Using Tectona Grandis Biomass to Produce Valuable Adsorbents for Pesticide Removal from Liquid Effluent
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Activated Carbon from Tectona Grandis
2.2. Structural and Chemical Characterization
2.2.1. Textural Characterization
2.2.2. Chemical Characterization
2.3. Pesticide Removal from the Liquid Phase
3. Results and Discussion
3.1. Activated Carbon Production by Chemical Activation
3.2. Activated Carbon Characterization
3.3. Pesticide Removal from the Aqueous Phase
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Batista, T.; Cansado, I.P.P.; Tita, B.; Ilhéu, A.; Metrogos, L.; Mourão, P.A.M.; Nabais, J.M.V.; Castanheiro, J.E.; Borges, C.; Matos, G. Dealing with Plastic Waste from Agriculture Activity. Agronomy 2022, 12, 134–145. [Google Scholar] [CrossRef]
- Cansado, I.P.P.; Mourão, P.A.M.; Nabais, J.M.V.; Tita, B.; Batista, T.; Rocha, T.; Borges, C.; Matos, G. Use of dirty plastic waste as precursors for activated carbon production—A contribution to the circular economy. Water Environ. J. 2021, 1, 96–104. [Google Scholar] [CrossRef]
- Heidarinejad, Z.; Dehghani, M.H.; Heidari, M.; Javedan, G.; Ali, I.; Sillanpää, M. Methods for preparation and activation of activated carbon: A review. Environ. Chem. Lett. 2020, 18, 393–415. [Google Scholar] [CrossRef]
- Hattab, M.T.; Ghagy, A.E. Disposal and treatment methods for pesticides containing wastewaters: Critical review and comparative analysis. J. Environ. Prot. 2012, 3, 431–453. [Google Scholar] [CrossRef]
- Fallah, Z.; Zare, E.N.; Ghomi, M.; Ahmadijokani, F.; Amini, M.; Tajbakhsh, M.; Arjmand, M.; Sharma, G.; Ali, H.; Ahmad, A.; et al. Toxicity and remediation of pharmaceuticals and pesticides using metal oxides and carbon nanomaterials. Chemosphere 2021, 275, 130055. [Google Scholar] [CrossRef]
- Mohanty, K.; Das, D.; Biswas, M.N. Adsorption of phenol from aqueous solutions using activated carbons prepared from Tectona Grandis sawdust by ZnCl2 activation. Chem. Eng. J. 2005, 115, 121–131. [Google Scholar] [CrossRef]
- Lima, R.V.; Araújo, A.C.C.; Pego, A.F.F.; Soares, B.C.D.; Bianchi, M.L.; Trugillo, P.F. Activated carbon quality produced by Tectona Grandis wastes: Activation methods and adsorption capacity. Rev. Matéria 2020, 25, 4–17. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, S.L.; Xie, T.; Cao, J. Activated carbon derived from waste tangerine seed for the high-performance adsorption of carbamate pesticides from water and plant. Bioresour. Technol. 2020, 316, 123929. [Google Scholar] [CrossRef]
- Bergna, D.; Romar, H.; Tuomikoski, S.; Runtti, H.; Kangas, T.; Tynjälä, P.; Lassi, U. Activated Carbon from Renewable Sources: Thermochemical conversion and activation of biomass and carbon residues from biomass gasification. In Waste Biomass Management; Springer: Cham, Switzerland, 2017; pp. 187–213. [Google Scholar] [CrossRef]
- Cansado, I.P.P.; Belo, C.R.; Mourão, P.A.M. Valorisation of Tectona Grandis tree sawdust through the production of high activated carbon for environmental applications. Bioresour. Technol. 2018, 249, 28–333. [Google Scholar] [CrossRef] [Green Version]
- State of the World’s Forest. Food and Agriculture Organization of the United Nations. Produced by the Electronic Publishing Policy and Support Branch, Communication Division; State of the Worlds Forest’s 2007; FAO: Rome, Italy, 2007; pp. 15–22. ISBN 978-92-5-105586-1. Available online: fao.org (accessed on 21 April 2022).
- Miranda, I.; Sousa, V.; Pereira, H. Wood properties of teak (Tectona grandis) from a mature unmanaged stand in East Timor. J. Wood Sci. Jpn. Wood Res. Soc. 2011, 58, 171–178. [Google Scholar] [CrossRef]
- Nazal, M.K.; Gijjapu, D.R.; Abuzaid, N. Effective removal of methylated phenol and chlorinated phenol from aqueous solutions using a new activated carbon derived from Halodule uninervis waste. Colloid Interface Sci. Commun. 2021, 41, 100369–100376. [Google Scholar] [CrossRef]
- Mashkoor, F.; Nasar, A.; Asiri, A.M. Exploring the Reusability of Synthetically Contaminated Wastewater Containing Crystal Violet Dye using Tectona grandis Sawdust as a Very Low-Cost Adsorbent. Sci. Rep. 2018, 8, 8314. [Google Scholar] [CrossRef] [PubMed]
- Yahya, M.A.; Al-Qodah, Z.; Ngah, C.W.Z. Agricultural bio-waste materials as potential sustainable precursors used for activated carbon production: A review. Renew. Sustain. Energy Rev. 2015, 46, 218–235. [Google Scholar] [CrossRef]
- Siabi, W.K.; Owusu-Ansah, E.D.-J.; Essandoh, H.M.K.; Asiedu, N.Y. Modelling the adsorption of iron and manganese by activated carbon from teak and shea charcoal for continuous low flow. Water-Energy Nexus 2021, 4, 88–94. [Google Scholar] [CrossRef]
- Mohammed, D.W.; Tanweer, A. A review on the utilization of wood biomass as a sustainable precursor for activated carbon production and application. Renew. Sustain. Energy Rev. 2018, 87, 1–21. [Google Scholar]
- Singh, J.; Mishra, V. Synthesis and characterization of activated carbon derived from Tectona grandis sawdust via green route. Environ. Prog. Sustain. Energy 2021, 40, e13525. [Google Scholar] [CrossRef]
- Jafar, A.A.; Balasubramanian, S. Adsorption of Pb(II) ions on teak leaves activated carbon—A kinetic and equilibrium study. Der Chem. Sin. 2005, 1, 35–43. [Google Scholar]
- Kongsomart, B.; Li, L.; Takarada, T. Preparation of activated carbons from teak sawdust using chicken dropping compost and empty fruit bunch. Int. J. Biomass Renew. 2015, 4, 1–7. [Google Scholar]
- Saputro, S.; Masykuri, M.; Mahardiani, L.; Mulyani, B.; Qorina, I.; Yoshimura, K.; Takehara, K.; Matsuoka, S. The usage of activated carbon from teak sawdust (tectona grandis l.f.) and zeolite for the adsorption of Cr(VI) and its analysis using solid-phase spectrophotometry (sps). International Conference on Advanced Materials for Better Future 2017. IOP Conf. Ser. Mater. Sci. Eng. 2017, 176, 012019–012025. [Google Scholar] [CrossRef]
- Zhu, G.-Z.; Deng, X.-L.; Hou, M.; Sun, K.; Zhang, Y.; Li, P.; Liang, F. Comparative study on characterization and adsorption properties of activated carbons by phosphoric acid activation from corncob and its acid and alkaline hydrolysis residues. Fuel Processing Technol. 2016, 144, 255–261. [Google Scholar] [CrossRef]
- Larasati, A.; Fowler, G.D.; Graham, N.J.D. Extending granular activated carbon (GAC) bed life: A column study of in-situ chemical regeneration of pesticide loaded activated carbon for water treatment. Chemosphere 2022, 286, 131888–131998. [Google Scholar] [CrossRef] [PubMed]
- Plano Estratégico do Minestério da Agricultura e Pescas da República Democrática de Timor Leste, 2014–2020; Ministry of Agriculture & Fisheries: Tykyo, Japan, 2012; pp. 18–25. Available online: spc.int (accessed on 21 April 2022).
- Hough, R.L. A world view of pesticides. Nat. Geosci. 2021, 14, 183–184. [Google Scholar] [CrossRef]
- Mali, H.; Shaha, C.; Raghunandana, B.H.; Prajapati, A.S.; Patel, D.H.; Trivedi, U.; Subramanian, R.B. Organophosphate pesticides an emerging environmental contaminant: Pollution, toxicity, bioremediation progress, and remaining challenges. J. Environ. Sci. 2022, 127, 234–250. [Google Scholar] [CrossRef]
- Cansado, I.P.P.; Mourão, P.A.M.; Falcão, A.I.; Ribeiro Carrott, M.M.L.; Carrott, P.J.M. The influence of the activated carbon post-treatment on the phenolic compound’s removal. Fuel Process. Technol. 2012, 103, 64–70. [Google Scholar] [CrossRef]
- Salman, J.M.; Hameed, B.H. Adsorption of 2,4-dichlorophenoxyacetic acid and carbofuran pesticides onto granular activated carbon. Desalination 2010, 256, 129–135. [Google Scholar] [CrossRef]
- Direção Geral de Alimentação e Veterinária. Lista de Pesticidas a Pesquisar em Água de Consumo Humano. Triénio 2022–2024. 2021, pp. 14–16. (In Portuguese). Available online: https://www.dgav.pt/wp-content/uploads/2021/08/DGAV_PCQA_2022-2024-19-08-2021.pdf (accessed on 21 April 2022).
- Belo, C.R.; Cansado, I.P.P.; Mourão, P.A.M. Synthetic polymers blend used in the production of high activated carbon for pesticides removals from the liquid phase. Environ. Technol. 2017, 38, 285–296. [Google Scholar] [CrossRef]
- Carrott, P.J.M.; Roberts, R.A.; Sing, K.S.W. Adsorption of nitrogen by porous and non-porous carbons. Carbon 1987, 25, 59–68. [Google Scholar] [CrossRef]
- Palomba, V.; Frazzica, A. Modelling of sorption systems for thermal energy storage, Advances in Thermal Energy Storage Systems. 2nd Ed. Methods and Applications. Woodhead Publ. Ser. Energy 2021, 453–475. [Google Scholar] [CrossRef]
- Adinata, D.; Wan Daud, W.M.A.; Aroua, M.K. Preparation and characterization of activated carbon from palm shell by chemical activation with K2CO3. Bioresour. Technol. 2007, 98, 145–149. [Google Scholar] [CrossRef]
- Castro, C.S. Preparation of Activated Carbon from Coffee Grounds: Use as Catalytic Support for the Removal of Organic Pollutants in the Aqueous Medium. Master’s Thesis, Lavras University, Lavras, Brazil, 2009. Available online: https://pt.scribd.com/document/311048911 (accessed on 21 April 2022).
- Yuen, Q.; Xu, S.; Gao, B. Activated carbons with well-developed mesoporosity prepared by activation with different alkali salts. Mater. Lett. 2015, 146, 34–36. [Google Scholar]
- Zhu, R.; Yu, Q.; Li, M.; Zhao, H.; Jin, S.; Huang, Y.; Fan, J.; Chen, J. Analysis of factors influencing pore structure development of agricultural and forestry waste-derived activated carbon for adsorption application in gas and liquid phases: A review. J. Environ. Chem. Eng. 2021, 9, 105905–105936. [Google Scholar] [CrossRef]
- Spaltro, A.; Pila, M.; Simonetti, S.; Álvarez-Torrellas, S.; García Rodríguez, J.; Ruiz, D.; Company, A.D.; Juan, A.; Allegretti, P. Adsorption and removal of phenoxy acetic herbicides from water by using commercial activated carbons: Experimental and computational studies. J. Contam. Hydrol. 2018, 218, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Derylo-Marczewska, A.; Blachnio, M.; Marczewski, A.W.; Swiatkowski, A.; Tarasiuk, B. Adsorption of selected herbicides from aqueous solutions on activated carbon. J. Therm. Anal. Calorim. 2010, 101, 785–794. [Google Scholar] [CrossRef] [Green Version]
- Foo, K.Y.; Hameed, B.H. Insights into the modelling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Kulaishin, S.A.; Vedenyapina, M.D.; Kurmysheva, A.Y. Influence of the Surface Characteristics of Activated Carbon on the Adsorption of Herbicides (A Review). Solid Fuel Chem. 2022, 56, 181–198. [Google Scholar] [CrossRef]
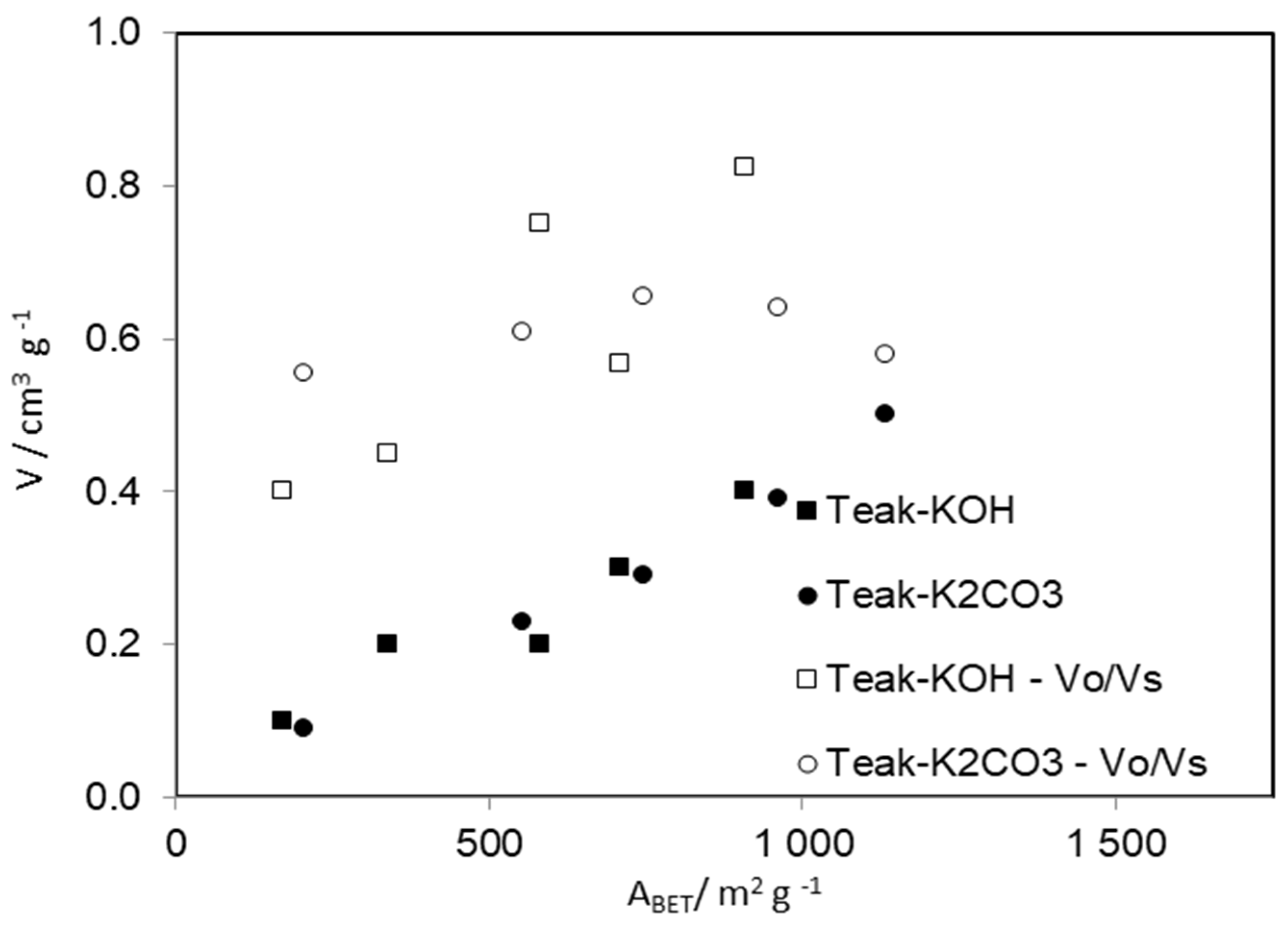





| Sample | ƞ/% | ABET/m2·g−1 | αs | DR | |||
|---|---|---|---|---|---|---|---|
| As/m2·g−1 | Vs/cm3·g−1 | Vo/cm3·g−1 | Eo/kJ·mol−1 | Lo/nm | |||
| Teak-KOH-1-2-700 | 17.5 | 709 | 65 | 0.30 | 0.17 | 18.6 | 1.35 |
| Teak-KOH-1-1-700 | 16.7 | 995 | 57 | 0.43 | 0.26 | 19.9 | 1.27 |
| Teak-KOH-2-1-700 | 24.1 | 337 | 36 | 0.20 | 0.09 | 14.9 | 3.05 |
| Teak-KOH-1-1-600 | 20.4 | 581 | 21 | 0.20 | 0.15 | 25.4 | 0.77 |
| Teak-KOH-1-1-450 | 25.0 | 170 | 27 | 0.10 | 0.04 | 14.9 | 3.10 |
| Sample | η/% | ABET/m2·g−1 | αs | DR | |||
|---|---|---|---|---|---|---|---|
| As/m2·g−1 | Vs/cm3·g−1 | Vo/cm3·g−1 | Eo/kJ·mol−1 | Lo/nm | |||
| Teak-K2CO3-1-2-700 | 20.2 | 1132 | 31 | 0.50 | 0.29 | 18.6 | 1.32 |
| Teak-K2CO3-1-1-700 | 24.0 | 962 | 98 | 0.39 | 0.25 | 22.9 | 0.84 |
| Teak-K2CO3-2-1-700 | 29.9 | 747 | 38 | 0.29 | 0.19 | 27.1 | 0.70 |
| Teak-K2CO3-1-1-600 | 16.0 | 554 | 32 | 0.23 | 0.14 | 21.7 | 1.17 |
| Teak-K2CO3-1-1-450 | 22.7 | 203 | 26 | 0.09 | 0.05 | 16.0 | 2.35 |
| Activated Carbon | N/wt% | C/wt% | H/wt% | S/wt% | O/* wt% | pH pzc |
|---|---|---|---|---|---|---|
| Teak-KOH-1-1-700 | --- | 81.8 | 1.1 | 0.19 | 23.2 | 8.6 |
| Teak-K2CO3-1-1-700 | --- | 80.2 | 1.9 | 0.10 | 22.4 | 8.4 |
| System | Endo/exo | nmax | nmL | KL | KF | nF | |
|---|---|---|---|---|---|---|---|
| mmol·g−1 | mmol·g−1 | mmol·g−1 | mmol·g−1 (dm3·mmol−1)1/nF | ||||
| MCPA | Teak-KOH-1-1-700 | Exo | 1.64 | 1.84 | 6.6 | 1.72 | 2.9 |
| Teak-K2CO3-1-1-700 | Exo | 1.88 | 1.96 | 8.0 | 1.94 | 2.5 | |
| 2,4-D | Teak-KOH-1:1-700 | Exo | 1.63 | 1.99 | 7.3 | 2.56 | 1.8 |
| Teak-K2CO3-1-1-700 | Exo | 1.67 | 1.66 | 8.4 | 2.44 | 1.8 | |
| Atrazine | Teak-KOH-1-1-700 | Exo | 0.78 | 0.99 | 22.4 | 1.55 | 3.2 |
| Teak-K2CO3-1-1-700 | Exo | 0.89 | 0.80 | 62.8 | 3.02 | 1.6 | |
| Diuron | Teak-KOH-1-1-700 | Exo | 1.10 | 1.19 | 49.6 | 2.71 | 2.6 |
| Teak-K2CO3-1-1-700 | Exo | 0.97 | 1.06 | 54.5 | 1.92 | 3.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Paixão Cansado, I.P.; Mourão, P.A.M.; Belo, C.R. Using Tectona Grandis Biomass to Produce Valuable Adsorbents for Pesticide Removal from Liquid Effluent. Materials 2022, 15, 5842. https://doi.org/10.3390/ma15175842
da Paixão Cansado IP, Mourão PAM, Belo CR. Using Tectona Grandis Biomass to Produce Valuable Adsorbents for Pesticide Removal from Liquid Effluent. Materials. 2022; 15(17):5842. https://doi.org/10.3390/ma15175842
Chicago/Turabian Styleda Paixão Cansado, Isabel Pestana, Paulo Alexandre Mira Mourão, and Cristóvão Ramiro Belo. 2022. "Using Tectona Grandis Biomass to Produce Valuable Adsorbents for Pesticide Removal from Liquid Effluent" Materials 15, no. 17: 5842. https://doi.org/10.3390/ma15175842






