Influence of Age and Dose on the Effect of Resveratrol for Glycemic Control in Type 2 Diabetes Mellitus: Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Results
2.1. Literature Search
2.2. Study Characteristics
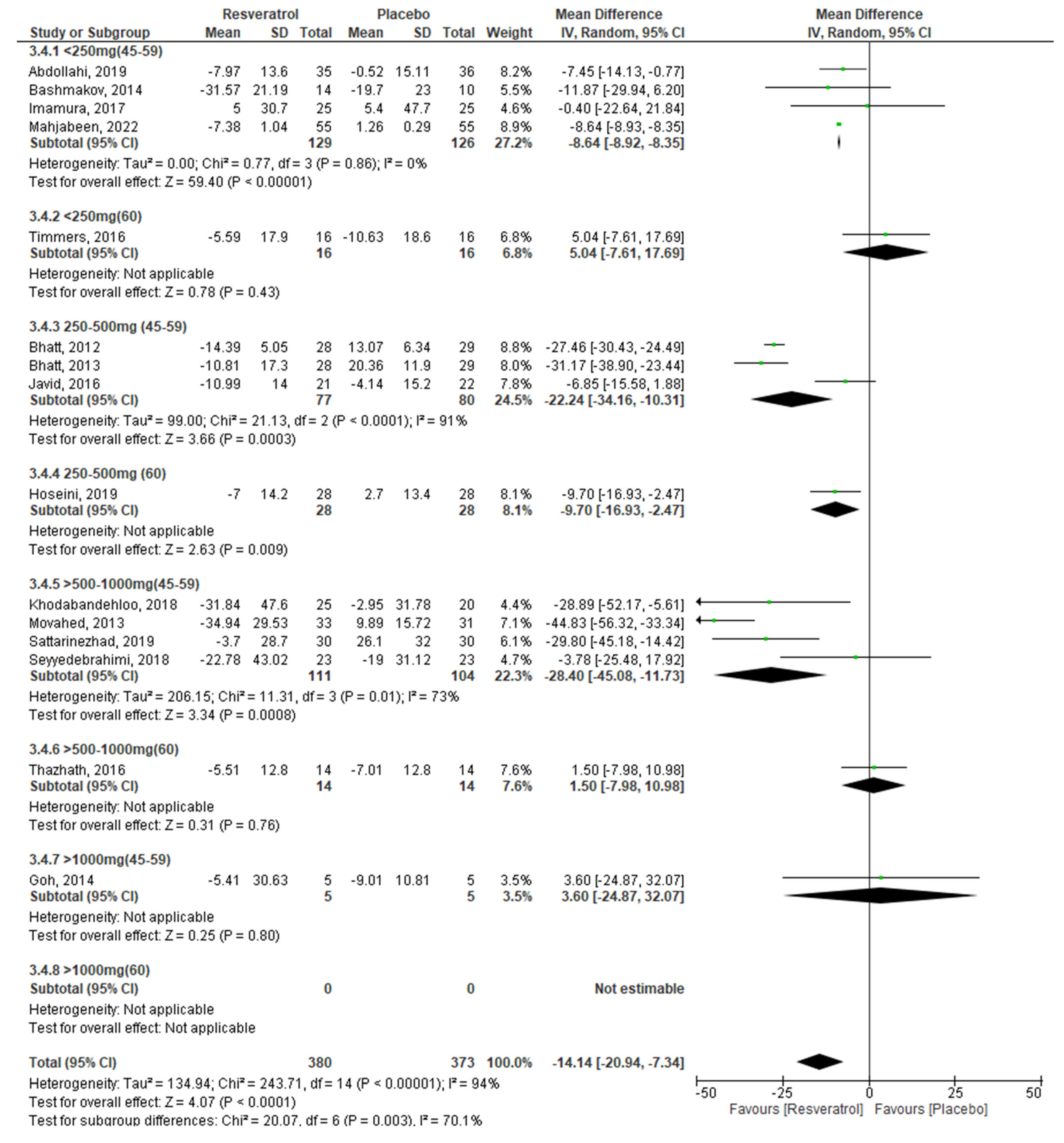
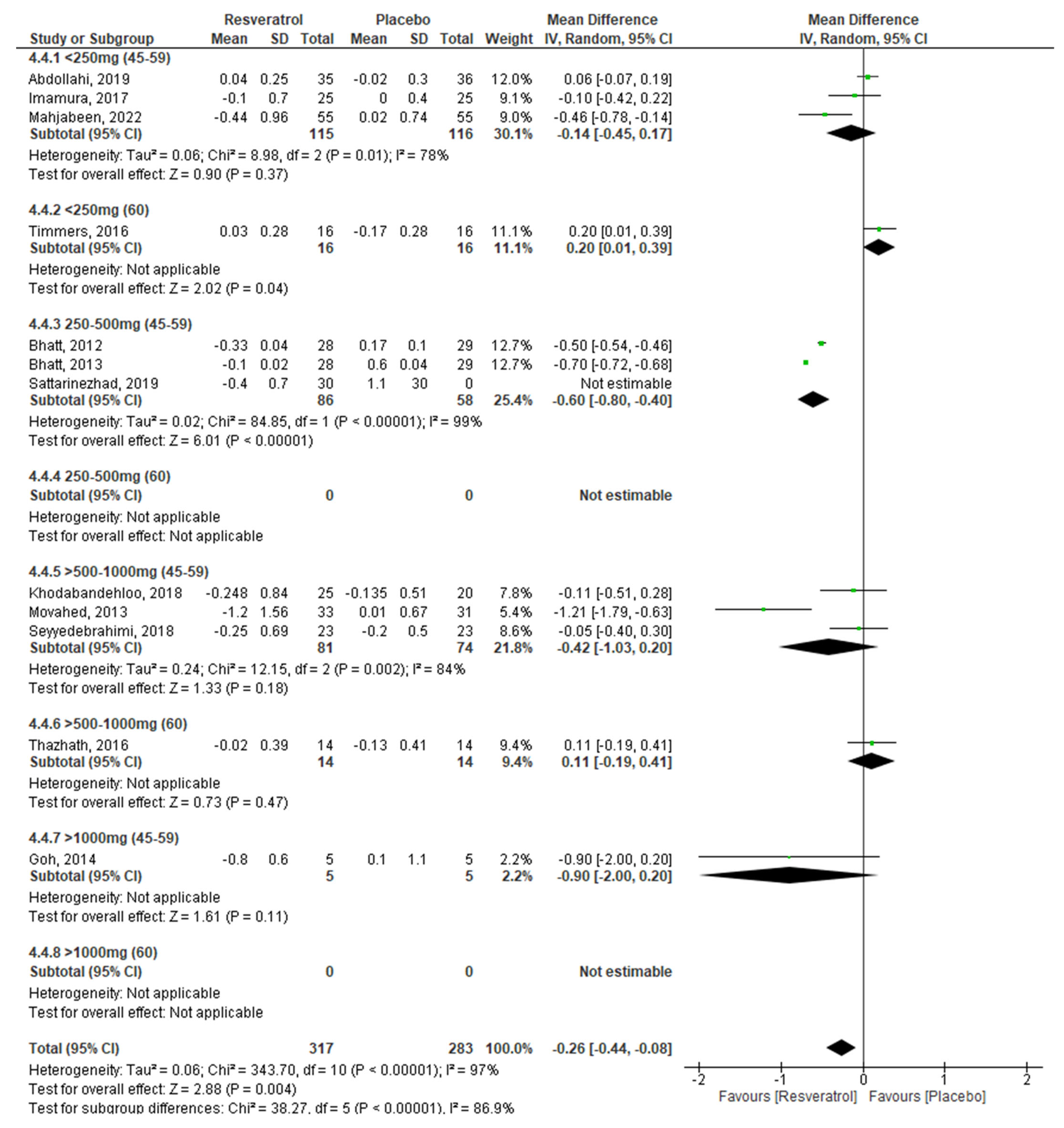
2.3. Meta-Analysis
3. Discussion
3.1. Effect of RV on Glycemic Control by Dose
3.2. Effect of RV on Glycemic Control by Age
3.3. Effect of RV on Glycemic Control by Age and Dose
4. Materials and Methods
4.1. Search Strategy
4.2. Selection of Studies
4.2.1. Selection Criteria
- -
- Randomized clinical trials (RCTs) blind, double blind, open, or crossover
- -
- Oral administration of RV as a coadyuvant in T2DM treatment
- -
- Studies with placebo group as comparator
- -
- Published in English and/or Spanish
- -
- Evaluate at least one of the following glycemic markers: serum glucose and insulin levels, HbA1c, and insulin resistance (HOMA-IR)
- -
- Intervention lasting at least 2 weeks
- -
- Carried out in individuals over 20 years of age with T2DM
4.2.2. Exclusion Criteria
- -
- Use of RV in combination with other compounds
- -
- Use of compounds derived from resveratrol
- -
- Investigations without a comparator
- -
- Conference summaries
4.3. Assessment of the Risk of Bias and Certainty of the Included Studies
4.4. Data Extraction
4.5. Statistic Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. Studies Retrieved from Databases that Were Excluded from the Systematic Review and Meta-Analysis
| Study | Reason for Exclusion |
|---|---|
| Abdollahi, et al. BMJ. 2019;9:e026337. Doi:10.1136/ bmjopen-2018-026337 | It is a protocol |
| Arcanjo, et al. Biochim Biophys Acta Gen. 2018;1862:1938-1947. Doi: 10.1016/j.bbagen.2018.06.007 | They do not evaluate glycemic parameters |
| Bo, et al. Acta Diabetol. 2018;55:331–3402018. Doi:10.1007/s00592-017-1097-4 | They report only pre-treatment means of glycemic parameters |
| Bo, et al. Nutr Diabetes. 2018;8:51. Doi: 10.1038/s41387-018-0059-4 | They do not evaluate glycemic parameters |
| Bo, et al. Acta Diabetol. 2017;54:499–507. Doi: 10.1007/s00592-017-0977-y | They do not report only pre- and post-treatment means of glycemic parameters |
| De Ligt, et al. Mol Metab. 2018;12: 39e47. Doi: 10.1016/j.molmet.2018.04.004 | They do not evaluate glycemic parameters |
| Froghi, et al. IJEM. 2018;20:169–176. | Language other than English or Spanish |
| Javid, et al. Diabetes Metab Syndr. 2019;13:2769–2774. Doi: 10.1016/j.dsx.2019.07.042 | They do not evaluate glycemic parameters |
| Pollack, et al. J Gerontol A Biol Sci Med Sci. 2017;72(12):1703–1709. Doi: 10.1093/gerona/glx041 | They report only pre-treatment means of glycemic parameters |
| Saldanha, et al. J Ren Nutr. 2016. Doi: 10.1053/j.jrn.2016.06.005 | They do not evaluate glycemic parameters |
| Tabatabaie, et al. Phytoter Res. 2020;34:2023–2031.Doi: 10.1002/ptr.6655 | They report only pre-treatment means of glycemic parameters |
| Tomé-Carneiro et al. Pharmacol Res. 2013. Doi: 10.1016/j.phrs.2013.03.011 | They use a grape extract |
| Toupchian, et al. Phytoter Res. 2021;35: 3205–3213. Doi: 10.1002/ptr.7031 | They do not evaluate glycemic parameters |
| Wong, et al. Nutr Metabol Cardiovasc Dis. 2016. Doi: 10.1016/j.numecd.2016.03.003 | They use a single dose of RV |
| Wong, et al. Nutrients. 2016, 8, 425. Doi:10.3390/nu8070425 | They use a single dose of RV |
| Zhang, et al. Arterioscler Thromb Vasc Biol. 2017;37:A164. Doi: 10.1161/atvb.37.suppl_1.164 | Only abstract available |
Appendix B. Documents Retrieved from Other Sources that Were Excluded from the Systematic Review and Meta-Analysis
| Reason for Exclusion | Document Excluded |
|---|---|
| They do not meet the selection criteria (n = 19) | NCT04449198, NCT03762096, NCT03436992, NCT01997762, NCT03436992, IRCT201710108129N11, CTRI/2017/04/008384, NCT04449198, NCT00823381, NCT01593605, NCT02565979, NCT02129595, NCT02247596, NCT02834078, NCT01451918, NCT01714102, NCT03597568, NCT01150955, NCT03866005 |
| They do not assess glycemic parameters (n = 8) | IRCT20171118037528N1, NCT01158417, NCT03762096, NCT01354977, NCT02502253, NCT02216552, NCT01302639, NCT02244879 |
| Unpublished data (n = 7) | SLCTR/2018/019, IRCT201601022394N19, NCT01038089, NCT05172947IRCT201411112394N14, NCT02502253, NCT02549924, |
| Published data included in review (n = 11) | IRCT2016100716876N2, NCT02704494 (Sattarinezhad, 2019) [35], IRCT2015080223336N2 (Khodabandehloo, 2018) [30], IRCT2015072523336N1 (Seyyedebrahimi, 2018) [31], IRCT2015012420765N1 (Javid, 2016) [29], ACTRN12613000717752 (Thazhath, 2016) [39], IRCT201111198129N1 (Movahed, 2013) [33], CTRI/2011/05/001731 (Bhatt, 2012; Bhatt, 2013) [27,28], ACTRN12610000565044 (Basmakov, 2014) [24], NCT01677611 (Goh, 2014) [34], NCT01638780 (Timmers, 2016) [36]. |
| Published data excluded from review (n = 3) | ACTRN12614000891628 (Wong, 2016), NCT01881347 (Zhang, 2017), NCT02244879 (Bo, 2018) |
| Language other than English or Spanish (n = 1) | IRCT20191203045588N1 |
| Ongoing (n = 2) | CTRI/2022/05/042806, ISRCTN15172592 |
Appendix C. Dose–Response Regression Analysis to Assess the Effect of RV on Glycemic Parameters
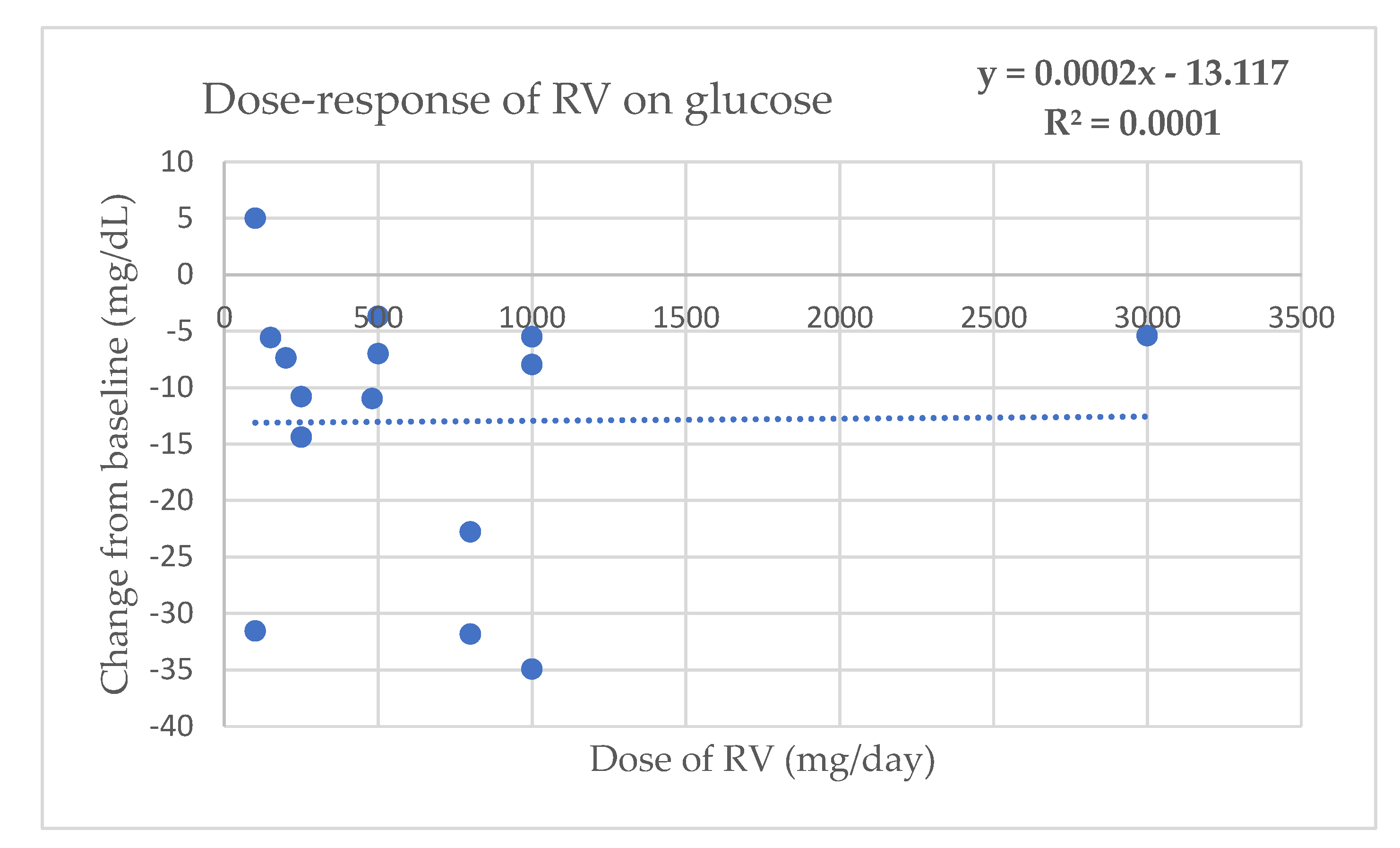


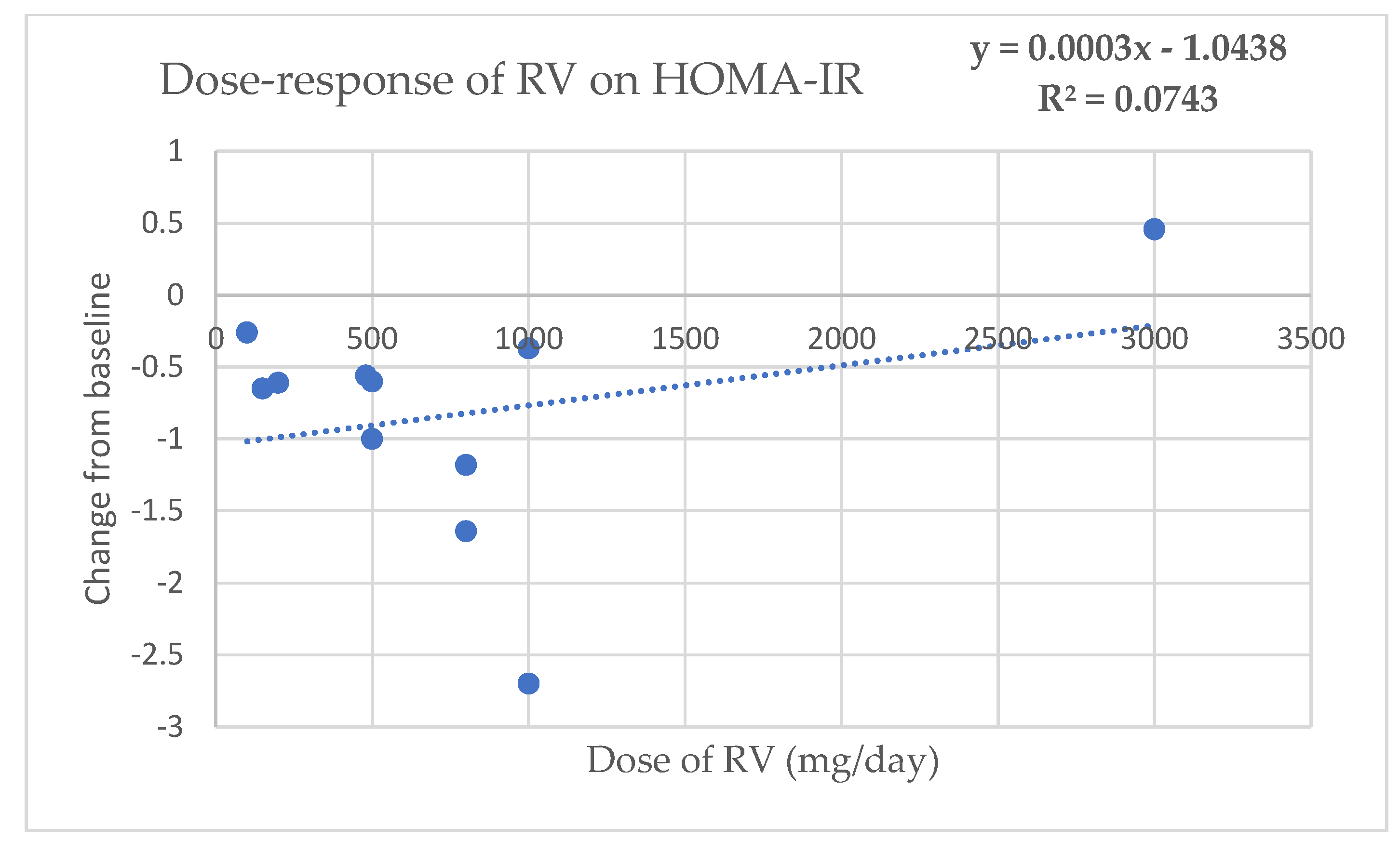
References
- Daryabor, G.; Atashzar, M.R.; Kabelitz, D.; Meri, S.; Kalantar, K. The effects of type 2 diabetes mellitus on organ metabolism and the immune system. Front. Immunol. 2020, 11, 1582. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Status Report on Noncommunicable Diseases 2014; World Health Organization: Geneva, Switzerland, 2014. [Google Scholar]
- Rauf, A.; Imran, M.; Suleria, H.A.R.; Ahmad, B.; Petersf, D.G.; Mubarak, M.S. A comprehensive review of the health perspectives of resveratrol. Food Funct. 2017, 8, 4284–4305. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.P.; Singh, R.; Singh, S.V.; Rai, V.; Kaschula, C.H.; Maiti, P.; Gupta, S.C. Health benefits of resveratrol: Evidence from clinical studies. Med. Res. Rev. 2019, 39, 1851–1891. [Google Scholar] [CrossRef] [PubMed]
- Galiniak, S.; Aebisher, D.; Bartusik-Aebisher, D. Health benefits of resveratrol administration. Acta Biochim. Pol. 2019, 66, 13–21. [Google Scholar] [CrossRef]
- Rouse, M.; Younès, A.; Egan, J.M. Resveratrol and curcumin enhance pancreatic β-cell function by inhibiting phosphodiesterase activity. J. Endocrinol. 2014, 223, 107–117. [Google Scholar] [CrossRef]
- Gowd, V.; Kang, Q.; Wang, Q.; Chen, F.; Cheng, K.W. Resveratrol: Evidence for its nephroprotective effect in diabetic nephropathy. Adv. Nutr. 2020, 11, 1555–1568. [Google Scholar] [CrossRef]
- Öztürka, E.; Karaboğa, A.K.A.; Yerer, M.B.; Bishayee, A. Resveratrol and diabetes: A critical review of clinical studies. Biomed. Pharmacother. 2017, 95, 230–234. [Google Scholar] [CrossRef]
- Oyenihi, O.R.; Oyenihi, A.B.; Adeyanju, A.A.; Oguntibeju, O.O. Antidiabetic effects of resveratrol: The way forward in its clinical utility. J. Diabetes Res. 2016, 2016, 9737483. [Google Scholar] [CrossRef]
- Mendoza-Núñez, V.M. Envejecimiento humano: Un marco conceptual para la intervención comunitaria. In Promoción de la Salud en la Mujer Adulta Mayor; Mendoza-Núñez, V.M., Martínez-Maldonado, M.L., Eds.; Instituto Nacional de Geriatría: Ciudad de México, Mexico, 2015; pp. 13–40. [Google Scholar]
- Yegorov, Y.E.; Poznyak, A.V.; Nikiforov, N.G.; Sobenin, I.A.; Orekhov, A.N. The link between chronic stress and accelerated aging. Biomedicines 2020, 8, 198. [Google Scholar] [CrossRef]
- García-Martínez, B.I.; Ruiz-Ramos, M.; Pedraza-Chaverri, J.; Santiago-Osorio, E.; Mendoza-Núñez, V.M. Hypoglycemic effect of resveratrol: A systematic review and meta-analysis. Antioxidants 2021, 10, 69. [Google Scholar] [CrossRef]
- Li, X.; Yang, T.; Sun, Z. Hormesis in health and chronic diseases. Trends Endocrinol. Metab. 2019, 30, 944–958. [Google Scholar] [CrossRef]
- Bhakta-Guha, D.; Efferth, T. Hormesis: Decoding two sides of the same coin. Pharmaceuticals 2015, 8, 865–883. [Google Scholar] [CrossRef]
- Mehdi, M.M.; Solanki, P.; Singh, P. Oxidative stress, antioxidants, hormesis and calorie restriction: The current perspective in the biology of aging. Arch. Gerontol. Geriatr. 2021, 95, 104413. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Iavicoli, I.; Calabrese, V. Hormesis: Why it is important to biogerontologists. Biogerontology 2012, 13, 215–235. [Google Scholar] [CrossRef]
- Calabrese, E.; Mattson, M.P. How does hormesis impact biology, toxicology, and medicine? Aging Mech. Dis. 2017, 3, 13. [Google Scholar] [CrossRef]
- Agathokleous, E.; Calabrese, E. Hormesis: The dose response for the 21st century: The future has arrived. Toxicology 2019, 425, 152249. [Google Scholar] [CrossRef]
- Retana-Ugalde, R.; Casanueva, E.; Altamirano-Lozano, M.; González-Torres, C.; Mendoza-Núñez, V.M. High dosage of ascorbic acid and alpha-tocopherol is not useful for diminishing oxidative stress and DNA damage in healthy elderly adults. Ann. Nutr. Metab. 2008, 52, 167–173. [Google Scholar] [CrossRef]
- Seals, D.R.; Justice, J.N.; LaRocca, T.J. Physiological geroscience: Targeting function to increase healthspan and achieve optimal longevity. J. Physiol. 2016, 594, 2001–2024. [Google Scholar] [CrossRef]
- Yabluchanskiy, A.; Ungvari, Z.; Tarantini, S. Advances and challenges in geroscience research: An update. Physiol. Int. 2018, 105, 298–308. [Google Scholar] [CrossRef]
- Kulkarni, A.S.; Aleksic, S.; Berger, D.M.; Sierra, F.; Kuchel, G.A.; Barzilai, N. Geroscience-guided repurposing of FDA-approved drugs to target aging: A proposed process and prioritization. Aging Cell 2022, 21, e13596. [Google Scholar] [CrossRef]
- Brasnyó, P.; Molnár, G.; Mohás, M.; Markó, L.; Laczy, B.; Cseh, J.; Mikolás, E.; Szijártó, I.A.; Mérei, A.; Halmai, R.; et al. Resveratrol improves insulin sensitivity, reduces oxidative stress and activates the Akt pathway in type 2 diabetic patients. Br. J. Nutr. 2011, 106, 383–389. [Google Scholar] [CrossRef]
- Bashmakov, Y.K.; Assaad-Khalil, S.H.; Abou, M.; Udumyan, R.; Megallaa, M.; Rohoma, K.H.; Zeitoun, M.; Petyaev, I.M. Resveratrol promotes foot ulcer size reduction in type 2 diabetes patients. ISRN Endocrinol. 2014, 2014, 816307. [Google Scholar] [CrossRef]
- Imamura, H.; Yamaguchi, T.; Nagayama, D.; Saiki, A.; Shirai, K.; Tatsuno, I. Resveratrol ameliorates arterial stiffness assessed by cardio-ankle vascular index in patients with type 2 diabetes mellitus. Int. Heart J. 2017, 58, 578–583. [Google Scholar] [CrossRef]
- Mahjabeen, W.; Khan, D.A.; Mirza, S.A. Role of resveratrol supplementation in regulation of glucose hemostasis, inflammation and oxidative stress in patients with diabetes mellitus type 2: A randomized, placebo-controlled trial. Complement. Ther. Med. 2022, 6, 102819. [Google Scholar] [CrossRef]
- Bhatt, J.K.; Thomas, S.; Nanjan, M.J. Resveratrol supplementation improves glycemic control in type 2 diabetes mellitus. Nutr. Res. 2012, 32, 537–541. [Google Scholar] [CrossRef]
- Bhatt, J.K.; Nanjan, M.J. Resveratrol supplementation in patients with type 2 diabetes mellitus: A prospective, open label, randomized controlled trial. Int. Res. J. Pharm. 2013, 4, 245–249. [Google Scholar] [CrossRef]
- Javid, A.Z.; Hormoznejad, R.; Yousefimanesh, H.; Zakerkish, M.; Haghighi-zadeh, M.H.; Dehghan, P.; Ravanbakhsh, M. The impact of resveratrol supplementation on blood glucose, insulin, insulin resistance, triglyceride, and periodontal markers in type 2 diabetic patients with chronic periodontitis. Phytother. Res. 2016, 31, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Khodabandehloo, H.; Seyyedebrahimi, S.; Esfahani, E.N.; Razi, F.; Meshkani, R. Resveratrol supplementation decreases blood glucose without changing the circulating CD14+ CD16+ monocytes and inflammatory cytokines in patients with type 2 diabetes: A randomized, double-blind, placebo-controlled study. Nutr. Res. 2018, 54, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Seyyedebrahimi, S.; Khodabandehloo, H.; Esfahani, E.N.; Meshkani, R. The effects of resveratrol on markers of oxidative stress in patients with type 2 diabetes: A randomized, double-blind, placebo-controlled clinical trial. Acta Diabetol. 2018, 55, 341–353. [Google Scholar] [CrossRef] [PubMed]
- Abdollahi, S.; Salehi-Abargouei, A.; Toupchian, O.; Sheikhha, M.H.; Fallahzadeh, H.; Rahmanian, M.; Tabatabaie, M.; Mozaffari-Khosravi, H. The effect of resveratrol supplementation on cardio-metabolic risk factors in patients with type 2 diabetes: A randomized, double-blind controlled trial. Phytother. Res. 2019, 33, 3153–3162. [Google Scholar] [CrossRef] [PubMed]
- Movahed, A.; Nabipour, I.; Lieben, X.L.; Thandapilly, S.J.; Yu, L.; Kalantarhormozi, M.; Rekabpour, S.J.; Netticadan, T. Antihyperglycemic effects of short-term resveratrol supplementation in type 2 diabetic patients. Evid. Based Complement. Alternat. Med. 2013, 2013, 851267. [Google Scholar] [CrossRef]
- Goh, K.P.; Lee, H.Y.; Lau, D.P.; Supaat, W.; Chan, Y.H.; Yung, A.F. Effects of resveratrol in patients with type 2 diabetes mellitus on skeletal muscle SIRT1 expression and energy expenditure. Int. J. Sport Nutr. Exerc. Metab. 2014, 24, 2–13. [Google Scholar] [CrossRef]
- Sattarinezhad, A.; Roozbeh, J.; Yeganeh, S.; Omrani, G.; Shams, M. Resveratrol reduces albuminuria in diabetic nephropathy: A randomized double-blind placebo-controlled clinical trial. Diabetes Metab. 2019, 45, 53–59. [Google Scholar] [CrossRef]
- Timmers, S.; De Ligt, M.; Phielix, E.; Van de Weijer, T.; Hansen, J.; Moonen-Kornips, E.; Schaart, G.; Kunz, I.; Hesselink, M.K.; Schrauwen-Hinderling, V.B.; et al. Resveratrol as add-on therapy in subjects with well-controlled type 2 diabetes: A randomized controlled trial. Diabetes Care 2016, 19, 2211–2217. [Google Scholar] [CrossRef]
- Bo, S.; Ponzo, V.; Ciccone, G.; Evangelista, A.; Saba, F.; Goitre, I.; Procopio, M.; Pagano, G.; Cassader, M.; Gambino, R. Six months of resveratrol supplementation has no measurable effect in type 2 diabetic patients. A randomized, double blind, placebo-controlled trial. Pharmacol. Res. 2016, 111, 896–905. [Google Scholar] [CrossRef]
- Hoseini, A.; Namazi, G.; Farrokhian, A.; Reiner, Z.; Aghadavod, E.; Bahmania, F.; Asemi, Z. The effects of resveratrol on metabolic status in patients with type 2 diabetes mellitus and coronary heart disease. Food Funct. 2019, 10, 6042–6051. [Google Scholar] [CrossRef]
- Thazhath, S.S.; Wu, T.; Bound, M.J.; Checklin, H.L.; Standfield, S.; Jones, K.L.; Horowitz, M.; Rayner, C.K. Administration of resveratrol for 5 wk has no effect on glucagon-like peptide 1 secretion, gastric emptying, or glycemic control in type 2 diabetes: A randomized controlled trial. Am. J. Clin. Nutr. 2016, 103, 66–70. [Google Scholar] [CrossRef]
- Anhe, F.F.; Desjardins, Y.; Pilon, G.; Dudonne, S.; Genovese, M.I.; Lajolo, F.M.; Marette, A. Polyphenols and type 2 diabetes: A prospective review. Pharma. Nutr. 2013, 1, 105–114. [Google Scholar] [CrossRef]
- Repossi, G.; Das, U.N.; Eynard, A.E. Molecular basis of the beneficial actions of resveratrol. Arch. Med. Res. 2020, 51, 105–114. [Google Scholar] [CrossRef]
- Huang, D.D.; Shi, G.; Jiang, Y.; Yao, C.; Zhu, C. A review on the potential of resveratrol in prevention and therapy of diabetes and diabetic complications. Biomed. Pharmacother. 2020, 125, 109767. [Google Scholar] [CrossRef]
- Sergi, C.; Chiu, B.; Feulefack, J.; Shen, F.; Chiu, B. Usefulness of resveratrol supplementation in decreasing cardiometabolic risk factors comparing subjects with metabolic syndrome and healthy subjects with or without obesity: Meta-analysis using multinational, randomised, controlled trials. Arch. Med. Sci. Atheroscler. Dis. 2020, 5, e98–e111. [Google Scholar] [CrossRef]
- Avogaro, A.; Fadini, G.P. Microvascular complications in diabetes: A growing concern for cardiologists. Int. J. Cardiol. 2019, 291, 29–35. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Microvascular complications and foot care: Standards of medical care in diabetes—2021. Diabetes Care 2021, 44 (Suppl.S1), S151–S167. [Google Scholar] [CrossRef]
- Oliveira, S.; Monteiro, A.T.; Silva, S.; Matafome, P. Curcumin derivatives for Type 2 Diabetes management and prevention of complications. Arch. Pharm. Res. 2020, 43, 567–581. [Google Scholar] [CrossRef]
- Dehdashtian, E.; Hossein, M.P.; Hemati, K.; Mehrzadi, S.; Hosseinzadeh, A. A therapeutic application of nutraceuticals in diabetic nephropathy: Current evidence and future implications. Diabetes Metab. Res. Rev. 2020, 36, e3336. [Google Scholar] [CrossRef]
- Zhang, T.; He, Q.; Liu, Y.; Chen, Z.; Hu, H. Efficacy and safety of resveratrol supplements on blood lipid and blood glucose control in patients with type 2 diabetes: A systematic review and meta-analysis of randomized controlled trials. Evid. Based Complement. Alternat. Med. 2021, 2021, 5644171. [Google Scholar] [CrossRef]
- Liu, K.; Zhou, R.; Wang, B.; Mi, M.T. Effect of resveratrol on glucose control and insulin sensitivity: A meta-analysis of 11 randomized controlled trials. Am. J. Clin. Nutr. 2014, 99, 1510–1519. [Google Scholar] [CrossRef]
- Hausenblas, H.A.; Schoulda, J.A.; Smoliga, J.M. Resveratrol treatment as an adjunct to pharmacological management in type 2 diabetes mellitus—systematic review and meta-analysis. Mol. Nutr. Food Res. 2015, 59, 147–159. [Google Scholar] [CrossRef]
- Shaito, A.; Posadino, A.M.; Younes, N.; Hasan, H.; Halabi, S.; Alhababi, D.; Al-Mohannadi, A.; Abdel-Rahman, W.M.; Eid, A.H.; Nasrallah, G.K.; et al. Potential adverse effects of resveratrol: Literature review. Int. J. Mol. Sci. 2020, 21, 2084. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Mattson, M.P.; Calabrese, V. Resveratrol commonly displays hormesis: Occurrence and biomedical significance. Hum. Exp. Toxicol. 2010, 29, 980–1015. [Google Scholar] [CrossRef]
- Lagouge, M.; Argmann, C.; Gerhart-Hines, Z.; Meziane, H.; Lerin, C.; Daussin, F.; Geny, B. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1α. Cell 2006, 127, 1109–1122. [Google Scholar] [CrossRef] [PubMed]
- Gambino, R.; Fanni, G.; Togliatto, G.; Ponzo, V.; Goitre, I.; Cassader, M.; Brizzi, M.F.; Bo, S. Rs12778366 single nucleotide polymorphism of Sirtuin 1 (SIRT1) and response to resveratrol supplementation in patients with type 2 diabetes mellitus. Acta Diabetol. 2019, 56, 963–966. [Google Scholar] [CrossRef] [PubMed]
- Price, N.L.; Gomes, A.P.; Ling, A.J.; Duarte, F.V.; Martin-Montalvo, A.; North, B.J.; Hubbard, B.P. SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab. 2012, 15, 675–690. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, E.J. Hormesis: Principles and applications. Homeopathy 2015, 104, 69e82. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Dhawan, G.; Kapoor, R.; Iavicoli, I.; Calabrese, V. Hormesis: A fundamental concept with widespread biological and biomedical applications. Gerontology 2016, 62, 530–535. [Google Scholar] [CrossRef]
- Calabrese, E.J. Hormesis: Path and progression to significance. Int. J. Mol. Sci. 2018, 19, 2871. [Google Scholar] [CrossRef]
- Kulkarni, S.S.; Cantó, C. The molecular targets of resveratrol. Biochim. Biophys. Acta 2015, 1852, 1114–1123. [Google Scholar] [CrossRef]
- Zhu, X.; Wu, C.; Qiu, S.; Yuan, X.; Li, L. Effects of resveratrol on glucose control and insulin sensitivity in subjects with type 2 diabetes: Systematic review and meta-analysis. Nutr. Metab. 2017, 14, 60. [Google Scholar] [CrossRef]
- Mukherjee, S.; Dudley, J.I.; Das, D.K. Dose-dependency of resveratrol in providing health benefits. Dose Response 2010, 8, 478–500. [Google Scholar] [CrossRef]
- Crandall, J.P.; Oram, V.; Trandafirescu, G.; Reid, M.; Kishore, P.; Hawkins, M.; Cohen, H.W.; Barzilai, N. Pilot study of resveratrol in older adults with impaired glucose tolerance. J. Gerontol. A Biol. Sci. Med. Sci. 2012, 67, 1307–1312. [Google Scholar] [CrossRef]
- Jeyaraman, M.M.; Al-Yousif, N.S.; Mann, A.S.; Dolinsky, V.W.; Rabbani, R.; Zarychanski, R.; Abou-Setta, A.M. Resveratrol for adults with type 2 diabetes mellitus. Cochrane Database Syst. Rev. 2020, 1, CD011919. [Google Scholar] [CrossRef]
- Guo, X.F.; Li, J.M.; Tang, J.; Li, D. Effects of resveratrol supplementation on risk factors of non-communicable diseases: A meta-analysis of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2018, 58, 3016–3029. [Google Scholar] [CrossRef]
- Rahman, S.; Islam, R. Mammalian Sirt1: Insights on its biological functions. Cell Commun. Signal 2011, 9, 11. [Google Scholar] [CrossRef]
- Ramsey, K.M.; Mills, K.F.; Satoh, A.; Imai, S.I. Age-associated loss of Sirt1-mediated enhancement of glucose-stimulated insulin secretion in beta cell-specific Sirt1-overexpressing (BESTO) mice. Aging Cell 2008, 7, 78–88. [Google Scholar] [CrossRef]
- Grassi, M.; Petraccia, L.; Mennuni, G.; Fontana, M.; Scarno, A.; Sabetta, A.; Fraioli, A. Changes, functional disorders, and diseases in the gastrointestinal tract of elderly. Nutr. Hosp. 2011, 26, 659–668. [Google Scholar] [CrossRef]
- Khan, S.S.; Singer, B.; Vaughan, D. Molecular and physiological manifestations and measurement of aging in humans. Aging Cell 2017, 16, 624–633. [Google Scholar] [CrossRef]
- Slawinska, N.; Krupa, R. Molecular aspects of senescence and organismal ageing-DNA damage response, telomeres, inflammation and chromatin. Int. J. Mol. Sci. 2021, 22, 590. [Google Scholar] [CrossRef]
- Herranz, N.; Gil, J. Mechanisms and functions of cellular senescence. J. Clin. Investig. 2018, 128, 1238–1246. [Google Scholar] [CrossRef]
- Malaquin, N.; Martinez, A.; Rodier, F. Keeping the senescence secretome under control: Molecular reins on the senescence-associated secretory phenotype. Exp. Gerontol. 2016, 82, 39–49. [Google Scholar] [CrossRef]
- Borghesan, M.; Hoogaars, W.M.H.; Varela-Eirin, M.; Talma, N.; Demaria, M. A senescence-centric view of aging: Implications for longevity and disease. Trends Cell Biol. 2020, 30, 777–791. [Google Scholar] [CrossRef]
- Webb, M.; Sideris, D.P. Intimate relations-Mitochondria and ageing. Int. J. Mols. Sci. 2020, 21, 7580. [Google Scholar] [CrossRef] [PubMed]
- Lambert, A.J.; Brand, M.D. Reactive oxygen species production by mitochondria. Methods Mol. Biol. 2009, 554, 165–181. [Google Scholar] [CrossRef] [PubMed]
- Di Nottia, M.; Verrigni, D.; Torraco, A.; Rizza, T.; Bertini, E.; Carrozzo, R. Mitochondrial dynamics: Molecular mechanisms, related primary mitochondrial disorders and therapeutic approaches. Genes 2021, 12, 247. [Google Scholar] [CrossRef]
- Blanco-Benítez, M.; Calderón-Fernández, A.; Canales-Cortés, S.; Alegre-Cortés, E.; Uribe-Carretero, E.; Paredes-Barquero, M.; Gimenez-Bejarano, A.; González, G.D.; Gómez-Suaga, P.; Ortega-Vidal, J.; et al. Biological effects of olive oil phenolic compounds on mitochondria. Mol. Cell Oncol. 2022, 9, 2044263. [Google Scholar] [CrossRef] [PubMed]
- Ashrafizadeh, M.; Javanmardi, S.; Moradi-Ozarlou, M.; Mohammadinejad, R.; Farkhondeh, T.; Samarghandian, S.; Garg, M. Natural products and phytochemical nanoformulations targeting mitochondria in oncotherapy: An updated review on resveratrol. Biosci. Rep. 2020, 40, BSR20200257. [Google Scholar] [CrossRef] [PubMed]
- Labbadia, J.; Morimoto, R.I. The biology of proteostasis in aging and disease. Annu. Rev. Biochem. 2015, 84, 435–464. [Google Scholar] [CrossRef] [PubMed]
- Walter, P.; Ron, D. The unfolded protein response: From stress pathway to homeostatic regulation. Science 2011, 334, 1081–1086. [Google Scholar] [CrossRef] [PubMed]
- Höhn, A.; König, J.; Grune, T. Protein oxidation in aging and removal of oxidized proteins. J. Proteom. 2013, 92, 132–159. [Google Scholar] [CrossRef]
- Vatner, S.; Zhang, J.; Oydanich, M.; Berkman, T.; Naftalovich, R.; Vatner, D. Healthful aging mediated by inhibition of oxidative stress. Ageing Res. Rev. 2020, 64, 101194. [Google Scholar] [CrossRef]
- Sies, H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal Transduct. Target Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- De la Fuente, M.; Miquel, J. An update of the oxidation-inflammation theory of aging: The involvement of the immune system in oxi-inflammaging. Curr. Pharm. Des. 2009, 15, 3003–3026. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- The Nordic Cochrane Centre. Review Manager (RevMan). Cochrane Collab, 2014. Available online: http://community.cochrane.org/tools/review-production-tools/revman-5/about-revman-5 (accessed on 5 August 2022).
- Li, N.; Yue, H.; Jia, M.; Liu, W.; Hou, H.; Huang, F.; Xu, T. Effect of low-ratio n-6/n-3 PUFA on blood glucose: A meta-analysis. Food Funct. 2019, 10, 4557–4565. [Google Scholar] [CrossRef]
- Hozo, S.P.; Djulbegovic, B.; Hozo, I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med. Res. Metodol. 2005, 5, 13. [Google Scholar] [CrossRef]


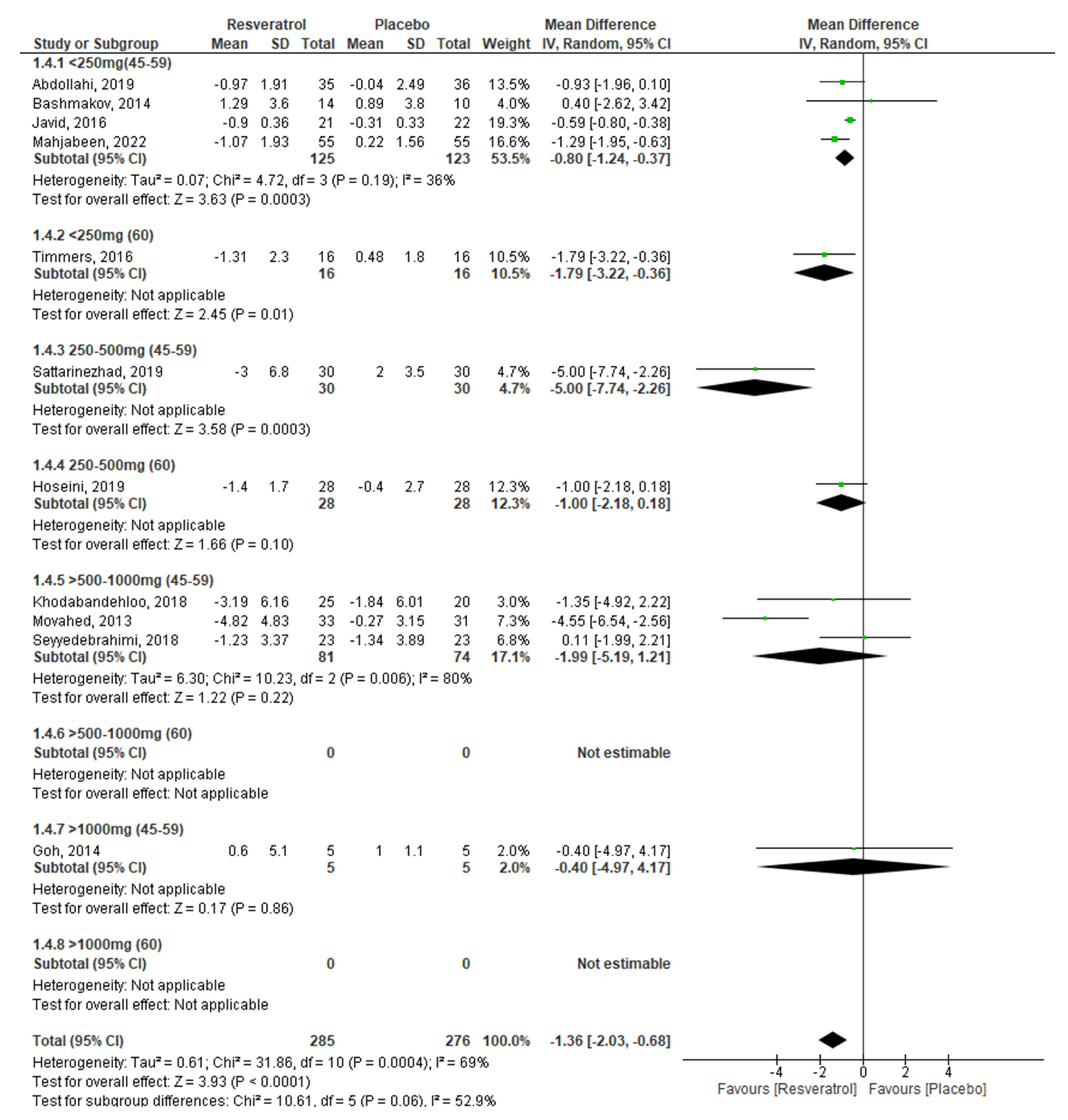
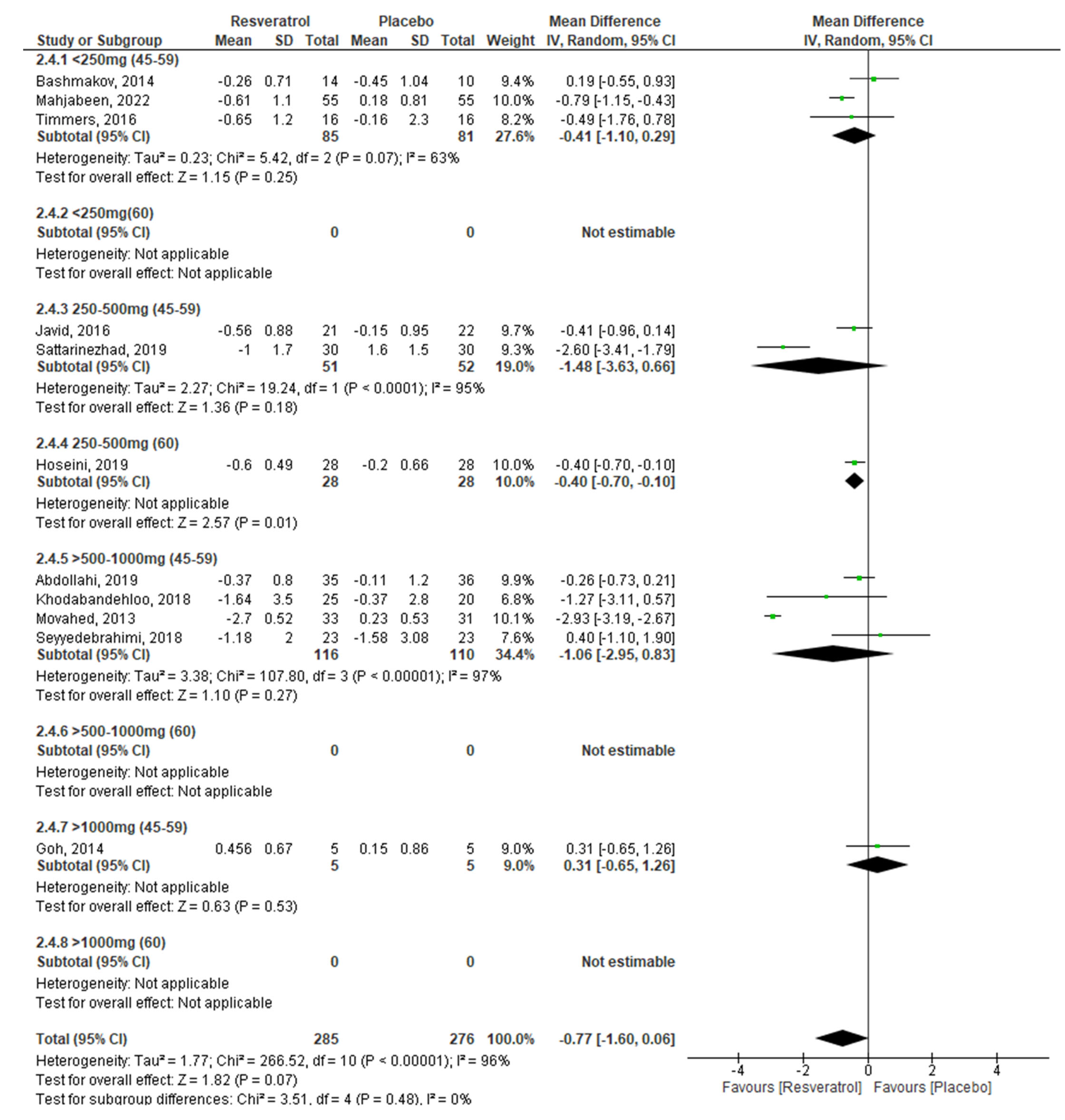
| First Author (Year) | Study Design | Intervention | Population | Glycemic Parameters | Findings | Certainty |
|---|---|---|---|---|---|---|
| Subjects 45–59 years old | ||||||
| Brasnyó et al. (2011) [23] | RCT double-blind | 10 mg/day 4 weeks | 19 men with T2DM 55 ± 9 years old | Insulin levels and HOMA-IR, | No changes in insulin levels, tendendency to decrease of HOMA-IR after RV administration | ⨁◯◯◯ Very low |
| Bashmakov et al. (2014) [24] | RCT parallel-blind | 100 mg/day 8 weeks | 24 subjects with diabetic food 56 ± 9 years old | Glucose e insulin levels, HOMA-IR | Non-significant decrease of glucose in both study groups. No changes in insulin and HOMA-IR. | ⨁◯◯◯ Very low |
| Imamura et al. (2017) [25] | RCT double-blind | 100 mg/day 3 months | 50 subjects with T2DM 57 ± 10 years old | Glucose levels and HbA1c | Non-significant changes after intervention | ⨁◯◯◯ Very low |
| Mahjabeen et al. (2022) [26] | RCT double-blind | 200 mg/day 24 weeks | 110 subjects with T2DM 50 ± 11 years old | Glucose and insulin levels, HbA1c and HOMA-IR | Significant decrease in glucose and HbA1c (p < 0.05). Significant decrease in insulin and HOMA-IR (p = 0.001) | ⨁⨁⨁◯ Moderate |
| Bhatt et al. (2012) [27] | RCT open-label | 250 mg/day 3 months | 57 subjects with T2DM 57 ± 9 years old | Glucose levels and HbA1c | Significant decrease in HbA1c (p < 0.05) after RV administration | ⨁⨁◯◯ Low |
| Bhatt et al. (2013) [28] | RCT open-label | 250 mg/day 6 months | 57 subjects with T2DM 57 ± 9 years old | Glucose levels and HbA1c | Non-significant decrease in HbA1c and glucose levels | ⨁⨁◯◯ Low |
| Javid et al. (2016) [29] | RCT double-blind | 480 mg/day 4 weeks | 43 subjects with T2DM and CP 50 ± 8 years old | Glucose and insulin levels, HOMA-IR | Significant decrease in insulin and HOMA-IR (p < 0.05). No significant changes in glucose levels | ⨁⨁⨁◯ Moderate |
| Khodabandenlhoo et al. (2018) [30] | RCT double-blind | 800 mg/day 8 weeks | 45 subjects wit T2DM 57 ± 9 years old | Glucose and insulin levels, HbA1c, HOMA-IR | Significant decrease in glucose levels (p < 0.05). No significant changes in HbA1c, insulin levels, and HOMA-IR | ⨁⨁◯◯ Low |
| Seyyedebrahimi et al. (2018) [31] | RCT double-blind | 800 mg/day 8 weeks | 46 subjects with T2DM 58 ± 6 years old | Glucose and insulin levels, HbA1c and HOMA-IR | Non-significant changes after RV administration | ⨁⨁◯◯ Low |
| Abdollahi et al. (2019) [32] | RCT double-blind | 1 g/day 8 weeks | 71 subjects with T2DM and overweight 50 ± 7 years old | Glucose and insulin levels, HbA1c, HOMA-IR | Significant decrease in glucose (p = 0.03) and insulin levels (p = 0.02), improvement in HOMA-IR (p = 0.01). No significant changes in HbA1c | ⨁⨁⨁◯ Moderate |
| Movahed et al. (2013) [33] | RCT double-blind | 1 g/day 45 days | 64 subjects with T2DM 52 ± 7 years old | Glucose and insulin levels, HbA1c and HOMA-IR | Significant decrease in glucose, insulin and HbA1c levels (p < 0.05). Improvement in HOMA-IR after RV administration | ⨁⨁◯◯ Low |
| Goh et al. (2014) [34] | RCT double-blind | 3 g/day 3 months | 10 subjects with TD2M 56 ± 6 years old | Glucose and insulin levels, HbA1c, HOMA-IR | Tendency to decrease in HbA1c; no significant changes in HOMA-IR. No changes in glucose and insulin levels | ⨁⨁⨁◯ Moderate |
| Sattarinezhad et al. (2019) [35] | RCT double-blind | 500 mg/day 3 months | 60 subjects with T2DM and albuminuria 57 ± 9 years old | Glucose and insulin levels, HbA1c and HOMA-IR | Significant decrease in glucose, insulin and HbA1c levels (p < 0.05). Improvement in HOMA-IR after RV administration | ⨁⨁⨁◯ Moderate |
| Subjects > 60 years old | ||||||
| Timmers et al. (2016) [36] | RCT double-blind cross-over | 150 mg/day 4 weeks | 16 subjects with T2DM 64 ± 4 years old | Glucose and insulin levels, HbA1c | Non-significant changes after RV administration | ⨁⨁◯◯ Low |
| Bo et al. (2016) [37] | RCT double-blind | 40, 500 mg/day 6 months | 179 subjects with T2DM 65 ± 8 years old | Glucose and insulin levels, HOMA-IR, HbA1c | Non-significant changes between study groups | ⨁⨁⨁◯ Moderate |
| Hoseini et al. (2019) [38] | RCT double-blind | 500 mg/day 4 weeks | 56 subjects with T2DM and CD 62 ± 9 years old | Glucose and insulin levels, HOMA-IR | Significant decrease in glucose and insulin levels (p = 0.01) and HOMA-IR (p = 0.001) | ⨁⨁⨁◯ Moderate |
| Thazhath et al. (2016) [39] | RCT double-blind cross-over | 1 g/day 5 weeks | 14 subjects with T2DM 68 ± 2 years old | Glucose levels and HbA1c | Non-significant changes in glucose and HbA1c | ⨁⨁◯◯ Low |
| Subgroup | No. of Trials | Effect Size | 95% CI | p-Value | Heterogeneity (I2) | p-Value for I2 |
|---|---|---|---|---|---|---|
| Glucose −14.13 (−20.90,−7.36) p < 0.0001 | ||||||
| RV dosage (I2 = 58%; p = 0.07) | ||||||
| <250 mg/day | 4 | −5.41 | −12.74, 1.93 | 0.15 | 42% | 0.16 |
| 250–500 mg/day | 5 | −20.72 | −30.62, −10.83 | <0.0001 | 90% | <0.00001 |
| >500–1000 mg/day | 5 | −16.40 | −34.05, 1.25 | 0.07 | 91% | <0.00001 |
| >1000 mg/day | 1 | 3.60 | −24.87, 32.07 | 0.80 | ---- | ---- |
| Age (I2 = 84%; p = 0.01) | ||||||
| 45–59 years | 12 | −17.73 | −25.93, −9.54 | <0.0001 | 95% | <0.00001 |
| ≥60 years | 3 | −2.00 | −11.29, 7.28 | 0.67 | 64% | 0.06 |
| HbA1c −0.27 (−0.44, −0.10) p = 0.002 | ||||||
| RV dosage (I2 = 78%; p = 0.003) | ||||||
| <250 mg/day | 4 | −0.04 | −0.27, 0.19 | 0.72 | 77% | 0.005 |
| 250–500 mg/day | 3 | −0.58 | −0.76, −0.39 | <0.0001 | 98% | 0.00001 |
| >500–1000 mg/day | 4 | −0.26 | −0.71, 0.19 | 0.25 | 81% | 0.001 |
| >1000 mg/day | 1 | −0.90 | −2.00, 0.20 | 0.11 | ---- | ---- |
| Age (I2 = 95%; p = 0.0001) | ||||||
| 45–59 years | 10 | −0.37 | −0.54, −0.20 | <0.0001 | 96% | 0.0001 |
| ≥60 years | 2 | 0.17 | 0.01, 0.34 | 0.04 | 0% | 0.62 |
| Insulin −1.36 (−2.03, −0.68) p < 0.0001 | ||||||
| RV dosage (I2 = 0; %; p = 0.92) | ||||||
| <250 mg/day | 4 | −1.22 | −1.73, −0.71 | <0.0001 | 0% | 0.56 |
| 250–500 mg/day | 3 | −1.55 | −3.08, −0.03 | 0.05 | 81% | 0.00 |
| >500–1000 mg/day | 3 | −1.99 | −5.19, 1.21 | 0.22 | 80% | 0.006 |
| >1000 mg/day | 1 | −0.40 | −4.97, 4.17 | 0.86 | ---- | ---- |
| Age (I2 = 0%; p = 0.92) | ||||||
| 45–59 years | 9 | −1.39 | −2.21, −0.56 | 0.001 | 73% | 0.0003 |
| ≥60 years | 2 | −1.32 | −2.23, −0.41 | 0.005 | 69% | <0.0001 |
| HOMA-IR −0.77 (−1.60, 0.06) p = 0.07 | ||||||
| RV dosage (I2 = 26%; p = 0.26) | ||||||
| <250 mg/day | 3 | −0.41 | −1.10, 0.29 | 0.25 | 63% | 0.07 |
| 250–500 mg/day | 3 | −1.08 | −2.16, 0.01 | 0.05 | 92% | 0.00001 |
| >500–1000 mg/day | 4 | −1.06 | −2.95, 0.83 | 0.27 | 97% | 0.00001 |
| >1000 mg/day | 1 | 0.31 | −0.65, 1.26 | 0.53 | ---- | ---- |
| Age (I2 = 0%; p = 0.41) | ||||||
| 45–59 years | 9 | −0.84 | −1.83, 0.15 | 0.10 | 96% | 0.00001 |
| ≥60 years | 2 | −0.40 | −0.70, −0.11 | 0.007 | 0% | 0.89 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Martínez, B.I.; Ruiz-Ramos, M.; Pedraza-Chaverri, J.; Santiago-Osorio, E.; Mendoza-Núñez, V.M. Influence of Age and Dose on the Effect of Resveratrol for Glycemic Control in Type 2 Diabetes Mellitus: Systematic Review and Meta-Analysis. Molecules 2022, 27, 5232. https://doi.org/10.3390/molecules27165232
García-Martínez BI, Ruiz-Ramos M, Pedraza-Chaverri J, Santiago-Osorio E, Mendoza-Núñez VM. Influence of Age and Dose on the Effect of Resveratrol for Glycemic Control in Type 2 Diabetes Mellitus: Systematic Review and Meta-Analysis. Molecules. 2022; 27(16):5232. https://doi.org/10.3390/molecules27165232
Chicago/Turabian StyleGarcía-Martínez, Beatriz Isabel, Mirna Ruiz-Ramos, José Pedraza-Chaverri, Edelmiro Santiago-Osorio, and Víctor Manuel Mendoza-Núñez. 2022. "Influence of Age and Dose on the Effect of Resveratrol for Glycemic Control in Type 2 Diabetes Mellitus: Systematic Review and Meta-Analysis" Molecules 27, no. 16: 5232. https://doi.org/10.3390/molecules27165232
APA StyleGarcía-Martínez, B. I., Ruiz-Ramos, M., Pedraza-Chaverri, J., Santiago-Osorio, E., & Mendoza-Núñez, V. M. (2022). Influence of Age and Dose on the Effect of Resveratrol for Glycemic Control in Type 2 Diabetes Mellitus: Systematic Review and Meta-Analysis. Molecules, 27(16), 5232. https://doi.org/10.3390/molecules27165232







