Immunomodulatory Mechanisms of Mesenchymal Stem Cells and Their Potential Clinical Applications
Abstract
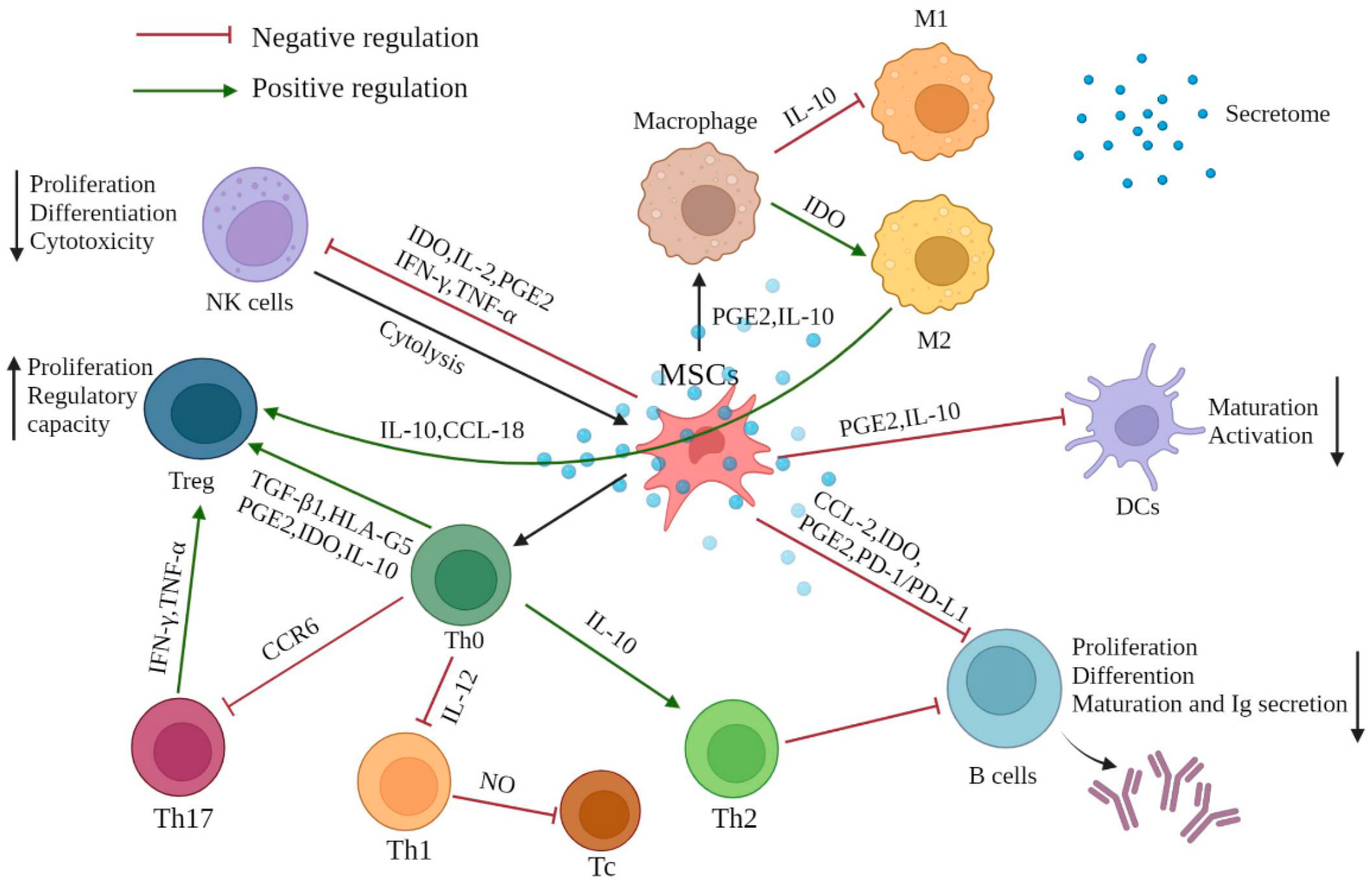
:1. Introduction
2. Immunomodulatory Effects of MSCs
2.1. Interaction of MSCs with Innate Immune Cells
2.1.1. Natural Killer Cells
2.1.2. Macrophages
2.1.3. Dendritic Cells
2.2. Interaction of MSCs with Adaptive Immune Cells
2.2.1. T Cells
2.2.2. B Cells
2.3. Preconditioning of Pro-Inflammatory Factors
2.4. Gene Engineered MSCs
3. Potential Clinical Applications of MSC-Mediated Immunomodulation
3.1. Systemic Lupus Erythematosus
3.2. Crohn’s Disease
3.3. Graft-versus-Host Disease
3.4. COVID-19 Pandemic
4. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Caplan, A.I. Mesenchymal stem cells. J. Orthop. Res. 1991, 9, 641–650. [Google Scholar] [CrossRef]
- Mahla, R.S. Stem Cells Applications in Regenerative Medicine and Disease Therapeutics. Int. J. Cell Biol. 2016, 2016, 6940283. [Google Scholar] [CrossRef]
- Ankrum, J.A.; Ong, J.F.; Karp, J.M. Mesenchymal stem cells: Immune evasive, not immune privileged. Nat. Biotechnol. 2014, 32, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Altemus, J.; Lightner, A.L. Mesenchymal stem cells and acellular products attenuate murine induced colitis. Stem Cell Res. Ther. 2020, 11, 515. [Google Scholar] [CrossRef] [PubMed]
- Golchin, A.; Seyedjafari, E.; Ardeshirylajimi, A. Mesenchymal Stem Cell Therapy for COVID-19: Present or Future. Stem Cell Rev. Rep. 2020, 16, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Sémont, A.; Mouiseddine, M.; François, A.; Demarquay, C.; Mathieu, N.; Chapel, A.; Saché, A.; Thierry, D.; Laloi, P.; Gourmelon, P. Mesenchymal stem cells improve small intestinal integrity through regulation of endogenous epithelial cell homeostasis. Cell Death Differ. 2010, 17, 952–961. [Google Scholar] [CrossRef]
- Ko, S.-F.; Yip, H.-K.; Zhen, Y.-Y.; Lee, C.-C.; Lee, C.-C.; Huang, C.-C.; Ng, S.-H.; Lin, J.-W. Adipose-Derived Mesenchymal Stem Cell Exosomes Suppress Hepatocellular Carcinoma Growth in a Rat Model: Apparent Diffusion Coefficient, Natural Killer T-Cell Responses, and Histopathological Features. Stem Cells Int. 2015, 2015, 853506. [Google Scholar] [CrossRef]
- Sotiropoulou, P.A.; Perez, S.A.; Gritzapis, A.D.; Baxevanis, C.N.; Papamichail, M. Interactions between human mesenchymal stem cells and natural killer cells. Stem Cells 2006, 24, 74–85. [Google Scholar] [CrossRef]
- Boissel, L.; Tuncer, H.H.; Betancur, M.; Wolfberg, A.; Klingemann, H. Umbilical Cord Mesenchymal Stem Cells Increase Expansion of Cord Blood Natural Killer Cells. Biol. Blood Marrow Transplant. 2008, 14, 1031–1038. [Google Scholar] [CrossRef]
- Moretta, A. Natural killer cells and dendritic cells: Rendezvous in abused tissues. Nat. Rev. Immunol. 2002, 2, 957–965. [Google Scholar] [CrossRef]
- Spaggiari, G.M.; Capobianco, A.; Becchetti, S.; Mingari, M.C.; Moretta, L. Mesenchymal stem cell-natural killer cell interactions: Evidence that activated NK cells are capable of killing MSCs, whereas MSCs can inhibit IL-2-induced NK-cell proliferation. Blood 2006, 107, 1484–1490. [Google Scholar] [CrossRef]
- Rasmusson, I.; Ringdén, O.; Sundberg, B.; Le Blanc, K. Mesenchymal stem cells inhibit the formation of cytotoxic T lymphocytes, but not activated cytotoxic T lymphocytes or natural killer cells. Transplantation 2003, 76, 1208–1213. [Google Scholar] [CrossRef]
- Götherström, C.; Lundqvist, A.; Duprez, I.R.; Childs, R.; Berg, L.; le Blanc, K. Fetal and adult multipotent mesenchymal stromal cells are killed by different pathways. Cytotherapy 2011, 13, 269–278. [Google Scholar] [CrossRef]
- An, J.H.; Li, Q.; Bhang, D.H.; Song, W.J.; Youn, H.Y. TNF-α and INF-γ primed canine stem cell-derived extracellular vesicles alleviate experimental murine colitis. Sci. Rep. 2020, 10, 2115. [Google Scholar] [CrossRef]
- Kawata, Y.; Tsuchiya, A.; Seino, S.; Watanabe, Y.; Kojima, Y.; Ikarashi, S.; Tominaga, K.; Yokoyama, J.; Yamagiwa, S.; Terai, S. Early injection of human adipose tissue-derived mesenchymal stem cell after inflammation ameliorates dextran sulfate sodium-induced colitis in mice through the induction of M2 macrophages and regulatory T cells. Cell Tissue Res. 2019, 376, 257–271. [Google Scholar] [CrossRef]
- Cao, X.; Duan, L.; Hou, H.; Liu, Y.; Chen, S.; Zhang, S.; Liu, Y.; Wang, C.; Qi, X.; Liu, N.; et al. IGF-1C hydrogel improves the therapeutic effects of MSCs on colitis in mice through PGE2-mediated M2 macrophage polarization. Theranostics 2020, 10, 7697–7709. [Google Scholar] [CrossRef]
- Zhang, Q.-Z.; Su, W.-R.; Shi, S.-H.; Wilder-Smith, P.; Xiang, A.P.; Wong, A.; Nguyen, A.L.; Kwon, C.W.; Le, A.D. Human Gingiva-Derived Mesenchymal Stem Cells Elicit Polarization of M2 Macrophages and Enhance Cutaneous Wound Healing. Stem Cells 2010, 28, 1856–1868. [Google Scholar] [CrossRef]
- Németh, K.; Leelahavanichkul, A.; Yuen, P.S.; Mayer, B.; Parmelee, A.; Doi, K.; Robey, P.G.; Leelahavanichkul, K.; Koller, B.H.; Brown, J.M.; et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E2–dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat. Med. 2009, 15, 42–49. [Google Scholar] [CrossRef]
- Choi, H.; Lee, R.H.; Bazhanov, N.; Oh, J.Y.; Prockop, D.J. Anti-inflammatory protein TSG-6 secreted by activated MSCs attenuates zymosan-induced mouse peritonitis by decreasing TLR2/NF-κB signaling in resident macrophages. Blood 2011, 118, 330–338. [Google Scholar] [CrossRef]
- Zhao, J.; Li, X.; Hu, J.; Chen, F.; Qiao, S.; Sun, X.; Gao, L.; Xie, J.; Xu, B. Mesenchymal stromal cell-derived exosomes attenuate myocardial ischaemia-reperfusion injury through miR-182-regulated macrophage polarization. Cardiovasc. Res. 2019, 115, 1205–1216. [Google Scholar] [CrossRef] [Green Version]
- Abbasi-Kenarsari, H.; Heidari, N.; Baghaei, K.; Amani, D.; Zali, M.R.; Khaligh, S.G.; Shafiee, A.; Hashemi, S.M. Synergistic therapeutic effect of mesenchymal stem cells and tolerogenic dendritic cells in an acute colitis mouse model. Int. Immunopharmacol. 2020, 88, 107006. [Google Scholar] [CrossRef]
- Ramasamy, R.; Fazekasova, H.; Lam, E.W.F.; Soeiro, I.; Lombardi, G.; Dazzi, F. Mesenchymal Stem Cells Inhibit Dendritic Cell Differentiation and Function by Preventing Entry into the Cell Cycle. Transplantation 2007, 83, 71–76. [Google Scholar] [CrossRef]
- Li, Y.-P.; Paczesny, S.; Lauret, E.; Poirault, S.; Bordigoni, P.; Mekhloufi, F.; Hequet, O.; Bertrand, Y.; Ou-Yang, J.-P.; Stoltz, J.-F.; et al. Human Mesenchymal Stem Cells License Adult CD34+ Hemopoietic Progenitor Cells to Differentiate into Regulatory Dendritic Cells through Activation of the Notch Pathway. J. Immunol. 2008, 180, 1598–1608. [Google Scholar] [CrossRef]
- Chiesa, S.; Morbelli, S.; Morando, S.; Massollo, M.; Marini, C.; Bertoni, A.; Frassoni, F.; Bartolomé, S.T.; Sambuceti, G.; Traggiai, E.; et al. Mesenchymal stem cells impair in vivo T-cell priming by dendritic cells. Proc. Natl. Acad. Sci. USA 2011, 108, 17384–17389. [Google Scholar] [CrossRef]
- Liu, X.; Qu, X.; Chen, Y.; Liao, L.; Cheng, K.; Shao, C.; Zenke, M.; Keating, A.; Zhao, R.C. Mesenchymal Stem/Stromal Cells Induce the Generation of Novel IL-10–Dependent Regulatory Dendritic Cells by SOCS3 Activation. J. Immunol. 2012, 189, 1182–1192. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Hao, Z.; Du, J.; Gao, Y.; Yang, S.; Zhou, Y. Bacteroides thetaiotaomicron relieves colon inflammation by activating aryl hydrocarbon receptor and modulating CD4+T cell homeostasis. Int. Immunopharmacol. 2021, 90, 107183. [Google Scholar] [CrossRef]
- Di Nicola, M.; Carlo-Stella, C.; Magni, M.; Milanesi, M.; Longoni, P.D.; Matteucci, P.; Grisanti, S.; Gianni, A.M. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood 2002, 99, 3838–3843. [Google Scholar] [CrossRef] [PubMed]
- Glennie, S.; Soeiro, I.; Dyson, P.J.; Lam, E.W.F.; Dazzi, F.; Lutsiak, M.E.C.; Semnani, R.T.; De Pascalis, R.; Kashmiri, S.V.S.; Schlom, J.; et al. Bone marrow mesenchymal stem cells induce division arrest anergy of activated T cells. Blood 2005, 105, 2821–2827. [Google Scholar] [CrossRef] [PubMed]
- Maccario, R.; Podestà, M.; Moretta, A.; Cometa, A.; Comoli, P.; Montagna, D.; Daudt, L.E.; Ibatici, A.; Piaggio, G.; Pozzi, S.; et al. Interaction of human mesenchymal stem cells with cells involved in alloantigen-specific immune response favors the differentiation of CD4+ T-cell subsets expressing a regulatory/suppressive phenotype. Haematologica 2005, 90, 516–525. [Google Scholar]
- Tse, W.T.; Pendleton, J.D.; Beyer, W.M.; Egalka, M.C.; Guinan, E.C. Suppression of allogeneic T-cell proliferation by human marrow stromal cells: Implications in transplantation. Transplantation 2003, 75, 389–397. [Google Scholar] [CrossRef]
- Majumdar, M.K.; Keane-Moore, M.; Buyaner, D.; Hardy, W.B.; Moorman, M.A.; McIntosh, K.R.; Mosca, J.D. Characterization and functionality of cell surface molecules on human mesenchymal stem cells. J. Biomed. Sci. 2003, 10, 228–241. [Google Scholar] [CrossRef]
- Ren, G.; Zhang, L.; Zhao, X.; Xu, G.; Zhang, Y.; Roberts, A.I.; Zhao, R.C.; Shi, Y. Mesenchymal Stem Cell-Mediated Immunosuppression Occurs via Concerted Action of Chemokines and Nitric Oxide. Cell Stem Cell 2008, 2, 141–150. [Google Scholar] [CrossRef]
- Ren, G.; Zhao, X.; Zhang, L.; Zhang, J.; L’Huillier, A.; Ling, W.; Roberts, A.I.; Le, A.D.; Shi, S.; Shao, C.; et al. Inflammatory Cytokine-Induced Intercellular Adhesion Molecule-1 and Vascular Cell Adhesion Molecule-1 in Mesenchymal Stem Cells Are Critical for Immunosuppression. J. Immunol. 2010, 184, 2321–2328. [Google Scholar] [CrossRef]
- Gieseke, F.; Kruchen, A.; Tzaribachev, N.; Bentzien, F.; Dominici, M.; Müller, I. Proinflammatory stimuli induce galectin-9 in human mesenchymal stromal cells to suppress T-cell proliferation. Eur. J. Immunol. 2013, 43, 2741–2749. [Google Scholar] [CrossRef]
- Sakaguchi, S.; Yamaguchi, T.; Nomura, T.; Ono, M. Regulatory T Cells and Immune Tolerance. Cell 2008, 133, 775–787. [Google Scholar] [CrossRef]
- Di Ianni, M.; Del Papa, B.; De Ioanni, M.; Moretti, L.; Bonifacio, E.; Cecchini, D.; Sportoletti, P.; Falzetti, F.; Tabilio, A. Mesenchymal cells recruit and regulate T regulatory cells. Exp. Hematol. 2008, 36, 309–318. [Google Scholar] [CrossRef]
- Melief, S.M.; Schrama, E.; Brugman, M.H.; Tiemessen, M.M.; Hoogduijn, M.J.; Fibbe, W.E.; Roelofs, H. Multipotent stromal cells induce human regulatory T cells through a novel pathway involving skewing of monocytes toward anti-inflammatory macrophages. Stem Cells 2013, 31, 1980–1991. [Google Scholar] [CrossRef]
- Saldanha-Araujo, F.; Haddad, R.; Farias, K.C.; Souza Ade, P.; Palma, P.V.; Araujo, A.G.; Orellana, M.D.; Voltarelli, J.C.; Covas, D.T.; Zago, M.A.; et al. Mesenchymal stem cells promote the sustained expression of CD69 on activated T lymphocytes: Roles of canonical and non-canonical NF-κB signalling. J. Cell Mol. Med. 2012, 16, 1232–1244. [Google Scholar] [CrossRef]
- Duffy, M.M.; Pindjakova, J.; Hanley, S.A.; McCarthy, C.; Weidhofer, G.A.; Sweeney, E.M.; English, K.; Shaw, G.; Murphy, J.M.; Barry, F.P.; et al. Mesenchymal stem cell inhibition of T-helper 17 cell- differentiation is triggered by cell-cell contact and mediated by prostaglandin E2 via the EP4 receptor. Eur. J. Immunol. 2011, 41, 2840–2851. [Google Scholar] [CrossRef]
- Latella, G.; Viscido, A. Controversial Contribution of Th17/IL-17 Toward the Immune Response in Intestinal Fibrosis. Dig. Dis. Sci. 2020, 65, 1299–1306. [Google Scholar] [CrossRef]
- Luz-Crawford, P.; Djouad, F.; Toupet, K.; Bony, C.; Franquesa, M.; Hoogduijn, M.J.; Jorgensen, C.; Noël, D. Mesenchymal Stem Cell-Derived Interleukin 1 Receptor Antagonist Promotes Macrophage Polarization and Inhibits B Cell Differentiation. Stem Cells 2016, 34, 483–492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rafei, M.; Hsieh, J.; Fortier, S.; Li, M.; Yuan, S.; Birman, E.; Forner, K.; Boivin, M.-N.; Doody, K.; Tremblay, M.; et al. Mesenchymal stromal cell–derived CCL2 suppresses plasma cell immunoglobulin production via STAT3 inactivation and PAX5 induction. Blood 2008, 112, 4991–4998. [Google Scholar] [CrossRef] [PubMed]
- Corcione, A.; Benvenuto, F.; Ferretti, E.; Giunti, D.; Cappiello, V.; Cazzanti, F.; Risso, M.; Gualandi, F.; Mancardi, G.L.; Pistoia, V.; et al. Human mesenchymal stem cells modulate B-cell functions. Blood 2006, 107, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Augello, A.; Tasso, R.; Negrini, S.M.; Amateis, A.; Indiveri, F.; Cancedda, R.; Pennesi, G. Bone marrow mesenchymal progenitor cells inhibit lymphocyte proliferation by activation of the programmed death 1 pathway. Eur. J. Immunol. 2005, 35, 1482–1490. [Google Scholar] [CrossRef] [PubMed]
- Schena, F.; Gambini, C.; Gregorio, A.; Mosconi, M.; Reverberi, D.; Gattorno, M.; Casazza, S.; Uccelli, A.; Moretta, L.; Martini, A.; et al. Interferon-γ-dependent inhibition of B cell activation by bone marrow-derived mesenchymal stem cells in a murine model of systemic lupus erythematosus. Arthritis Rheumatol. 2010, 62, 2776–2786. [Google Scholar] [CrossRef] [PubMed]
- Gerdoni, E.; Gallo, B.; Casazza, S.; Musio, S.; Bonanni, I.; Pedemonte, E.; Mantegazza, R.; Frassoni, F.; Mancardi, G.; Pedotti, R.; et al. Mesenchymal stem cells effectively modulate pathogenic immune response in experimental autoimmune encephalomyelitis. Ann. Neurol. 2007, 61, 219–227. [Google Scholar] [CrossRef]
- Inoue, S.; Popp, F.C.; Koehl, G.E.; Piso, P.; Schlitt, H.J.; Geissler, E.K.; Dahlke, M.H. Immunomodulatory Effects of Mesenchymal Stem Cells in a Rat Organ Transplant Model. Transplantation 2006, 81, 1589–1595. [Google Scholar] [CrossRef]
- Rosado, M.M.; Bernardo, M.E.; Scarsella, M.; Conforti, A.; Giorda, E.; Biagini, S.; Cascioli, S.; Rossi, F.; Guzzo, I.; Vivarelli, M.; et al. Inhibition of B-Cell Proliferation and Antibody Production by Mesenchymal Stromal Cells Is Mediated by T Cells. Stem Cells Dev. 2015, 24, 93–103. [Google Scholar] [CrossRef]
- Kim, D.S.; Jang, I.K.; Lee, M.W.; Ko, Y.J.; Lee, D.H.; Lee, J.W.; Sung, K.W.; Koo, H.H.; Yoo, K.H. Enhanced Immunosuppressive Properties of Human Mesenchymal Stem Cells Primed by Interferon-γ. EBioMedicine 2018, 28, 261–273. [Google Scholar] [CrossRef]
- Groh, M.E.; Maitra, B.; Szekely, E.; Koç, O.N. Human mesenchymal stem cells require monocyte-mediated activation to suppress alloreactive T cells. Exp. Hematol. 2005, 33, 928–934. [Google Scholar] [CrossRef]
- Boland, L.; Burand, A.J.; Brown, A.J.; Boyt, D.; Lira, V.A.; Ankrum, J.A. IFN-γ and TNF-α Pre-licensing Protects Mesenchymal Stromal Cells from the Pro-inflammatory Effects of Palmitate. Mol. Ther. 2018, 26, 860–873. [Google Scholar] [CrossRef] [Green Version]
- Noronha, N.C.; Mizukami, A.; Caliari-Oliveira, C.; Cominal, J.G.; Rocha, J.L.M.; Covas, D.T.; Swiech, K.; Malmegrim, K.C.R. Priming approaches to improve the efficacy of mesenchymal stromal cell-based therapies. Stem Cell Res. Ther. 2019, 10, 131. [Google Scholar] [CrossRef]
- Mei, S.H.J.; McCarter, S.D.; Deng, Y.; Parker, C.H.; Liles, W.C.; Stewart, D.J. Prevention of LPS-Induced Acute Lung Injury in Mice by Mesenchymal Stem Cells Overexpressing Angiopoietin 1. PLoS Med. 2007, 4, e269. [Google Scholar] [CrossRef]
- Florian, M.; Wang, J.-P.; Deng, Y.; Souza-Moreira, L.; Stewart, D.J.; Mei, S.H.J. Gene engineered mesenchymal stem cells: Greater transgene expression and efficacy with minicircle vs. plasmid DNA vectors in a mouse model of acute lung injury. Stem Cell Res. Ther. 2021, 12, 184. [Google Scholar] [CrossRef]
- Shu, P.; Sun, D.L.; Shu, Z.X.; Tian, S.; Pan, Q.; Wen, M.C.; Xi, J.Y.; Ye, S.N. Therapeutic Applications of Genes and Gene-Engineered Mesenchymal Stem Cells for Femoral Head Necrosis. Hum. Gene Ther. 2020, 31, 286–296. [Google Scholar] [CrossRef]
- Serra, J.; Alves, C.P.A.; Brito, L.; Monteiro, G.A.; Cabral, J.M.; Prazeres, D.M.F.; Da Silva, C.L. Engineering of Human Mesenchymal Stem/Stromal Cells with Vascular Endothelial Growth Factor–Encoding Minicircles for Angiogenic Ex Vivo Gene Therapy. Hum. Gene Ther. 2019, 30, 316–329. [Google Scholar] [CrossRef]
- Pan, L.; Lu, M.-P.; Wang, J.-H.; Xu, M.; Yang, S.-R. Immunological pathogenesis and treatment of systemic lupus erythematosus. World J. Pediatr. 2020, 16, 19–30. [Google Scholar] [CrossRef]
- Shi, D.; Li, X.; Chen, H.; Che, N.; Zhou, S.; Lu, Z.; Shi, S.; Sun, L. High level of reactive oxygen species impaired mesenchymal stem cell migration via overpolymerization of F-actin cytoskeleton in systemic lupus erythematosus. Pathol. Biol. 2014, 62, 382–390. [Google Scholar] [CrossRef]
- Zhu, Y.; Feng, X. Genetic contribution to mesenchymal stem cell dysfunction in systemic lupus erythematosus. Stem Cell Res. Ther. 2018, 9, 149. [Google Scholar] [CrossRef]
- Tang, X.; Li, W.; Wen, X.; Zhang, Z.; Chen, W.; Yao, G.; Chen, H.; Wang, D.; Shi, S.; Sun, L. Transplantation of dental tissue-derived mesenchymal stem cells ameliorates nephritis in lupus mice. Ann. Transl. Med. 2019, 7, 132. [Google Scholar] [CrossRef]
- Kamen, D.L.; Wallace, C.; Li, Z.; Wyatt, M.; Paulos, C.; Wei, C.; Wang, H.; Wolf, B.J.; Nietert, P.J.; Gilkeson, G. Safety, immunological effects and clinical response in a phase I trial of umbilical cord mesenchymal stromal cells in patients with treatment refractory SLE. Lupus Sci. Med. 2022, 9, e000704. [Google Scholar] [CrossRef] [PubMed]
- Jang, E.; Jeong, M.; Kim, S.; Jang, K.; Kang, B.-K.; Lee, D.Y.; Bae, S.-C.; Kim, K.S.; Youn, J. Infusion of Human Bone Marrow-Derived Mesenchymal Stem Cells Alleviates Autoimmune Nephritis in a Lupus Model by Suppressing Follicular Helper T-Cell Development. Cell Transplant. 2016, 25, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Gohil, K.; Carramusa, B. Ulcerative colitis and Crohn’s disease. Pharm. Ther. 2014, 39, 576–577. [Google Scholar]
- Faleiro, R.; Liu, J.; Karunarathne, D.; Edmundson, A.; Winterford, C.; Nguyen, T.H.; Simms, L.A.; Radford-Smith, G.; Wykes, M. Crohn’s disease is facilitated by a disturbance of programmed death-1 ligand 2 on blood dendritic cells. Clin. Transl. Immunol. 2019, 8, e01071. [Google Scholar] [CrossRef]
- González, M.A.; Gonzalez–Rey, E.; Rico, L.; Büscher, D.; Delgado, M. Adipose-Derived Mesenchymal Stem Cells Alleviate Experimental Colitis by Inhibiting Inflammatory and Autoimmune Responses. Gastroenterology 2009, 136, 978–989. [Google Scholar] [CrossRef]
- Xie, M.; Qin, H.; Luo, Q.; He, X.; He, X.; Lan, P.; Lian, L. Comparison of Adipose-Derived and Bone Marrow Mesenchymal Stromal Cells in a Murine Model of Crohn’s Disease. Am. J. Dig. Dis. 2016, 62, 115–123. [Google Scholar] [CrossRef]
- Mannon, P.J. Remestemcel-L: Human mesenchymal stem cells as an emerging therapy for Crohn’s disease. Expert Opin. Biol. Ther. 2011, 11, 1249–1256. [Google Scholar] [CrossRef]
- Ciccocioppo, R.; Bernardo, M.E.; Sgarella, A.; Maccario, R.; Avanzini, M.A.; Ubezio, C.; Minelli, A.; Alvisi, C.; Vanoli, A.; Calliada, F.; et al. Autologous bone marrow-derived mesenchymal stromal cells in the treatment of fistulising Crohn’s disease. Gut 2011, 60, 788–798. [Google Scholar] [CrossRef]
- Gao, J.-G.; Yu, M.-S.; Zhang, M.-M.; Gu, X.-W.; Ren, Y.; Zhou, X.-X.; Chen, D.; Yan, T.-L.; Li, Y.-M.; Jin, X. Adipose-derived mesenchymal stem cells alleviate TNBS-induced colitis in rats by influencing intestinal epithelial cell regeneration, Wnt signaling, and T cell immunity. World J. Gastroenterol. 2020, 26, 3750–3766. [Google Scholar] [CrossRef]
- Panes, J.; Garcia-Olmo, D.; Van Assche, G.; Colombel, J.F.; Reinisch, W.; Baumgart, D.C.; Dignass, A.; Nachury, M.; Ferrante, M.; Kazemi-Shirazi, L.; et al. Expanded allogeneic adipose-derived mesenchymal stem cells (Cx601) for complex perianal fistulas in Crohn’s disease: A phase 3 randomised, double-blind controlled trial. Lancet 2016, 388, 1281–1290. [Google Scholar] [CrossRef]
- Garcia-Olmo, D.; Gilaberte, I.; Binek, M.; D’hoore, A.J.; Lindner, D.; Selvaggi, F.; Spinelli, A.; Panés, J. Follow-up Study to Evaluate the Long-term Safety and Efficacy of Darvadstrocel (Mesenchymal Stem Cell Treatment) in Patients With Perianal Fistulizing Crohn’s Disease: ADMIRE-CD Phase 3 Randomized Controlled Trial. Dis. Colon Rectum 2022, 65, 713–720. [Google Scholar] [CrossRef]
- Molendijk, I.; Bonsing, B.A.; Roelofs, H.; Peeters, K.C.; Wasser, M.N.; Dijkstra, G.; van der Woude, C.J.; Duijvestein, M.; Veenendaal, R.A.; Zwaginga, J.-J.; et al. Allogeneic Bone Marrow–Derived Mesenchymal Stromal Cells Promote Healing of Refractory Perianal Fistulas in Patients With Crohn’s Disease. Gastroenterology 2015, 149, 918–927.e6. [Google Scholar] [CrossRef] [Green Version]
- Dietz, A.B.; Dozois, E.J.; Fletcher, J.G.; Butler, G.W.; Radel, D.; Lightner, A.L.; Dave, M.; Friton, J.; Nair, A.; Camilleri, E.T.; et al. Autologous Mesenchymal Stem Cells, Applied in a Bioabsorbable Matrix, for Treatment of Perianal Fistulas in Patients with Crohn’s Disease. Gastroenterology 2017, 153, 59–62.e2. [Google Scholar] [CrossRef] [PubMed]
- Fujii, S.; Miura, Y.; Fujishiro, A.; Shindo, T.; Shimazu, Y.; Hirai, H.; Tahara, H.; Takaori-Kondo, A.; Ichinohe, T.; Maekawa, T. Graft-Versus-Host Disease Amelioration by Human Bone Marrow Mesenchymal Stromal/Stem Cell-Derived Extracellular Vesicles Is Associated with Peripheral Preservation of Naive T Cell Populations. Stem Cells 2018, 36, 434–445. [Google Scholar] [CrossRef]
- Tang, B.; Li, X.; Liu, Y.; Chen, X.; Li, X.; Chu, Y.; Zhu, H.; Liu, W.; Xu, F.; Zhou, F.; et al. The Therapeutic Effect of ICAM-1-Overexpressing Mesenchymal Stem Cells on Acute Graft-Versus-Host Disease. Cell. Physiol. Biochem. 2018, 46, 2624–2635. [Google Scholar] [CrossRef]
- Chen, W.; Li, M.; Li, Z.; Yan, Z.; Cheng, H.; Pan, B.; Cao, J.; Chen, C.; Zeng, L.; Xu, K. CXCR4-transduced mesenchymal stem cells protect mice against graft-versus-host disease. Immunol. Lett. 2012, 143, 161–169. [Google Scholar] [CrossRef]
- Muroi, K.; Miyamura, K.; Okada, M.; Yamashita, T.; Murata, M.; Ishikawa, T.; Uike, N.; Hidaka, M.; Kobayashi, R.; Imamura, M.; et al. Bone marrow-derived mesenchymal stem cells (JR-031) for steroid-refractory grade III or IV acute graft-versus-host disease: A phase II/III study. Int. J. Hematol. 2015, 103, 243–250. [Google Scholar] [CrossRef]
- Baron, F.; Lechanteur, C.; Willems, E.; Bruck, F.; Baudoux, E.; Seidel, L.; Vanbellinghen, J.-F.; Hafraoui, K.; Lejeune, M.; Gothot, A.; et al. Cotransplantation of Mesenchymal Stem Cells Might Prevent Death from Graft-versus-Host Disease (GVHD) without Abrogating Graft-versus-Tumor Effects after HLA-Mismatched Allogeneic Transplantation following Nonmyeloablative Conditioning. Biol. Blood Marrow Transplant. 2010, 16, 838–847. [Google Scholar] [CrossRef]
- Gao, L.; Zhang, Y.; Hu, B.; Liu, J.; Kong, P.; Lou, S.; Su, Y.; Yang, T.; Li, H.; Liu, Y.; et al. Phase II Multicenter, Randomized, Double-Blind Controlled Study of Efficacy and Safety of Umbilical Cord-Derived Mesenchymal Stromal Cells in the Prophylaxis of Chronic Graft-Versus-Host Disease After HLA-Haploidentical Stem-Cell Transplantation. J. Clin. Oncol. 2016, 34, 2843–2850. [Google Scholar] [CrossRef]
- Bloor, A.J.C.; Patel, A.; Griffin, J.E.; Gilleece, M.H.; Radia, R.; Yeung, D.T.; Drier, D.; Larson, L.S.; Uenishi, G.I.; Hei, D.; et al. Production, safety and efficacy of iPSC-derived mesenchymal stromal cells in acute steroid-resistant graft versus host disease: A phase I, multicenter, open-label, dose-escalation study. Nat. Med. 2020, 26, 1720–1725. [Google Scholar] [CrossRef]
- Kurtzberg, J.; Abdel-Azim, H.; Carpenter, P.; Chaudhury, S.; Horn, B.; Mahadeo, K.; Nemecek, E.; Neudorf, S.; Prasad, V.; Prockop, S.; et al. A Phase 3, Single-Arm, Prospective Study of Remestemcel-L, Ex Vivo Culture-Expanded Adult Human Mesenchymal Stromal Cells for the Treatment of Pediatric Patients Who Failed to Respond to Steroid Treatment for Acute Graft-versus-Host Disease. Biol. Blood Marrow Transplant. 2020, 26, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; McGoogan, J.M. Characteristics of and Important Lessons from the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72314 Cases from the Chinese Center for Disease Control and Prevention. JAMA 2020, 323, 1239–1242. [Google Scholar] [CrossRef] [PubMed]
- Douaud, G.; Lee, S.; Alfaro-Almagro, F.; Arthofer, C.; Wang, C.; McCarthy, P.; Lange, F.; Andersson, J.L.R.; Griffanti, L.; Duff, E.; et al. SARS-CoV-2 is associated with changes in brain structure in UK Biobank. Nature 2022, 604, 697–707. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, S.; Liu, J.; Liang, B.; Wang, X.; Wang, H.; Li, W.; Tong, Q.; Yi, J.; Zhao, L.; et al. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine 2020, 55, 102763. [Google Scholar] [CrossRef]
- Kavianpour, M.; Saleh, M.; Verdi, J. The role of mesenchymal stromal cells in immune modulation of COVID-19: Focus on cytokine storm. Stem Cell Res. Ther. 2020, 11, 404. [Google Scholar] [CrossRef]
- Shao, M.; Xu, Q.; Wu, Z.; Chen, Y.; Shu, Y.; Cao, X.; Chen, M.; Zhang, B.; Zhou, Y.; Yao, R.; et al. Exosomes derived from human umbilical cord mesenchymal stem cells ameliorate IL-6-induced acute liver injury through miR-455-3p. Stem Cell Res. Ther. 2020, 11, 37. [Google Scholar] [CrossRef]
- Lu, Z.; Chang, W.; Meng, S.; Xu, X.; Xie, J.; Guo, F.; Yang, Y.; Qiu, H.; Liu, L. Mesenchymal stem cells induce dendritic cell immune tolerance via paracrine hepatocyte growth factor to alleviate acute lung injury. Stem Cell Res. Ther. 2019, 10, 372. [Google Scholar] [CrossRef]
- Leng, Z.; Zhu, R.; Hou, W.; Feng, Y.; Yang, Y.; Han, Q.; Shan, G.; Meng, F.; Du, D.; Wang, S.; et al. Transplantation of ACE2- Mesenchymal Stem Cells Improves the Outcome of Patients with COVID-19 Pneumonia. Aging Dis. 2020, 11, 216–228. [Google Scholar] [CrossRef]
- Fujita, Y.; Kosaka, N.; Araya, J.; Kuwano, K.; Ochiya, T. Extracellular vesicles in lung microenvironment and pathogenesis. Trends Mol. Med. 2015, 21, 533–542. [Google Scholar] [CrossRef]
- Sengupta, V.; Sengupta, S.; Lazo, A.; Woods, P.; Nolan, A.; Bremer, N. Exosomes Derived from Bone Marrow Mesenchymal Stem Cells as Treatment for Severe COVID-19. Stem Cells Dev. 2020, 29, 747–754. [Google Scholar] [CrossRef]
- Shi, L.; Huang, H.; Lu, X.; Yan, X.; Jiang, X.; Xu, R.; Wang, S.; Zhang, C.; Yuan, X.; Xu, Z.; et al. Effect of human umbilical cord-derived mesenchymal stem cells on lung damage in severe COVID-19 patients: A randomized, double-blind, placebo-controlled phase 2 trial. Signal Transduct. Target. Ther. 2021, 6, 58. [Google Scholar] [CrossRef]
- Monsel, A.; Hauw-Berlemont, C.; Mebarki, M.; Heming, N.; Mayaux, J.; Tchoumba, O.N.; Diehl, J.-L.; Demoule, A.; Annane, D.; Marois, C.; et al. Treatment of COVID-19-associated ARDS with mesenchymal stromal cells: A multicenter randomized double-blind trial. Crit. Care 2022, 26, 48. [Google Scholar] [CrossRef]
- Karyana, M.; Djaharuddin, I.; Rif’Ati, L.; Arif, M.; Choi, M.K.; Angginy, N.; Yoon, A.; Han, J.; Josh, F.; Arlinda, D.; et al. Safety of DW-MSC infusion in patients with low clinical risk COVID-19 infection: A randomized, double-blind, placebo-controlled trial. Stem Cell Res. Ther. 2022, 13, 134. [Google Scholar] [CrossRef]
- Liu, H.; Kemeny, D.M.; Heng, B.C.; Ouyang, H.W.; Melendez, A.J.; Cao, T. The Immunogenicity and Immunomodulatory Function of Osteogenic Cells Differentiated from Mesenchymal Stem Cells. J. Immunol. 2006, 176, 2864–2871. [Google Scholar] [CrossRef]
- Garcia-Olmo, D.; Garcia-Arranz, M.; Herreros, D.; Pascual, I.; Peiro, C.; Rodriguez-Montes, J.A. A phase I clinical trial of the treatment of Crohn’s fistula by adipose mesenchymal stem cell transplantation. Dis. Colon Rectum 2005, 48, 1416–1423. [Google Scholar] [CrossRef]
- Camilleri, E.T.; Gustafson, M.P.; Dudakovic, A.; Riester, S.M.; Garces, C.G.; Paradise, C.R.; Takai, H.; Karperien, M.; Cool, S.; Sampen, H.-J.I.; et al. Identification and validation of multiple cell surface markers of clinical-grade adipose-derived mesenchymal stromal cells as novel release criteria for good manufacturing practice-compliant production. Stem Cell Res. Ther. 2016, 7, 107. [Google Scholar] [CrossRef]
- Kebriaei, P.; Isola, L.; Bahceci, E.; Holland, K.; Rowley, S.; McGuirk, J.; Devetten, M.; Jansen, J.; Herzig, R.; Schuster, M.; et al. Adult Human Mesenchymal Stem Cells Added to Corticosteroid Therapy for the Treatment of Acute Graft-versus-Host Disease. Biol. Blood Marrow Transplant. 2009, 15, 804–811. [Google Scholar] [CrossRef] [Green Version]
| Disease Type | Interventions | Number of Patients | Study Phase | NCT Number | Outcome | Ref. |
|---|---|---|---|---|---|---|
| SLE | UC-MSCs | 6 | Phase 1 | NCT03171194 | Increased GARP-TGFβ | [61] |
| SLE | Pooled allogenic olfactory mucosa-MSCs | 7 | Phase 1/2 | NCT04184258 | No results posted | Not provided |
| SLE | BM-MSCs | 7 | Phase 1 | NCT03174587 | Decreased generation of autoantibodies | [62] |
| CD | Autologous AT-MSCs | 15 | Phase 1/2 | NCT01157650 | Recovered external opening | [95] |
| CD | UC-MSCs | 82 | Phase 1/2 | NCT02445547 | No results posted | Not provided |
| CD | Allogenic BM-MSCs | 21 | Phase 1/2 | NCT01144962 | Perianal fistula healed gradually, no associated adverse events | [72] |
| CD | Cx601 AT-MSCs | 278 | Phase 3 | NCT01541579 | Long-term safety, well tolerated | [71] |
| CD | MSC-AFP | 5 | Phase 1 | NCT03220243 | No results posted | Not provided |
| CD | MSC-AFP | 20 | Phase1 | NCT01915927 | Decreased length and diameter of fistula tract | [73] |
| CD | PROCHYMAL® adult human MSCs | 98 | Phase 3 | NCT00543374 | No results posted | Not provided |
| CD | PROCHYMAL® adult human MSCs | 330 | Phase 3 | NCT00482092 | No results posted | Not provided |
| CD | PROCHYMAL® adult human MSCs | 73 | Phase 3 | NCT01233960 | No results posted | Not provided |
| CD | PROCHYMALTM adult human MSCs | 10 | Phase 2 | NCT00294112 | No results posted | Not provided |
| GVHD | Allogenic MSCs | 15 | Phase 1/2 | NCT01956903 | No results posted | Not provided |
| GVHD | AT-MSCs | 19 | Phase 1/2 | NCT01222039 | No results posted | Not provided |
| GVHD | BM-MSCs | 10 | Phase 1/2 | NCT02824653 | No results posted | Not provided |
| GVHD | BM-MSCs | 30 | Phase 2 | NCT00504803 | Promoted engraftment and prevent GVHD | [78] |
| GVHD | PROCHYMAL® adult human MSCs | 260 | Phase 3 | NCT00366145 | Well tolerated and no associated toxicities | [96] |
| GVHD | PROCHYMAL® adult human MSCs | 32 | Phase 2 | NCT00136903 | No associated toxicities or ectopic tissue formations | [97] |
| GVHD | BM-MSCs | 10 | Phase 1 | NCT01318330 | No results posted | Not provided |
| GVHD | Mesenchymoangioblast-MSCs | 16 | Phase 1 | NCT02923375 | Safe, well tolerated and no serious adverse events | [80] |
| GVHD | PROCHYMAL® adult human MSCs | 11 | Phase 2 | NCT00284986 | No results posted | Not provided |
| GVHD | MSC (hPPL) | 50 | Phase 1/2 | NCT00827398 | No results posted | Not provided |
| GVHD | UC-MSCs | 10 | Phase 1/2 | NCT00823316 | No results posted | Not provided |
| GVHD | PROCHYMAL® adult human MSCs | 192 | Phase 3 | NCT00562497 | No results posted | Not provided |
| GVHD | MSCs infusion in Haplo-SCT | 6 | Phase 3 | NCT03106662 | No results posted | Not provided |
| GVHD | PROCHYMAL® adult human MSCs | 55 | Phase 3 | NCT02336230 | Improved response rate and increased survival | [81] |
| COVID-19 | MSCs secretome | 40 | Phase 3 | NCT05122234 | No results posted | Not provided |
| COVID-19 | UC-MSCs | 100 | Phase 2 | NCT04288102 | Improved lung lesion volume, reduced solid component lesion volume | [91] |
| COVID-19 | AT-MSCs | 56 | Phase 2 | NCT04349631 | No results posted | Not provided |
| COVID-19 | AT-MSCs | 55 | Phase 2 | NCT04348435 | No results posted | Not provided |
| COVID-19 | Wharton’s Jelly MSCs | 30 | Phase 2 | NCT04625738 | No results posted | Not provided |
| COVID-19 | UC-MSCs | 40 | Phase 1 | NCT04573270 | No results posted | Not provided |
| COVID-19 | Pooled allogenic olfactory mucosa-MSCs | 32 | Phase 1/2 | NCT04382547 | No results posted | Not provided |
| COVID-19 | UC-MSCs + Heparin | 24 | Phase 1/2 | NCT04355728 | No results posted | Not provided |
| COVID-19 | Allogenic MSCs | 9 | Phase 1 | NCT04535856 | No progression of severity | [93] |
| COVID-19 | Allogenic AT-MSCs | 26 | Phase 1/2 | NCT04366323 | No results posted | Not provided |
| COVID-19 | BM-MSCs extracellular vesicles | 120 | Phase 2 | NCT04493242 | Oxygenation restored, cytokine storm reduced and immunity modulated | [90] |
| COVID-19 | Armed Forces BM-MSCs | 600 | Not applicable | NCT04492501 | No results posted | Not provided |
| COVID-19 | AT-MSCs | 6 | Phase 1 | NCT04522986 | No results posted | Not provided |
| COVID-19 | UC-MSCs | 30 | Phase 1/2 | NCT04392778 | No results posted | Not provided |
| COVID-19 | Allogenic MSCs | 24 | Phase 2 | NCT04361942 | No results posted | Not provided |
| COVID-19 | MSCs exosomes | 30 | Phase 1/2 | NCT04491240 | No results posted | Not provided |
| COVID-19 | UC-MSCs | 15 | Phase 1/2 | NCT04400032 | No results posted | Not provided |
| COVID-19 | UC-MSCs | 47 | Phase 1/2 | NCT04333368 | No obvious difference in PaO2/FiO2 changes between UC-MSCs infusion group and placebo-treated group | [92] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.; Wu, Q.; Tam, P.K.H. Immunomodulatory Mechanisms of Mesenchymal Stem Cells and Their Potential Clinical Applications. Int. J. Mol. Sci. 2022, 23, 10023. https://doi.org/10.3390/ijms231710023
Huang Y, Wu Q, Tam PKH. Immunomodulatory Mechanisms of Mesenchymal Stem Cells and Their Potential Clinical Applications. International Journal of Molecular Sciences. 2022; 23(17):10023. https://doi.org/10.3390/ijms231710023
Chicago/Turabian StyleHuang, Yutong, Qiang Wu, and Paul Kwong Hang Tam. 2022. "Immunomodulatory Mechanisms of Mesenchymal Stem Cells and Their Potential Clinical Applications" International Journal of Molecular Sciences 23, no. 17: 10023. https://doi.org/10.3390/ijms231710023
APA StyleHuang, Y., Wu, Q., & Tam, P. K. H. (2022). Immunomodulatory Mechanisms of Mesenchymal Stem Cells and Their Potential Clinical Applications. International Journal of Molecular Sciences, 23(17), 10023. https://doi.org/10.3390/ijms231710023






