Simultaneous Detection and Analysis of Free Amino Acids and Glutathione in Different Shrimp
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Instruments
2.2. Chromatographic Conditions
2.3. Preparation of Standard Stock Solution
2.4. Sample Preparation
2.5. Method Validation
2.6. Statistical Analysis
3. Results and Discussion
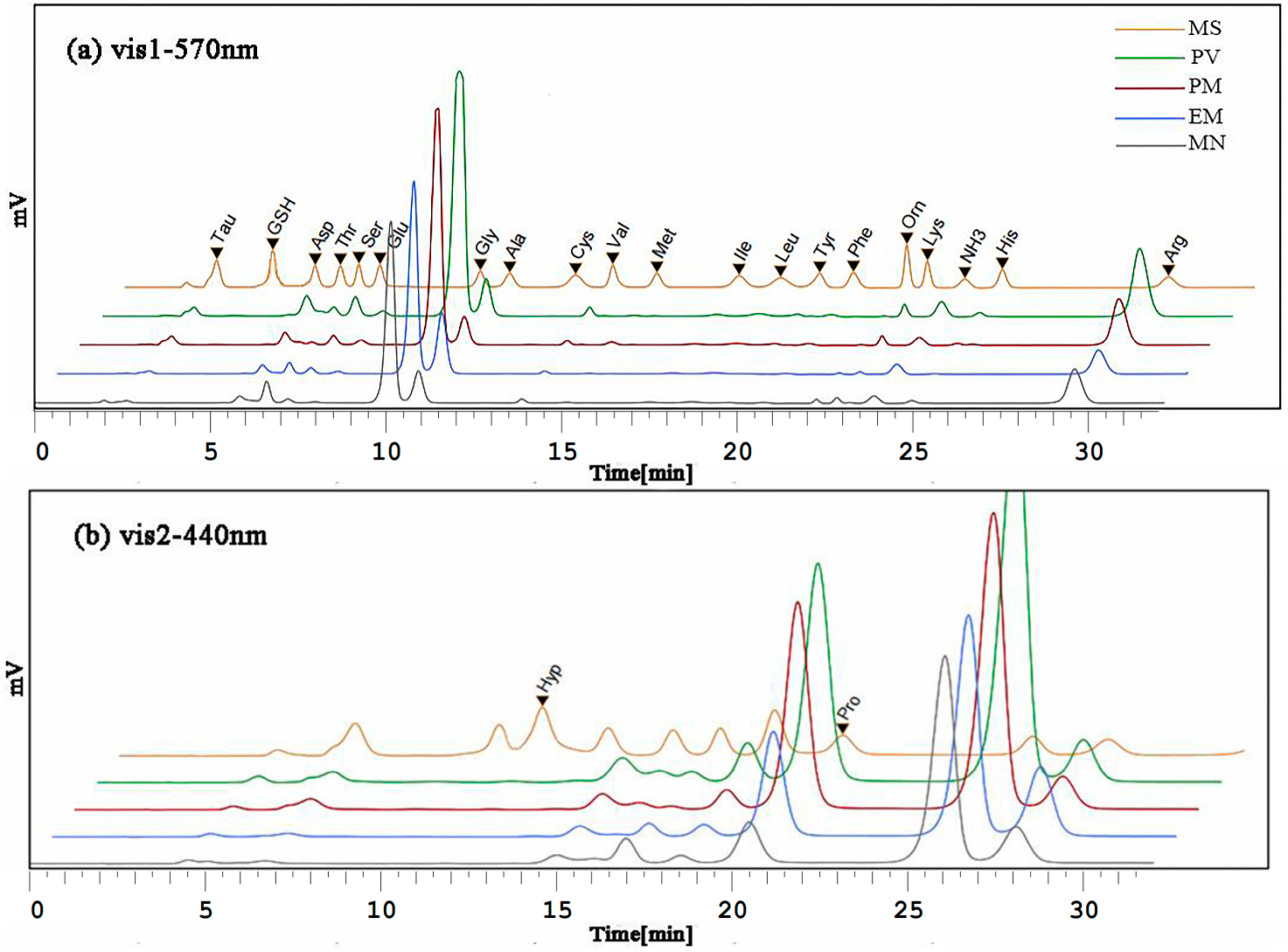
3.1. Optimization of Chromatographic Conditions
3.2. Selection of Extractant
3.3. Method Validation
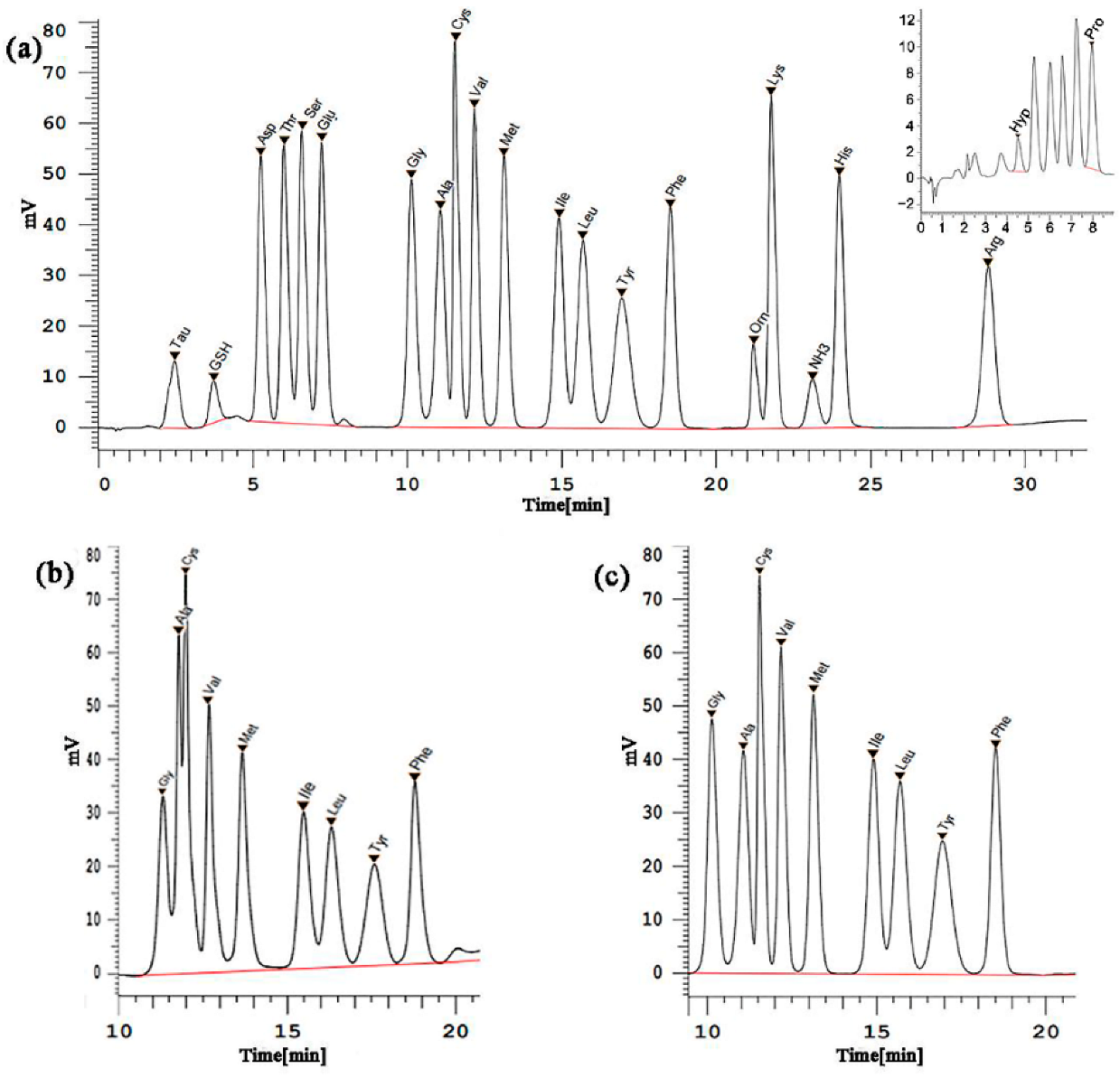
3.3.1. Mixed Standard Solution and Sample Chromatogram
3.3.2. Linearity and Detection Limit
3.3.3. Precision and Recovery
3.4. Analysis of FAAs and GSH in Different Shrimp
3.4.1. Analysis of FAAs Profile and GSH Content
3.4.2. Taste Effect and Taste Activity Value of Amino Acids
3.4.3. PCA and Comprehensive Evaluation of Different Species of Shrimp
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Calanche, J.; Tomas, A.; Martinez, S.; Jover, M.; Alonso, V.; Roncalés, P.; Beltrán, J.A. Relation of quality and sensory perception with changes in free amino acids of thawed seabream (Sparus aurata). Food Res. Int. 2019, 119, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Wang, A.-L.; Xian, J.-A. Variation of free amino acid and carbohydrate concentrations in white shrimp, Litopenaeus vannamei: Effects of continuous cold stress. Aquaculture 2011, 317, 182–186. [Google Scholar] [CrossRef]
- Chen, J.; Huang, X.; Zheng, J.; Sun, Y.; Dong, X.; Zhou, D.; Zhu, B.; Qin, L. Comprehensive metabolomic and lipidomic profiling of the seasonal variation of blue mussels (Mytilus edulis L.): Free amino acids, 5′-nucleotides, and lipids. LWT 2021, 149, 111835. [Google Scholar] [CrossRef]
- Jiang, W.; Wu, P.; Tang, R.; Liu, Y.; Kuang, S.; Jiang, J.; Tang, L.; Tang, W.; Zhang, Y.; Zhou, X.; et al. Nutritive values, flavor amino acids, healthcare fatty acids and flesh quality improved by manganese referring to up-regulating the antioxidant capacity and signaling molecules TOR and Nrf2 in the muscle of fish. Food Res. Int. 2016, 89, 670–678. [Google Scholar] [CrossRef] [PubMed]
- Araya, M.; García, S.; Rengel, J.; Pizarro, S.; Álvarez, G. Determination of free and protein amino acid content in microalgae by HPLC-DAD with pre-column derivatization and pressure hydrolysis. Mar. Chem. 2021, 234, 103999. [Google Scholar] [CrossRef]
- Chen, S.; Fu, Y.; Bian, X.; Zhao, M.; Zuo, Y.; Ge, Y.; Xiao, Y.; Xiao, J.; Li, N.; Wu, J.-L. Investigation and dynamic profiling of oligopeptides, free amino acids and derivatives during Pu-erh tea fermentation by ultra-high performance liquid chromatography tandem mass spectrometry. Food Chem. 2022, 371, 131176. [Google Scholar] [CrossRef]
- Li, J.; Zhao, A.; Li, D.; He, Y. Comparative study of the free amino acid compositions and contents in three different botanical origins of Coptis herb. Biochem. Syst. Ecol. 2019, 83, 117–120. [Google Scholar] [CrossRef]
- Łozowicka, B.; Kaczyński, P.; Iwaniuk, P. Analysis of 22 free amino acids in honey from Eastern Europe and Central Asia using LC-MS/MS technique without derivatization step. J. Food Compos. Anal. 2021, 98, 103837. [Google Scholar] [CrossRef]
- Zhou, C.; Le, Y.; Zheng, Y.; Wang, J.; Li, G.; Bai, Y.; Li, C.; Xu, X.; Zhou, G.; Cao, J. Characterizing the effect of free amino acids and volatile compounds on excessive bitterness and sourness in defective dry-cured ham. LWT 2020, 123, 109071. [Google Scholar] [CrossRef]
- Sukhovskaya, I.V.; Borvinskaya, E.V.; Smirnov, L.P.; Kochneva, A.A. Role of glutathione in functioning of the system of antioxidant protection in fish (review). Inland Water Biol. 2017, 10, 97–102. [Google Scholar] [CrossRef]
- Piechowiak, T. Effect of ozone treatment on glutathione (GSH) status in selected berry fruit. Phytochemistry 2021, 187, 112767. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Wang, H.; Chen, Y.; Ding, J.; Shan, G. Fluorescence sensing analysis for rapid detection of serum glutathione based on degrading AuNCs@Lys-MnO2 fluorescence resonance energy transfer system. Microchem. J. 2020, 159, 105556. [Google Scholar] [CrossRef]
- Huang, M.; Wang, Y.; Song, M.; Chen, F. Bovine serum albumin-encapsulated gold nanoclusters-Cu2+ synergize and promote calcein chemiluminescence for glutathione detection in human whole blood. Microchem. J. 2021, 170, 106749. [Google Scholar] [CrossRef]
- Davis, R.; Abebe, A.; Boyd, C.; McNevin, A. Exploring the relationship between production intensity and land use: A meta-analytic approach with shrimp aquaculture. J. Environ. Manag. 2021, 300, 113719. [Google Scholar] [CrossRef]
- Rahi, M.L.; Mahmud, S.; Dilruba, K.J.; Sabbir, W.; Aziz, D.; Hurwood, D.A. Temperature induced changes in physiological traits and expression of selected candidate genes in black tiger shrimp (Penaeus monodon) larvae. Aquac. Rep. 2021, 19, 100620. [Google Scholar] [CrossRef]
- Yuan, Y.; Luo, J.; Zhu, T.; Jin, M.; Jiao, L.; Sun, P.; Ward, T.L.; Ji, F.; Xu, G.; Zhou, Q. Alteration of growth performance, meat quality, antioxidant and immune capacity of juvenile Litopenaeus vannamei in response to different dietary dosage forms of zinc: Comparative advantages of zinc amino acid complex. Aquaculture 2020, 522, 735120. [Google Scholar] [CrossRef]
- Xiong, Y.; Li, Q.; Ding, Z.; Zheng, J.; Zhou, D.; Wei, S.; Han, X.; Cheng, X.; Li, X.; Xue, Y. Dietary α-lipoic acid requirement and its effects on antioxidant status, carbohydrate metabolism, and intestinal microflora in oriental river prawn Macrobrachium nipponense (De Haan). Aquaculture 2021, 547, 737531. [Google Scholar] [CrossRef]
- Zhang, Z.; Xiao, H.; Zhang, X.; Zhou, P. Conformation, allergenicity and human cell allergy sensitization of tropomyosin from Exopalaemon modestus: Effects of deglycosylation and Maillard reaction. Food Chem. 2019, 276, 520–527. [Google Scholar] [CrossRef]
- Baek, J.H.; Lee, S.Y.; Oh, S.W. Enhancing safety and quality of shrimp by nanoparticles of sodium alginate-based edible coating containing grapefruit seed extract. Int. J. Biol. Macromol. 2021, 189, 84–90. [Google Scholar] [CrossRef]
- Omar, M.M.A.; Elbashir, A.A.; Schmitz, O.J. Capillary electrophoresis method with UV-detection for analysis of free amino acids concentrations in food. Food Chem. 2017, 214, 300–307. [Google Scholar] [CrossRef]
- Mompó-Roselló, O.; Vergara-Barberán, M.; Simó-Alfonso, E.F.; Herrero-Martínez, J.M. In syringe hybrid monoliths modified with gold nanoparticles for selective extraction of glutathione in biological fluids prior to its determination by HPLC. Talanta 2020, 209, 120566. [Google Scholar] [CrossRef] [PubMed]
- Öztürk Er, E.; Özbek, B.; Bakırdere, S. Determination of seventeen free amino acids in human urine and plasma samples using quadruple isotope dilution mass spectrometry combined with hydrophilic interaction liquid chromatography—Tandem mass spectrometry. J. Chromatogr. A 2021, 1641, 461970. [Google Scholar] [CrossRef]
- Sun, X.; Heinrich, P.; Berger, R.S.; Oefner, P.J.; Dettmer, K. Quantification and 13C-Tracer analysis of total reduced glutathione by HPLC-QTOFMS/MS. Anal. Chim. Acta 2019, 1080, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Odani, S.; Koide, T.; Ono, T.; Aoyagi, Y. Analysis of strongly acidic amino acids by the conventional amino acid analyzer: Application to determination of protein-bound cysteine and glutathione. Anal. Biochem. 1988, 171, 305–309. [Google Scholar] [CrossRef]
- Smon, A.; Cuk, V.; Brecelj, J.; Murko, S.; Groselj, U.; Zerjav Tansek, M.; Battelino, T.; Repic Lampret, B. Comparison of liquid chromatography with tandem mass spectrometry and ion-exchange chromatography by post-column ninhydrin derivatization for amino acid monitoring. Clin. Chim. Acta 2019, 495, 446–450. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Ji, W.; Jiang, H.; Shi, Y.; He, L.; Gu, Z.; Zhu, S. Comparison of biochemical composition and non-volatile taste active compounds in raw, high hydrostatic pressure-treated and steamed oysters Crassostrea hongkongensis. Food Chem. 2021, 344, 128632. [Google Scholar] [CrossRef]
- Felisiak, K.; Szymczak, M.; Kołakowski, E. Identification of non-protein nitrogen compounds separated by CZE without derivatization from TCA extract from salted herring meat. J. Food Compos. Anal. 2019, 77, 108–114. [Google Scholar] [CrossRef]
- Yu, D.; Xu, Y.; Regenstein, J.M.; Xia, W.; Yang, F.; Jiang, Q.; Wang, B. The effects of edible chitosan-based coatings on flavor quality of raw grass carp (Ctenopharyngodon idellus) fillets during refrigerated storage. Food Chem. 2018, 242, 412–420. [Google Scholar] [CrossRef]
- Bowden, N.A.; Sevillano, D.M.; Sanders, J.P.M.; Bruins, M.E. Modelling the effects of ethanol on the solubility of the proteinogenic amino acids with the NRTL, Gude and Jouyban-Acree models. Fluid Phase Equilib. 2018, 459, 158–169. [Google Scholar] [CrossRef]
- Chilakala, S.; Mehtab, V.; Tallapally, M.; Vemula, M.; Shaikh, A.S.; Chenna, S.; Upadhyayula, V. SEC-MS/MS determination of amino acids from mango fruits and application of the method for studying amino acid perturbations due to post harvest ripening. LWT 2021, 138, 110680. [Google Scholar] [CrossRef]
- Chen, J.; Chen, J. Study on the free amino acid levels in the hemolymph, gill, hepatopancreas and muscle of Penaeus monodon exposed to elevated ambient ammonia. Aquat. Toxicol. 2000, 50, 27–37. [Google Scholar] [CrossRef]
- Cheng, X.; Li, M.; Leng, X.; Wen, H.; Wu, F.; Yu, L.; Jiang, M.; Lu, X.; Gao, W.; Zhang, W.; et al. Creatine improves the flesh quality of Pacific white shrimp (Litopenaeus vannamei) reared in freshwater. Food Chem. 2021, 354, 129498. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.-N.; Wang, A.-L.; Bao, L.; Wang, J.P.; Liu, Y.; Sun, R.-Y. Changes of protein-bound and free amino acids in the muscle of the freshwater prawn Macrobrachium nipponense in different salinities. Aquaculture 2004, 233, 561–571. [Google Scholar] [CrossRef]
- El-Sayed, A.-F.M. Is dietary taurine supplementation beneficial for farmed fish and shrimp? a comprehensive review. Rev. Aquac. 2015, 6, 241–255. [Google Scholar] [CrossRef]
- Wu, G.; Bazer, F.W.; Burghardt, R.C.; Johnson, G.A.; Kim, S.W.; Knabe, D.A.; Li, P.; Li, X.; McKnight, J.R.; Satterfield, M.C. Proline and hydroxyproline metabolism: Implications for animal and human nutrition. Amino Acids 2011, 40, 1053–1063. [Google Scholar] [CrossRef]
- Liu, Y.; He, G.; Wang, Q.; Mai, K.; Xu, W.; Zhou, H. Hydroxyproline supplementation on the performances of high plant protein source based diets in turbot (Scophthalmus maximus L.). Aquaculture 2014, 433, 476–480. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, M. Non-volatile taste active compounds in the meat of Chinese mitten crab (Eriocheir sinensis). Food Chem. 2007, 104, 1200–1205. [Google Scholar] [CrossRef]
- Liu, C.; Meng, F.; Tang, X.; Wang, A.; Zhi, P. Comparison of nonvolatile taste active compounds of wild and cultured mud crab Scylla paramamosain. Fish. Sci. 2018, 84, 897–907. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, W.; Xing, L. Effects of ultrasound on the taste components from aqueous extract of unsmoked bacon. Food Chem. 2021, 365, 130411. [Google Scholar] [CrossRef]
- Akitomi, H.; Tahara, Y.; Yasuura, M.; Kobayashi, Y.; Ikezaki, H.; Toko, K. Quantification of tastes of amino acids using taste sensors. Sens. Actuators B Chem. 2013, 179, 276–281. [Google Scholar] [CrossRef]


| Reagent | B1 | B2 | B3 | B4 | B5 |
|---|---|---|---|---|---|
| pH (nominal) | 3.3 | 3.2 | 4.0 | 4.9 | - |
| Sodium citrate·2H2O (mL) | 6.19 | 7.74 | 13.31 | 26.67 | - |
| NaOH (mL) | 7.00 | 15.00 | 4.00 | - | 8 g (solid) |
| NaCl (g) | 5.66 | 7.07 | 3.74 | 54.35 | - |
| Sodium citrate·H2O (mL) | 19.80 | 22.00 | 12.80 | 6.10 | - |
| Ethanol (mL) | 135.00 | 25.00 | 9.00 | - | 100.00 |
| Brij-35 (mL) | 4.00 | ||||
| Octoic acid (mL) | 0.10 | ||||
| Ultra-pure water constant Volume (L) | 1.00 | ||||
| Time (min) | B1 (%) | B2 (%) | B3 (%) | B4 (%) | B5 (%) | Separation Column Temperature (°C) | R1 (%) | R2 (%) | R3 (%) |
|---|---|---|---|---|---|---|---|---|---|
| 0 | 100 | 0 | 0 | 0 | 0 | 57 | 50 | 50 | 0 |
| 2.7 | 100 | 0 | 0 | 0 | 0 | - | - | - | |
| 2.8 | 0 | 100 | 0 | 0 | 0 | - | - | - | |
| 5.5 | 0 | 100 | 0 | 0 | 0 | - | - | - | |
| 5.6 | 0 | 0 | 100 | 0 | 0 | - | - | - | |
| 13.6 | 0 | 0 | 100 | 0 | 0 | - | - | - | |
| 13.7 | 0 | 0 | 0 | 100 | 0 | - | - | - | |
| 30.8 | 0 | 0 | 0 | 100 | 0 | - | - | - | |
| 30.9 | 0 | 0 | 0 | 0 | 100 | - | - | ||
| 33.7 | 0 | 0 | 0 | 0 | 100 | 50 | 50 | 0 | |
| 33.8 | 0 | 0 | 0 | 0 | 100 | 0 | 0 | 100 | |
| 34.0 | 0 | 0 | 0 | 0 | 100 | - | - | - | |
| 34.8 | 0 | 100 | 0 | 0 | 0 | - | - | - | |
| 35.9 | 0 | 100 | 0 | 0 | 0 | - | - | - | |
| 38.1 | 100 | 0 | 0 | 0 | 0 | - | - | - | |
| 39.0 | 100 | 0 | 0 | 0 | 0 | 0 | 0 | 100 | |
| 39.1 | 100 | 0 | 0 | 0 | 0 | 50 | 50 | 0 | |
| 55.0 | 100 | 0 | 0 | 0 | 0 | - | - | - |
| Analytes | Correlation Coefficient of Determination (R2) | Linear Range (µmol·L−1) | LOQ (µmol·L−1) | LOD (µmol·L−1) | Average Spike Recovery (%) | Precision (%RSD, n = 5) | |
|---|---|---|---|---|---|---|---|
| Intra-Day | Inter-Day | ||||||
| Tau | 0.9992 | 0.40~58.20 | 0.38 | 0.10 | 90.42 | 0.46 | 1.23 |
| GSH | 0.9994 | 0.35~30.21 | 0.30 | 0.12 | 88.26 | 0.51 | 1.82 |
| Asp | 0.9993 | 0.65~60.32 | 0.60 | 0.20 | 94.25 | 0.52 | 1.63 |
| Thr | 0.9997 | 0.75~64.72 | 0.52 | 0.22 | 95.38 | 0.53 | 1.68 |
| Ser | 0.9991 | 0.74~65.85 | 0.74 | 0.24 | 87.61 | 0.71 | 2.10 |
| Glu | 0.9999 | 0.86~68.92 | 0.83 | 0.23 | 92.33 | 0.71 | 2.35 |
| Gly | 0.9993 | 0.85~67.62 | 0.85 | 0.27 | 100.80 | 0.38 | 1.32 |
| Ala | 0.9997 | 0.91~78.64 | 0.91 | 0.25 | 87.31 | 0.36 | 1.26 |
| Cys | 0.9992 | 1.12~75.82 | 0.67 | 0.16 | 87.92 | 0.67 | 2.38 |
| Val | 0.9993 | 1.05~73.16 | 0.94 | 0.25 | 86.42 | 0.46 | 1.53 |
| Met | 0.9996 | 0.65~68.59 | 0.54 | 0.12 | 89.57 | 0.53 | 1.85 |
| Ile | 0.9994 | 0.85~81.57 | 0.76 | 0.23 | 99.25 | 0.62 | 2.10 |
| Leu | 0.9992 | 0.72~83.24 | 0.60 | 0.13 | 102.33 | 0.31 | 1.63 |
| Tyr | 0.9996 | 0.54~63.84 | 0.21 | 0.07 | 95.79 | 0.56 | 1.94 |
| Phe | 0.9993 | 0.61~68.43 | 0.43 | 0.16 | 103.64 | 0.61 | 2.32 |
| Orn | 0.9991 | 0.52~56.16 | 0.52 | 0.19 | 95.10 | 0.37 | 1.74 |
| Lys | 0.9993 | 0.47~55.63 | 0.47 | 0.12 | 89.32 | 0.46 | 1.83 |
| His | 0.9998 | 0.84~68.37 | 0.80 | 0.27 | 87.67 | 0.35 | 1.82 |
| Arg | 0.9997 | 0.82~88.49 | 0.65 | 0.19 | 87.56 | 0.39 | 1.42 |
| Hyp | 0.9994 | 0.80~79.65 | 0.72 | 0.18 | 99.51 | 0.52 | 1.82 |
| Pro | 0.9992 | 2.40~98.36 | 2.40 | 0.73 | 92.19 | 0.62 | 2.01 |
| Component | PV | PM | EM | MN | |||||
|---|---|---|---|---|---|---|---|---|---|
| Content (mg/100 g) | Proportion/% | Content (mg/100 g) | Proportion/% | Content (mg/100 g) | Proportion/% | Content (mg/100 g) | Proportion/% | ||
| 1 | Tau | 67.63 ± 0.35 a | 1.60 | 34.36 ± 2.39 b | 1.02 | 8.77 ± 0.88 c | 0.39 | 11.22 ± 0.13 c | 0.50 |
| 2 | GSH | 13.76 ± 0.14 a | - | 9.54 ± 0.27 b | - | ND | - | ND | - |
| 3 | Asp | 7.37 ± 0.07 a | 0.17 | 2.42 ± 0.13 b | 0.07 | 2.18 ± 0.03 c | 0.09 | ND | - |
| 4 | Thr ▲ | 158.92 ± 1.58 a | 3.76 | 103.2 ± 6.78 b | 3.08 | 57.52 ± 0.88 d | 2.60 | 65.19 ± 0.18 c | 2.92 |
| 5 | Ser | 38.41 ± 0.38 c | 0.90 | 11.59 ± 0.65 d | 0.34 | 45.49 ± 0.85 b | 2.05 | 103.33 ± 0.76 a | 4.66 |
| 6 | Glu | 106.59 ± 0.95 a | 2.52 | 55.01 ± 3.66 b | 1.64 | 35.79 ± 0.56 c | 1.62 | 25.34 ± 0.16 d | 1.14 |
| 7 | Gly | 1479.21 ± 13.37 a | 35.01 | 1158.97 ± 72.05 b | 34.64 | 821.39 ± 14.24 d | 37.18 | 900.71 ± 6.83 c | 40.62 |
| 8 | Ala | 195.12 ± 1.58 c | 4.61 | 143.99 ± 9.03 d | 4.30 | 327.47 ± 6.1 a | 14.82 | 216.19 ± 1.2 b | 9.73 |
| 9 | Cys | 27.44 ± 2.79 a | 0.64 | 5.08 ± 0.27 a | 0.15 | ND | - | ND | - |
| 10 | Val ▲ | 36.64 ± 0.38 a | 0.86 | 20.43 ± 1.34 b | 0.61 | 13.08 ± 0.36 c | 0.59 | 19.67 ± 0.29 b | 0.89 |
| 11 | Met ▲ | 7.07 ± 0.13 b | 0.16 | 21.31 ± 1.51 a | 0.63 | 5.55 ± 0.34 b | 0.25 | 6.39 ± 0.22 b | 0.29 |
| 12 | Ile ▲ | 15.41 ± 0.29 a | 0.36 | 9.38 ± 0.64 b | 0.28 | 7.58 ± 0.19 c | 0.34 | 8.22 ± 0.18 c | 0.37 |
| 13 | Leu ▲ | 25.85 ± 0.22 a | 0.61 | 16.4 ± 1.09 b | 0.49 | 12.22 ± 0.44 d | 0.55 | 13.54 ± 0.23 c | 0.61 |
| 14 | Tyr | 23.51 ± 0.25 a | 0.55 | 15.54 ± 0.71 b | 0.46 | 3.99 ± 0.22 c | 0.18 | 8.96 ± 0.22 d | 0.41 |
| 15 | Phe ▲ | 18.3 ± 0.36 a | 0.43 | 12.89 ± 0.83 b | 0.38 | 5.62 ± 0.24 c | 0.25 | 5.55 ± 0.22 c | 0.25 |
| 16 | Orn | 4.87 ± 0.03 c | 0.11 | 4.79 ± 0.18 c | 0.14 | 6.99 ± 0.09 b | 0.31 | 14.8 ± 0.05 a | 0.66 |
| 17 | Lys ▲ | 48.26 ± 0.53 a | 1.14 | 38.12 ± 2.62 b | 1.13 | 9.98 ± 0.11 d | 0.45 | 30.02 ± 0.18 c | 1.35 |
| 18 | His | 22.92 ± 0.17 a | 0.54 | 10.5 ± 0.69 c | 0.31 | 3.98 ± 0.03 d | 0.18 | 21.73 ± 0.02 b | 0.97 |
| 19 | Arg | 880.04 ± 8.46 a | 20.83 | 628.18 ± 42.91 b | 18.77 | 315.03 ± 4.93 d | 14.26 | 533.58 ± 4.63 c | 24.09 |
| 20 | Hyp | 11.51 ± 1.61 a | 0.27 | ND | - | ND | - | ND | - |
| 21 | Pro | 1076.86 ± 10.47 a | 25.49 | 1053.77 ± 66.8 a | 31.49 | 526.14 ± 9.6 b | 23.81 | 231.4 ± 2.22 c | 10.45 |
| EAAs | 310.49 ± 3.28 a | 7.34 | 221.75 ± 14.84 b | 6.62 | 111.58 ± 2.59 d | 5.05 | 148.61 ± 1.53 c | 6.72 | |
| TFAAs | 4250.95 ± 36.96 a | 100 | 3346.00 ± 214.36 b | 100 | 2208.88 ± 38.41 c | 100 | 2215.95 ± 17.8 c | 100 | |
| FAAs | Taste Attribute | Taste Threshold (mg/100 g) | TAV | |||
|---|---|---|---|---|---|---|
| PV | PM | EM | MN | |||
| Asp | Umami (+) | 100 | 0.08 | 0.03 | 0.03 | ND |
| Glu | Umami (+) | 30 | 3.56 | 1.84 | 1.20 | 0.85 |
| Thr | Sweet (+) | 260 | 0.62 | 0.40 | 0.23 | 0.26 |
| Ser | Sweet (+) | 150 | 0.26 | 0.08 | 0.31 | 0.69 |
| Gly | Sweet (+) | 130 | 11.38 | 8.92 | 6.32 | 6.93 |
| Ala | Sweet (+) | 60 | 3.26 | 2.40 | 5.46 | 3.61 |
| Arg | Bitter/sweet (+) | 50 | 17.61 | 12.57 | 6.31 | 10.68 |
| Pro | Sweet/bitter (+) | 300 | 3.59 | 3.52 | 1.76 | 0.78 |
| Val | Sweet/bitter (−) | 40 | 0.92 | 0.52 | 0.33 | 0.50 |
| Met | Bitter/sweet/sulfurous (−) | 30 | 0.24 | 0.72 | 0.19 | 0.22 |
| Ile | Bitter (−) | 90 | 0.18 | 0.11 | 0.09 | 0.10 |
| Leu | Bitter (−) | 190 | 0.14 | 0.09 | 0.07 | 0.08 |
| Phe | Bitter (−) | 90 | 0.21 | 0.15 | 0.07 | 0.07 |
| Lys | Sweet/bitter (−) | 50 | 0.97 | 0.77 | 0.20 | 0.61 |
| His | Bitter (−) | 20 | 1.15 | 0.53 | 0.20 | 1.09 |
| Class | F1 | F2 | F | Order |
|---|---|---|---|---|
| PV | 2.29 | 1.20 | 2.14 | 1 |
| PM | 0.96 | −0.40 | 0.80 | 2 |
| EM | −1.67 | −1.24 | −1.60 | 4 |
| MN | −1.59 | 0.44 | −1.34 | 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, Y.; Xu, M.; Jin, Y.; Deng, S.; Tao, N.; Qiu, W. Simultaneous Detection and Analysis of Free Amino Acids and Glutathione in Different Shrimp. Foods 2022, 11, 2599. https://doi.org/10.3390/foods11172599
Jin Y, Xu M, Jin Y, Deng S, Tao N, Qiu W. Simultaneous Detection and Analysis of Free Amino Acids and Glutathione in Different Shrimp. Foods. 2022; 11(17):2599. https://doi.org/10.3390/foods11172599
Chicago/Turabian StyleJin, Yinzhe, Minhua Xu, Yingshan Jin, Shanggui Deng, Ningping Tao, and Weiqiang Qiu. 2022. "Simultaneous Detection and Analysis of Free Amino Acids and Glutathione in Different Shrimp" Foods 11, no. 17: 2599. https://doi.org/10.3390/foods11172599
APA StyleJin, Y., Xu, M., Jin, Y., Deng, S., Tao, N., & Qiu, W. (2022). Simultaneous Detection and Analysis of Free Amino Acids and Glutathione in Different Shrimp. Foods, 11(17), 2599. https://doi.org/10.3390/foods11172599