Enhanced Photoelectrochemical Activity of TiO2 Nanotubes Decorated with Lanthanide Ions for Hydrogen Production
Abstract
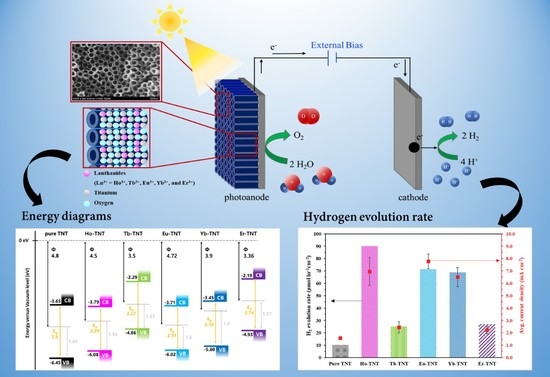
:1. Introduction
2. Results and Discussion
2.1. Morphology and Surface Characteristics of Lanthanide-Doped TiO2
2.2. Electronic Structure and Optical Properties of Lanthanide-Doped TNTs
2.3. Photoelectrochemical Characterization
2.4. Photoelectrochemical Performance of Lanthanide-Doped TNT
3. Materials and Methods
3.1. Preparation of TiO2 Nanotubes
3.2. Preparation of Lanthanide-Doped TiO2 Nanotubes
3.3. Characterization
3.4. Electrochemical Characteristics
3.5. Photoelectrochemical Water Splitting Experiment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Khribich, A.; Kacem, R.H.; Dakhlaoui, A. Causality nexus of renewable energy consumption and social development: Evidence from high-income countries. Renew. Energy 2021, 169, 14–22. [Google Scholar] [CrossRef]
- Rivero, M.J.; Iglesias, O.; Ribao, P.; Ortiz, I. Kinetic performance of TiO2/Pt/reduced graphene oxide composites in the photocatalytic hydrogen production. Int. J. Hydrog. Energy 2019, 44, 101–109. [Google Scholar] [CrossRef]
- Zou, X.; Zhang, Y. Noble metal-free hydrogen evolution catalysts for water splitting. Chem. Soc. Rev. 2015, 44, 5148–5180. [Google Scholar] [CrossRef]
- Police, A.K.R.; Chennaiahgari, M.; Boddula, R.; Vattikuti, S.V.P.; Mandari, K.K.; Chan, B. Single-step hydrothermal synthesis of wrinkled graphene wrapped TiO2 nanotubes for photocatalytic hydrogen production and supercapacitor applications. Mater. Res. Bull. 2018, 98, 314–321. [Google Scholar] [CrossRef]
- Breeze, P. Solar power. In Power Generation Technologies; Elsevier: Amsterdam, The Netherlands, 2019; pp. 293–321. [Google Scholar] [CrossRef]
- Wang, Y.-Y.; Chen, Y.-X.; Barakat, T.; Zeng, Y.-J.; Liu, J.; Siffert, S.; Su, B.-L. Recent advances in non-metal doped titania for solar-driven photocatalytic/photoelectrochemical water-splitting. J. Energy Chem. 2022, 66, 529–559. [Google Scholar] [CrossRef]
- Hu, H.; Qian, D.; Lin, P.; Ding, Z.; Cui, C. Oxygen vacancies mediated in-situ growth of noble-metal (Ag. Au, Pt) nanoparticles on 3D TiO2 hierarchical spheres for efficient photocatalytic hydrogen evolution from water splitting. Int. J. Hydrog. Energy 2020, 45, 629–639. [Google Scholar] [CrossRef]
- Lu, Q.; Wei, Z.; Li, C.; Ma, J.; Li, L. Photocatalytic degradation of methyl orange by noble metal Ag modified semiconductor Zn2SnO4. Mater. Sci. Semicond. Process 2022, 138, 106290. [Google Scholar] [CrossRef]
- Kovalevskiy, N.; Selishchev, D.; Svintsitskiy, D.; Selishcheva, S.; Berezin, A.; Kozlov, D. Synergistic effect of polychromatic radiation on visible light activity of N-doped TiO2 photocatalyst. Catal. Commun. 2020, 134, 105841. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiong, X.; Han, Y.; Yuan, H.; Deng, S.; Xiao, H.; Shen, F.; Wu, X. Application of titanium dioxide-loaded activated carbon fiber in a pulsed discharge reactor for degradation of methyl orange. Chem. Eng. J. 2010, 162, 1045–1049. [Google Scholar] [CrossRef]
- Bai, L.; Huang, H.; Yu, S.; Zhang, D.; Huang, H.; Zhang, Y. Role of transition metal oxides in g-C3N4-based heterojunctions for photocatalysis and supercapacitors. J. Energy Chem. 2022, 64, 214–235. [Google Scholar] [CrossRef]
- Barmeh, A.; Nilforoushan, M.R.; Otroj, S. Wetting and photocatalytic properties of Ni-doped TiO2 coating on glazed ceramic tiles under visible light. Thin Solid Film. 2018, 666, 137–142. [Google Scholar] [CrossRef]
- Cho, H.; Joo, H.; Kim, H.; Kim, J.E.; Kang, K.S.; Yoon, J. Enhanced photocatalytic activity of TiO2 nanotubes decorated with erbium and reduced graphene oxide. Appl. Surf. Sci. 2021, 565, 150459. [Google Scholar] [CrossRef]
- Yoon, J.; Bae, S.; Shim, E.; Joo, H. Pyrococcus furiosus-immobilized anodized tubular titania cathode in a hydrogen production system. J. Power Sources 2009, 189, 1296–1301. [Google Scholar] [CrossRef]
- Nie, J.; Mo, Y.; Zheng, B.; Yuan, H.; Xiao, D. Electrochemical fabrication of lanthanum-doped TiO2 nanotube array electrode and investigation of its photoelectrochemical capability. Electrochim. Acta 2013, 90, 589–596. [Google Scholar] [CrossRef]
- Mazierski, P.; Lisowski, W.; Grzyb, T.; Winiarski, M.J.; Klimczuk, T.; Mikołajczyk, A.; Flisikowski, J.; Hirsch, A.; Kołakowska, A.; Puzyn, T.; et al. Enhanced photocatalytic properties of lanthanide-TiO2 nanotubes: An experimental and theoretical study. Appl. Catal. B 2017, 205, 376–385. [Google Scholar] [CrossRef]
- Khoshnavazi, R.; Sohrabi, H.; Bahrami, L.; Amiri, M. Photocatalytic activity inhancement of TiO2 nanoparticles with lanthanide ions and sandwich-type polyoxometalates. J. Sol-Gel Sci. Technol. 2017, 83, 332–341. [Google Scholar] [CrossRef]
- Avram, D.; Patrascu, A.A.; Istrate, M.C.; Cojocaru, B.; Tiseanu, C. Lanthanide doped TiO2: Coexistence of discrete and continuous dopant distribution in anatase phase. J. Alloys Compd. 2021, 851, 156849. [Google Scholar] [CrossRef]
- Yu, J.; Yang, Y.; Zhang, C.; Fan, R.; Su, T. Preparation of YbF3-Ho@TiO2 core-shell sub-microcrystal spheres and their application to the electrode of dye-sensitized solar cells. New J. Chem. 2020, 44, 10545–10553. [Google Scholar] [CrossRef]
- Kumaravel, V.; Mathew, S.; Bartlett, J.; Pillai, S.C. Photocatalytic hydrogen production using metal doped TiO2: A review of recent advances. Appl. Catal. B 2019, 244, 1021–1064. [Google Scholar] [CrossRef]
- Lee, D.Y.; Kim, J.T.; Park, J.H.; Kim, Y.H.; Lee, I.K.; Lee, M.H.; Kim, B.Y. Effect of Er doping on optical band gap energy of TiO2 thin films prepared by spin coating. Curr. Appl. Phys. 2013, 13, 1301–1305. [Google Scholar] [CrossRef]
- Reszczyńska, J.; Grzyb, T.; Sobczak, J.W.; Lisowski, W.; Gazda, M.; Ohtani, B.; Zaleska, A. Lanthanide co-doped TiO2: The effect of metal type and amount on surface properties and photocatalytic activity. Appl. Surf. Sci. 2014, 307, 333–345. [Google Scholar] [CrossRef]
- Xu, R.; Li, Y.; Feng, S.; Wang, J.; Zhang, J.; Zhang, X.; Bian, C.; Fu, W.; Li, Z.; Yang, H. Enhanced performance of planar perovskite solar cells using Ce-doped TiO2 as electron transport layer. J. Mater. Sci. 2020, 55, 5681–5689. [Google Scholar] [CrossRef]
- Agresti, A.; Pazniak, A.; Pescetelli, S.; di Vito, A.; Rossi, D.; Pecchia, A.; Auf der Maur, M.; Liedl, A.; Larciprete, R.; Kuznetsov, D.V.; et al. Titanium-carbide MXenes for work function and interface engineering in perovskite solar cells. Nat. Mater. 2019, 18, 1228–1234. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Wang, Y.; Sun, M.; Hanif, A.; Wu, H.; Gu, Q.; Ok, Y.S.; Tsang, D.C.W.; Li, J.; Yu, J.; et al. Thermally treated zeolitic imidazolate framework-8 (ZIF-8) for visible light photocatalytic degradation of gaseous formaldehyde. Chem. Sci. 2020, 11, 6670–6681. [Google Scholar] [CrossRef]
- Shi, L.; Xu, C.; Sun, X.; Zhang, H.; Liu, Z.; Qu, X.; Du, F. Facile fabrication of hierarchical BiVO4/TiO2 heterostructures for enhanced photocatalytic activities under visible-light irradiation. J. Mater. Sci. 2018, 53, 11329–11342. [Google Scholar] [CrossRef]
- Kalanur, S.S. Structural, optical, band edge and enhanced photoelectrochemical water splitting properties of Tin-doped WO3. Catalysts 2019, 9, 456. [Google Scholar] [CrossRef] [Green Version]
- Maheu, C.; Cardenas, L.; Puzenat, E.; Afanasiev, P.; Geantet, C. UPS and UV spectroscopies combined to position the energy levels of TiO2 anatase and rutile nanopowders. Phys. Chem. Chem. Phys. 2018, 20, 25629–25637. [Google Scholar] [CrossRef]
- Xu, G.; Xu, Z.; Shi, Z.; Pei, L.; Yan, S.; Gu, Z.; Zou, Z. Silicon photoanodes partially covered by Ni@Ni(OH)2 core–shell particles for photoelectrochemical water oxidation. ChemSusChem 2017, 10, 2897–2903. [Google Scholar] [CrossRef]
- Zhang, H.; Ebaid, M.; Min, J.W.; Ng, T.K.; Ooi, B.S. Enhanced photoelectrochemical performance of InGaN-based nanowire photoanodes by optimizing the ionized dopant concentration. J. Appl. Phys. 2018, 124, 083105. [Google Scholar] [CrossRef]
- Liu, C.; Wang, F.; Zhang, J.; Wang, K.; Qiu, Y.; Liang, Q.; Chen, Z. Efficient photoelectrochemical water splitting by g-C3N4/TiO2 nanotube array heterostructures. Nano-Micro Lett. 2018, 10, 37. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Wang, F.; Zhu, S.; Xu, Y.; Liang, Q.; Chen, Z. Controlled charge-dynamics in cobalt-doped TiO2 nanowire photoanodes for enhanced photoelectrochemical water splitting. J. Colloid Interface Sci. 2018, 530, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Marchesi, L.F.; Freitas, R.G.; Spada, E.R.; Paula, F.R.; Góes, M.S.; Garcia, J.R. Photoelectrochemical characterization of ITO/TiO2 electrodes obtained by cathodic electrodeposition from aqueous solution. J. Solid State Electrochem. 2015, 19, 2205–2211. [Google Scholar] [CrossRef] [Green Version]
- Raja, K.S.; Mahajan, V.K.; Misra, M. Determination of photo conversion efficiency of nanotubular titanium oxide photo-electrochemical cell for solar hydrogen generation. J. Power Sources 2006, 159, 1258–1265. [Google Scholar] [CrossRef]
- Samad, N.A.A.; Lai, C.W.; Lau, K.S.; Hamid, S.B.A. Efficient solar-induced photoelectrochemical response using coupling semiconductor TiO2-ZnO nanorod film. Materials 2016, 9, 937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Z.; Springer, M.A.; Geng, W.; Zhu, X.; Li, T.; Li, M.; Jing, Y.; Heine, T. Rational design of two-dimensional binary polymers from Heterotriangulenes for photocatalytic water splitting. J. Phys. Chem. Lett. 2021, 12, 8134–8140. [Google Scholar] [CrossRef] [PubMed]
- Altmaier, M.; Xavier, G.; David, F.; Gunnar, B. Intercomparison of Redox Determination Methods on Designed and Near-Natural Aqueous Systems. Volume 7572 of KIT Scientific Reports; KIT Scientific Publishing: Karlsruhe, Germany, 2011. [Google Scholar] [CrossRef]
- Mortazavi, B.; Javvaji, B.; Shojaei, F.; Rabczuk, T.; Shapeev, A.V.; Zhuang, X. Exceptional piezoelectricity, high thermal conductivity and stiffness and promising photocatalysis in two-dimensional MoSi2N4 family confirmed by first-principles. Nano Energy 2021, 82, 105716. [Google Scholar] [CrossRef]
- Ayon, S.A.; Billah, M.M.; Nishat, S.S.; Kabir, A. Enhanced photocatalytic activity of Ho3+ doped ZnO NPs synthesized by modified sol–gel method: An experimental and theoretical investigation. J. Alloys Compd. 2021, 856, 158217. [Google Scholar] [CrossRef]
- Pan, T.-M.; Chen, C.-H.; Liu, J.-H. Structural properties and electrical characteristics of Ho2O3 and HoTixOy gate dielectrics for a-InGaZnO thin-film transistors. RSC Adv. 2014, 4, 29300–29304. [Google Scholar] [CrossRef]
- Cho, H.; Joo, H.; Kim, H.; Kim, J.E.; Kang, K.S.; Yoon, J. Improved photoelectrochemical properties of TiO2 nanotubes doped with Er and effects on hydrogen production from water splitting. Chemosphere 2021, 267, 129289. [Google Scholar] [CrossRef]








| Sample Label | EDS | XRD | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elemental Composition (Atomic %) | Lattice Parameter | |||||||||||
| Ti | O | Ho | Tb | Eu | Yb | Er | Size (Å) | a (Å) | b (Å) | c (Å) | Cell Volume (Å3) | |
| pure TNT | 31.13 | 68.87 | - | - | - | - | - | 351 | 3.78 | 3.78 | 9.49 | 135.42 |
| Ho-TNT | 28.44 | 69.35 | 2.22 | - | - | - | - | 289 | 3.78 | 3.78 | 9.48 | 135.35 |
| Tb-TNT | 29.53 | 70.03 | - | 0.44 | - | - | - | 279 | 3.78 | 3.78 | 9.50 | 135.69 |
| Eu-TNT | 30.59 | 68.26 | - | - | 1.15 | - | - | 323 | 3.78 | 3.78 | 9.48 | 135.29 |
| Yb-TNT | 29.2 | 69.59 | - | - | - | 1.2 | - | 337 | 3.78 | 3.78 | 9.48 | 135.29 |
| Er-TNT | 27.94 | 71.19 | - | - | - | - | 0.87 | 341 | 3.78 | 3.78 | 9.48 | 135.38 |
| Sample Label | Work Function (eV) | Valence Band (eV) | Conduction Band (eV) | Band Gap (eV) |
|---|---|---|---|---|
| pure TNT | 4.8 | −6.45 | −3.65 | 2.8 |
| Ho-TNT | 4.53 | −6.08 | −3.79 | 2.29 |
| Tb-TNT | 3.53 | −4.86 | −2.29 | 2.57 |
| Eu-TNT | 4.72 | −6.02 | −3.71 | 2.31 |
| Yb-TNT | 3.90 | −5.80 | −3.45 | 2.35 |
| Er-TNT | 3.36 | −4.93 | −2.19 | 2.74 |
| Sample Label | Light Off (V vs. Hg/HgO) | Light On (V vs. Hg/HgO) | OCP(V vs. Hg/HgO) | Maximum Current Density (mAcm−2) | Photo-Conversion Efficiency (%) |
|---|---|---|---|---|---|
| pure TNT | −0.16 | −0.61 | 0.46 | 1.03 | 0.29 |
| Ho-TNT | −0.25 | −0.87 | 0.63 | 23.6 | 2.68 |
| Tb-TNT | −0.25 | −0.82 | 0.57 | 8.15 | 1.55 |
| Eu-TNT | −0.17 | −0.77 | 0.61 | 18.6 | 2.21 |
| Yb-TNT | −0.17 | −0.83 | 0.66 | 14.6 | 2.19 |
| Er-TNT | −0.14 | −0.71 | 0.57 | 7.21 | 1.59 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, H.; Joo, H.; Kim, H.; Kim, J.-E.; Kang, K.-S.; Jung, H.; Yoon, J. Enhanced Photoelectrochemical Activity of TiO2 Nanotubes Decorated with Lanthanide Ions for Hydrogen Production. Catalysts 2022, 12, 866. https://doi.org/10.3390/catal12080866
Cho H, Joo H, Kim H, Kim J-E, Kang K-S, Jung H, Yoon J. Enhanced Photoelectrochemical Activity of TiO2 Nanotubes Decorated with Lanthanide Ions for Hydrogen Production. Catalysts. 2022; 12(8):866. https://doi.org/10.3390/catal12080866
Chicago/Turabian StyleCho, Hyekyung, Hyunku Joo, Hansung Kim, Ji-Eun Kim, Kyoung-Soo Kang, Hyeonmin Jung, and Jaekyung Yoon. 2022. "Enhanced Photoelectrochemical Activity of TiO2 Nanotubes Decorated with Lanthanide Ions for Hydrogen Production" Catalysts 12, no. 8: 866. https://doi.org/10.3390/catal12080866
APA StyleCho, H., Joo, H., Kim, H., Kim, J.-E., Kang, K.-S., Jung, H., & Yoon, J. (2022). Enhanced Photoelectrochemical Activity of TiO2 Nanotubes Decorated with Lanthanide Ions for Hydrogen Production. Catalysts, 12(8), 866. https://doi.org/10.3390/catal12080866