Ascorbic Acid (Vitamin C) as a Cosmeceutical to Increase Dermal Collagen for Skin Antiaging Purposes: Emerging Combination Therapies
Abstract
:1. Introduction
2. Biochemical Properties of AA
3. AA in Collagen Metabolism
4. Clinical Evidence for Dermal Collagen-Enhancing and Skin Antiaging Effects of AA
5. Clinical Studies Using a Combined Composition of AA and Other Active Ingredients
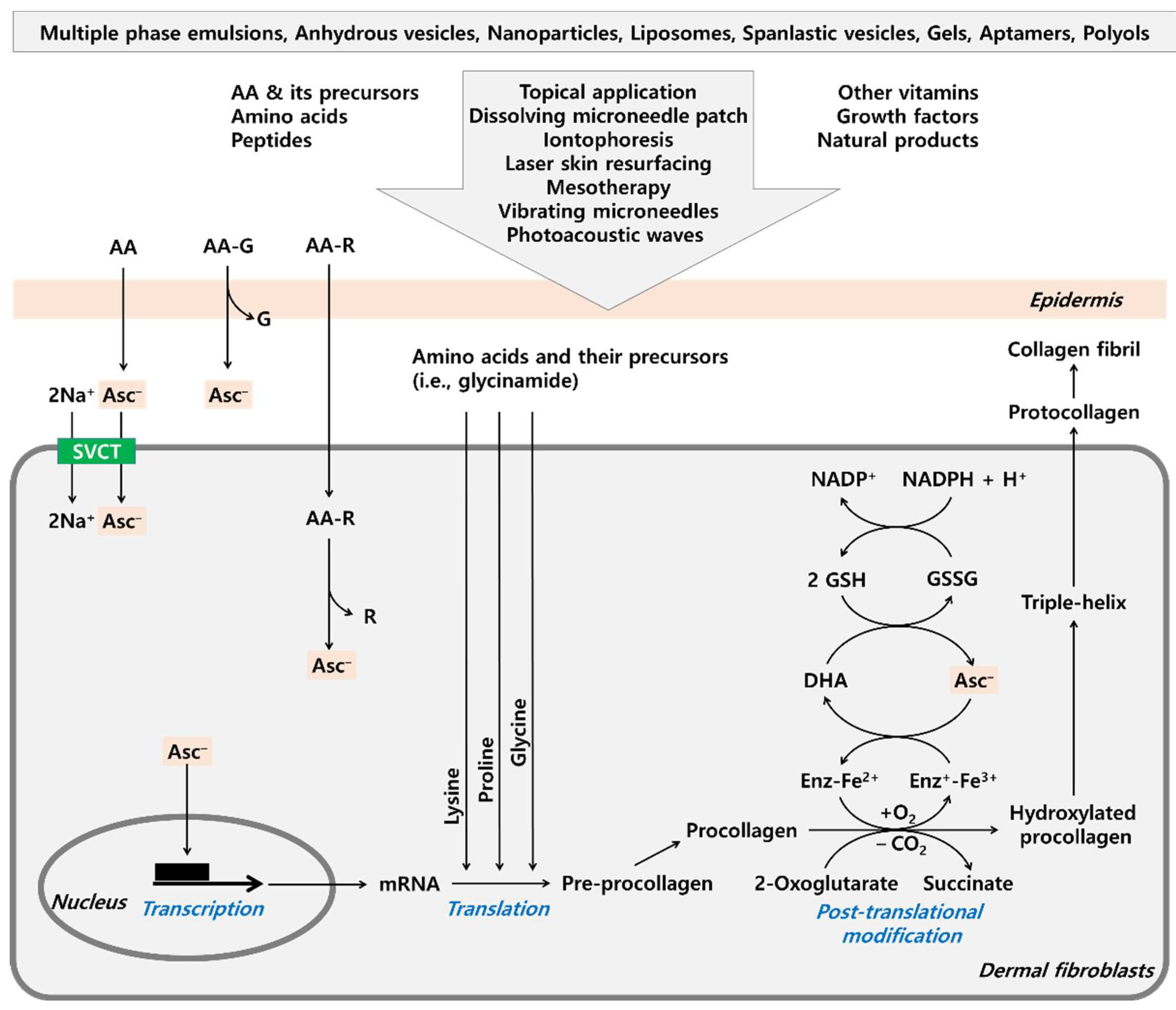
6. Various Approaches to Maximize the Efficacy of AA
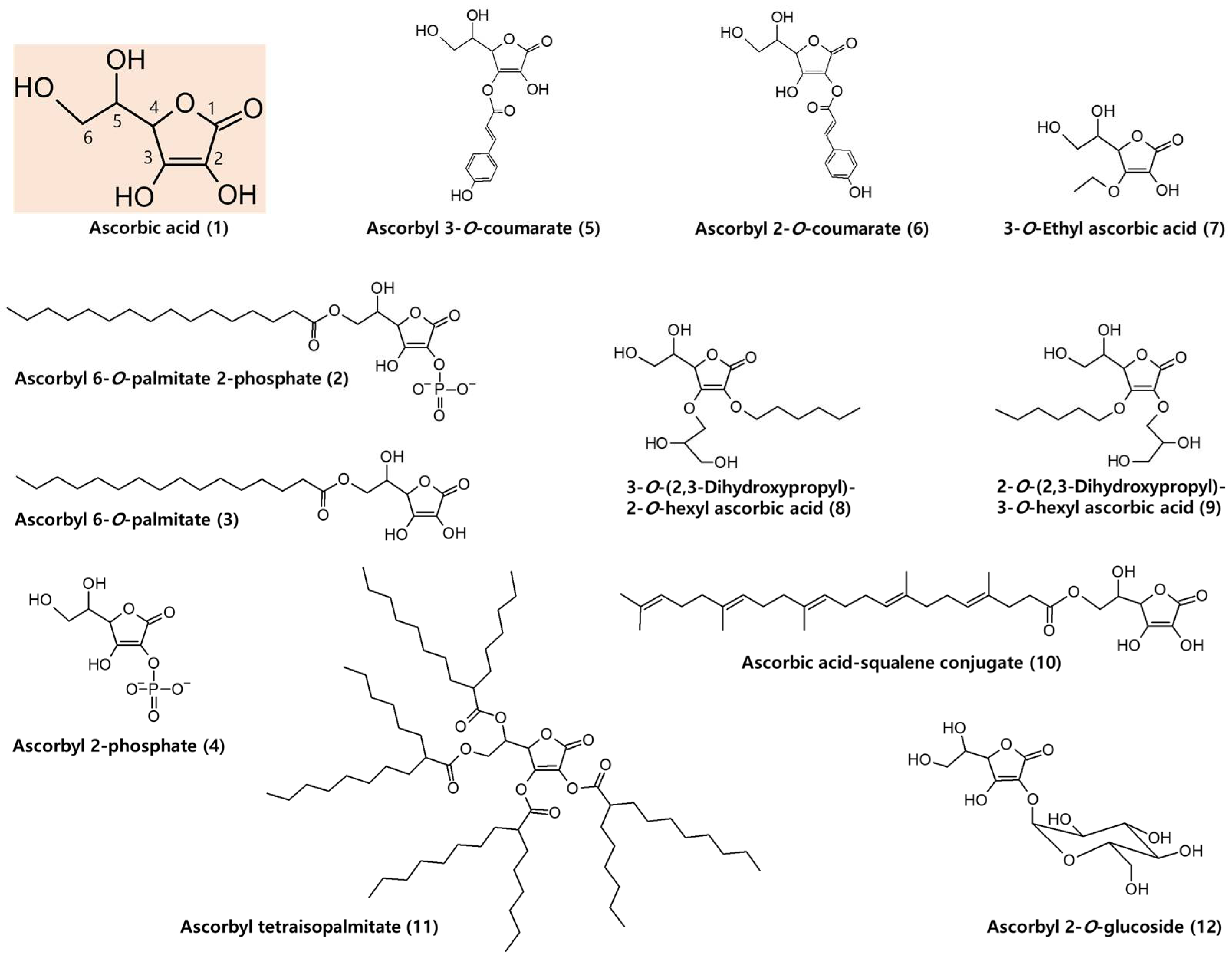
6.1. AA Derivatives with Added Advantages
6.2. Formulations to Improve the Stability and Skin Absorption of AA
6.3. Use of Medical Devices to Enhance Skin Absorption of AA
6.4. Antioxidant Effects of AA and Combination with Other Antioxidants
6.5. Combination of AA with Amino Acids to Synergistically Increase Collagen Production
7. Discussion
8. Conclusions
Funding
Conflicts of Interest
References
- Padayatty, S.J.; Levine, M. Vitamin C: The known and the unknown and Goldilocks. Oral Dis. 2016, 22, 463–493. [Google Scholar] [CrossRef] [PubMed]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Shen, J.; Griffiths, P.T.; Campbell, S.J.; Utinger, B.; Kalberer, M.; Paulson, S.E. Ascorbate oxidation by iron, copper and reactive oxygen species: Review, model development, and derivation of key rate constants. Sci. Rep. 2021, 11, 7417. [Google Scholar] [CrossRef] [PubMed]
- Njus, D.; Kelley, P.M.; Tu, Y.J.; Schlegel, H.B. Ascorbic acid: The chemistry underlying its antioxidant properties. Free Radic. Biol. Med. 2020, 159, 37–43. [Google Scholar] [CrossRef]
- Kuiper, C.; Vissers, M.C. Ascorbate as a co-factor for Fe- and 2-oxoglutarate dependent dioxygenases: Physiological activity in tumor growth and progression. Front. Oncol. 2014, 4, 359. [Google Scholar] [CrossRef] [PubMed]
- Vasta, J.D.; Raines, R.T. Human Collagen Prolyl 4-Hydroxylase Is Activated by Ligands for Its Iron Center. Biochemistry 2016, 55, 3224–3233. [Google Scholar] [CrossRef]
- Takeshita, N.; Kawade, N.; Suzuki, W.; Hara, S.; Horio, F.; Ichinose, H. Deficiency of ascorbic acid decreases the contents of tetrahydrobiopterin in the liver and the brain of ODS rats. Neurosci. Lett. 2020, 715, 134656. [Google Scholar] [CrossRef]
- Sun, B. The mechanics of fibrillar collagen extracellular matrix. Cell Rep. Phys. Sci. 2021, 2, 100515. [Google Scholar] [CrossRef]
- Cole, M.A.; Quan, T.; Voorhees, J.J.; Fisher, G.J. Extracellular matrix regulation of fibroblast function: Redefining our perspective on skin aging. J. Cell Commun. Signal. 2018, 12, 35–43. [Google Scholar] [CrossRef]
- Ferreira, A.M.; Gentile, P.; Chiono, V.; Ciardelli, G. Collagen for bone tissue regeneration. Acta Biomater. 2012, 8, 3191–3200. [Google Scholar] [CrossRef]
- Ramshaw, J.A.; Shah, N.K.; Brodsky, B. Gly-X-Y tripeptide frequencies in collagen: A context for host-guest triple-helical peptides. J. Struct. Biol. 1998, 122, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Samad, N.; Sikarwar, A. Collagen: New Dimension in Cosmetic and Healthcare. Int. J. Biochem. Res. Rev. 2016, 14, 1–8. [Google Scholar] [CrossRef]
- Nassa, M.; Anand, P.; Jain, A.; Chhabra, A.; Jaiswal, A.; Malhotra, U.; Rani, V. Analysis of human collagen sequences. Bioinformation 2012, 8, 26–33. [Google Scholar] [CrossRef]
- Morikawa, M.; Derynck, R.; Miyazono, K. TGF-beta and the TGF-beta Family: Context-Dependent Roles in Cell and Tissue Physiology. Cold Spring Harb. Perspect. Biol. 2016, 8, a021873. [Google Scholar] [CrossRef] [PubMed]
- Pittayapruek, P.; Meephansan, J.; Prapapan, O.; Komine, M.; Ohtsuki, M. Role of Matrix Metalloproteinases in Photoaging and Photocarcinogenesis. Int. J. Mol. Sci. 2016, 17, 868. [Google Scholar] [CrossRef] [PubMed]
- Jablonska-Trypuc, A.; Matejczyk, M.; Rosochacki, S. Matrix metalloproteinases (MMPs), the main extracellular matrix (ECM) enzymes in collagen degradation, as a target for anticancer drugs. J. Enzym. Inhib. Med. Chem. 2016, 31, 177–183. [Google Scholar] [CrossRef]
- Passeron, T.; Krutmann, J.; Andersen, M.L.; Katta, R.; Zouboulis, C.C. Clinical and biological impact of the exposome on the skin. J. Eur. Acad. Dermatol. Venereol. 2020, 34 (Suppl. 4), 4–25. [Google Scholar] [CrossRef]
- Rittie, L.; Fisher, G.J. Natural and sun-induced aging of human skin. Cold Spring Harb. Perspect. Med. 2015, 5, a015370. [Google Scholar] [CrossRef]
- Shin, J.W.; Kwon, S.H.; Choi, J.Y.; Na, J.I.; Huh, C.H.; Choi, H.R.; Park, K.C. Molecular Mechanisms of Dermal Aging and Antiaging Approaches. Int. J. Mol. Sci. 2019, 20, 2126. [Google Scholar] [CrossRef]
- Sandhu, S.V.; Gupta, S.; Bansal, H.; Singla, K.; Yadav, N.S. Collagen in Health and Disease. J. Orofac. Res. 2012, 2, 153–159. [Google Scholar] [CrossRef]
- Reilly, D.M.; Lozano, J. Skin collagen through the lifestages: Importance for skin health and beauty. Plast. Aesthet. Res. 2021, 8, 2. [Google Scholar] [CrossRef]
- Cheng, W.; Yan-Hua, R.; Fang-Gang, N.; Guo-An, Z. The content and ratio of type I and III collagen in skin differ with age and injury. Afr. J. Biotechnol. 2011, 10, 2524–2529. [Google Scholar]
- Quan, T.H.; Fisher, G.J. Role of Age-Associated Alterations of the Dermal Extracellular Matrix Microenvironment in Human Skin Aging: A Mini-Review. Gerontology 2015, 61, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Imokawa, G.; Ishida, K. Biological mechanis.sms underlying the ultraviolet radiation-induced formation of skin wrinkling and sagging I: Reduced skin elasticity, highly associated with enhanced dermal elastase activity, triggers wrinkling and sagging. Int. J. Mol. Sci. 2015, 16, 7753–7775. [Google Scholar] [CrossRef]
- Maione-Silva, L.; de Castro, E.G.; Nascimento, T.L.; Cintra, E.R.; Moreira, L.C.; Cintra, B.A.S.; Valadares, M.C.; Lima, E.M. Ascorbic acid encapsulated into negatively charged liposomes exhibits increased skin permeation, retention and enhances collagen synthesis by fibroblasts. Sci. Rep. 2019, 9, 522. [Google Scholar] [CrossRef]
- Geesin, J.C.; Darr, D.; Kaufman, R.; Murad, S.; Pinnell, S.R. Ascorbic acid specifically increases type I and type III procollagen messenger RNA levels in human skin fibroblast. J. Investig. Dermatol. 1988, 90, 420–424. [Google Scholar] [CrossRef]
- Tajima, S.; Pinnell, S.R. Ascorbic acid preferentially enhances type I and III collagen gene transcription in human skin fibroblasts. J. Dermatol. Sci. 1996, 11, 250–253. [Google Scholar] [CrossRef]
- Pullar, J.M.; Carr, A.C.; Vissers, M.C.M. The Roles of Vitamin C in Skin Health. Nutrients 2017, 9, 866. [Google Scholar] [CrossRef]
- Wang, K.; Jiang, H.; Li, W.; Qiang, M.; Dong, T.; Li, H. Role of Vitamin C in Skin Diseases. Front. Physiol. 2018, 9, 819. [Google Scholar] [CrossRef]
- Stamford, N.P. Stability, transdermal penetration, and cutaneous effects of ascorbic acid and its derivatives. J. Cosmet. Dermatol. 2012, 11, 310–317. [Google Scholar] [CrossRef]
- Viviani, A.; Fambrini, M.; Giordani, T.; Pugliesi, C. L-Ascorbic acid in plants: From biosynthesis to its role in plant development and stress response. Agrochimica 2021, 65, 151–171. [Google Scholar] [CrossRef]
- Linster, C.L.; Van Schaftingen, E. Vitamin C—Biosynthesis, recycling and degradation in mammals. FEBS J. 2007, 274, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Nishikimi, M.; Kawai, T.; Yagi, K. Guinea-Pigs Possess a Highly Mutated Gene for L-Gulono-Gamma-Lactone Oxidase, the Key Enzyme for L-Ascorbic-Acid Biosynthesis Missing in This Species. J. Biol. Chem. 1992, 267, 21967–21972. [Google Scholar] [CrossRef]
- Lykkesfeldt, J.; Tveden-Nyborg, P. The Pharmacokinetics of Vitamin C. Nutrients 2019, 11, 2412. [Google Scholar] [CrossRef]
- May, J.M. The SLC23 family of ascorbate transporters: Ensuring that you get and keep your daily dose of vitamin C. Br. J. Pharmacol. 2011, 164, 1793–1801. [Google Scholar] [CrossRef] [PubMed]
- Rumsey, S.C.; Kwon, O.; Xu, G.W.; Burant, C.F.; Simpson, I.; Levine, M. Glucose transporter isoforms GLUT1 and GLUT3 transport dehydroascorbic acid. J. Biol. Chem. 1997, 272, 18982–18989. [Google Scholar] [CrossRef]
- May, J.M.; Qu, Z.C. Transport and intracellular accumulation of vitamin C in endothelial cells: Relevance to collagen synthesis. Arch. Biochem. Biophys. 2005, 434, 178–186. [Google Scholar] [CrossRef]
- Al Fata, N.; George, S.; Andre, S.; Renard, C.M.G.C. Determination of reaction orders for ascorbic acid degradation during sterilization using a new experimental device: The thermoresistometer Mastia (R). LWT-Food Sci. Technol. 2017, 85, 487–492. [Google Scholar] [CrossRef]
- Jiang, D.; Li, X.; Liu, L.; Yagnik, G.B.; Zhou, F. Reaction rates and mechanism of the ascorbic acid oxidation by molecular oxygen facilitated by Cu(II)-containing amyloid-beta complexes and aggregates. J. Phys. Chem. B 2010, 114, 4896–4903. [Google Scholar] [CrossRef]
- Kramarenko, G.G.; Hummel, S.G.; Martin, S.M.; Buettner, G.R. Ascorbate reacts with singlet oxygen to produce hydrogen peroxide. Photochem. Photobiol. 2006, 82, 1634–1637. [Google Scholar] [CrossRef]
- Nimse, S.B.; Pal, D. Free radicals, natural antioxidants, and their reaction mechanisms. RSC Adv. 2015, 5, 27986–28006. [Google Scholar] [CrossRef] [Green Version]
- Martinez, S.; Hausinger, R.P. Catalytic Mechanisms of Fe(II)-and 2-Oxoglutarate-dependent Oxygenases. J. Biol. Chem. 2015, 290, 20702–20711. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Leissing, T.M.; Chowdhury, R.; Hopkinson, R.J.; Schofield, C.J. 2-Oxoglutarate-Dependent Oxygenases. Annu. Rev. Biochem. 2018, 87, 585–620. [Google Scholar] [CrossRef]
- Navas, P.; Sun, I.; Crane, F.L.; Morre, D.M.; Morre, D.J. Monoascorbate free radical-dependent oxidation-reduction reactions of liver Golgi apparatus membranes. J. Bioenerg. Biomembr. 2010, 42, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.N.; Brock, J.; Liu, D.; Board, P.G.; Oakley, A.J. Structural Insights into the Dehydroascorbate Reductase Activity of Human Omega-Class Glutathione Transferases. J. Mol. Biol. 2012, 420, 190–203. [Google Scholar] [CrossRef]
- Deshmukh, S.N.; Dive, A.M.; Moharil, R.; Munde, P. Enigmatic insight into collagen. J. Oral Maxillofac. Pathol. 2016, 20, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Ricard-Blum, S. The collagen family. Cold Spring Harb. Perspect. Biol. 2011, 3, a004978. [Google Scholar] [CrossRef]
- Ito, S.; Nagata, K. Roles of the endoplasmic reticulum-resident, collagen-specific molecular chaperone Hsp47 in vertebrate cells and human disease. J. Biol. Chem. 2019, 294, 2133–2141. [Google Scholar] [CrossRef]
- Matsui, Y.; Hirata, Y.; Wada, I.; Hosokawa, N. Visualization of Procollagen IV Reveals ER-to-Golgi Transport by ERGIC-independent Carriers. Cell Struct. Funct. 2020, 45, 107–119. [Google Scholar] [CrossRef]
- Malhotra, V.; Erlmann, P. The pathway of collagen secretion. Annu. Rev. Cell Dev. Biol. 2015, 31, 109–124. [Google Scholar] [CrossRef]
- Asgari, M.; Latifi, N.; Heris, H.K.; Vali, H.; Mongeau, L. In vitro fibrillogenesis of tropocollagen type III in collagen type I affects its relative fibrillar topology and mechanics. Sci. Rep. 2017, 7, 1392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Markolovic, S.; Wilkins, S.E.; Schofield, C.J. Protein Hydroxylation Catalyzed by 2-Oxoglutarate-dependent Oxygenases. J. Biol. Chem. 2015, 290, 20712–20722. [Google Scholar] [CrossRef]
- Rappu, P.; Salo, A.M.; Myllyharju, J.; Heino, J. Role of prolyl hydroxylation in the molecular interactions of collagens. Essays Biochem. 2019, 63, 325–335. [Google Scholar] [PubMed]
- Yamauchi, M.; Terajima, M.; Shiiba, M. Lysine Hydroxylation and Cross-Linking of Collagen. Methods Mol. Biol. 2019, 1934, 309–324. [Google Scholar] [PubMed]
- Salo, A.M.; Myllyharju, J. Prolyl and lysyl hydroxylases in collagen synthesis. Exp. Dermatol. 2021, 30, 38–49. [Google Scholar] [CrossRef]
- Olmedo, J.M.; Yiannias, J.A.; Windgassen, E.B.; Gornet, N.K. Scurvy: A disease almost forgotten. Int. J. Dermatol. 2006, 45, 909–913. [Google Scholar] [CrossRef]
- Murad, S.; Grove, D.; Lindberg, K.A.; Reynolds, G.; Sivarajah, A.; Pinnell, S.R. Regulation of collagen synthesis by ascorbic acid. Proc. Natl. Acad. Sci. USA 1981, 78, 2879–2882. [Google Scholar] [CrossRef]
- Pinnell, S.R. Regulation of collagen biosynthesis by ascorbic acid: A review. Yale J. Biol. Med. 1985, 58, 553–559. [Google Scholar]
- Pinnell, S.R.; Murad, S.; Darr, D. Induction of Collagen-Synthesis by Ascorbic-Acid—A Possible Mechanism. Arch. Dermatol. 1987, 123, 1684. [Google Scholar] [CrossRef]
- Chojkier, M.; Houglum, K.; Solisherruzo, J.; Brenner, D.A. Stimulation of Collagen Gene-Expression by Ascorbic-Acid in Cultured Human-Fibroblasts—A Role for Lipid-Peroxidation. J. Biol. Chem. 1989, 264, 16957–16962. [Google Scholar] [CrossRef]
- Houglum, K.P.; Brenner, D.A.; Chojkier, M. Ascorbic-Acid Stimulation of Collagen Biosynthesis Independent of Hydroxylation. Am. J. Clin. Nutr. 1991, 54, S1141–S1143. [Google Scholar] [CrossRef]
- Darr, D.; Combs, S.; Pinnell, S. Ascorbic-Acid and Collagen-Synthesis—Rethinking a Role for Lipid-Peroxidation. Arch. Biochem. Biophys. 1993, 307, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Strowitzki, M.J.; Cummins, E.P.; Taylor, C.T. Protein Hydroxylation by Hypoxia-Inducible Factor (HIF) Hydroxylases: Unique or Ubiquitous? Cells 2019, 8, 384. [Google Scholar] [CrossRef] [PubMed]
- Li, L.Y.; Artlett, C.M.; Jimenez, S.A.; Hall, D.J.; Varga, J. Positive Regulation of Human Alpha-1(I) Collagen Promoter Activity by Transcription Factor Sp1. Gene 1995, 164, 229–234. [Google Scholar] [CrossRef]
- Goto, T.; Matsui, Y.; Fernandes, R.J.; Hanson, D.A.; Kubo, T.; Yukata, K.; Michigami, T.; Komori, T.; Fujita, T.; Yang, L.; et al. Sp1 family of transcription factors regulates the human alpha 2 (XI) collagen gene (COL11A2) in Saos-2 osteoblastic cells. J. Bone Miner. Res. 2006, 21, 661–673. [Google Scholar] [CrossRef]
- Rhie, G.E.; Shin, M.H.; Seo, J.Y.; Choi, W.W.; Cho, K.H.; Kim, K.H.; Park, K.C.; Eun, H.C.; Chung, J.H. Aging- and photoaging-dependent changes of enzymic and nonenzymic antioxidants in the epidermis and dermis of human skin in vivo. J. Investig. Dermatol. 2001, 117, 1212–1217. [Google Scholar] [CrossRef]
- Nusgens, B.V.; Humbert, P.; Rougier, A.; Colige, A.C.; Haftek, M.; Lambert, C.A.; Richard, A.; Creidi, P.; Lapiere, C.M. Topically applied vitamin C enhances the mRNA level of collagens I and III, their processing enzymes and tissue inhibitor of matrix metalloproteinase 1 in the human dermis. J. Investig. Dermatol. 2001, 116, 853–859. [Google Scholar] [CrossRef]
- Humbert, P.G.; Haftek, M.; Creidi, P.; Lapiere, C.; Nusgens, B.; Richard, A.; Schmitt, D.; Rougier, A.; Zahouani, H. Topical ascorbic acid on photoaged skin. Clinical, topographical and ultrastructural evaluation: Double-blind study vs. placebo. Exp. Dermatol. 2003, 12, 237–244. [Google Scholar] [CrossRef]
- Xu, T.H.; Chen, J.Z.; Li, Y.H.; Wu, Y.; Luo, Y.J.; Gao, X.H.; Chen, H.D. Split-face study of topical 23.8% L-ascorbic acid serum in treating photo-aged skin. J. Drugs Dermatol. 2012, 11, 51–56. [Google Scholar] [PubMed]
- Lee, C.; Yang, H.; Kim, S.; Kim, M.; Kang, H.; Kim, N.; An, S.; Koh, J.; Jung, H. Evaluation of the anti-wrinkle effect of an ascorbic acid-loaded dissolving microneedle patch via a double-blind, placebo-controlled clinical study. Int. J. Cosmet. Sci. 2016, 38, 375–381. [Google Scholar] [CrossRef]
- Machado, B.H.B.; Frame, J.; Zhang, J.; Najlah, M. Comparative Study on the Outcome of Periorbital Wrinkles Treated with Laser-Assisted Delivery of Vitamin C or Vitamin C Plus Growth Factors: A Randomized, Double-blind, Clinical Trial. Aesthet. Plast. Surg. 2021, 45, 1020–1032. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, G.; Wainwright, L.J.; Holland, R.; Barrett, K.E.; Casey, J. Wrinkle reduction in post-menopausal women consuming a novel oral supplement: A double-blind placebo-controlled randomized study. Int. J. Cosmet. Sci. 2014, 36, 22–31. [Google Scholar] [CrossRef]
- Crisan, D.; Roman, I.; Crisan, M.; Scharffetter-Kochanek, K.; Badea, R. The role of vitamin C in pushing back the boundaries of skin aging: An ultrasonographic approach. Clin. Cosmet. Investig. Dermatol. 2015, 8, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Garre, A.; Narda, M.; Valderas-Martinez, P.; Piquero, J.; Granger, C. Antiaging effects of a novel facial serum containing L-Ascorbic acid, proteoglycans, and proteoglycan-stimulating tripeptide: Ex vivo skin explant studies and in vivo clinical studies in women. Clin. Cosmet. Investig. Dermatol. 2018, 11, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Rattanawiwatpong, P.; Wanitphakdeedecha, R.; Bumrungpert, A.; Maiprasert, M. Anti-aging and brightening effects of a topical treatment containing vitamin C, vitamin E, and raspberry leaf cell culture extract: A split-face, randomized controlled trial. J. Cosmet. Dermatol. 2020, 19, 671–676. [Google Scholar] [CrossRef]
- Lintner, K.; Gerstein, F.; Solish, N. A serum containing vitamins C & E and a matrix-repair tripeptide reduces facial signs of aging as evidenced by Primos(R) analysis and frequently repeated auto-perception. J. Cosmet. Dermatol. 2020, 19, 3262–3269. [Google Scholar]
- Escobar, S.; Valois, A.; Nielsen, M.; Closs, B.; Kerob, D. Effectiveness of a formulation containing peptides and vitamin C in treating signs of facial ageing: Three clinical studies. Int. J. Cosmet. Sci. 2021, 43, 131–135. [Google Scholar] [CrossRef]
- Golubitskii, G.B.; Budko, E.V.; Basova, E.M.; Kostarnoi, A.V.; Ivanov, V.M. Stability of ascorbic acid in aqueous and aqueous-organic solutions for quantitative determination. J. Anal. Chem. 2007, 62, 742–747. [Google Scholar] [CrossRef]
- Kim, S.; Lee, T.G. Stabilization of L-ascorbic acid in cosmetic emulsions. J. Ind. Eng. Chem. 2018, 57, 193–198. [Google Scholar] [CrossRef]
- Ahmad, I.; Sheraz, M.A.; Ahmed, S.; Shaikh, R.H.; Vaid, F.H.M.; Khattak, S.U.R.; Ansari, S.A. Photostability and Interaction of Ascorbic Acid in Cream Formulations. AAPS Pharmscitech 2011, 12, 917–923. [Google Scholar] [CrossRef]
- Lee, A.-R.C.; Tojo, K. Characterization of Skin Permeation of Vitamin C: Theoretical Analysis of Penetration Profiles and Differential Scanning Calorimetry Study. Chem. Pharm. Bull. 1998, 46, 174–177. [Google Scholar] [CrossRef] [PubMed]
- Taira, N.; Katsuyama, Y.; Yoshioka, M.; Muraoka, O.; Morikawa, T. Structural Requirements of Alkylglyceryl-l-Ascorbic Acid Derivatives for Melanogenesis Inhibitory Activity. Int. J. Mol. Sci. 2018, 19, 1144. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, S.; Sakaguchi, I.; Ito, S.; Kato, E.; Watanabe, K.; Izuo, N.; Shimizu, T. Topical Application of Trisodium Ascorbyl 6-Palmitate 2-Phosphate Actively Supplies Ascorbate to Skin Cells in an Ascorbate Transporter-Independent Manner. Nutrients 2017, 9, 645. [Google Scholar] [CrossRef] [Green Version]
- Kwak, J.Y.; Park, S.; Seok, J.K.; Liu, K.H.; Boo, Y.C. Ascorbyl coumarates as multifunctional cosmeceutical agents that inhibit melanogenesis and enhance collagen synthesis. Arch. Dermatol Res. 2015, 307, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Iliopoulos, F.; Sil, B.C.; Moore, D.J.; Lucas, R.A.; Lane, M.E. 3-O-ethyl-l-ascorbic acid: Characterisation and investigation of single solvent systems for delivery to the skin. Int. J. Pharm. X 2019, 1, 100025. [Google Scholar] [CrossRef] [PubMed]
- Zerbinati, N.; Sommatis, S.; Maccario, C.; Di Francesco, S.; Capillo, M.C.; Rauso, R.; Herrera, M.; Bencini, P.L.; Guida, S.; Mocchi, R. The Anti-Ageing and Whitening Potential of a Cosmetic Serum Containing 3-O-ethyl-l-ascorbic Acid. Life 2021, 11, 406. [Google Scholar] [CrossRef]
- Gref, R.; Delomenie, C.; Maksimenko, A.; Gouadon, E.; Percoco, G.; Lati, E.; Desmaele, D.; Zouhiri, F.; Couvreur, P. Vitamin C-squalene bioconjugate promotes epidermal thickening and collagen production in human skin. Sci. Rep. 2020, 10, 16883. [Google Scholar] [CrossRef]
- Xiao, L.; Kaneyasu, K.; Saitoh, Y.; Terashima, Y.; Kowata, Y.; Miwa, N. Cytoprotective Effects of the Lipoidic-Liquiform Pro-Vitamin C Tetra-Isopalmitoyl-Ascorbate (VC-IP) against Ultraviolet-A Ray-Induced Injuries in Human Skin Cells Together with Collagen Retention, MMP Inhibition and p53 Gene Repression. J. Cell Biochem. 2009, 106, 589–598. [Google Scholar] [CrossRef]
- Yokota, M.; Yahagi, S. Evaluation of the anti-wrinkle effect of a lipophilic pro-vitamin C derivative, tetra-isopalmitoyl ascorbic acid. J. Cosmet. Dermatol. 2021, 21, 3503–3514. [Google Scholar] [CrossRef]
- Yamamoto, I.; Muto, N.; Murakami, K.; Akiyama, J.I. Collagen-Synthesis in Human Skin Fibroblasts Is Stimulated by a Stable Form of Ascorbate, 2-O-Alpha-D-Glucopyranosyl-L-Ascorbic Acid. J. Nutr. 1992, 122, 871–877. [Google Scholar] [CrossRef]
- Taniguchi, M.; Arai, N.; Kohno, K.; Ushio, S.; Fukuda, S. Anti-oxidative and anti-aging activities of 2-O-alpha-glucopyranosyl-L-ascorbic acid on human dermal fibroblasts. Eur. J. Pharmacol. 2012, 674, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Roure, R.; Nollent, V.; Dayan, L.; Camel, E.; Bertin, C. A Double-Blind, 12-Week Study to Evaluate the Antiaging Efficacy of a Cream Containing the NF kappa B Inhibitor 4-Hexyl-1, 3-Phenylenediol and Ascorbic Acid-2 Glucoside in Adult Females. J. Drugs Dermatol. 2016, 15, 750–758. [Google Scholar] [PubMed]
- Starr, N.J.; Hamid, K.A.; Wibawa, J.; Marlow, I.; Bell, M.; Perez-Garcia, L.; Barrett, D.A.; Scurr, D.J. Enhanced vitamin C skin permeation from supramolecular hydrogels, illustrated using in situ ToF-SIMS 3D chemical profiling. Int. J. Pharm. 2019, 563, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Jacques, C.; Genies, C.; Bacqueville, D.; Tourette, A.; Borotra, N.; Chaves, F.; Sanches, F.; Gaudry, A.L.; Bessou-Touya, S.; Duplan, H. Ascorbic acid 2-glucoside: An ascorbic acid pro-drug with longer-term antioxidant efficacy in skin. Int. J. Cosmet. Sci. 2021, 43, 691–702. [Google Scholar] [CrossRef]
- Farahmand, S.; Tajerzadeh, H.; Farboud, E.S. Formulation and evaluation of a vitamin C multiple emulsion. Pharm. Dev. Technol. 2006, 11, 255–261. [Google Scholar] [CrossRef]
- Heber, G.K.; Markovic, B.; Hayes, A. An immunohistological study of anhydrous topical ascorbic acid compositions on ex vivo human skin. J. Cosmet. Dermatol. 2006, 5, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Adachi, T.; Nakamura, Y. Aptamers: A Review of Their Chemical Properties and Modifications for Therapeutic Application. Molecules 2019, 24, 4229. [Google Scholar] [CrossRef]
- Choi, S.; Han, J.; Kim, J.H.; Kim, A.R.; Kim, S.H.; Lee, W.; Yoon, M.Y.; Kim, G.; Kim, Y.S. Advances in dermatology using DNA aptamer “Aptamin C” innovation: Oxidative stress prevention and effect maximization of vitamin C through antioxidation. J. Cosmet. Dermatol. 2020, 19, 970–976. [Google Scholar] [CrossRef]
- Golonka, I.; Kizior, B.; Szyja, B.M.; Damek, M.P.; Musiał, W. Assessment of the Influence of the Selected Range of Visible Light Radiation on the Durability of the Gel with Ascorbic Acid and Its Derivative. Int. J. Mol. Sci. 2022, 23, 8759. [Google Scholar] [CrossRef]
- Stevanovic, M.M.; Jordovic, B.; Uskokovic, D.P. Preparation and characterization of poly(D, L-lactide-co-glycolide) nanoparticles containing ascorbic acid. J. Biomed. Biotechnol. 2007, 2007, 084965. [Google Scholar] [CrossRef]
- Duarah, S.; Durai, R.D.; Narayanan, V.B. Nanoparticle-in-gel system for delivery of vitamin C for topical application. Drug Deliv. Transl. Res. 2017, 7, 750–760. [Google Scholar] [CrossRef] [PubMed]
- Serrano, G.; Almudever, P.; Serrano, J.M.; Milara, J.; Torrens, A.; Exposito, I.; Cortijo, J. Phosphatidylcholine liposomes as carriers to improve topical ascorbic acid treatment of skin disorders. Clin. Cosmet. Investig. Dermatol. 2015, 8, 591–599. [Google Scholar] [PubMed]
- Iliopoulos, F.; Hossain, A.S.M.M.A.; Sil, B.C.; Moore, D.J.; Lucas, R.A.; Lane, M.E. Topical Delivery of 3-O-ethyl l-ascorbic Acid from Complex Solvent Systems. Sci. Pharm. 2020, 88, 19. [Google Scholar] [CrossRef] [Green Version]
- Elhabak, M.; Ibrahim, S.; Abouelatta, S.M. Topical delivery of l-ascorbic acid spanlastics for stability enhancement and treatment of UVB induced damaged skin. Drug Deliv. 2021, 28, 445–453. [Google Scholar] [CrossRef]
- Yin, X.; Chen, K.W.; Cheng, H.; Chen, X.; Feng, S.; Song, Y.D.; Liang, L. Chemical Stability of Ascorbic Acid Integrated into Commercial Products: A Review on Bioactivity and Delivery Technology. Antioxidants 2022, 11, 153. [Google Scholar] [CrossRef] [PubMed]
- Ebihara, M.; Akiyama, M.; Ohnishi, Y.; Tajima, S.; Komata, K.; Mitsui, Y. Iontophoresis promotes percutaneous absorption of L-ascorbic acid in rat skin. J. Dermatol. Sci. 2003, 32, 217–222. [Google Scholar] [CrossRef]
- Hori, Y.; Akimoto, R.; Hori, A.; Kato, K.; Chino, D.; Matsumoto, S.; Kamiya, S.; Watanabe, Y. Skin collagen reproduction increased by ascorbic acid derivative iontophoresis by frequent-reversal bipolar electric stimulation. J. Cosmet. Sci. 2009, 60, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, C.Y.; Huang, C.H.; Hu, S.; Ko, Y.S.; Sung, H.C.; Chen, C.C.; Huang, S.Y. Fractional Carbon Dioxide Laser Treatment to Enhance Skin Permeation of Ascorbic Acid 2-Glucoside with Minimal Skin Disruption. Dermatol. Surg. 2012, 38, 1284–1293. [Google Scholar] [CrossRef]
- Lee, C.A.; Baek, J.S.; Kwag, D.G.; Lee, H.J.; Park, J.; Cho, C.W. Enhancement of skin permeation of vitamin C using vibrating microneedles. Transl. Clin. Pharmacol. 2017, 25, 15–20. [Google Scholar] [CrossRef]
- Iraji, F.; Nasimi, M.; Asilian, A.; Faghihi, G.; Mozafarpoor, S.; Hafezi, H. Efficacy of mesotherapy with tranexamic acid and ascorbic acid with and without glutathione in treatment of melasma: A split face comparative trial. J. Cosmet. Dermatol. 2019, 18, 1416–1421. [Google Scholar] [CrossRef]
- Balevi, A.; Ustuner, P.; Ozdemir, M. Salicylic acid peeling combined with vitamin C mesotherapy versus salicylic acid peeling alone in the treatment of mixed type melasma: A comparative study. J. Cosmet. Laser Ther. 2017, 19, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Melo-Guimaro, S.; Cardoso, R.; Joao, C.P.; Santos, J.; Melro, E.; Arnaut, L.G.; Pereira, J.C.; Serpa, C. Efficient dermal delivery of ascorbic acid 2-glucoside with photoacoustic waves. Int. J. Cosmet. Sci. 2022, 44, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Boo, Y.C. Mechanistic Basis and Clinical Evidence for the Applications of Nicotinamide (Niacinamide) to Control Skin Aging and Pigmentation. Antioxidants 2021, 10, 1315. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, S.; Funakoshi, T.; Sato, Y.; Saito, N.; Ohsawa, H.; Kurita, K.; Nagata, K.; Yoshida, M.; Ishigami, A. Protective effect of pre- and post-vitamin C treatments on UVB-irradiation-induced skin damage. Sci. Rep. 2018, 8, 16199. [Google Scholar] [CrossRef] [PubMed]
- Seok, J.K.; Lee, J.W.; Kim, Y.M.; Boo, Y.C. Punicalagin and (-)-Epigallocatechin-3-Gallate Rescue Cell Viability and Attenuate Inflammatory Responses of Human Epidermal Keratinocytes Exposed to Airborne Particulate Matter PM10. Skin Pharmacol. Physiol. 2018, 31, 134–143. [Google Scholar] [CrossRef]
- Lee, S.; Koh, J.-S.; Ha, B.-J.; Boo, Y. Quercus glauca extract and rutin inhibit the UVB-induced expression of matrix metalloproteinase-1 in human dermal fibroblasts. J. Korean Soc. Appl. Biol. Chem. 2010, 53, 677–684. [Google Scholar] [CrossRef]
- Lee, J.E.; Oh, J.; Song, D.; Lee, M.; Hahn, D.; Boo, Y.C.; Kang, N.J. Acetylated Resveratrol and Oxyresveratrol Suppress UVB-Induced MMP-1 Expression in Human Dermal Fibroblasts. Antioxidants 2021, 10, 1252. [Google Scholar] [CrossRef]
- Hantke, B.; Lahmann, C.; Venzke, K.; Fischer, T.; Kocourek, A.; Windsor, L.J.; Bergemann, J.; Stab, F.; Tschesche, H. Influence of flavonoids and vitamins on the MMP- and TIMP-expression of human dermal fibroblasts after UVA irradiation. Photochem. Photobiol. Sci. 2002, 1, 826–833. [Google Scholar] [CrossRef]
- Kim, S.; Kim, J.; Lee, Y.I.; Jang, S.; Song, S.Y.; Lee, W.J.; Lee, J.H. Particulate matter-induced atmospheric skin aging is aggravated by UVA and inhibited by a topical L-ascorbic acid compound. Photodermatol. Photoimmunol. Photomed. 2022, 38, 123–131. [Google Scholar] [CrossRef]
- Pandel, R.; Poljsak, B.; Godic, A.; Dahmane, R. Skin photoaging and the role of antioxidants in its prevention. ISRN Dermatol. 2013, 2013, 930164. [Google Scholar] [CrossRef]
- Fuchs, J.; Kern, H. Modulation of UV-light-induced skin inflammation by alpha-tocopherol and L-ascorbic acid: A clinical study using solar simulated radiation. Free Radic. Biol. Med. 1998, 25, 1006–1012. [Google Scholar] [CrossRef]
- Gianeti, M.D.; Gaspar, L.R.; de Camargo, F.B.; Campos, P.M.B.G.M. Benefits of Combinations of Vitamin A, C and E Derivatives in the Stability of Cosmetic Formulations. Molecules 2012, 17, 2219–2230. [Google Scholar] [CrossRef] [PubMed]
- Gegotek, A.; Ambrozewicz, E.; Jastrzab, A.; Jarocka-Karpowicz, I.; Skrzydlewska, E. Rutin and ascorbic acid cooperation in antioxidant and antiapoptotic effect on human skin keratinocytes and fibroblasts exposed to UVA and UVB radiation. Arch. Dermatol. Res. 2019, 311, 203–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boo, Y.C. Metabolic Basis and Clinical Evidence for Skin Lightening Effects of Thiol Compounds. Antioxidants 2022, 11, 503. [Google Scholar] [CrossRef]
- Lima, X.T.; Alora-Palli, M.B.; Beck, S.; Kimball, A.B. A double-blinded, randomized, controlled trial to quantitate photoprotective effects of an antioxidant combination product. J. Clin. Aesthet. Dermatol. 2012, 5, 29–32. [Google Scholar]
- Neves, J.R.; Grether-Beck, S.; Krutmann, J.; Correia, P.; Goncalves, J.E., Jr.; Sant’Anna, B.; Kerob, D. Efficacy of a topical serum containing L-ascorbic acid, neohesperidin, pycnogenol, tocopherol, and hyaluronic acid in relation to skin aging signs. J. Cosmet. Dermatol 2022. [Google Scholar] [CrossRef]
- Zhang, P.; Omaye, S.T. Antioxidant and prooxidant roles for beta-carotene, alpha-tocopherol and ascorbic acid in human lung cells. Toxicol. In Vitro 2001, 15, 13–24. [Google Scholar] [CrossRef]
- Zillich, O.V.; Schweiggert-Weisz, U.; Eisner, P.; Kerscher, M. Polyphenols as active ingredients for cosmetic products. Int. J. Cosmet. Sci. 2015, 37, 455–464. [Google Scholar] [CrossRef]
- Karna, E.; Szoka, L.; Huynh, T.Y.L.; Palka, J.A. Proline-dependent regulation of collagen metabolism. Cell Mol. Life Sci. 2020, 77, 1911–1918. [Google Scholar] [CrossRef]
- Bellon, G.; Monboisse, J.C.; Randoux, A.; Borel, J.P. Effects of Preformed Proline and Proline Amino-Acid Precursors (Including Glutamine) on Collagen-Synthesis in Human Fibroblast-Cultures. Biochim. Biophys. Acta 1987, 930, 39–47. [Google Scholar] [CrossRef]
- Kay, E.J.; Koulouras, G.; Zanivan, S. Regulation of Extracellular Matrix Production in Activated Fibroblasts: Roles of Amino Acid Metabolism in Collagen Synthesis. Front. Oncol. 2021, 11, 719922. [Google Scholar] [CrossRef] [PubMed]
- Krupsky, M.; Kuang, P.P.; Goldstein, R.H. Regulation of type I collagen mRNA by amino acid deprivation in human lung fibroblasts. J. Biol. Chem. 1997, 272, 13864–13868. [Google Scholar] [CrossRef] [PubMed]
- Karna, E.; Miltyk, W.; Wolczynski, S.; Palka, J.A. The potential mechanism for glutamine-induced collagen biosynthesis in cultured human skin fibroblasts. Comp. Biochem. Phys. B 2001, 130, 23–32. [Google Scholar] [CrossRef]
- Szoka, L.; Karna, E.; Hlebowicz-Sarat, K.; Karaszewski, J.; Palka, J.A. Exogenous proline stimulates type I collagen and HIF-1 alpha expression and the process is attenuated by glutamine in human skin fibroblasts. Mol. Cell. Biochem. 2017, 435, 197–206. [Google Scholar] [CrossRef] [Green Version]
- de Paz-Lugo, P.; Lupianez, J.A.; Melendez-Hevia, E. High glycine concentration increases collagen synthesis by articular chondrocytes in vitro: Acute glycine deficiency could be an important cause of osteoarthritis. Amino Acids 2018, 50, 1357–1365. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.E.; Boo, Y.C. Combination of Glycinamide and Ascorbic Acid Synergistically Promotes Collagen Production and Wound Healing in Human Dermal Fibroblasts. Biomedicines 2022, 10, 1029. [Google Scholar] [CrossRef] [PubMed]
- Kavishe, R.A.; Koenderink, J.B.; Alifrangis, M. Oxidative stress in malaria and artemisinin combination therapy: Pros and Cons. FEBS J. 2017, 284, 2579–2591. [Google Scholar] [CrossRef]
- Braccini, F.; Dohan Ehrenfest, D.M. Advantages of combined therapies in cosmetic medicine for the treatment of face aging: Botulinum toxin, fillers and mesotherapy. Rev. Laryngol. Otol. Rhinol. 2010, 131, 89–95. [Google Scholar]
- Pinnell, S.R.; Yang, H.; Omar, M.; Monteiro-Riviere, N.; DeBuys, H.V.; Walker, L.C.; Wang, Y.; Levine, M. Topical L-ascorbic acid: Percutaneous absorption studies. Dermatol. Surg. 2001, 27, 137–142. [Google Scholar] [CrossRef]
- Marosz, A.; Chlubek, D. The risk of abuse of vitamin supplements. Ann. Acad. Med. Stetin. 2014, 60, 60–64. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boo, Y.C. Ascorbic Acid (Vitamin C) as a Cosmeceutical to Increase Dermal Collagen for Skin Antiaging Purposes: Emerging Combination Therapies. Antioxidants 2022, 11, 1663. https://doi.org/10.3390/antiox11091663
Boo YC. Ascorbic Acid (Vitamin C) as a Cosmeceutical to Increase Dermal Collagen for Skin Antiaging Purposes: Emerging Combination Therapies. Antioxidants. 2022; 11(9):1663. https://doi.org/10.3390/antiox11091663
Chicago/Turabian StyleBoo, Yong Chool. 2022. "Ascorbic Acid (Vitamin C) as a Cosmeceutical to Increase Dermal Collagen for Skin Antiaging Purposes: Emerging Combination Therapies" Antioxidants 11, no. 9: 1663. https://doi.org/10.3390/antiox11091663





