Mesenchymal Stem Cell Therapy for Inflammatory Skin Diseases: Clinical Potential and Mode of Action
Abstract
:1. Introduction
2. Properties of MSCs
2.1. Generalities
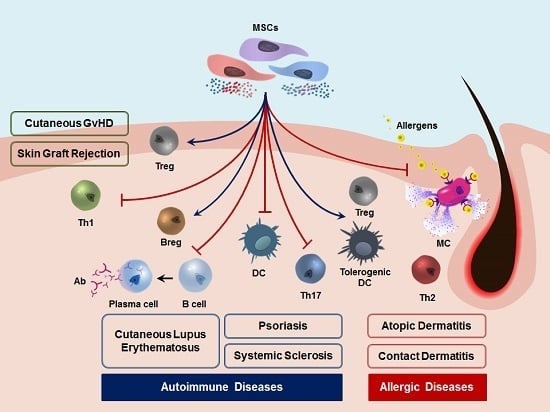
2.2. Immunological Properties of MSCs
2.3. Therapeutic Application of MSCs
3. Preclinical and Clinical Studies of MSCs in Inflammatory Dermatoses
3.1. Autoimmune Skin Diseases
3.1.1. Cutaneous GvHD
3.1.2. Cutaneous Lupus Erythematosus
3.1.3. Systemic Sclerosis/Scleroderma
3.1.4. Psoriasis
3.2. Allergic Skin Diseases
3.2.1. Atopic Dermatitis/Eczema
3.2.2. Allergic Contact Dermatitis
4. Conclusions and Future Perspectives
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ACD | Allergic contact dermatitis |
| AD | Atopic dermatitis |
| APC | Antigen presenting cell |
| AT | Adipose tissue |
| BM | Bone marrow |
| Breg | Regulatory B lymphocyte |
| CD | Clusters of differentiation |
| CHS | Contact hypersensitivity |
| CLE | Cutaneous lupus erythematosus |
| COX-2 | Cyclooxygenase 2 |
| CTL | Cytotoxic T lymphocyte |
| DC | Dendritic cell |
| Df | Dermatophagoides farinae |
| DTH | Delayed type hypersensitivity |
| EASI | Eczema Area and Severity Index |
| FcεRI | High affinity immunoglobulin E receptor |
| GvHD | Graft-versus-host disease |
| HClO | Hypochlorous acid |
| HGF | Hepatocyte growth factor |
| HLA | Human leukocyte antigen |
| ICD | Irritant contact dermatitis |
| IDO | Indoleamine 2,3-dioxygenase |
| IFN | Interferon |
| Ig | Immunoglobulin |
| IL | Interleukin |
| IV | Intravenous or intravenously |
| IMQ | Imiquimod |
| LPS | Lipopolysaccharide |
| MC | Mast cell |
| MHC | Major histocompatibility complex |
| MSC | Mesenchymal stem cell |
| NK | Natural killer |
| NO | Nitric oxide |
| OVA | Ovalbumin |
| PASI | Psoriasis Area and Severity Index |
| PGE2 | Prostaglandin E2 |
| SC | Subcutaneous or subcutaneously |
| SLE | Systemic lupus erythematosus |
| SSc | Systemic sclerosis |
| TGF | Transforming growth factor |
| Th | T helper lymphocyte |
| TLR | Toll-like receptor |
| TNF | Tumor necrosis factor |
| Treg | Regulatory T lymphocyte |
| UC | Umbilical cord |
| UCB | Umbilical cord blood |
References
- Odhiambo, J.A.; Williams, H.C.; Clayton, T.O.; Robertson, C.F.; Asher, M.I.; ISAAC Phase Three Study Group. Global variations in prevalence of eczema symptoms in children from ISAAC Phase Three. J. Allergy Clin. Immunol. 2009, 124, 1251–1258. [Google Scholar] [CrossRef] [PubMed]
- Silverberg, J.I.; Hanifin, J.M. Adult eczema prevalence and associations with asthma and other health and demographic factors: A US population-based study. J. Allergy Clin. Immunol. 2013, 132, 1132–1138. [Google Scholar] [CrossRef] [PubMed]
- Williams, H.; Flohr, C. How epidemiology has challenged 3 prevailing concepts about atopic dermatitis. J. Allergy Clin. Immunol. 2006, 118, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Chehregani, A.; Majde, A.; Moin, M.; Gholami, M.; Shariatzadeh, M.A.; Nassiri, H. Increasing allergy potency of Zinnia pollen grains in polluted areas. Ecotoxicol. Environ. Saf. 2004, 58, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Sanchez, D.; Penichet-Garcia, M.; Saxon, A. Diesel exhaust particles directly induce activated mast cells to degranulate and increase histamine levels and symptom severity. J. Allergy Clin. Immunol. 2000, 106, 1140–1146. [Google Scholar] [CrossRef] [PubMed]
- Eichenfield, L.F.; Tom, W.L.; Berger, T.G.; Krol, A.; Paller, A.S.; Schwarzenberger, K.; Bergman, J.N.; Chamlin, S.L.; Cohen, D.E.; Cooper, K.D.; et al. Guidelines of care for the management of atopic dermatitis: Section 2. Management and treatment of atopic dermatitis with topical therapies. J. Am. Acad. Dermatol. 2014, 71, 116–132. [Google Scholar] [CrossRef] [PubMed]
- Ring, J.; Alomar, A.; Bieber, T.; Deleuran, M.; Fink-Wagner, A.; Gelmetti, C.; Gieler, U.; Lipozencic, J.; Luger, T.; Oranje, A.P.; et al. Guidelines for treatment of atopic eczema (atopic dermatitis) part I. J. Eur. Acad. Dermatol. Venereol. 2012, 26, 1045–1060. [Google Scholar] [CrossRef] [PubMed]
- Montes-Torres, A.; Llamas-Velasco, M.; Perez-Plaza, A.; Solano-Lopez, G.; Sanchez-Perez, J. Biological treatments in atopic dermatitis. J. Clin. Med. 2015, 4, 593–613. [Google Scholar] [CrossRef] [PubMed]
- Connick, P.; Kolappan, M.; Crawley, C.; Webber, D.J.; Patani, R.; Michell, A.W.; Du, M.Q.; Luan, S.L.; Altmann, D.R.; Thompson, A.J.; et al. Autologous mesenchymal stem cells for the treatment of secondary progressive multiple sclerosis: An open-label phase 2a proof-of-concept study. Lancet Neurol. 2012, 11, 150–156. [Google Scholar] [CrossRef] [Green Version]
- Karussis, D.; Karageorgiou, C.; Vaknin-Dembinsky, A.; Gowda-Kurkalli, B.; Gomori, J.M.; Kassis, I.; Bulte, J.W.; Petrou, P.; Ben-Hur, T.; Abramsky, O.; et al. Safety and immunological effects of mesenchymal stem cell transplantation in patients with multiple sclerosis and amyotrophic lateral sclerosis. Arch. Neurol. 2010, 67, 1187–1194. [Google Scholar] [CrossRef] [PubMed]
- Lalu, M.M.; McIntyre, L.; Pugliese, C.; Fergusson, D.; Winston, B.W.; Marshall, J.C.; Granton, J.; Stewart, D.J. Safety of cell therapy with mesenchymal stromal cells (SafeCell): A systematic review and meta-analysis of clinical trials. PLoS ONE 2012, 7, e47559. [Google Scholar] [CrossRef] [PubMed]
- Le Blanc, K.; Frassoni, F.; Ball, L.; Locatelli, F.; Roelofs, H.; Lewis, I.; Lanino, E.; Sundberg, B.; Bernardo, M.E.; Remberger, M.; et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: A phase II study. Lancet 2008, 371, 1579–1586. [Google Scholar] [CrossRef]
- Khosrotehrani, K. Mesenchymal stem cell therapy in skin: Why and what for? Exp. Dermatol. 2013, 22, 307–310. [Google Scholar] [CrossRef] [PubMed]
- Friedenstein, A.J.; Chailakhjan, R.K.; Lalykina, K.S. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Prolif. 1970, 3, 393–403. [Google Scholar] [CrossRef]
- Hass, R.; Kasper, C.; Bohm, S.; Jacobs, R. Different populations and sources of human mesenchymal stem cells (MSC): A comparison of adult and neonatal tissue-derived MSC. Cell. Commun. Signal. 2011, 9, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoogduijn, M.J.; Betjes, M.G.; Baan, C.C. Mesenchymal stromal cells for organ transplantation: Different sources and unique characteristics? Curr. Opin. Organ. Transplant. 2014, 19, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Campagnoli, C.; Roberts, I.A.; Kumar, S.; Bennett, P.R.; Bellantuono, I.; Fisk, N.M. Identification of mesenchymal stem/progenitor cells in human first-trimester fetal blood, liver, and bone marrow. Blood 2001, 98, 2396–2402. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.L.; Liu, Y.J.; Yang, S.G.; Zhao, Q.J.; Wang, X.; Gong, W.; Han, Z.B.; Xu, Z.S.; Lu, Y.X.; Liu, D.; et al. Isolation and characterization of human umbilical cord mesenchymal stem cells with hematopoiesis-supportive function and other potentials. Haematologica 2006, 91, 1017–1026. [Google Scholar] [PubMed]
- Lee, O.K.; Kuo, T.K.; Chen, W.M.; Lee, K.D.; Hsieh, S.L.; Chen, T.H. Isolation of multipotent mesenchymal stem cells from umbilical cord blood. Blood 2004, 103, 1669–1675. [Google Scholar] [CrossRef] [PubMed]
- Zuk, P.A.; Zhu, M.; Ashjian, P.; de Ugarte, D.A.; Huang, J.I.; Mizuno, H.; Alfonso, Z.C.; Fraser, J.K.; Benhaim, P.; Hedrick, M.H. Human adipose tissue is a source of multipotent stem cells. Mol. Biol. Cell 2002, 13, 4279–4295. [Google Scholar] [CrossRef] [PubMed]
- Scherjon, S.A.; Kleijburg-van der Keur, C.; Noort, W.A.; Claas, F.H.; Willemze, R.; Fibbe, W.E.; Kanhai, H.H. Amniotic fluid as a novel source of mesenchymal stem cells for therapeutic transplantation. Blood 2003, 102, 1548–1549. [Google Scholar]
- Barlow, S.; Brooke, G.; Chatterjee, K.; Price, G.; Pelekanos, R.; Rossetti, T.; Doody, M.; Venter, D.; Pain, S.; Gilshenan, K.; et al. Comparison of human placenta- and bone marrow-derived multipotent mesenchymal stem cells. Stem Cells Dev. 2008, 17, 1095–1107. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.T.; Gronthos, S.; Shi, S. Mesenchymal stem cells derived from dental tissues vs. those from other sources: Their biology and role in regenerative medicine. J. Dent. Res. 2009, 88, 792–806. [Google Scholar] [CrossRef] [PubMed]
- Sellheyer, K.; Krahl, D. Skin mesenchymal stem cells: Prospects for clinical dermatology. J. Am. Acad Dermatol. 2010, 63, 859–865. [Google Scholar] [CrossRef] [PubMed]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Kopen, G.C.; Prockop, D.J.; Phinney, D.G. Marrow stromal cells migrate throughout forebrain and cerebellum, and they differentiate into astrocytes after injection into neonatal mouse brains. Proc. Nat. Acad. Sci. USA 1999, 96, 10711–10716. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Shah, B.; Moioli, E.K.; Mao, J.J. CTGF directs fibroblast differentiation from human mesenchymal stem/stromal cells and defines connective tissue healing in a rodent injury model. J. Clin. Investig. 2010, 120, 3340–3349. [Google Scholar] [CrossRef] [PubMed]
- Russell, K.C.; Phinney, D.G.; Lacey, M.R.; Barrilleaux, B.L.; Meyertholen, K.E.; O’Connor, K.C. In vitro high-capacity assay to quantify the clonal heterogeneity in trilineage potential of mesenchymal stem cells reveals a complex hierarchy of lineage commitment. Stem Cells 2010, 28, 788–798. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The international society for cellular therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.S.; Xin, Z.C.; Dai, J.; Lue, T.F. Commonly used mesenchymal stem cell markers and tracking labels: Limitations and challenges. Histol. Histopathol. 2013, 28, 1109–1116. [Google Scholar] [PubMed]
- Koppula, P.R.; Chelluri, L.K.; Polisetti, N.; Vemuganti, G.K. Histocompatibility testing of cultivated human bone marrow stromal cells—A promising step towards pre-clinical screening for allogeneic stem cell therapy. Cell. Immunol. 2009, 259, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Tredget, E.E.; Liu, C.; Wu, Y. Analysis of allogenicity of mesenchymal stem cells in engraftment and wound healing in mice. PLoS ONE 2009, 4, e7119. [Google Scholar] [CrossRef] [PubMed]
- Bonfield, T.L.; Nola, M.T.; Lennon, D.P.; Caplan, A.I. Defining human mesenchymal stem cell efficacy in vivo. J. Inflamm 2010, 7, 51. [Google Scholar] [CrossRef] [PubMed]
- Nauta, A.J.; Westerhuis, G.; Kruisselbrink, A.B.; Lurvink, E.G.; Willemze, R.; Fibbe, W.E. Donor-derived mesenchymal stem cells are immunogenic in an allogeneic host and stimulate donor graft rejection in a nonmyeloablative setting. Blood 2006, 108, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Deak, E.; Seifried, E.; Henschler, R. Homing pathways of mesenchymal stromal cells (MSCs) and their role in clinical applications. Int. Rev. Immunol. 2010, 29, 514–529. [Google Scholar] [CrossRef] [PubMed]
- Uccelli, A.; Moretta, L.; Pistoia, V. Mesenchymal stem cells in health and disease. Nat. Rev. Immunol. 2008, 8, 726–736. [Google Scholar] [CrossRef] [PubMed]
- Glennie, S.; Soeiro, I.; Dyson, P.J.; Lam, E.W.; Dazzi, F. Bone marrow mesenchymal stem cells induce division arrest anergy of activated T cells. Blood 2005, 105, 2821–2827. [Google Scholar] [CrossRef] [PubMed]
- Corcione, A.; Benvenuto, F.; Ferretti, E.; Giunti, D.; Cappiello, V.; Cazzanti, F.; Risso, M.; Gualandi, F.; Mancardi, G.L.; Pistoia, V.; et al. Human mesenchymal stem cells modulate B cell functions. Blood 2006, 107, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Liu, R.; Shi, D.; Liu, X.; Chen, Y.; Dou, X.; Zhu, X.; Lu, C.; Liang, W.; Liao, L.; et al. Mesenchymal stem cells induce mature dendritic cells into a novel Jagged-2-dependent regulatory dendritic cell population. Blood 2009, 113, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Sotiropoulou, P.A.; Perez, S.A.; Gritzapis, A.D.; Baxevanis, C.N.; Papamichail, M. Interactions between human mesenchymal stem cells and natural killer cells. Stem Cells 2006, 24, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Xie, N.; Li, W.; Yuan, B.; Shi, Y.; Wang, Y. Immunobiology of mesenchymal stem cells. Cell. Death Differ. 2014, 21, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Di Nicola, M.; Carlo-Stella, C.; Magni, M.; Milanesi, M.; Longoni, P.D.; Matteucci, P.; Grisanti, S.; Gianni, A.M. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood 2002, 99, 3838–3843. [Google Scholar] [CrossRef] [PubMed]
- Bartholomew, A.; Sturgeon, C.; Siatskas, M.; Ferrer, K.; McIntosh, K.; Patil, S.; Hardy, W.; Devine, S.; Ucker, D.; Deans, R.; et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp. Hematol. 2002, 30, 42–48. [Google Scholar] [CrossRef]
- Le Blanc, K.; Rasmusson, I.; Sundberg, B.; Gotherstrom, C.; Hassan, M.; Uzunel, M.; Ringden, O. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet 2004, 363, 1439–1441. [Google Scholar] [CrossRef]
- Yanez, R.; Lamana, M.L.; Garcia-Castro, J.; Colmenero, I.; Ramirez, M.; Bueren, J.A. Adipose tissue-derived mesenchymal stem cells have in vivo immunosuppressive properties applicable for the control of the graft-versus-host disease. Stem Cells 2006, 24, 2582–2591. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Yang, J.; Cao, G.; Fan, H.; Guo, C.; Ma, Y.E.; Qian, Y.; Chen, L.; Li, X.; Chang, C. Xenogeneic immunosuppression of human umbilical cord mesenchymal stem cells in a major histocompatibility complex-mismatched allogeneic acute graft-versus-host disease murine model. Eur. J. Haematol. 2011, 87, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Akiyama, K.; Zhang, H.; Yamaza, T.; Hou, Y.; Zhao, S.; Xu, T.; Le, A.; Shi, S. Mesenchymal stem cell transplantation reverses multiorgan dysfunction in systemic lupus erythematosus mice and humans. Stem Cells 2009, 27, 1421–1432. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Zhang, H.; Hua, B.; Wang, H.; Lu, L.; Shi, S.; Hou, Y.; Zeng, X.; Gilkeson, G.S.; Sun, L. Allogenic mesenchymal stem cells transplantation in refractory systemic lupus erythematosus: A pilot clinical study. Ann. Rheum. Dis. 2010, 69, 1423–1429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzalez, M.A.; Gonzalez-Rey, E.; Rico, L.; Buscher, D.; Delgado, M. Treatment of experimental arthritis by inducing immune tolerance with human adipose-derived mesenchymal stem cells. Arthritis Rheum. 2009, 60, 1006–1019. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, L.; Cong, X.; Liu, G.; Zhou, J.; Bai, B.; Li, Y.; Bai, W.; Li, M.; Ji, H.; et al. Human umbilical cord mesenchymal stem cell therapy for patients with active rheumatoid arthritis: Safety and efficacy. Stem Cells Dev. 2013, 22, 3192–3202. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.J.; Zhang, J.F.; Sun, B.; Peng, H.S.; Kong, Q.F.; Bai, S.S.; Liu, Y.M.; Wang, G.Y.; Wang, J.H.; Li, H.L. Reciprocal effect of mesenchymal stem cell on experimental autoimmune encephalomyelitis is mediated by transforming growth factor-β and interleukin-6. Clin. Exp. Immunol. 2009, 158, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, K.; Keane-Myers, A.; Brown, J.M.; Metcalfe, D.D.; Gorham, J.D.; Bundoc, V.G.; Hodges, M.G.; Jelinek, I.; Madala, S.; Karpati, S.; et al. Bone marrow stromal cells use TGF-β to suppress allergic responses in a mouse model of ragweed-induced asthma. Proc. Nat. Acad. Sci. USA 2010, 107, 5652–5657. [Google Scholar] [CrossRef] [PubMed]
- Kavanagh, H.; Mahon, B.P. Allogeneic mesenchymal stem cells prevent allergic airway inflammation by inducing murine regulatory T cells. Allergy 2011, 66, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.Q.; Deng, M.X.; He, J.; Zeng, Q.X.; Wen, W.; Wong, D.S.; Tse, H.F.; Xu, G.; Lian, Q.; Shi, J.; et al. Human pluripotent stem cell-derived mesenchymal stem cells prevent allergic airway inflammation in mice. Stem Cells 2012, 30, 2692–2699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Na, K.; Yoo, H.S.; Zhang, Y.X.; Choi, M.S.; Lee, K.; Yi, T.G.; Song, S.U.; Jeon, M.S. Bone marrow-derived clonal mesenchymal stem cells inhibit ovalbumin-induced atopic dermatitis. Cell. Death Dis. 2014, 5, e1345. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Yun, J.W.; Shin, T.H.; Lee, S.H.; Lee, B.C.; Yu, K.R.; Seo, Y.; Lee, S.; Kang, T.W.; Choi, S.W.; et al. Human umbilical cord blood mesenchymal stem cell-derived PGE2 and TGF-β1 alleviate atopic dermatitis by reducing mast cell degranulation. Stem Cells 2015, 33, 1254–1266. [Google Scholar] [CrossRef] [PubMed]
- Su, W.R.; Zhang, Q.Z.; Shi, S.H.; Nguyen, A.L.; Le, A.D. Human gingiva-derived mesenchymal stromal cells attenuate contact hypersensitivity via prostaglandin E2-dependent mechanisms. Stem Cells 2011, 29, 1849–1860. [Google Scholar] [CrossRef] [PubMed]
- Perez-Simon, J.A.; Lopez-Villar, O.; Andreu, E.J.; Rifon, J.; Muntion, S.; Diez Campelo, M.; Sanchez-Guijo, F.M.; Martinez, C.; Valcarcel, D.; Canizo, C.D. Mesenchymal stem cells expanded in vitro with human serum for the treatment of acute and chronic graft-versus-host disease: Results of a phase I/II clinical trial. Haematologica 2011, 96, 1072–1076. [Google Scholar] [CrossRef] [PubMed]
- Christopeit, M.; Schendel, M.; Foll, J.; Muller, L.P.; Keysser, G.; Behre, G. Marked improvement of severe progressive systemic sclerosis after transplantation of mesenchymal stem cells from an allogeneic haploidentical-related donor mediated by ligation of CD137L. Leukemia 2008, 22, 1062–1064. [Google Scholar] [CrossRef] [PubMed]
- Ringden, O.; Erkers, T.; Nava, S.; Uzunel, M.; Iwarsson, E.; Conrad, R.; Westgren, M.; Mattsson, J.; Kaipe, H. Fetal membrane cells for treatment of steroid-refractory acute graft-versus-host disease. Stem Cells 2013, 31, 592–601. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Guo, M.; Bian, C.; Sun, Z.; Yang, Z.; Zeng, Y.; Ai, H.; Zhao, R.C. Efficacy of bone marrow-derived mesenchymal stem cells in the treatment of sclerodermatous chronic graft-versus-host disease: Clinical report. Biol. Blood Marrow Transplant. 2010, 16, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Sah, S.K.; Park, K.H.; Yun, C.O.; Kang, K.S.; Kim, T.Y. Effects of human mesenchymal stem cells transduced with superoxide dismutase on imiquimod-induced psoriasis-like skin inflammation in mice. Antioxid. Redox Signal. 2016, 24, 233–248. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Lee, J.H.; Roh, K.H.; Jun, H.J.; Kang, K.S.; Kim, T.Y. Clinical trial of human umbilical cord blood-derived stem cells for the treatment of moderate-to-severe atopic dermatitis: Phase I/IIa studies. Stem Cells 2017, 35, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zhao, Y.; Ge, L. Therapeutic effects of human gingiva-derived mesenchymal stromal cells on murine contact hypersensitivity via prostaglandin E2-EP3 signaling. Stem Cell. Res. Ther. 2016, 7, 103. [Google Scholar] [CrossRef] [PubMed]
- Scuderi, N.; Ceccarelli, S.; Onesti, M.G.; Fioramonti, P.; Guidi, C.; Romano, F.; Frati, L.; Angeloni, A.; Marchese, C. Human adipose-derived stromal cells for cell-based therapies in the treatment of systemic sclerosis. Cell. Transplant. 2013, 22, 779–795. [Google Scholar] [CrossRef] [PubMed]
- Shin, T.H.; Lee, B.C.; Choi, S.W.; Shin, J.H.; Kang, I.; Lee, J.Y.; Kim, J.J.; Lee, H.K.; Jung, J.E.; Choi, Y.W.; et al. Human adipose tissue-derived mesenchymal stem cells alleviate atopic dermatitis via regulation of B lymphocyte maturation. Oncotarget 2017, 8, 512–522. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Che, N.; Gu, Z.; Huang, J.; Wang, D.; Liang, J.; Hou, Y.; Gilkeson, G.; Lu, L.; Sun, L. Allogenic mesenchymal stem cell transplantation ameliorates nephritis in lupus mice via inhibition of B cell activation. Cell Transplant. 2013, 22, 2279–2290. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.W.; Hung, S.P.; Wu, H.H.; Wu, W.M.; Yang, A.H.; Tsai, H.L.; Yang, L.Y.; Lee, O.K. Therapeutic effects of umbilical cord blood-derived mesenchymal stem cell transplantation in experimental lupus nephritis. Cell Transplant. 2011, 20, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Maria, A.T.; Toupet, K.; Bony, C.; Pirot, N.; Vozenin, M.C.; Petit, B.; Roger, P.; Batteux, F.; Le Quellec, A.; Jorgensen, C.; et al. Antifibrotic, antioxidant, and immunomodulatory effects of mesenchymal stem cells in HOCI-induced systemic sclerosis. Arthritis Rheumatol. 2016, 68, 1013–1025. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.H.; Kim, J.S.; Yoon, I.H.; Shin, J.S.; Nam, H.Y.; Yang, S.H.; Kim, S.J.; Park, C.G. Immunomodulation of delayed-type hypersensitivity responses by mesenchymal stem cells is associated with bystander T cell apoptosis in the draining lymph node. J. Immunol. 2010, 185, 4022–4029. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Niu, J.W.; Ning, H.M.; Pan, X.; Li, X.B.; Li, Y.; Wang, D.H.; Hu, L.D.; Sheng, H.X.; Xu, M.; et al. Treatment of psoriasis with mesenchymal stem cells. Am. J. Med. 2016, 129, e13–e14. [Google Scholar] [CrossRef] [PubMed]
- De Jesus, M.M.; Santiago, J.S.; Trinidad, C.V.; See, M.E.; Semon, K.R.; Fernandez, M.O., Jr.; Chung, F.S. Autologous adipose-derived mesenchymal stromal cells for the treatment of psoriasis vulgaris and psoriatic arthritis: A case report. Cell. Transplant. 2016, 25, 2063–2069. [Google Scholar] [CrossRef] [PubMed]
- Carrion, F.; Nova, E.; Ruiz, C.; Diaz, F.; Inostroza, C.; Rojo, D.; Monckeberg, G.; Figueroa, F.E. Autologous mesenchymal stem cell treatment increased T regulatory cells with no effect on disease activity in two systemic lupus erythematosus patients. Lupus 2010, 19, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Li, J.; Zhang, Y.; Zhang, M.; Chen, J.; Li, X.; Hu, X.; Jiang, S.; Shi, S.; Sun, L. Umbilical cord mesenchymal stem cell transplantation in active and refractory systemic lupus erythematosus: A multicenter clinical study. Arthritis Res. Ther. 2014, 16, R79. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Wang, D.; Liang, J.; Zhang, H.; Feng, X.; Wang, H.; Hua, B.; Liu, B.; Ye, S.; Hu, X.; et al. Umbilical cord mesenchymal stem cell transplantation in severe and refractory systemic lupus erythematosus. Arthritis Rheum. 2010, 62, 2467–2475. [Google Scholar] [CrossRef] [PubMed]
- Keyszer, G.; Christopeit, M.; Fick, S.; Schendel, M.; Taute, B.M.; Behre, G.; Muller, L.P.; Schmoll, H.J. Treatment of severe progressive systemic sclerosis with transplantation of mesenchymal stromal cells from allogeneic related donors: Report of five cases. Arthritis Rheum. 2011, 63, 2540–2542. [Google Scholar] [CrossRef] [PubMed]
- Prasad, V.K.; Lucas, K.G.; Kleiner, G.I.; Talano, J.A.; Jacobsohn, D.; Broadwater, G.; Monroy, R.; Kurtzberg, J. Efficacy and safety of ex vivo cultured adult human mesenchymal stem cells (prochymal) in pediatric patients with severe refractory acute graft-versus-host disease in a compassionate use study. Biol. Blood Marrow Transplant. 2011, 17, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, J.L.; Levine, J.E.; Reddy, P.; Holler, E. Graft-versus-host disease. Lancet 2009, 373, 1550–1561. [Google Scholar] [CrossRef]
- Bruggen, M.C.; Klein, I.; Greinix, H.; Bauer, W.; Kuzmina, Z.; Rabitsch, W.; Kalhs, P.; Petzelbauer, P.; Knobler, R.; Stingl, G.; et al. Diverse T cell responses characterize the different manifestations of cutaneous graft-versus-host disease. Blood 2014, 123, 290–299. [Google Scholar] [CrossRef] [PubMed]
- English, K.; Ryan, J.M.; Tobin, L.; Murphy, M.J.; Barry, F.P.; Mahon, B.P. Cell contact, prostaglandin E2 and transforming growth factor β1 play non-redundant roles in human mesenchymal stem cell induction of CD4+CD25high forkhead box p3+ regulatory T cells. Clin. Exp. Immunol. 2009, 156, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, S.; Pittenger, M.F. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 2005, 105, 1815–1822. [Google Scholar] [CrossRef] [PubMed]
- Aksu, A.E.; Horibe, E.; Sacks, J.; Ikeguchi, R.; Breitinger, J.; Scozio, M.; Unadkat, J.; Feili-Hariri, M. Co-infusion of donor bone marrow with host mesenchymal stem cells treats GVHD and promotes vascularized skin allograft survival in rats. Clin. Immunol. 2008, 127, 348–358. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.; Zhang, L.; Zhao, X.; Xu, G.; Zhang, Y.; Roberts, A.I.; Zhao, R.C.; Shi, Y. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell 2008, 2, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Meisel, R.; Zibert, A.; Laryea, M.; Gobel, U.; Daubener, W.; Dilloo, D. Human bone marrow stromal cells inhibit allogeneic T cell responses by indoleamine 2,3-dioxygenase-mediated tryptophan degradation. Blood 2004, 103, 4619–4621. [Google Scholar] [CrossRef] [PubMed]
- Selmani, Z.; Naji, A.; Zidi, I.; Favier, B.; Gaiffe, E.; Obert, L.; Borg, C.; Saas, P.; Tiberghien, P.; Rouas-Freiss, N.; et al. Human leukocyte antigen-G5 secretion by human mesenchymal stem cells is required to suppress T lymphocyte and natural killer function and to induce CD4+CD25highFOXP3+ regulatory T cells. Stem Cells 2008, 26, 212–222. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, M.; Sueblinvong, V.; Eisenhauer, P.; Ziats, N.P.; LeClair, L.; Poynter, M.E.; Steele, C.; Rincon, M.; Weiss, D.J. Bone marrow-derived mesenchymal stromal cells inhibit Th2-mediated allergic airways inflammation in mice. Stem Cells 2011, 29, 1137–1148. [Google Scholar] [CrossRef] [PubMed]
- Park, H.K.; Cho, K.S.; Park, H.Y.; Shin, D.H.; Kim, Y.K.; Jung, J.S.; Park, S.K.; Roh, H.J. Adipose-derived stromal cells inhibit allergic airway inflammation in mice. Stem Cells Dev. 2010, 19, 1811–1818. [Google Scholar] [CrossRef] [PubMed]
- Mareschi, K.; Castiglia, S.; Sanavio, F.; Rustichelli, D.; Muraro, M.; Defedele, D.; Bergallo, M.; Fagioli, F. Immunoregulatory effects on T lymphocytes by human mesenchymal stromal cells isolated from bone marrow, amniotic fluid, and placenta. Exp. Hematol. 2016, 44, 138–150. [Google Scholar] [CrossRef] [PubMed]
- Pianta, S.; Bonassi Signoroni, P.; Muradore, I.; Rodrigues, M.F.; Rossi, D.; Silini, A.; Parolini, O. Amniotic membrane mesenchymal cells-derived factors skew T cell polarization toward TREG and downregulate Th1 and Th17 cells subsets. Stem Cell Rev. 2015, 11, 394–407. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; Liu, X.; Cheng, K.; Yang, R.; Zhao, R.C. Mesenchymal stem cells inhibit Th17 cell differentiation by IL-10 secretion. Exp. Hematol. 2012, 40, 761–770. [Google Scholar] [CrossRef] [PubMed]
- Duffy, M.M.; Pindjakova, J.; Hanley, S.A.; McCarthy, C.; Weidhofer, G.A.; Sweeney, E.M.; English, K.; Shaw, G.; Murphy, J.M.; Barry, F.P.; et al. Mesenchymal stem cell inhibition of T-helper 17 cell- differentiation is triggered by cell–cell contact and mediated by prostaglandin E2 via the EP4 receptor. Eur. J. Immunol. 2011, 41, 2840–2851. [Google Scholar] [CrossRef] [PubMed]
- Carrion, F.; Nova, E.; Luz, P.; Apablaza, F.; Figueroa, F. Opposing effect of mesenchymal stem cells on Th1 and Th17 cell polarization according to the state of CD4+ T cell activation. Immunol. Lett. 2011, 135, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Ghannam, S.; Pene, J.; Moquet-Torcy, G.; Jorgensen, C.; Yssel, H. Mesenchymal stem cells inhibit human Th17 cell differentiation and function and induce a T regulatory cell phenotype. J. Immunol. 2010, 185, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Luz-Crawford, P.; Kurte, M.; Bravo-Alegria, J.; Contreras, R.; Nova-Lamperti, E.; Tejedor, G.; Noel, D.; Jorgensen, C.; Figueroa, F.; Djouad, F.; et al. Mesenchymal stem cells generate a CD4+CD25+FOXP3+ regulatory T cell population during the differentiation process of Th1 and Th17 cells. Stem Cell Res. Ther. 2013, 4, 65. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, K.; Chen, C.; Wang, D.; Xu, X.; Qu, C.; Yamaza, T.; Cai, T.; Chen, W.; Sun, L.; Shi, S. Mesenchymal-stem cell-induced immunoregulation involves fas-ligand-/fas-mediated T cell apoptosis. Cell Stem Cell 2012, 10, 544–555. [Google Scholar] [CrossRef] [PubMed]
- Melief, S.M.; Schrama, E.; Brugman, M.H.; Tiemessen, M.M.; Hoogduijn, M.J.; Fibbe, W.E.; Roelofs, H. Multipotent stromal cells induce human regulatory T cells through a novel pathway involving skewing of monocytes toward anti-inflammatory macrophages. Stem Cells 2013, 31, 1980–1991. [Google Scholar] [CrossRef] [PubMed]
- Tipnis, S.; Viswanathan, C.; Majumdar, A.S. Immunosuppressive properties of human umbilical cord-derived mesenchymal stem cells: Role of B7-H1 and IDO. Immunol. Cell Biol. 2010, 88, 795–806. [Google Scholar] [CrossRef] [PubMed]
- Asari, S.; Itakura, S.; Ferreri, K.; Liu, C.P.; Kuroda, Y.; Kandeel, F.; Mullen, Y. Mesenchymal stem cells suppress B cell terminal differentiation. Exp. Hematol. 2009, 37, 604–615. [Google Scholar] [CrossRef] [PubMed]
- Augello, A.; Tasso, R.; Negrini, S.M.; Amateis, A.; Indiveri, F.; Cancedda, R.; Pennesi, G. Bone marrow mesenchymal progenitor cells inhibit lymphocyte proliferation by activation of the programmed death 1 pathway. Eur. J. Immunol. 2005, 35, 1482–1490. [Google Scholar] [CrossRef] [PubMed]
- Healy, M.E.; Bergin, R.; Mahon, B.P.; English, K. Mesenchymal stromal cells protect against caspase 3-mediated apoptosis of CD19+ peripheral b cells through contact-dependent upregulation of VEGF. Stem Cells Dev. 2015, 24, 2391–2402. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Chen, X.; Liu, Q.; Zhang, X.; Huang, K.; Liu, L.; Li, H.; Zhou, M.; Huang, F.; Fan, Z.; et al. Mesenchymal stromal cells infusions improve refractory chronic graft versus host disease through an increase of CD5+ regulatory B cells producing interleukin 10. Leukemia 2015, 29, 636–646. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.R.; Yang, Z.X.; Han, Z.B.; Meng, L.; Liang, L.; Feng, X.M.; Yang, S.G.; Chi, Y.; Chen, D.D.; Wang, Y.W.; et al. Mesenchymal stem cells support proliferation and terminal differentiation of B cells. Cell. Physiol. Biochem. 2012, 30, 1526–1537. [Google Scholar] [CrossRef] [PubMed]
- Che, N.; Li, X.; Zhou, S.; Liu, R.; Shi, D.; Lu, L.; Sun, L. Umbilical cord mesenchymal stem cells suppress B cell proliferation and differentiation. Cell. Immunol. 2012, 274, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Franquesa, M.; Mensah, F.K.; Huizinga, R.; Strini, T.; Boon, L.; Lombardo, E.; DelaRosa, O.; Laman, J.D.; Grinyo, J.M.; Weimar, W.; et al. Human adipose tissue-derived mesenchymal stem cells abrogate plasmablast formation and induce regulatory B cells independently of T helper cells. Stem Cells 2015, 33, 880–891. [Google Scholar] [CrossRef] [PubMed]
- Djouad, F.; Charbonnier, L.M.; Bouffi, C.; Louis-Plence, P.; Bony, C.; Apparailly, F.; Cantos, C.; Jorgensen, C.; Noel, D. Mesenchymal stem cells inhibit the differentiation of dendritic cells through an interleukin-6-dependent mechanism. Stem Cells 2007, 25, 2025–2032. [Google Scholar] [CrossRef] [PubMed]
- Spaggiari, G.M.; Abdelrazik, H.; Becchetti, F.; Moretta, L. MSCs inhibit monocyte-derived DC maturation and function by selectively interfering with the generation of immature DCs: Central role of MSC-derived prostaglandin E2. Blood 2009, 113, 6576–6583. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.X.; Zhang, Y.; Liu, B.; Zhang, S.X.; Wu, Y.; Yu, X.D.; Mao, N. Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood 2005, 105, 4120–4126. [Google Scholar] [CrossRef] [PubMed]
- Chiesa, S.; Morbelli, S.; Morando, S.; Massollo, M.; Marini, C.; Bertoni, A.; Frassoni, F.; Bartolome, S.T.; Sambuceti, G.; Traggiai, E.; et al. Mesenchymal stem cells impair in vivo T cell priming by dendritic cells. Proc. Natl. Acad. Sci. USA 2011, 108, 17384–17389. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, R.; Fazekasova, H.; Lam, E.W.; Soeiro, I.; Lombardi, G.; Dazzi, F. Mesenchymal stem cells inhibit dendritic cell differentiation and function by preventing entry into the cell cycle. Transplantation 2007, 83, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Nauta, A.J.; Kruisselbrink, A.B.; Lurvink, E.; Willemze, R.; Fibbe, W.E. Mesenchymal stem cells inhibit generation and function of both CD34+-derived and monocyte-derived dendritic cells. J. Immunol. 2006, 177, 2080–2087. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.W.; Chen, H.Y.; Wang, L.T.; Wang, F.H.; Fang, L.W.; Lai, H.Y.; Chen, H.H.; Lu, J.; Hung, M.S.; Cheng, Y.; et al. Mesenchymal stem cells tune the development of monocyte-derived dendritic cells toward a myeloid-derived suppressive phenotype through growth-regulated oncogene chemokines. J. Immunol. 2013, 190, 5065–5077. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.M.; Nemeth, K.; Kushnir-Sukhov, N.M.; Metcalfe, D.D.; Mezey, E. Bone marrow stromal cells inhibit mast cell function via a COX2-dependent mechanism. Clin. Exp. Allergy 2011, 41, 526–534. [Google Scholar] [CrossRef] [PubMed]
- Tsokos, G.C. Systemic lupus erythematosus. N. Engl. J. Med. 2011, 365, 2110–2121. [Google Scholar] [CrossRef] [PubMed]
- Wahren-Herlenius, M.; Dorner, T. Immunopathogenic mechanisms of systemic autoimmune disease. Lancet 2013, 382, 819–831. [Google Scholar] [CrossRef]
- Shao, W.H.; Cohen, P.L. Disturbances of apoptotic cell clearance in systemic lupus erythematosus. Arthritis Res. Ther. 2011, 13, 202. [Google Scholar] [CrossRef] [PubMed]
- Rosado, M.M.; Bernardo, M.E.; Scarsella, M.; Conforti, A.; Giorda, E.; Biagini, S.; Cascioli, S.; Rossi, F.; Guzzo, I.; Vivarelli, M.; et al. Inhibition of B cell proliferation and antibody production by mesenchymal stromal cells is mediated by T cells. Stem Cells Dev. 2015, 24, 93–103. [Google Scholar] [CrossRef] [PubMed]
- LeRoy, E.C.; Medsger, T.A., Jr. Criteria for the classification of early systemic sclerosis. J. Rheumatol. 2001, 28, 1573–1576. [Google Scholar] [PubMed]
- Gabrielli, A.; Avvedimento, E.V.; Krieg, T. Scleroderma. N. Engl. J. Med. 2009, 360, 1989–2003. [Google Scholar] [CrossRef] [PubMed]
- Fuschiotti, P. Current perspectives on the immunopathogenesis of systemic sclerosis. Immunotargets Ther. 2016, 5, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Van Rhijn-Brouwer, F.C.; Gremmels, H.; Fledderus, J.O.; Radstake, T.R.; Verhaar, M.C.; van Laar, J.M. Cellular therapies in systemic sclerosis: Recent progress. Curr. Rheumatol. Rep. 2016, 18, 12. [Google Scholar] [CrossRef] [PubMed]
- Kumamoto, M.; Nishiwaki, T.; Matsuo, N.; Kimura, H.; Matsushima, K. Minimally cultured bone marrow mesenchymal stem cells ameliorate fibrotic lung injury. Eur. Respir. J. 2009, 34, 740–748. [Google Scholar] [CrossRef] [PubMed]
- Moodley, Y.; Atienza, D.; Manuelpillai, U.; Samuel, C.S.; Tchongue, J.; Ilancheran, S.; Boyd, R.; Trounson, A. Human umbilical cord mesenchymal stem cells reduce fibrosis of bleomycin-induced lung injury. Am. J. Pathol. 2009, 175, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Zhang, Y.F.; Liu, Y.G.; Zhou, J.J.; Li, Z.K.; Wu, C.G.; Qi, H.W. Therapeutic effects of bone marrow-derived mesenchymal stem cells engraftment on bleomycin-induced lung injury in rats. Transplant. Proc. 2008, 40, 1700–1705. [Google Scholar] [CrossRef] [PubMed]
- Christophers, E. Psoriasis—Epidemiology and clinical spectrum. Clin. Exp. Dermatol. 2001, 26, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Parisi, R.; Symmons, D.P.; Griffiths, C.E.; Ashcroft, D.M. Global epidemiology of psoriasis: A systematic review of incidence and prevalence. J. Invest. Dermatol. 2013, 133, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, C.E.; Barker, J.N. Pathogenesis and clinical features of psoriasis. Lancet 2007, 370, 263–271. [Google Scholar] [CrossRef]
- Palfreeman, A.C.; McNamee, K.E.; McCann, F.E. New developments in the management of psoriasis and psoriatic arthritis: A focus on apremilast. Drug Des. Dev. Ther. 2013, 7, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Boehncke, W.H.; Schon, M.P. Psoriasis. Lancet 2015, 386, 983–994. [Google Scholar] [CrossRef]
- Lowes, M.A.; Kikuchi, T.; Fuentes-Duculan, J.; Cardinale, I.; Zaba, L.C.; Haider, A.S.; Bowman, E.P.; Krueger, J.G. Psoriasis vulgaris lesions contain discrete populations of Th1 and Th17 T cells. J. Investig. Dermatol. 2008, 128, 1207–1211. [Google Scholar] [CrossRef] [PubMed]
- Nestle, F.O.; Conrad, C.; Tun-Kyi, A.; Homey, B.; Gombert, M.; Boyman, O.; Burg, G.; Liu, Y.J.; Gilliet, M. Plasmacytoid predendritic cells initiate psoriasis through interferon-α production. J. Exp. Med. 2005, 202, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Nestle, F.O.; Kaplan, D.H.; Barker, J. Psoriasis. N. Engl. J. Med. 2009, 361, 496–509. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, C.E.; Strober, B.E.; van de Kerkhof, P.; Ho, V.; Fidelus-Gort, R.; Yeilding, N.; Guzzo, C.; Xia, Y.; Zhou, B.; Li, S.; et al. Comparison of ustekinumab and etanercept for moderate-to-severe psoriasis. N. Engl. J. Med. 2010, 362, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, C.L.; Kimball, A.B.; Papp, K.A.; Yeilding, N.; Guzzo, C.; Wang, Y.; Li, S.; Dooley, L.T.; Gordon, K.B.; investigators, P.S. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-Week results from a randomised, double-blind, placebo-controlled trial (phoenix 1). Lancet 2008, 371, 1665–1674. [Google Scholar] [CrossRef]
- Bohgaki, T.; Atsumi, T.; Koike, T. Multiple autoimmune diseases after autologous stem cell transplantation. N. Engl. J. Med. 2007, 357, 2734–2736. [Google Scholar] [CrossRef] [PubMed]
- Koike, K.; Kohda, K.; Kuga, T.; Nakazawa, O.; Ando, M.; Takayanagi, N.; Matsunaga, T.; Sakamaki, S.; Niitsu, Y. Ulcerative colitis after autologous peripheral blood stem cell transplantation for non-Hodgkin‘s lymphoma. Bone Marrow Transplant. 2001, 28, 619–621. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, Y.; Yamamoto, Y.; Ito, T.; Matsumoto, N.; Ichiyoshi, H.; Katsurada, T.; Date, M.; Ohga, S.; Kitajima, H.; Ikehara, S.; et al. Transfer of autoimmune thyroiditis and resolution of palmoplantar pustular psoriasis following allogeneic bone marrow transplantation. Bone Marrow Transplant. 1997, 19, 1041–1043. [Google Scholar] [CrossRef] [PubMed]
- Loh, Y.S.; Hwang, W.Y.; Ratnagopal, P. Autologous haematopoietic stem cell transplantation for the treatment of multiple sclerosis. Ann. Acad. Med. Singapore 2007, 36, 421–426. [Google Scholar] [PubMed]
- Liu, R.; Wang, Y.; Zhao, X.; Yang, Y.; Zhang, K. Lymphocyte inhibition is compromised in mesenchymal stem cells from psoriatic skin. Eur. J. Dermatol. 2014, 24, 560–567. [Google Scholar] [PubMed]
- Campanati, A.; Orciani, M.; Consales, V.; Lazzarini, R.; Ganzetti, G.; di Benedetto, G.; di Primio, R.; Offidani, A. Characterization and profiling of immunomodulatory genes in resident mesenchymal stem cells reflect the Th1–Th17/Th2 imbalance of psoriasis. Arch. Dermatol. Res. 2014, 306, 915–920. [Google Scholar] [CrossRef] [PubMed]
- Wittkowski, A.; Richards, H.L.; Griffiths, C.E.; Main, C.J. The impact of psychological and clinical factors on quality of life in individuals with atopic dermatitis. J. Psychosom. Res. 2004, 57, 195–200. [Google Scholar] [CrossRef]
- Yaghmaie, P.; Koudelka, C.W.; Simpson, E.L. Mental health comorbidity in patients with atopic dermatitis. J. Allergy Clin. Immunol. 2013, 131, 428–433. [Google Scholar] [CrossRef] [PubMed]
- Dalgard, F.J.; Gieler, U.; Tomas-Aragones, L.; Lien, L.; Poot, F.; Jemec, G.B.; Misery, L.; Szabo, C.; Linder, D.; Sampogna, F.; et al. The psychological burden of skin diseases: A cross-sectional multicenter study among dermatological out-patients in 13 european countries. J. Investig. Dermatol. 2015, 135, 984–991. [Google Scholar] [CrossRef] [PubMed]
- Drucker, A.M.; Wang, A.R.; Li, W.Q.; Sevetson, E.; Block, J.K.; Qureshi, A.A. The burden of atopic dermatitis: Summary of a report for the national eczema association. J. Investig. Dermatol. 2017, 137, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Perez, J.; Dauden-Tello, E.; Mora, A.M.; Lara Surinyac, N. Impact of atopic dermatitis on health-related quality of life in spanish children and adults: The pseda study. Actas Dermosifiliogr. 2013, 104, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Spergel, J.M.; Paller, A.S. Atopic dermatitis and the atopic march. J. Allergy Clin. Immunol. 2003, 112, S118–S127. [Google Scholar] [CrossRef] [PubMed]
- Ricci, G.; Patrizi, A.; Baldi, E.; Menna, G.; Tabanelli, M.; Masi, M. Long-term follow-up of atopic dermatitis: Retrospective analysis of related risk factors and association with concomitant allergic diseases. J. Am. Acad. Dermatol. 2006, 55, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Bantz, S.K.; Zhu, Z.; Zheng, T. The atopic march: Progression from atopic dermatitis to allergic rhinitis and asthma. J. Clin. Cell. Immunol. 2014, 5, 202. [Google Scholar] [PubMed]
- Boguniewicz, M.; Leung, D.Y. Atopic dermatitis: A disease of altered skin barrier and immune dysregulation. Immunol. Rev. 2011, 242, 233–246. [Google Scholar] [CrossRef] [PubMed]
- Schneider, L.; Tilles, S.; Lio, P.; Boguniewicz, M.; Beck, L.; LeBovidge, J.; Novak, N.; Bernstein, D.; Blessing-Moore, J.; Khan, D.; et al. Atopic dermatitis: A practice parameter update 2012. J. Allergy Clin. Immunol. 2013, 131, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Simon, D.; Braathen, L.R.; Simon, H.U. Eosinophils and atopic dermatitis. Allergy 2004, 59, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Suarez-Farinas, M.; Dhingra, N.; Gittler, J.; Shemer, A.; Cardinale, I.; de Guzman Strong, C.; Krueger, J.G.; Guttman-Yassky, E. Intrinsic atopic dermatitis shows similar Th2 and higher Th17 immune activation compared with extrinsic atopic dermatitis. J. Allergy Clin. Immunol. 2013, 132, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Moy, A.P.; Murali, M.; Kroshinsky, D.; Duncan, L.M.; Nazarian, R.M. Immunologic overlap of helper T cell subtypes 17 and 22 in erythrodermic psoriasis and atopic dermatitis. JAMA Dermatol. 2015, 151, 753–760. [Google Scholar] [CrossRef] [PubMed]
- Wollenberg, A.; Kraft, S.; Hanau, D.; Bieber, T. Immunomorphological and ultrastructural characterization of langerhans cells and a novel, inflammatory dendritic epidermal cell (IDEC) population in lesional skin of atopic eczema. J. Investig. Dermatol. 1996, 106, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Werfel, T.; Allam, J.P.; Biedermann, T.; Eyerich, K.; Gilles, S.; Guttman-Yassky, E.; Hoetzenecker, W.; Knol, E.; Simon, H.U.; Wollenberg, A.; et al. Cellular and molecular immunologic mechanisms in patients with atopic dermatitis. J. Allergy Clin. Immunol. 2016, 138, 336–349. [Google Scholar] [CrossRef] [PubMed]
- Kimber, I.; Basketter, D.A.; Gerberick, G.F.; Dearman, R.J. Allergic contact dermatitis. Int. Immunopharmacol. 2002, 2, 201–211. [Google Scholar] [CrossRef]
- Fonacier, L.S.; Dreskin, S.C.; Leung, D.Y. Allergic skin diseases. J. Allergy Clin. Immunol. 2010, 125, S138–S149. [Google Scholar] [CrossRef] [PubMed]
- Vocanson, M.; Hennino, A.; Rozieres, A.; Poyet, G.; Nicolas, J.F. Effector and regulatory mechanisms in allergic contact dermatitis. Allergy 2009, 64, 1699–1714. [Google Scholar] [CrossRef] [PubMed]
- Krampera, M. Mesenchymal stromal cell “licensing”: A multistep process. Leukemia 2011, 25, 1408–1414. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, E.M.; Gordon, P.L.; Koo, W.K.; Marx, J.C.; Neel, M.D.; McNall, R.Y.; Muul, L.; Hofmann, T. Isolated allogeneic bone marrow-derived mesenchymal cells engraft and stimulate growth in children with osteogenesis imperfecta: Implications for cell therapy of bone. Proc. Natl. Acad. Sci. USA 2002, 99, 8932–8937. [Google Scholar] [CrossRef] [PubMed]
- Von Bahr, L.; Batsis, I.; Moll, G.; Hagg, M.; Szakos, A.; Sundberg, B.; Uzunel, M.; Ringden, O.; Le Blanc, K. Analysis of tissues following mesenchymal stromal cell therapy in humans indicates limited long-term engraftment and no ectopic tissue formation. Stem Cells 2012, 30, 1575–1578. [Google Scholar] [CrossRef] [PubMed]
- Ankrum, J.A.; Ong, J.F.; Karp, J.M. Mesenchymal stem cells: Immune evasive, not immune privileged. Nat. Biotechnol. 2014, 32, 252–260. [Google Scholar] [CrossRef] [PubMed]

| Model | Animals (Strain) | MSCs | Reference | |||
|---|---|---|---|---|---|---|
| Source | Route | Effect | Mechanisms & Note | |||
| AD (OVA-induced) | Mouse (BALB/c) | Mouse BM | IV | Y | T cell-suppression via NO; B cell-suppression via CSR | [55] |
| AD (Df-induced) | Mouse (Nc/Nga) | Human UCB | SC | Y | Inhibition of MC degranulation through PGE2 and TGF-β1 | [56] |
| AD (Df-induced) | Mouse (Nc/Nga) | Human AT | IV | Y | B cell-suppression via COX-2 | [66] |
| Psoriasis (IMQ-induced) | Mouse (C57BL/6) | Human UCB | SC | Y | Inhibition of various effector cells; SOD3-transduced MSC | [62] |
| SLE | Mouse (MRL/lpr) | Mouse BM | IV | Y | B cell-suppression via BAFF | [67] |
| SLE | Mouse (NZB/W F1) | Human UCB | IV | Y | - | [68] |
| SSc (HClO-induced) | Mouse (BALB/c) | Mouse BM | IV | Y | Diffuse SSc | [69] |
| GvHD | Mouse (B6D2F1) | Mouse AT | IV | Y | T cell-suppression | [45] |
| Acute GvHD | Mouse (DBA/2) | Human UC | IV | Y | T cell-suppression; TGF-β1 and IDO | [46] |
| Cutaneous DTH (DNFB-induced) | Mouse (C57BL/6) | Mouse BM | IV | Y | Induction of activated T cell; apoptosis in dLN | [70] |
| CHS | Mouse (BALB/c) | Human Gingiva | IV | Y | Suppression of DCs and MCs through PGE2 | [57] |
| CHS | Mouse (BALB/c) | Human Gingiva/AT/BM | IV/Local | Y | PGE2-EP3 signaling | [64] |
| Disease | Type | Size | Periods | MSC Sources | Responses & Note | Reference |
|---|---|---|---|---|---|---|
| Moderate-to-severe AD (NCT01927005) | Phase I; Phase IIa | 7 Adults; 27 Adults | 4 and 12 weeks | AlloUCB | 6/11 (55%) :EASI50 in high dose treated group | [63] |
| Moderate-to-severe Psoriasis vulgaris (NCT02491658) | Case report | 2 Adults | 4–5 years | AlloUC | 2/2 CR; No adverse effects | [71] |
| Psoriasis vulgaris | Case report | 1 Adult | 292 days | AlloAT | Reduction in PASI | [72] |
| Refractory SLE (NCT00698191) | Pilot Study | 15 Adults | 17.2 ± 9.5 months | AlloBM | Reduction in SLEDAI; Remission of skin rash | [48] |
| SLE | Case report | 2 Adults | 14 weeks | AutoBM | No clinical effect | [73] |
| Active and refractory SLE (NCT01741857) | Multicenter clinical study | 40 Adults | 1 year | AlloUC | 37/40 (92.5%) survival; 7/40 (17.5%) relapse; after 6 months | [74] |
| Refractory SLE (NCT00698191) | Case report | 4 Adults | 12–18 months | AlloBM | Recovery | [75] |
| Severe progressive SSc | Case report | 5 Adults | 4–44 months | AlloBM | 2/5 (40%) improvement in MRSS | [76] |
| Severe progressive SSc | Case report | 1 Adult | 6 months | AlloBM | Marked improvement; by CD137L ligation | [59] |
| SSc | Case report | 6 Adults | 1 year | AutoAT (w/HA) | 4/6: significant; 1/5: moderate; No related complications | [65] |
| Steroid-resistant, severe, acute GvHD | Phase II | 30 Adults; 25 Children | 60 months | AlloBM | 30/55 (54.5%) CR; 9/55 (16.4%) PR | [12] |
| Sever refractory acute GVHD | Open-label | 12 Children | 2 years | AlloBM | 7/12 (58.3%) CR; 2/12 (16.7%) PR | [77] |
| Acute GvHD; chronic GvHD (NCT00447460) | Phase I/II | 10 Adults; 8 Adults | 3 days–1 year | AlloBM | 1/10 CR, 6/10 PR; 1/8 CR, 3/8 PR | [58] |
| Sclerodermatous chronic GvHD | Case report | 4 Adults | 4.6–23 months | AlloBM | Gradually improved | [61] |
| Cells | MSCs | Effects | Mechanism | |
|---|---|---|---|---|
| T cells | mBMSCs [37,83]; hBMSCs [42,81,84,85] | Proliferation↓ [37,42,83,84,85]; Differentiation↓ [37] | Cell cycle arrest at G1 [37]; TGF-β1, HGF [42]; iNOS [83]; IDO [84]; HLA-G5 [85] | |
| Th cells | Th1 | mBMSCs [70]; hBMSCs [81]; hUCB-MSCs [62] | Differentiation↓ [37,55,62]; Cytokine production↓ [55,62,81] | PGE2 [81] |
| Th2 | mBMSCs [52,55,86]; mAT-MSCs [87]; hBMSCs [81]; hAM-MSCs [88,89]; hUCB-MSCs [62] | Activation↑ [81]; Differentiation↓ [62,70,88]; Cytokine production↓ [52,55,62,88]; No change [89] | TGF-β1 [52]; IFN-γ [86,87] | |
| Th17 | mBMSCs [90,91,92]; hBMSCs [80,93]; hUCB-MSCs [62] | Th17 differentiation↓ [62,90,91,92,93]; Th17 differentiation↑ [92]; Cytokine production↓ [62,93] | IL-10 [90]; PGE2 [93]; CCR6/CCL20, CD18/CD54L [91]; COX-2 [91] | |
| Treg cells | mBMSCs [94,95]; hBMSCs [80,81,85,96]; hUC-MSCs [97] | Treg induction↑ [80,81,93,94,95,96,97]; IL-10 production↑ [80,81,93,94,96,97] | Cell contact, PGE2, TGF-β1 [80]; IDO [97]; HLA-G5 [85]; Monocyte regulation [96]; FAS/FASL-mediated T cell apoptosis↑ [95] | |
| B cells | mBMSCs [55,98,99]; hBMSCs [38,100,101]; hUC-MSCs [102,103]; hAT-MSCs [104]; hUCB-MSCs [66] | Proliferation↓ [38,55,66,98,103]; Proliferation↑ [102]; Differentiation↓ [38,55,66,98,103,104]; Differentiation↑ [102]; Antibody production↓ [38,98]; Antibody production↑ [102]; Chemotactic ability↓ [38]; Apoptosis↓ [87,100]; Breg induction↑ [101,104] | Cell cycle arrest at G0/G1 [38]; PGE2 [102]; VEGF [100]; IDO [101]; Unknown soluble factors [38,103]; PD-1/PD-L1 [99]; COX-2 [66] | |
| DCs | mBMSCs [105]; hBMSCs [81,106,107,108,109,110]; hAD-MSCs [111] | Early DC maturation↓ [106,107]; Proliferation↓ [109,110]; Differentiation↓ [105]; T cell priming ability↓ [108]; Tolerogenic DC induction↑ [111]; mDC generation↓ [81] | PGE2 [106]; Cell cycle arrest at G0 state [109]; TLR4 [108]; GRO-γ [111]; IL-6 [105] | |
| MCs | mBMSCs [112]; hUCB-MSCs [56]; hGMSCs [56] | Degranulation↓ [56,112]; Cytokine production↓ [57,112] | COX-2-dependent cell contact [112]; PGE2 [56,57]; TGF-β1 [56] | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, T.-H.; Kim, H.-S.; Choi, S.W.; Kang, K.-S. Mesenchymal Stem Cell Therapy for Inflammatory Skin Diseases: Clinical Potential and Mode of Action. Int. J. Mol. Sci. 2017, 18, 244. https://doi.org/10.3390/ijms18020244
Shin T-H, Kim H-S, Choi SW, Kang K-S. Mesenchymal Stem Cell Therapy for Inflammatory Skin Diseases: Clinical Potential and Mode of Action. International Journal of Molecular Sciences. 2017; 18(2):244. https://doi.org/10.3390/ijms18020244
Chicago/Turabian StyleShin, Tae-Hoon, Hyung-Sik Kim, Soon Won Choi, and Kyung-Sun Kang. 2017. "Mesenchymal Stem Cell Therapy for Inflammatory Skin Diseases: Clinical Potential and Mode of Action" International Journal of Molecular Sciences 18, no. 2: 244. https://doi.org/10.3390/ijms18020244
APA StyleShin, T.-H., Kim, H.-S., Choi, S. W., & Kang, K.-S. (2017). Mesenchymal Stem Cell Therapy for Inflammatory Skin Diseases: Clinical Potential and Mode of Action. International Journal of Molecular Sciences, 18(2), 244. https://doi.org/10.3390/ijms18020244