; Simonyan, A.H.; Dobrzhanskaya, A.V.; Shelud’ko, N.S.; Borovikov, Y.S. 40-kDa protein from thin filaments of the mussel Crenomytilus grayanus changes the conformation of F-actin during the ATPase cycle. Biochemistry (Moscow) 2013, 78, 273–281. [Google Scholar] [CrossRef]
- Funabara, D.; Watabe, S.; Kanoh, S. Molecular characterization of calponin in the catch muscle of the Yesso scallop Mizuhopecten yessoensis. Fish. Sci. 2015, 81, 155–162. [Google Scholar] [CrossRef]
- Matusovsky, O.S.; Dobrzhanskaya, A.V.; Pankova, V.V.; Kiselev, K.V.; Girich, U.V.; Shelud’ko, N.S. Crenomytilus grayanus 40 kDa calponin-like protein: cDNA cloning, sequence analysis, tissue expression, and post-translational modifications. Comp. Biochem. Physiol. Part. D Genom. Proteom. 2017, 22, 98–108. [Google Scholar] [CrossRef]
- Funabara, D.; Watanabe, D.; Satoh, N.; Kanoh, S. Genome-wide survey of genes encoding muscle proteins in the pearl oyster, Pinctada fucata. Zoolog. Sci. 2013, 30, 817–825. [Google Scholar] [CrossRef]
- Vyatchin, I.G.; Shevchenko, U.V.; Lazarev, S.S.; Matusovsky, O.S.; Shelud’ko, N.S. Troponin-like regulation in muscle thin filaments of the mussel Crenomytilus grayanus (Bivalvia: Mytiloida). Biochim. Biophys. Acta 2015, 1854, 1444–1450. [Google Scholar] [CrossRef] [PubMed]
- Shelud’ko, N.S.; Vyatchin, I.G.; Lazarev, S.S.; Shevchenko, U.V. Hybrid and non-hybrid actomyosins reconstituted with actin, myosin and tropomyosin from skeletal and catch muscles. Biochem. Biophys. Res. Commun. 2015, 464, 611–615. [Google Scholar] [CrossRef]
- Shelud’ko, N.S.; Girich, U.V.; Lazarev, S.S.; Vyatchin, I.G. Non-Straub type actin from molluscan catch muscle. Biochem. Biophys. Res. Commun. 2016, 474, 384–387. [Google Scholar] [CrossRef] [PubMed]
- Otani, O.; Hikichi, S.; Nishita, K.; Sekii, T.; Arai, K. A Loss of Actin fron shell-fish Myofibrils and Fish myosin B during Wash-Treatment. NIippon Suisan Gakkaishi 1983, 49, 415–424. [Google Scholar] [CrossRef]
- Shelud’ko, N.; Permjakova, T.; Tuturova, K.; Neverkina, O.; Drozdov, A. Myorod, a thick filament protein in molluscan smooth muscles: Isolation, polymerization and interaction with myosin. J. Muscle Res. Cell Motil. 2001, 22, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Margossian, S.S.; Lowey, S. Preparation of myosin and its subfragments from rabbit skeletal muscle. Methods Enzymol. 1982, 85, 55–72. [Google Scholar]
- Rees, M.; Young, M. Studies on the Isolation and Molecular Properties of Homogeneous Globular Actin: Evidence for a A single Polypeptide Chain Structure. J. Biol. Chem. 1967, 242, 4449–4458. [Google Scholar] [CrossRef]
- Itzhaki, R.; Gill, D. A microbiuret method for estimating proteins. Anal.Biochem. 1964, 9, 401–410. [Google Scholar] [CrossRef]
- Taussky, H.H.; Shorr, E. A microcolorimetric method for the determination of inorganic phosphorus. J. Biol. Chem. 1953, 202, 675–685. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lazarev, S.S.; Shevchenko, U.V.; Dyachuk, V.A.; Vyatchin, I.G. A Preparative Method for the Isolation of Calponin from Molluscan Catch Muscle. Int. J. Mol. Sci. 2022, 23, 7993. https://doi.org/10.3390/ijms23147993
Lazarev SS, Shevchenko UV, Dyachuk VA, Vyatchin IG. A Preparative Method for the Isolation of Calponin from Molluscan Catch Muscle. International Journal of Molecular Sciences. 2022; 23(14):7993. https://doi.org/10.3390/ijms23147993
Chicago/Turabian StyleLazarev, Stanislav S., Ulyana V. Shevchenko, Vyacheslav A. Dyachuk, and Ilya G. Vyatchin. 2022. "A Preparative Method for the Isolation of Calponin from Molluscan Catch Muscle" International Journal of Molecular Sciences 23, no. 14: 7993. https://doi.org/10.3390/ijms23147993
APA StyleLazarev, S. S., Shevchenko, U. V., Dyachuk, V. A., & Vyatchin, I. G. (2022). A Preparative Method for the Isolation of Calponin from Molluscan Catch Muscle. International Journal of Molecular Sciences, 23(14), 7993. https://doi.org/10.3390/ijms23147993





