In Vitro DNA-Binding, Anti-Oxidant and Anticancer Activity of Indole-2-Carboxylic Acid Dinuclear Copper(II) Complexes
Abstract
:1. Introduction
2. Results and Discussion
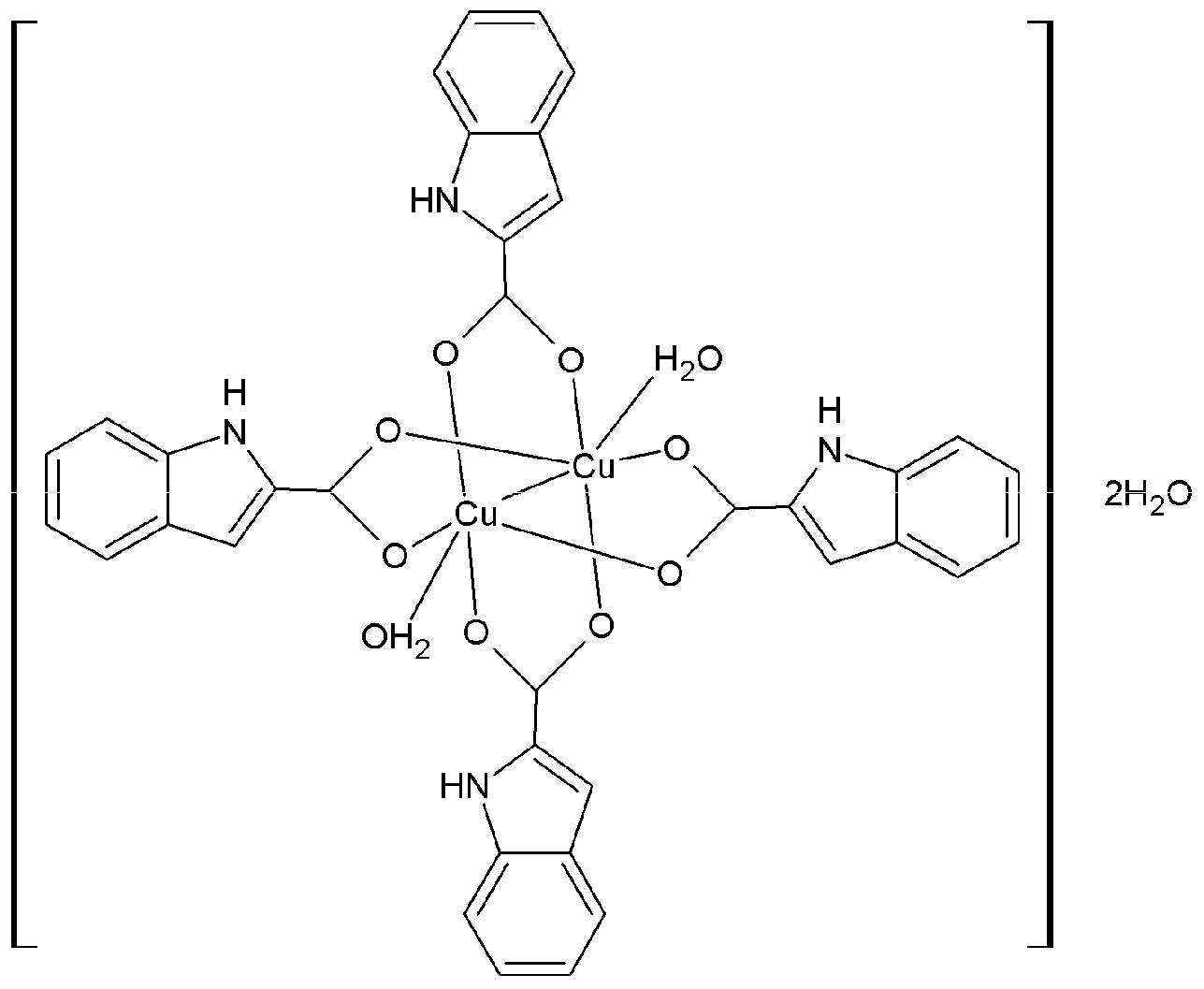
2.1. Synthesis and Characterization of ICA-Cu Complexes
2.1.1. Infrared Spectra
2.1.2. UV-Vis Absorption Spectra
2.1.3. 1H-NMR Spectra
2.1.4. Thermogravimetric Analysis
2.1.5. Elemental Analysis
2.1.6. Molar Conductivity
2.2. DNA Binding Studies
2.2.1. Fluorescence Spectroscopy Studies
2.2.2. Studies of Competitive DNA-Binding Ability with EB
2.2.3. Effect of Ionic Strength
2.2.4. Quenching Experiment of [Fe(CN)6]3−
2.2.5. Viscosity Measurement
2.3. Antioxidant Activity
2.4. In Vitro Cytotoxicity Studies
3. Experimental Section
3.1. Materials and Methods
3.2. Synthesis of the Complexes
3.3. DNA Binding Experiments
3.4. Antioxidant Experiments
3.5. In Vitro Anticancer Activity Experiments
3.6. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chen, W.Q.; Zheng, R.S.; Baade, P.D.; Zhang, S.W.; Zeng, H.M.; Bray, F.; Jemal, A.; Yu, X.Q.; He, J. Cancer Statistics in China, 2015. CA Cancer J. Clin. 2016, 66, 115–132. [Google Scholar] [CrossRef] [PubMed]
- Rebecca, L.S.; Kimberly, D.M.; Ahmedin, J. Cancer Statistics, 2016. CA Cancer J. Clin. 2016, 66, 7–30. [Google Scholar]
- Garbutcheon-Singh, K.B.; Grant, M.P.; Harper, B.W.; Krause-Heuer, A.M.; Manohar, M.; Orkey, N.; Aldrich-Wright, J.R. Transition Metal Based Anticancer Drugs. Curr. Top. Med. Chem. 2011, 11, 521–542. [Google Scholar] [CrossRef] [PubMed]
- Eckhardt, S. Recent progress in the development of anticancer agents. Curr. Med. Chem. Anticancer Agents 2002, 2, 419–439. [Google Scholar] [CrossRef] [PubMed]
- Kelland, L.R. Preclinical perspectives on platinum resistance. Drugs Exp. Clin. Res. 2000, 59 (Suppl. 4), 1–8. [Google Scholar] [CrossRef]
- Chen, D.; Milacic, V.; Frezza, M.; Dou, Q.P. Metal complexes, their celluar targets and potential for cancer therapy. Curr. Pharm. Des. 2009, 15, 777–791. [Google Scholar] [CrossRef] [PubMed]
- Milacic, V.; Chen, D.; Ronconi, L.; Landis-Piwowar, K.R.; Fregona, D.; Dou, Q.P. A novel anticancer gold(III) dithiocarbamate compound inhibits the activity of a purified 20S proteasome and 26S proteasome in human breast cancer cell cultures and xenografts. Cancer Res. 2006, 66, 10478–10486. [Google Scholar] [CrossRef] [PubMed]
- Cattaruzza, L.; Fregona, D.; Mongiat, M.; Ronconi, L.; Fassina, A.; Colombatti, A.; Aldinucci, D. Antitumor activity of gold(III)-dithiocarbamato derivatives on prostate cancer cells and xenografts. Int. J. Cancer 2011, 128, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Frezza, M.; Shakya, R.; Cui, Q.C.; Milacic, V.; Verani, C.N.; Dou, Q.P. Inhibition of the proteasome activity by gallium(III) complexes contributes to their anti prostate tumor effects. Cancer Res. 2007, 67, 9258–9265. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Bi, C.F.; Fan, Y.H.; Wang, H.N.; Bao, Y. Cefepime, a fourth-generation cephalosporin, in complex with manganese, inhibits proteasome activity and induces the apoptosis of human breast cancer cells. Int. J. Mol. Med. 2015, 36, 1143–1150. [Google Scholar] [CrossRef] [PubMed]
- Labbe, S.; Thiele, D.J. Pipes and wiring: The regulation of copper uptake and distribution in yeast. Trends Microbiol. 1999, 7, 500–505. [Google Scholar] [CrossRef]
- Tapiero, H.; Townsend, D.M.; Tew, K.D. Trace elements in human physiology and pathology. Biomed. Pharmacother. 2003, 57, 386–398. [Google Scholar] [CrossRef]
- Kuo, H.W.; Chen, S.F.; Wu, C.C.; Chen, D.R.; Lee, J.H. Serum and tissue trace elements in patients with breast cancer in Taiwan. Biol. Trace Elem. Res. 2002, 89, 1–11. [Google Scholar] [CrossRef]
- Nayak, S.B.; Bhat, V.R.; Upadhyay, D.; Udupa, S.L. Copper and ceruloplasmin status in serum of prostate and colon cancer patients. Indian J. Physiol. Pharmacol. 2003, 47, 108–110. [Google Scholar] [PubMed]
- Diez, M.; Arroyo, M.; Cerdan, F.J.; Munoz, M.; Martin, M.A.; Balibrea, J.L. Serum and tissue trace metal levels in lung cancer. Oncology 1989, 46, 230–234. [Google Scholar] [CrossRef] [PubMed]
- Turecky, L.; Kalina, P.; Uhlikova, E.; Namerova, S.; Krizko, J. Serum ceruloplasmin and copper levels in patients with primary brain tumors. Klin. Wochenschr. 1984, 62, 187–189. [Google Scholar] [CrossRef] [PubMed]
- Finney, L.; Vogt, S.; Fukai, T.; Glesne, D. Copper and angiogenesis: Unravelling a relationship key to cancer progression. Clin. Exp. Pharmacol. Physiol. 2009, 36, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Rau, K.M.; Huang, C.C.; Chiu, T.J.; Chen, Y.Y.; Lu, C.C.; Liu, C.T.; Pei, S.N.; Wei, Y.C. Neovascularization evaluated by CD105 correlates well with prognostic factors in breast cancers. Exp. Ther. Med. 2012, 4, 231–236. [Google Scholar] [PubMed]
- Daniel, K.G.; Chen, D.; Orlu, S.; Cui, Q.C.; Miller, F.R.; Dou, Q.P. Clioquinol and pyrrolidine dithiocarbamate complex with copper to form proteasome inhibitors and apoptosis inducers in human breast cancer cells. Breast Cancer Res. 2005, 7, R897–R908. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Cui, Q.C.; Yang, H.; Dou, Q.P. Disulfiram, a clinically used anti-alcoholism drug and copper-binding agent, induces apoptotic cell death in breast cancer cultures and xenografts via inhibition of the proteasome activity. Cancer Res. 2006, 66, 10425–10433. [Google Scholar] [CrossRef] [PubMed]
- Zhai, S.; Yang, L.; Cui, Q.C.; Sun, Y.; Dou, Q.P.; Yan, B. Tumor cellular proteasome inhibition and growth suppression by 8-hydroxyquinoline and clioquinol requires their capabilities to bind copper and transport copper into cells. J. Biol. Inorg. Chem. 2010, 15, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Duff, B.; Thangella, V.R.; Creaven, B.S.; Walsh, M.; Egan, D.A. Anti-cancer activity and mutagenic potential of novel copper(II) quinolinone Schiff base complexes in hepatocarcinoma cells. Eur. J. Pharmacol. 2012, 689, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Adsule, S.; Barve, V.; Chen, D.; Ahmed, F.; Dou, Q.P.; Padhye, S.; Sarkar, F.H. Novel Schiff base copper complexes of quinoline-2 carboxyaldehyde as proteasome inhibitors in human prostate cancer cells. J. Med. Chem. 2006, 49, 7242–7246. [Google Scholar] [CrossRef] [PubMed]
- Kumar, L.; Bala, S.; Jeet, K. The Diverse Pharmacological Importance of Indole Derivatives: A Review. Int. J. Res. Pharm. Sci. 2012, 2, 23–33. [Google Scholar]
- Rahman-Abdel, F. Synthesis of some new indole derivatives containing pyrazoles with potential antitumor activity. Arkivoc 2010, 11, 177–187. [Google Scholar]
- Kumar, D.; Kumar, N.; Singh, T. Synthesis of pharmacologically active 2-phenyl sulpha/substituted indole. Int. J. Eng. Sci. Technol. 2010, 2, 2553–2557. [Google Scholar]
- Zhang, Z.; Bi, C.F.; Schmitt, S.M.; Fan, Y.H.; Dong, L.L.; Zuo, J.; Dou, Q.P. 1,10-Phenanthroline promotes copper complexes into tumor cells and induces apoptosis by inhibiting the proteasome activity. J. Biol. Inorg. Chem. 2012, 17, 1257–1267. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Bi, C.F.; Buac, D.; Fan, Y.H.; Zhang, X.; Zuo, J.; Zhang, P.F.; Zhang, N.; Dong, L.L.; Dou, Q.P. Organic cadmium complexes as proteasome inhibitors and apoptosis inducers in human breast cancer cells. J. Inorg. Biochem. 2013, 123, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, H.Y.; Wang, Q.B.; Yan, M.C.; Wang, H.N.; Bi, C.F.; Sun, S.S.; Fan, Y.H. Anticancer activity and computational modeling of ternary copper(II) complexes with 3-indolecarboxylic acid and 1,10-phenanthroline. Int. J. Oncol. 2016, 49, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Kazuo, N.; Huang, D.R.; Wang, Q.R. Infrared and Raman Spectra of Inorganic and Coordination Compounds; Chemical Industry Press: Beijing, China, 1988. [Google Scholar]
- Gong, Q.J.; Jin, W.J.; Dong, C.; Liu, C.S. Synthesis of New Fluorescence Reagent: 4-Aminoantipyrine Aromatic Schiff Bases. Appl. Chem. 2000, 17, 227–229. [Google Scholar]
- Modukuru, N.K.; Snow, K.J.; Perrin, B.S., Jr.; Thota, J.; Kumar, C.V. Contributions of a long side chain to the binding affinity of an anthracene derivative to DNA. J. Phys. Chem. B 2005, 109, 11810–11818. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.C.; Zhang, K.J.; Lei, R.X.; Liu, J.N.; Zhou, T.L.; Yang, Z.Y. DNA-binding and anti-oxidation properties of binuclear lanthanide(III) complexes of 8-hydroxyquinoline-7-carbaldehyde-(isonicotinyl) hydrazone. J. Coord. Chem. 2012, 65, 2041–2054. [Google Scholar] [CrossRef]
- Palchaudhuri, R.; Hergenrother, P. DNA as a target for anticancer compounds: Methods to determine the mode of binding and the mechanism of action. Curr. Opin. Biotechnol. 2007, 18, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Ali, I.; Wani, W.A.; Saleem, K.; Wesselinova, D. Syntheses, DNA Binding and Anticancer Profiles of L-Glutamic Acid Ligand and its Copper(II) and Ruthenium(III) Complexes. Med. Chem. 2013, 9, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Skyrianou, K.C.; Psycharis, V.; Raptopoulou, C.P.; Kessissoglou, D.P.; Psomas, G. Nickel-quinolones interaction. Part 4—Structure and biological evaluation of nickel(II)-enrofloxacin complexes compared to zinc(II) analogues. J. Inorg. Biochem. 2011, 105, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Anjomshoa, M.; Torkzadeh-Mahani, M. In vitro DNA and BSA-binding, cell imaging and anticancer activity against human carcinoma cell lines of mixed ligand copper(II) complexes. Spectrochim. Acta A 2015, 150, 390–402. [Google Scholar] [CrossRef] [PubMed]
- Eshkourfu, R.; Čobeljić, B.; Vujčić, M.; Turel, I.; Pevec, A.; Sepčić, K.; Zec, M.; Radulović, S.; Srdić-Radić, T.; Mitić, D.; et al. Synthesis, characterization, cytotoxic activity and DNA binding Ni(II) complex with the 6-hydroxy chromone-3-carbaldehyde thiosemicarbazone. J. Organomet. Chem. 2009, 694, 4069–4075. [Google Scholar]
- Fox, J.T.; Sakamuru, S.; Huang, R.L.; Teneva, N.; Simmons, S.O.; Xia, M.H.; Tice, R.R.; Austin, C.P.; Myung, K. High-throughput genotoxicity assay identifies antioxidants as inducers of DNA damage response and cell death. Proc. Natl. Acad. Sci. USA 2012, 109, 5423–5428. [Google Scholar] [CrossRef] [PubMed]
- Li, G.Y.; Du, K.J.; Wang, J.Q.; Liang, J.W.; Kou, J.F.; Hou, X.J.; Ji, L.N.; Chao, H. Synthesis, crystal structure, DNA interaction and anticancer activity of tridentate copper(II) complexes. J. Inorg. Biochem. 2013, 119, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Xing, H.U.; Zhang, G.W.; Li, W.B.; He, L. Studies on the Interaction of Aminocarb with Calf Thymus DNA. J. Anal. Sci. 2010, 26, 195–198. [Google Scholar]
- Wang, Y.; Yang, Z.Y.; Wang, B.D. Synthesis, characterization and anti-oxidative activity of cobalt(II), nickel(II) and iron(II) Schiff base complexes. Transit. Met. Chem. 2005, 30, 879–883. [Google Scholar] [CrossRef]
- Li, X.C.; Lin, J.; Gao, Y.X.; Han, W.J.; Gao, D.F. Antioxidant activity and mechanism of Rhizoma Cimicifugae. Chem. Cent. J. 2012, 6, 140. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds ICA-Cu are available from the authors.








| Ligand/Complex | ν-NH- | νas(COO−) | νs(COO−) | νCu-O |
|---|---|---|---|---|
| ICA | 3355.75 | 1696.19 | 1200.91 | - |
| ICA-Cu | 3437.61 | 1510.92 | 1344.34 | 484.64 |
| Ligand/Complex | λmax |
|---|---|
| ICA | 292 |
| ICA-Cu | 298 |
| Ligand/Complex | H-3 | H-4 | H-5 | H-6 | H-7 | -COOH | -NH- |
|---|---|---|---|---|---|---|---|
| ICA | 7.252 (m) | 7.649 (m) | 7.051 (m) | 7.238 (m) | 7.442 (m) | 11.753 (s) | 12.943 (s) |
| ICA-Cu | 7.229 (m) | 7.519 (m) | 6.082 (m) | 7.042 (m) | 7.388 (m) | - | 14.476 (s) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Yan, M.; Wang, Q.; Wang, H.; Wang, Z.; Zhao, J.; Li, J.; Zhang, Z. In Vitro DNA-Binding, Anti-Oxidant and Anticancer Activity of Indole-2-Carboxylic Acid Dinuclear Copper(II) Complexes. Molecules 2017, 22, 171. https://doi.org/10.3390/molecules22010171
Wang X, Yan M, Wang Q, Wang H, Wang Z, Zhao J, Li J, Zhang Z. In Vitro DNA-Binding, Anti-Oxidant and Anticancer Activity of Indole-2-Carboxylic Acid Dinuclear Copper(II) Complexes. Molecules. 2017; 22(1):171. https://doi.org/10.3390/molecules22010171
Chicago/Turabian StyleWang, Xiangcong, Maocai Yan, Qibao Wang, Huannan Wang, Zhengyang Wang, Jiayi Zhao, Jing Li, and Zhen Zhang. 2017. "In Vitro DNA-Binding, Anti-Oxidant and Anticancer Activity of Indole-2-Carboxylic Acid Dinuclear Copper(II) Complexes" Molecules 22, no. 1: 171. https://doi.org/10.3390/molecules22010171
APA StyleWang, X., Yan, M., Wang, Q., Wang, H., Wang, Z., Zhao, J., Li, J., & Zhang, Z. (2017). In Vitro DNA-Binding, Anti-Oxidant and Anticancer Activity of Indole-2-Carboxylic Acid Dinuclear Copper(II) Complexes. Molecules, 22(1), 171. https://doi.org/10.3390/molecules22010171





