Increased mRNA Levels of ADAM17, IFITM3, and IFNE in Peripheral Blood Cells Are Present in Patients with Obesity and May Predict Severe COVID-19 Evolution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients Consent Statement
2.2. Participants
2.3. Anthropometric Measurements
2.4. Sample Collection
2.5. Real-Time qPCR Analysis in Whole Blood Cells
2.6. Analysis of Blood Parameters
2.7. Statistical Analysis
3. Results
3.1. Subject Characteristics
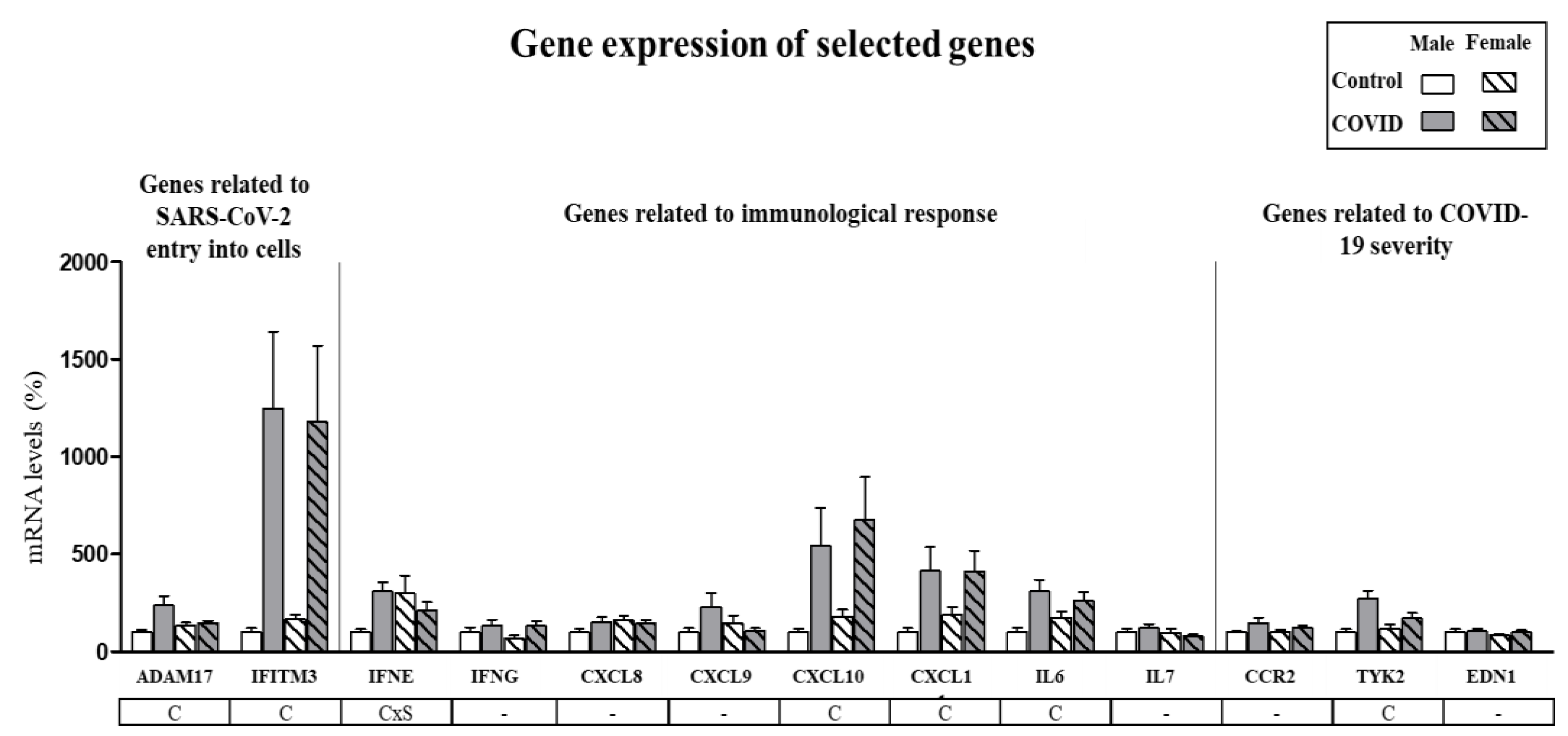
3.2. Assessment of Sex-Specific Differences in Gene Expression of Selected Genes
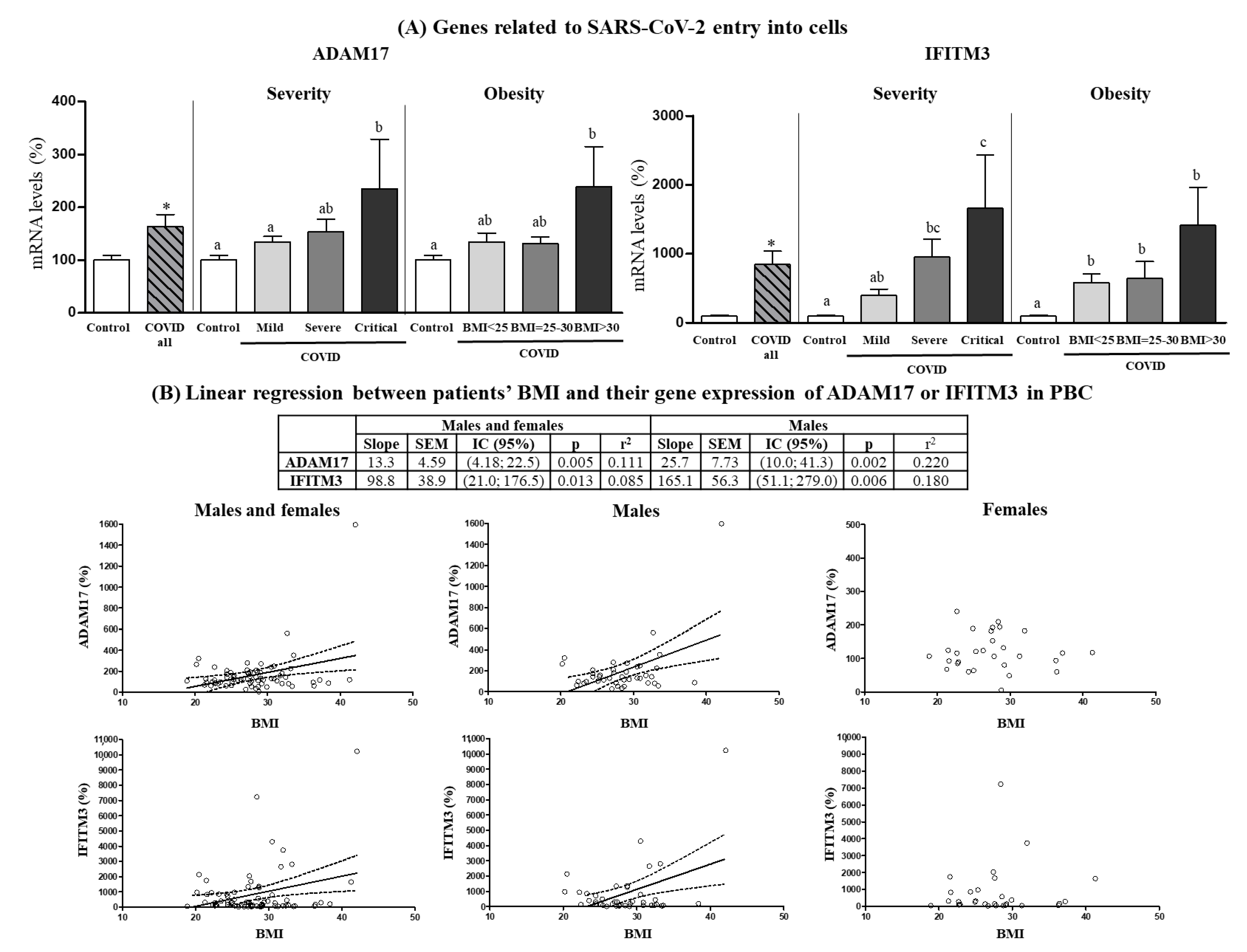
3.3. Expression Levels in PBCs of SARS-CoV-2 Cell Entry-Related Genes According to COVID-19 Severity and Obesity
3.4. Expression Levels in PBCs of Immunological Response-Related Genes According to COVID-19 Severity and Obesity
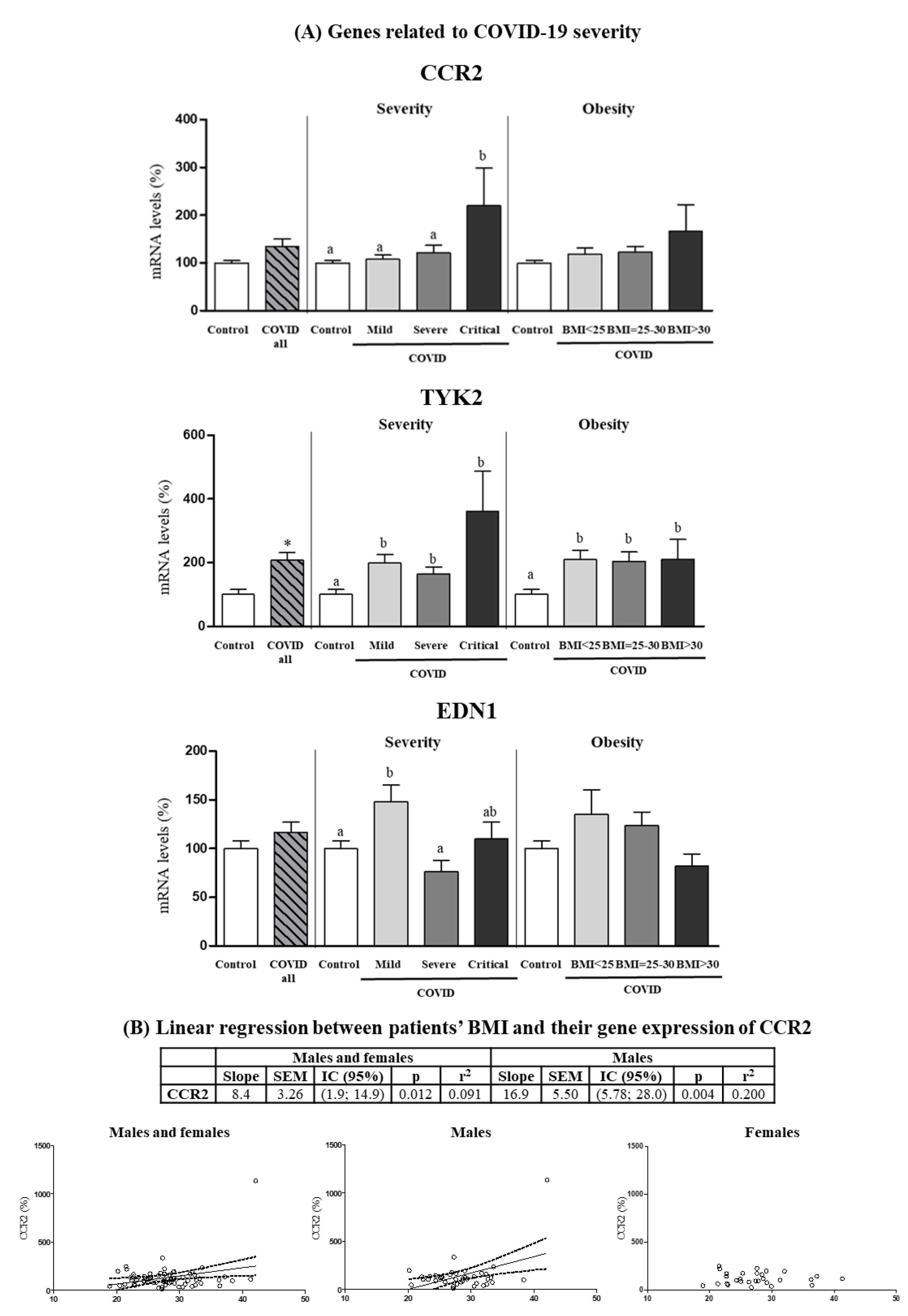
3.5. Expression Levels in PBCs of Other Genes Related to COVID-19 Severity
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- COVID-19 Excess Mortality Collaborators. Estimating excess mortality due to the COVID-19 pandemic: A systematic analysis of COVID-19-related mortality, 2020–2021. Lancet 2022, 399, 1513–1536. [Google Scholar] [CrossRef]
- Dessie, Z.G.; Zewotir, T. Mortality-related risk factors of COVID-19: A systematic review and meta-analysis of 42 studies and 423,117 patients. BMC Infect. Dis. 2021, 21, 855. [Google Scholar] [CrossRef] [PubMed]
- Epidemiology Working Group for NCIP Epidemic Response, Chinese Center for Disease Control and Prevention. The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China. Zhonghua Liu Xing Bing Xue Za Zhi 2020, 41, 145–151. [Google Scholar] [CrossRef]
- Sattar, N.; McInnes, I.B.; McMurray, J.J.V. Obesity Is a Risk Factor for Severe COVID-19 Infection: Multiple Potential Mechanisms. Circulation 2020, 142, 4–6. [Google Scholar] [CrossRef]
- Popkin, B.M.; Du, S.; Green, W.D.; Beck, M.A.; Algaith, T.; Herbst, C.H.; Alsukait, R.F.; Alluhidan, M.; Alazemi, N.; Shekar, M. Individuals with obesity and COVID-19: A global perspective on the epidemiology and biological relationships. Obes. Rev. 2020, 21, e13128. [Google Scholar] [CrossRef]
- Yang, J.; Tian, C.; Chen, Y.; Zhu, C.; Chi, H.; Li, J. Obesity aggravates COVID-19: An updated systematic review and meta-analysis. J. Med. Virol. 2021, 93, 2662–2674. [Google Scholar] [CrossRef]
- Huang, Y.; Lu, Y.; Huang, Y.M.; Wang, M.; Ling, W.; Sui, Y.; Zhao, H.L. Obesity in patients with COVID-19: A systematic review and meta-analysis. Metabolism 2020, 113, 154378. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.B.; June, C.H. Cytokine release syndrome in severe COVID-19. Science 2020, 368, 473–474. [Google Scholar] [CrossRef]
- Konieczna, J.; Sanchez, J.; van Schothorst, E.M.; Torrens, J.M.; Bunschoten, A.; Palou, M.; Pico, C.; Keijer, J.; Palou, A. Identification of early transcriptome-based biomarkers related to lipid metabolism in peripheral blood mononuclear cells of rats nutritionally programmed for improved metabolic health. Genes Nutr. 2014, 9, 366. [Google Scholar] [CrossRef]
- Cifre, M.; Palou, A.; Oliver, P. Impaired CPT1A Gene Expression Response to Retinoic Acid Treatment in Human PBMC as Predictor of Metabolic Risk. Nutrients 2020, 12, 2269. [Google Scholar] [CrossRef]
- Reynés, B.; Priego, T.; Cifre, M.; Oliver, P.; Palou, A. Peripheral Blood Cells, a Transcriptomic Tool in Nutrigenomic and Obesity Studies: Current State of the Art. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1006–1020. [Google Scholar] [CrossRef] [PubMed]
- Oliver, P.; Reynes, B.; Caimari, A.; Palou, A. Peripheral blood mononuclear cells: A potential source of homeostatic imbalance markers associated with obesity development. Pflügers Arch. 2013, 465, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, J.; Picó, C.; Ahrens, W.; Foraita, R.; Fraterman, A.; Moreno, L.A.; Russo, P.; Siani, A.; Palou, A. Transcriptome analysis in blood cells from children reveals potential early biomarkers of metabolic alterations. Int. J. Obes. 2017, 41, 1481–1488. [Google Scholar] [CrossRef] [PubMed]
- Cifre, M.; Palou, A.; Oliver, P. Cognitive impairment in metabolically-obese, normal-weight rats: Identification of early biomarkers in peripheral blood mononuclear cells. Mol. Neurodegener. 2018, 13, 14. [Google Scholar] [CrossRef]
- Caimari, A.; Oliver, P.; Keijer, J.; Palou, A. Peripheral blood mononuclear cells as a model to study the response of energy homeostasis-related genes to acute changes in feeding conditions. OMICS 2010, 14, 129–141. [Google Scholar] [CrossRef]
- Szostaczuk, N.; van Schothorst, E.M.; Sánchez, J.; Priego, T.; Palou, M.; Bekkenkamp-Grovenstein, M.; Faustmann, G.; Obermayer-Pietsch, B.; Tiran, B.; Roob, J.M.; et al. Identification of blood cell transcriptome-based biomarkers in adulthood predictive of increased risk to develop metabolic disorders using early life intervention rat models. FASEB J. 2020, 34, 9003–9017. [Google Scholar] [CrossRef]
- Izquierdo, A.G.; Carreira, M.C.; Boughanem, H.; Moreno-Navarrete, J.M.; Nicoletti, C.F.; Oliver, P.; de Luis, D.; Nonino, C.B.; Portillo, M.P.; Martinez-Olmos, M.A.; et al. Adipose tissue and blood leukocytes ACE2 DNA methylation in obesity and after weight loss. Eur. J. Clin. Investig. 2022, 52, e13685. [Google Scholar] [CrossRef]
- Jackson, C.B.; Farzan, M.; Chen, B.; Choe, H. Mechanisms of SARS-CoV-2 entry into cells. Nat. Rev. Mol. Cell Biol. 2022, 23, 3–20. [Google Scholar] [CrossRef]
- Brass, A.L.; Huang, I.C.; Benita, Y.; John, S.P.; Krishnan, M.N.; Feeley, E.M.; Ryan, B.J.; Weyer, J.L.; van der Weyden, L.; Fikrig, E.; et al. The IFITM proteins mediate cellular resistance to influenza A H1N1 virus, West Nile virus, and dengue virus. Cell 2009, 139, 1243–1254. [Google Scholar] [CrossRef]
- Lambert, D.W.; Yarski, M.; Warner, F.J.; Thornhill, P.; Parkin, E.T.; Smith, A.I.; Hooper, N.M.; Turner, A.J. Tumor necrosis factor-alpha convertase (ADAM17) mediates regulated ectodomain shedding of the severe-acute respiratory syndrome-coronavirus (SARS-CoV) receptor, angiotensin-converting enzyme-2 (ACE2). J. Biol. Chem. 2005, 280, 30113–30119. [Google Scholar] [CrossRef]
- Patel, V.B.; Clarke, N.; Wang, Z.; Fan, D.; Parajuli, N.; Basu, R.; Putko, B.; Kassiri, Z.; Turner, A.J.; Oudit, G.Y. Angiotensin II induced proteolytic cleavage of myocardial ACE2 is mediated by TACE/ADAM-17: A positive feedback mechanism in the RAS. J. Mol. Cell. Cardiol. 2014, 66, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Prokop, J.W.; Hartog, N.L.; Chesla, D.; Faber, W.; Love, C.P.; Karam, R.; Abualkheir, N.; Feldmann, B.; Teng, L.; McBride, T.; et al. High-Density Blood Transcriptomics Reveals Precision Immune Signatures of SARS-CoV-2 Infection in Hospitalized Individuals. Front. Immunol. 2021, 12, 694243. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Chen, D.; Yuan, D.; Lausted, C.; Choi, J.; Dai, C.L.; Voillet, V.; Duvvuri, V.R.; Scherler, K.; Troisch, P.; et al. Multi-Omics Resolves a Sharp Disease-State Shift between Mild and Moderate COVID-19. Cell 2020, 183, 1479–1495. [Google Scholar] [CrossRef] [PubMed]
- Daamen, A.R.; Bachali, P.; Owen, K.A.; Kingsmore, K.M.; Hubbard, E.L.; Labonte, A.C.; Robl, R.; Shrotri, S.; Grammer, A.C.; Lipsky, P.E. Comprehensive transcriptomic analysis of COVID-19 blood, lung, and airway. Sci. Rep. 2021, 11, 7052. [Google Scholar] [CrossRef]
- Hasan, M.I.; Rahman, M.H.; Islam, M.B.; Islam, M.Z.; Hossain, M.A.; Moni, M.A. Systems Biology and Bioinformatics approach to Identify blood based signatures molecules and drug targets of patient with COVID-19. Inform. Med. Unlocked 2022, 28, 100840. [Google Scholar] [CrossRef]
- Pairo-Castineira, E.; Clohisey, S.; Klaric, L.; Bretherick, A.D.; Rawlik, K.; Pasko, D.; Walker, S.; Parkinson, N.; Fourman, M.H.; Russell, C.D.; et al. Genetic mechanisms of critical illness in COVID-19. Nature 2021, 591, 92–98. [Google Scholar] [CrossRef]
- Abraham, G.R.; Kuc, R.E.; Althage, M.; Greasley, P.J.; Ambery, P.; Maguire, J.J.; Wilkinson, I.B.; Hoole, S.P.; Cheriyan, J.; Davenport, A.P. Endothelin-1 is increased in the plasma of patients hospitalised with Covid-19. J. Mol. Cell. Cardiol. 2022, 167, 92–96. [Google Scholar] [CrossRef]
- García-Gasalla, M.; Ferrer, J.M.; Fraile-Ribot, P.A.; Ferre-Beltrán, A.; Rodríguez, A.; Martínez-Pomar, N.; Ramon-Clar, L.; Iglesias, A.; Losada-López, I.; Fanjul, F.; et al. Predictive Immunological, Virological, and Routine Laboratory Markers for Critical COVID-19 on Admission. Can. J. Infect. Dis. Med. Microbiol. 2021, 2021, 9965850. [Google Scholar] [CrossRef]
- Costa, A.; Reynés, B.; Konieczna, J.; Martín, M.; Fiol, M.; Palou, A.; Romaguera, D.; Oliver, P. Use of human PBMC to analyse the impact of obesity on lipid metabolism and metabolic status: A proof-of-concept pilot study. Sci. Rep. 2021, 11, 18329. [Google Scholar] [CrossRef]
- Kwon, M.J.; Oh, E.; Lee, S.; Roh, M.R.; Kim, S.E.; Lee, Y.; Choi, Y.L.; In, Y.H.; Park, T.; Koh, S.S.; et al. Identification of novel reference genes using multiplatform expression data and their validation for quantitative gene expression analysis. PLoS ONE 2009, 4, e6162. [Google Scholar] [CrossRef]
- Li, M.Y.; Li, L.; Zhang, Y.; Wang, X.S. Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infect. Dis. Poverty 2020, 9, 45. [Google Scholar] [CrossRef] [PubMed]
- Gkogkou, E.; Barnasas, G.; Vougas, K.; Trougakos, I.P. Expression profiling meta-analysis of ACE2 and TMPRSS2, the putative anti-inflammatory receptor and priming protease of SARS-CoV-2 in human cells, and identification of putative modulators. Redox Biol. 2020, 36, 101615. [Google Scholar] [CrossRef] [PubMed]
- Haga, S.; Yamamoto, N.; Nakai-Murakami, C.; Osawa, Y.; Tokunaga, K.; Sata, T.; Sasazuki, T.; Ishizaka, Y. Modulation of TNF-alpha-converting enzyme by the spike protein of SARS-CoV and ACE2 induces TNF-alpha production and facilitates viral entry. Proc. Natl. Acad. Sci. USA 2008, 105, 7809–7814. [Google Scholar] [CrossRef] [PubMed]
- Heurich, A.; Hofmann-Winkler, H.; Gierer, S.; Liepold, T.; Jahn, O.; Pöhlmann, S. TMPRSS2 and ADAM17 cleave ACE2 differentially and only proteolysis by TMPRSS2 augments entry driven by the severe acute respiratory syndrome coronavirus spike protein. J. Virol. 2014, 88, 1293–1307. [Google Scholar] [CrossRef]
- Schreiber, B.; Patel, A.; Verma, A. Shedding Light on COVID-19: ADAM17 the Missing Link? Am. J. Ther. 2020, 28, e358–e360. [Google Scholar] [CrossRef]
- Zipeto, D.; Palmeira, J.D.F.; Argañaraz, G.A.; Argañaraz, E.R. ACE2/ADAM17/TMPRSS2 Interplay May Be the Main Risk Factor for COVID-19. Front. Immunol. 2020, 11, 576745. [Google Scholar] [CrossRef]
- Palau, V.; Riera, M.; Soler, M.J. ADAM17 inhibition may exert a protective effect on COVID-19. Nephrol. Dial. Transplant. 2020, 35, 1071–1072. [Google Scholar] [CrossRef]
- South, A.M.; Diz, D.I.; Chappell, M.C. COVID-19, ACE2, and the cardiovascular consequences. Am. J. Physiol. Heart Circ. Physiol. 2020, 318, H1084–H1090. [Google Scholar] [CrossRef]
- Gheblawi, M.; Wang, K.; Viveiros, A.; Nguyen, Q.; Zhong, J.C.; Turner, A.J.; Raizada, M.K.; Grant, M.B.; Oudit, G.Y. Angiotensin-Converting Enzyme 2: SARS-CoV-2 Receptor and Regulator of the Renin-Angiotensin System: Celebrating the 20th Anniversary of the Discovery of ACE2. Circ. Res. 2020, 126, 1456–1474. [Google Scholar] [CrossRef]
- Seltzer, S. Linking ACE2 and angiotensin II to pulmonary immunovascular dysregulation in SARS-CoV-2 infection. Int. J. Infect. Dis. 2020, 101, 42–45. [Google Scholar] [CrossRef]
- Oudit, G.Y.; Herzenberg, A.M.; Kassiri, Z.; Wong, D.; Reich, H.; Khokha, R.; Crackower, M.A.; Backx, P.H.; Penninger, J.M.; Scholey, J.W. Loss of angiotensin-converting enzyme-2 leads to the late development of angiotensin II-dependent glomerulosclerosis. Am. J. Pathol. 2006, 168, 1808–1820. [Google Scholar] [CrossRef]
- Black, R.A.; Rauch, C.T.; Kozlosky, C.J.; Peschon, J.J.; Slack, J.L.; Wolfson, M.F.; Castner, B.J.; Stocking, K.L.; Reddy, P.; Srinivasan, S.; et al. A metalloproteinase disintegrin that releases tumour-necrosis factor-alpha from cells. Nature 1997, 385, 729–733. [Google Scholar] [CrossRef]
- Chen, L.Y.C.; Biggs, C.M.; Jamal, S.; Stukas, S.; Wellington, C.L.; Sekhon, M.S. Soluble interleukin-6 receptor in the COVID-19 cytokine storm syndrome. Cell Rep. Med. 2021, 2, 100269. [Google Scholar] [CrossRef]
- Galván-Román, J.M.; Rodríguez-García, S.C.; Roy-Vallejo, E.; Marcos-Jiménez, A.; Sánchez-Alonso, S.; Fernández-Díaz, C.; Alcaraz-Serna, A.; Mateu-Albero, T.; Rodríguez-Cortes, P.; Sánchez-Cerrillo, I.; et al. IL-6 serum levels predict severity and response to tocilizumab in COVID-19: An observational study. J. Allergy Clin. Immunol. 2021, 147, 72–80.e8. [Google Scholar] [CrossRef]
- Menghini, R.; Fiorentino, L.; Casagrande, V.; Lauro, R.; Federici, M. The role of ADAM17 in metabolic inflammation. Atherosclerosis 2013, 228, 12–17. [Google Scholar] [CrossRef]
- Matthews, J.; Villescas, S.; Herat, L.; Schlaich, M.; Matthews, V. Implications of ADAM17 activation for hyperglycaemia, obesity and type 2 diabetes. Biosci. Rep. 2021, 41, BSR20210029. [Google Scholar] [CrossRef]
- Dou, H.; Feher, A.; Davila, A.C.; Romero, M.J.; Patel, V.S.; Kamath, V.M.; Gooz, M.B.; Rudic, R.D.; Lucas, R.; Fulton, D.J.; et al. Role of Adipose Tissue Endothelial ADAM17 in Age-Related Coronary Microvascular Dysfunction. Arter. Thromb. Vasc. Biol. 2017, 37, 1180–1193. [Google Scholar] [CrossRef]
- Gelling, R.W.; Yan, W.; Al-Noori, S.; Pardini, A.; Morton, G.J.; Ogimoto, K.; Schwartz, M.W.; Dempsey, P.J. Deficiency of TNFalpha converting enzyme (TACE/ADAM17) causes a lean, hypermetabolic phenotype in mice. Endocrinology 2008, 149, 6053–6064. [Google Scholar] [CrossRef]
- Tchernof, A.; Després, J.P. Pathophysiology of human visceral obesity: An update. Physiol. Rev. 2013, 93, 359–404. [Google Scholar] [CrossRef]
- Hachim, M.Y.; Al Heialy, S.; Hachim, I.Y.; Halwani, R.; Senok, A.C.; Maghazachi, A.A.; Hamid, Q. Interferon-Induced Transmembrane Protein (IFITM3) Is Upregulated Explicitly in SARS-CoV-2 Infected Lung Epithelial Cells. Front. Immunol. 2020, 11, 1372. [Google Scholar] [CrossRef]
- Shi, G.; Kenney, A.D.; Kudryashova, E.; Zani, A.; Zhang, L.; Lai, K.K.; Hall-Stoodley, L.; Robinson, R.T.; Kudryashov, D.S.; Compton, A.A.; et al. Opposing activities of IFITM proteins in SARS-CoV-2 infection. EMBO J. 2021, 40, e106501. [Google Scholar] [CrossRef] [PubMed]
- Prelli Bozzo, C.; Nchioua, R.; Volcic, M.; Koepke, L.; Krüger, J.; Schütz, D.; Heller, S.; Stürzel, C.M.; Kmiec, D.; Conzelmann, C.; et al. IFITM proteins promote SARS-CoV-2 infection and are targets for virus inhibition in vitro. Nat. Commun. 2021, 12, 4584. [Google Scholar] [CrossRef] [PubMed]
- Delshad, M.; Tavakolinia, N.; Pourbagheri-Sigaroodi, A.; Safaroghli-Azar, A.; Bagheri, N.; Bashash, D. The contributory role of lymphocyte subsets, pathophysiology of lymphopenia and its implication as prognostic and therapeutic opportunity in COVID-19. Int. Immunopharmacol. 2021, 95, 107586. [Google Scholar] [CrossRef] [PubMed]
- Wee, Y.S.; Weis, J.J.; Gahring, L.C.; Rogers, S.W.; Weis, J.H. Age-related onset of obesity corresponds with metabolic dysregulation and altered microglia morphology in mice deficient for Ifitm proteins. PLoS ONE 2015, 10, e0123218. [Google Scholar] [CrossRef]
- Coperchini, F.; Chiovato, L.; Ricci, G.; Croce, L.; Magri, F.; Rotondi, M. The cytokine storm in COVID-19: Further advances in our understanding the role of specific chemokines involved. Cytokine Growth Factor Rev. 2021, 58, 82–91. [Google Scholar] [CrossRef]
- Suhre, K.; Sarwath, H.; Engelke, R.; Sohail, M.U.; Cho, S.J.; Whalen, W.; Alvarez-Mulett, S.; Krumsiek, J.; Choi, A.M.K.; Schmidt, F. Identification of Robust Protein Associations With COVID-19 Disease Based on Five Clinical Studies. Front. Immunol. 2021, 12, 781100. [Google Scholar] [CrossRef]
- Zhang, N.; Zhao, Y.D.; Wang, X.M. CXCL10 an important chemokine associated with cytokine storm in COVID-19 infected patients. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 7497–7505. [Google Scholar] [CrossRef]
- Dhar, S.K.; Vishnupriyan, K.; Damodar, S.; Gujar, S.; Das, M. IL-6 and IL-10 as predictors of disease severity in COVID-19 patients: Results from meta-analysis and regression. Heliyon 2021, 7, e06155. [Google Scholar] [CrossRef]
- Jøntvedt Jørgensen, M.; Holter, J.C.; Christensen, E.E.; Schjalm, C.; Tonby, K.; Pischke, S.E.; Jenum, S.; Skeie, L.G.; Nur, S.; Lind, A.; et al. Increased interleukin-6 and macrophage chemoattractant protein-1 are associated with respiratory failure in COVID-19. Sci. Rep. 2020, 10, 21697. [Google Scholar] [CrossRef]
- Ahnach, M.; Zbiri, S.; Nejjari, S.; Ousti, F.; Elkettani, C. C-reactive protein as an early predictor of COVID-19 severity. J. Med. Biochem. 2020, 39, 500–507. [Google Scholar] [CrossRef]
- Eder, K.; Baffy, N.; Falus, A.; Fulop, A.K. The major inflammatory mediator interleukin-6 and obesity. Inflamm. Res. 2009, 58, 727–736. [Google Scholar] [CrossRef]
- Jaffe, E.A.; Armellino, D.; Lam, G.; Cordon-Cardo, C.; Murray, H.W.; Evans, R.L. IFN-gamma and IFN-alpha induce the expression and synthesis of Leu 13 antigen by cultured human endothelial cells. J. Immunol. 1989, 143, 3961–3966. [Google Scholar] [PubMed]
- Lau, S.L.; Yuen, M.L.; Kou, C.Y.; Au, K.W.; Zhou, J.; Tsui, S.K. Interferons induce the expression of IFITM1 and IFITM3 and suppress the proliferation of rat neonatal cardiomyocytes. J. Cell. Biochem. 2012, 113, 841–847. [Google Scholar] [CrossRef]
- Sposito, B.; Broggi, A.; Pandolfi, L.; Crotta, S.; Clementi, N.; Ferrarese, R.; Sisti, S.; Criscuolo, E.; Spreafico, R.; Long, J.M.; et al. The interferon landscape along the respiratory tract impacts the severity of COVID-19. Cell 2021, 184, 4953–4968.e16. [Google Scholar] [CrossRef]
- Galbraith, M.D.; Kinning, K.T.; Sullivan, K.D.; Araya, P.; Smith, K.P.; Granrath, R.E.; Shaw, J.R.; Baxter, R.; Jordan, K.R.; Russell, S.; et al. Specialized interferon action in COVID-19. Proc. Natl. Acad. Sci. USA 2022, 119, e2116730119. [Google Scholar] [CrossRef]
- Akamatsu, M.A.; de Castro, J.T.; Takano, C.Y.; Ho, P.L. Off balance: Interferons in COVID-19 lung infections. EBioMedicine 2021, 73, 103642. [Google Scholar] [CrossRef]
- Lee, J.S.; Shin, E.C. The type I interferon response in COVID-19: Implications for treatment. Nat. Rev. Immunol. 2020, 20, 585–586. [Google Scholar] [CrossRef]
- Kim, Y.M.; Shin, E.C. Type I and III interferon responses in SARS-CoV-2 infection. Exp. Mol. Med. 2021, 53, 750–760. [Google Scholar] [CrossRef] [PubMed]
- Marks, Z.R.C.; Campbell, N.; deWeerd, N.A.; Lim, S.S.; Gearing, L.J.; Bourke, N.M.; Hertzog, P.J. Properties and functions of the novel type I interferon epsilon. Semin. Immunol. 2019, 43, 101328. [Google Scholar] [CrossRef]
- Afsar, C.U.; Afsar, S. SARS-CoV-2 (Covid-19): Interferon-epsilon may be responsible of decreased mortality in females. J. Reprod. Immunol. 2020, 141, 103154. [Google Scholar] [CrossRef]
- Liu, T.; Balzano-Nogueira, L.; Lleo, A.; Conesa, A. Transcriptional Differences for COVID-19 Disease Map Genes between Males and Females Indicate a Different Basal Immunophenotype Relevant to the Disease. Genes 2020, 11, 1447. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.J.; Ashkar, A.A. The Dual Nature of Type I and Type II Interferons. Front. Immunol. 2018, 9, 2061. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Zhu, J.; Liu, D.; Sun, Y.; Wu, C. An integrative multiomics analysis identifies putative causal genes for COVID-19 severity. Genet. Med. 2021, 23, 2076–2086. [Google Scholar] [CrossRef] [PubMed]
- Sharif-Zak, M.; Abbasi-Jorjandi, M.; Asadikaram, G.; Ghoreshi, Z.A.; Rezazadeh-Jabalbarzi, M.; Afsharipur, A.; Rashidinejad, H.; Khajepour, F.; Jafarzadeh, A.; Arefinia, N.; et al. CCR2 and DPP9 expression in the peripheral blood of COVID-19 patients: Influences of the disease severity and gender. Immunobiology 2022, 227, 152184. [Google Scholar] [CrossRef]
- Khalil, B.A.; Elemam, N.M.; Maghazachi, A.A. Chemokines and chemokine receptors during COVID-19 infection. Comput. Struct. Biotechnol. J. 2021, 19, 976–988. [Google Scholar] [CrossRef]
- Weisberg, S.P.; Hunter, D.; Huber, R.; Lemieux, J.; Slaymaker, S.; Vaddi, K.; Charo, I.; Leibel, R.L.; Ferrante, A.W. CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J. Clin. Investig. 2006, 116, 115–124. [Google Scholar] [CrossRef]
- Lee, S.J.; Kang, J.S.; Kim, H.M.; Lee, E.S.; Lee, J.H.; Chung, C.H.; Lee, E.Y. CCR2 knockout ameliorates obesity-induced kidney injury through inhibiting oxidative stress and ER stress. PLoS ONE 2019, 14, e0222352. [Google Scholar] [CrossRef]
- Tamura, Y.; Sugimoto, M.; Murayama, T.; Ueda, Y.; Kanamori, H.; Ono, K.; Ariyasu, H.; Akamizu, T.; Kita, T.; Yokode, M.; et al. Inhibition of CCR2 ameliorates insulin resistance and hepatic steatosis in db/db mice. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 2195–2201. [Google Scholar] [CrossRef]
- Marroqui, L.; Dos Santos, R.S.; Fløyel, T.; Grieco, F.A.; Santin, I.; Op de Beeck, A.; Marselli, L.; Marchetti, P.; Pociot, F.; Eizirik, D.L. TYK2, a Candidate Gene for Type 1 Diabetes, Modulates Apoptosis and the Innate Immune Response in Human Pancreatic β-Cells. Diabetes 2015, 64, 3808–3817. [Google Scholar] [CrossRef]
- Baranova, A.; Cao, H.; Zhang, F. Unraveling Risk Genes of COVID-19 by Multi-Omics Integrative Analyses. Front. Med. 2021, 8, 738687. [Google Scholar] [CrossRef]
- de Rojas, I.; Hernández, I.; Montrreal, L.; Quintela, I.; Calero, M.; Royo, J.L.; Huerto Vilas, R.; González-Pérez, A.; Franco-Macías, E.; Macías, J.; et al. Genomic Characterization of Host Factors Related to SARS-CoV-2 Infection in People with Dementia and Control Populations: The GR@ACE/DEGESCO Study. J. Pers. Med. 2021, 11, 1318. [Google Scholar] [CrossRef] [PubMed]



| Control | COVID All | COVID by Severity | COVID by BMI | |||||
|---|---|---|---|---|---|---|---|---|
| COVID (Mild) | COVID (Severe) | COVID (Critical) | COVID (BMI < 25) | COVID (BMI = 25–30) | COVID (BMI > 30) | |||
| Number of volunteers | 47 | 73 | 33 | 24 | 16 | 20 | 32 | 21 |
| Male/Female | 15/32 | 43/30 | 15/18 | 15/9 | 13/3 | 9/11 | 19/13 | 15/6 |
| Anthropometric Measurements | ||||||||
| Age (years) | 47.6 ± 1.8 | 58.3 ± 1.7 * | 54.1 ± 2.6 | 61.8 ± 2.7 | 61.6 ± 3.0 | 57.9 ± 3.1 | 58.7 ± 2.8 | 58.0 ± 2.8 |
| Weight (kg) | 73.5 ± 2.7 | 77.5 ± 1.6 | 73.9 ± 2.4 | 78.1 ± 2.8 | 83.8 ± 3.3 # | 64.7± 1.8 a | 75.9 ± 1.4 b | 91.9 ± 2.6 c |
| Height (cm) | 166 ± 1 | 165 ± 2 | 164 ± 1.6 | 166 ± 1.4 | 163 ± 7 | 168 ± 1.9 | 165 ± 1.5 | 160 ± 5.5 |
| BMI (kg/m2) | 26.7 ± 0.1 | 28.1 ± 0.6 | 27.4 ± 0.9 | 28.3 ± 0.9 | 29.2 ± 1.2 | 22.7 ± 0.4 a | 27.6 ± 0.2 b | 33.9 ± 0.7 c |
| COVID by Severity | COVID by BMI | |||||
|---|---|---|---|---|---|---|
| COVID (Mild) | COVID (Severe) | COVID (Critical) | COVID (BMI < 25) | COVID (BMI = 25–30) | COVID (BMI > 30) | |
| Number of volunteers | 33 | 24 | 16 | 20 | 32 | 21 |
| Male/Female | 15/18 | 15/9 | 13/3 | 9/11 | 19/13 | 15/6 |
| Circulating Parameters | ||||||
| Hemoglobin (g/dL) | 13.8 ± 0.3 | 14.1 ± 0.3 | 13.9 ± 0.4 | 13.4 ± 0.4 a | 13.8 ± 0.2 ab | 14.6 ± 0.3 b |
| D-dimer (ng/mL) | 184 ± 19 | 981 ± 333 | 561 ± 159 | 343 ± 123 | 529 ± 184 | 686 ± 279 |
| Lactate dehydrogenase (U/L) | 230 ± 17 a | 345 ± 26 b | 447 ± 37 c | 277 ± 34 | 334 ± 27 | 340 ± 33 |
| Bilirubin (mg/dL) | 0.8 ± 0.1 | 0.9 ± 0.1 | 1.0± 0.1 | 1.0 ± 0.1 a | 0.8 ± 0.1 b | 0.9 ± 0.1 ab |
| GPT (U/L) | 36.6 ± 8.1 | 54.0 ± 9.9 | 39.8 ± 4.9 | 31.3 ± 7.5 | 43.2 ± 5.7 | 54.3 ± 13.1 |
| GOT (U/L) | 29.5 ± 4.3 a | 49.1 ±7.9 b | 47.1 ± 5.5 ab | 36.1 ± 7.0 | 39.4 ± 5.0 | 43.1 ± 7.2 |
| GGT (U/L) | 42.0 ± 6.7 a | 81.5 ± 15.6 b | 76.5 ± 15.6 ab | 55.5 ± 16.2 | 61.5 ± 10.1 | 71.7± 12.7 |
| Urea (mg/dL) | 30.3 ± 1.8 a | 40.0 ± 4.0 b | 39.3 ± 3.1 b | 36.8 ± 3.2 | 32.5 ± 2.0 | 38.9 ± 4.5 |
| Glucose (mg/dL) | 111 ± 12 | 130 ± 12 | 136 ± 12 | 108 ± 8 | 128 ± 13 | 130 ± 13 |
| C-reactive protein (mg/dL) | 4.39 ± 1.29 a | 12.2 ± 1.74 b | 12.3 ± 2.36 b | 7.57 ± 1.73 | 8.84 ± 1.68 | 9.51 ± 2.12 |
| Interleukin 6 (pg/mL) | 49 ± 9.9 | 140 ± 47 | 304 ± 172 | 120 ± 49 | 160 ± 86 | 98 ± 43 |
| Ferritin (ng/mL) | 325 ± 93 a | 984 ± 294 b | 1002 ± 159 b | 319 ± 80 | 887 ± 223 | 764 ± 186 |
| Vitamin D (ng/mL) | 14.7 ± 1.7 | 22.0 ± 3.8 | 14.5 ± 1.8 | 21.5 ± 4.1 | 14.9 ± 1.3 | 15.5 ± 2.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pomar, C.A.; Bonet, M.L.; Ferre-Beltrán, A.; Fraile-Ribot, P.A.; García-Gasalla, M.; Riera, M.; Picó, C.; Palou, A. Increased mRNA Levels of ADAM17, IFITM3, and IFNE in Peripheral Blood Cells Are Present in Patients with Obesity and May Predict Severe COVID-19 Evolution. Biomedicines 2022, 10, 2007. https://doi.org/10.3390/biomedicines10082007
Pomar CA, Bonet ML, Ferre-Beltrán A, Fraile-Ribot PA, García-Gasalla M, Riera M, Picó C, Palou A. Increased mRNA Levels of ADAM17, IFITM3, and IFNE in Peripheral Blood Cells Are Present in Patients with Obesity and May Predict Severe COVID-19 Evolution. Biomedicines. 2022; 10(8):2007. https://doi.org/10.3390/biomedicines10082007
Chicago/Turabian StylePomar, Catalina A., M. Luisa Bonet, Adrián Ferre-Beltrán, Pablo A. Fraile-Ribot, Mercedes García-Gasalla, Melchor Riera, Catalina Picó, and Andreu Palou. 2022. "Increased mRNA Levels of ADAM17, IFITM3, and IFNE in Peripheral Blood Cells Are Present in Patients with Obesity and May Predict Severe COVID-19 Evolution" Biomedicines 10, no. 8: 2007. https://doi.org/10.3390/biomedicines10082007
APA StylePomar, C. A., Bonet, M. L., Ferre-Beltrán, A., Fraile-Ribot, P. A., García-Gasalla, M., Riera, M., Picó, C., & Palou, A. (2022). Increased mRNA Levels of ADAM17, IFITM3, and IFNE in Peripheral Blood Cells Are Present in Patients with Obesity and May Predict Severe COVID-19 Evolution. Biomedicines, 10(8), 2007. https://doi.org/10.3390/biomedicines10082007










