Health Benefits and Risks of Consuming Spices on the Example of Black Pepper and Cinnamon
Abstract
:1. Introduction
2. Characteristics of Selected Spices and Its Relevance to the Consumer
2.1. Black Pepper [Piper nigrum L.]—Origin, Types and Properties
2.2. Cinnamon (Cinnamomum spp.)—Characteristics, Properties, Use
3. Materials and Method
3.1. Used Chemicals
3.2. Total Phenolic Content
3.3. DPPH Free Radical Scavenging
3.4. Piperine Assay
3.5. Essential Oil Content
3.6. Coumarin Content
3.7. Statistical ANALYSIS
4. Results and Discussion
4.1. Analysis of Black Pepper (Piper nigrum L.)
4.1.1. Antioxidant Properties of Black Pepper (Piper nigrum L.)
4.1.2. Piperine Content in Black Pepper (Piper nigrum L.)
4.1.3. Essential Oil Content in Black Pepper (Piper nigrum L.)
4.2. Analysis of Cinnamon (Cinnamonum sp.)
4.2.1. Antioxidant Properties of Cinnamon (Cinnamonum sp.)
4.2.2. Coumarin Content in Cinnamon (Cinnamonum spp.)
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Embuscado, M.E. Spices and herbs: Natural sources of antioxidants—A mini review. J. Funct. Foods 2015, 18, 811–819. [Google Scholar] [CrossRef]
- Yashin, A.; Yashin, Y.; Xia, X.; Nemzer, B. Antioxidant Activity of Spices and Their Impact on Human Health: A Review. Antioxidants 2017, 6, 70. [Google Scholar] [CrossRef]
- Abdel-Aziz, S.M.; Abd El-Hack, M.E.; Alagawany, M.; Abdel-Moneim, A.-M.E.; Mohammed, N.G.; Khafaga, A.F.; Bin-Jumah Aeron, A.; Kahil, T.A. Health Benefits and Possible Risks off Herbal Medicine. In Microbes in Food and Health; Garg, N., Abdel-Aziz, S.M., Aeron, A., Eds.; Springer International Publishing: Cham, Switzerland, 2016. [Google Scholar]
- Vázquez-Fresno, R.; Rosana, A.R.R.; Sajed, T.; Onookome-Okome, T.; Wishart, N.A.; Wishart, D.S. Herbs and Spices—Biomarkers of Intake Based on Human Intervention Studies—A Systematic Review. Genes Nutr. 2019, 14, 18. [Google Scholar] [CrossRef] [PubMed]
- Directive 2004/24/EC of the European Parliament and of the Council of 31 March 2004. “Herbal Substances Are: All, Mainly Whole, Divided or Cut Plants, Parts of Plants, Algae, Fungi, Lichen in Un-Processed Form, Usually Dried, Sometimes Fresh”. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX:32004L0024 (accessed on 4 May 2022).
- Śmiechowska, M.; Newerli-Guz, J.; Skotnicka, M. Spices and seasoning mixes in European Union-innovations and ensuring safety. Foods 2021, 10, 2289. [Google Scholar] [CrossRef]
- Chironi, S.; Bacarella, S.; Altamore, L.; Columba, P.; Ingrassia, M. Consumption of spices and ethnic contamination in the daily diet of Italians -consumers’ preferences and modification of eating habits. J. Ethn. Foods 2021, 8, 6. [Google Scholar] [CrossRef]
- Bortnowska, G.; Kałużna-Zajączkowska, J. Preferencje wyboru przypraw sypkich do potraw przez osoby pracujące zawodowo z uwzględnieniem innowacyjnych zmian w ich produkcji. Rocz. Państw. Zakł. Hig. 2011, 62, 445–452. [Google Scholar] [PubMed]
- Krishnamoorthy, B.; Parthasarathy, V.A. Improvement of Black Pepper. CAB Rev. 2009, 4, 85. Available online: https://cabidigitallibrary.org/doi/10.1079/PAVSNNR20105003 (accessed on 4 May 2022). [CrossRef]
- Korikanthimath, V.S.; Dhas, P.H.A. Processing and quality of black pepper—A review. J. Spices Aromat. Crops 2003, 12, 1–13. [Google Scholar]
- Ganapathi, T.; Patil, S.V.; Rajakumar, G.R. Processing of black pepper (Piper nigrum L.) through solarisation. Int. J. Agric. Sci. 2018, 14, 211–214. [Google Scholar] [CrossRef]
- Pickova, D.; Ostry, V.; Malir, J.; Toman, J.; Malir, F. A Review on Mycotoxins and Microfungi in Spices in the Light of the Last Five Years. Toxins 2020, 12, 789. [Google Scholar] [CrossRef]
- Jalili, M.; Jinap, S.; Noranizam, M.A. Aflatoxins and ochratoxins a reduction in black and white pepper by gamma radiation. Radiat. Phys. Chem. 2012, 81, 1786–1788. [Google Scholar] [CrossRef]
- Ee, K.P.; Shang, C.Y. Novel Farming Innovation for High Production of Black Pepper (Piper nigrum L.) Planting Materials. J. Agric. Sci. Technol. B 2017, 7, 301–308. [Google Scholar]
- Sulok, K.M.T.; Ahmed, O.H.; Khew, C.Y.; Zehnder, J.A.M. Introducing Natural Farming in Black Pepper (Piper nigrum L.) Cultivation. Int. J. Agron. 2018, 2018, 9312537. [Google Scholar] [CrossRef]
- Nguyen, S.D.; Trinh, T.H.T.; Tra, T.D.; Nguyen, T.V.; Chuyen, H.V.; Ngo, V.A.; Nguyen, A.D. Combined Application of Rhizosphere Bacteria with Endophytic Bacteria Suppresses Root Diseases and Increases Productivity of Black Pepper (Piper nigrum L.). Agriculture 2021, 11, 15. [Google Scholar] [CrossRef]
- Karmawati, E.; Ardana, I.K.; Siswanto; Soetopo, D. Factors effecting pepper production and quality in several production center. IOP Conf. Ser. Earth Environ. Sci. 2020, 418, 012051. [Google Scholar] [CrossRef]
- Hu, L.; Yin, C.; Ma, S.; Liu, Z. Assessing the authenticity of black pepper using diffuse reflectance midinfrared Fourier transform spectroscopy coupled with chemometrics. Comput. Electron. Agric. 2018, 154, 491–500. [Google Scholar] [CrossRef]
- Rivera-Pérez, A.; Romero-González, R.; Frenich, A.G. Application of an innovative metabolomics approach to discriminate geographical origin and processing of black pepper by untargeted UHPLC-Q-Orbitrap-HRMS analysis and mid-level data Fusion. Food Res. Int. 2021, 150, 110722. [Google Scholar] [CrossRef]
- Cantarelli, M.Á.; Moldes, C.A.; Marchevsky, E.J.; Azcarate, S.M.; Camiña, J.M. Low-cost analytic method for the identification of Cinnamon adulteration. Microchem. J. 2020, 159, 1055132020. [Google Scholar] [CrossRef]
- Modupalli, N.; Naik, M.; Sunil, C.K.; Natarajan, V. Emerging non-destructive methods for quality and safety monitoring of spices. Trends Food Sci. Technol. 2021, 108, 133–147. [Google Scholar] [CrossRef]
- Milenković, A.N.; Stanojević, L.P. Black pepper—Chemical composition and biological activities. Adv. Technol. 2021, 10, 40–50. [Google Scholar] [CrossRef]
- Hammouti, B.; Dahmani, M.; Yahyi, A.; Ettouhami, A.; Messali, M.; Asehraou, A.; Bouyanzer, A.; Warad, I.; Touzani, R. Black Pepper, the “King of Spices”: Chemical composition to applications. Arab. J. Chem. Environ. Res. 2019, 6, 12–56. [Google Scholar]
- Bermawie, N.; Wahyuni, S.; Heryanto, R.; Darwati, I. Morphological characteristics, yield and quality of black pepper Ciinten variety in three agro ecological conditions. IOP Conf. Ser. Earth Environ. Sci. 2019, 292, 012065. [Google Scholar] [CrossRef]
- Chen, S.-A.; Yang, K.; Xiang, J.-Y.; Kwaku, O.R.; Han, J.-X.; Zhu, X.-A.; Huang, Y.-T.; Liu, L.-J.; Shen, S.-B.; Li, H.-Z.; et al. Comparison of Chemical Compositions of the Pepper EOs from Different Cultivars and Their AChE Inhibitory Activity. Nat. Prod. Commun. 2020, 15, 1934578X20971469. [Google Scholar] [CrossRef]
- Lee, J.-G.; Kim, D.-W.; Shin, Y.; Kim, Y.-J. Comparative study of the bioactive compounds, Lee flavours and minerals present in black pepper before and after removing the outer skin. LW-Food Sci. Technol. 2020, 125, 109356. [Google Scholar] [CrossRef]
- Aswar, U.; Shintre, S.; Chepurwar, S.; Aswar, M. Antiallergic effect of piperine on ovalbumin-induced allergic rhinitis in mice. Pharm. Biol. 2015, 53, 1358–1366. [Google Scholar] [CrossRef]
- Kim, S.-H.; Lee, Y.-C. Piperine inhibits eosinophil infiltration and airway hyperresponsiveness by suppressing T cell activity and Th2 cytokine production in the ovalbumin-induced asthma model. J. Physiol. Pharmacol. 2009, 61, 353–359. [Google Scholar] [CrossRef]
- Bukhari, I.A.; Alhumayyd, M.S.; Mahesar, A.L.; Gilani, A.H. The analgesic and anticonvulsant effects of piperine in mice. J. Physiol. Pharmacol. 2013, 64, 789–794. [Google Scholar]
- Jeena, K.; Liju, V.B.; Umadevi, N.P.; Kuttan, R. Antioxidant, anti-inflammatory and antinociceptive properties of black pepper essential oil (Piper nigrum L.). J. Essent. Oil Bear. Plants 2014, 17, 1–12. [Google Scholar] [CrossRef]
- Tasleem, F.; Ashar, I.; Ali, S.N.; Perveen, S.; Mahmood, Z.A. Analgesic and anti-inflammatory activities of Piper nigrum L. Asian Pac. J. Trop. Med. 2014, 7 (Suppl. S1), 461–468. [Google Scholar] [CrossRef]
- Bang, J.S.; Oh, D.H.; Choi, H.M.; Sur, B.-J.; Lim, S.-J.; Kim, J.Y.; Yang, H.-I.; Yoo, M.C.; Hahm, D.-H.; Kim, K.S. Anti-inflammatory and antiarthritic effects of piperine in human interleukin 1β-stimulated fibroblast-like synoviocytes and in rat arthritis models. Arthritis Res. Ther. 2009, 11, R49. [Google Scholar] [CrossRef]
- Umar, S.; Sarwar, A.H.M.G.; Umar, K.; Ahmad, N.; Sajad, M.M.; Ahmad, S.; Katiyar, C.K.; Khan, H.A. Piperine ameliorates oxidative stress, inflammation and histological out-come in collagen induced arthritis. Cell. Immunol. 2013, 284, 51–59. [Google Scholar] [CrossRef]
- Banerjee, S.; Katiyar, P.; Kumar, V.; Saini, S.S.; Varshney, R.; Krishnan, V.; Sircar, D.; Roy, P. Black pepper and piperine induce anticancer effects on leukemia cell line. Toxicol. Res. 2021, 10, 232–248. [Google Scholar] [CrossRef]
- Greenshields, A.L.; Doucette, C.D.; Sutton, K.M.; Madera, L.; Annan, H.; Yaffe, P.B.; Knickle, A.F.; Dong, Z.; Hoskin, D.W. Piperine inhibits the growth and motility of triple-negative breast cancer cells. Cancer Lett. 2015, 357, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Morsy, F.N.S.; Abd El-Salam, E.A. Antimicrobial and Antiproliferative Activities of Black Pepper (Piper nigrum L.) Essential Oil and Oleoresin. J. Essent. Oil Bear. Plants 2017, 20, 779–790. [Google Scholar] [CrossRef]
- Prashant, A.; Rangaswamy, C.; Yadav, A.K.; Reddy, V.; Sowmya, M.N.; Madhunapantula, S. In vitro anticancer activity of ethanolic extracts of Piper nigrum against colorectal carcinoma cell lines. Int. J. Appl. Basic Med. Res. 2017, 7, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Atal, S.; Atal, S.; Vyas, S.; Phadnis, P. Bio-enhancing Effect of Piperine with Metformin on Lowering Blood Glucose Level in Alloxan Induced Diabetic Mice. Pharmacogn. Res. 2016, 8, 56–60. [Google Scholar] [CrossRef]
- Kharbanda, C.; Alam, M.S.; Hamid, H.; Javed, K.; Bano, S.; Ali, Y.; Dhulap, A.; Alam, P.; Pasha, M.A.Q. Novel piperine derivatives with antidiabetic effect as PPAR-γ agonists. Chem. Biol. Drug Des. 2016, 88, 354–362. [Google Scholar] [CrossRef]
- Oboh, G.; Ademosun, A.O.; Odubanjo, O.V.; Akinbola, I.A. Antioxidative Properties and Inhibition of Key Enzymes Relevant to Type-2 Diabetes and Hypertension by Essential Oils from Black Pepper. Adv. Pharmacol. Sci. 2013, 2013, 926047. [Google Scholar] [CrossRef] [PubMed]
- Sarfraz, M.; Khaliq, T.; Hafizur, R.M.; Raza, S.A.; Ullah, H. Effect of black pepper, turmeric and ajwa date on the endocrine pancreas of the experimentally induced diabetes in wister albino rats: A histological and immunohistochemical study. Endocr. Metab. Sci. 2021, 4, 100098. [Google Scholar] [CrossRef]
- Emon, N.U.; Alam, S.; Rudra, S.; Riya, S.R.; Paul, A.; Hossen, S.M.M.; Kulsum, U.; Ganguly, A. Antide-pressant, anxiolytic, antipyretic, and thrombolytic profiling of methanol extract of the aerial part of Piper nigrum: In vivo, in vitro, and in silico approaches. Food Sci. Nutr. 2020, 9, 833–846. [Google Scholar] [CrossRef]
- Ghosh, S.; Kumar, A.; Sachan, N.; Chandra, P. Anxiolytic and antidepressant-like effects of essential oil from the fruits of Piper nigrum Linn. (Black pepper) in mice: Involvement of serotonergic but not GABAergic transmission system. Heliyon 2021, 7, e06884. [Google Scholar] [CrossRef]
- Hritcu, L.; Noumedem, J.A.; Cioanca, O.; Hancianu, M.; Postu, P.; Mihasan, M. Anxiolytic and antidepressant profile of the methanolic extract of Piper nigrum fruits in beta-amyloid (1–42) rat model of Alzheimer’s disease. Behav. Brain Funct. 2015, 11, 13. [Google Scholar] [CrossRef]
- Mao, Q.-Q.; Huang, Z.; Zhong, X.-M.; Xian, Y.-F.; Ip, S.-P. Piperine reverses the effects of corti-costerone on behawior and hippocampal BDNF expression in mice. Neurochem. Int. 2014, 74, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Hlavačková, L.; Janegová, A.; Uličná, O.; Janega, P.; Černá, A.; Babál, P. Spice up the hypertension diet—curcumin and piperine prevent remodeling of aorta in experimental L-NAME induced hypertension. Nutr. Metab. 2011, 8, 72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, K.P.; Lee, K.; Park, H.-W.; Kim, H.; Hong, H. Piperine inhibits platelet-derived growth factor-BB-induced proliferation and migration in vascular smooth muscle cells. J. Med. Food 2015, 18, 208–215. [Google Scholar] [CrossRef]
- Taqvi, S.I.H.; Shah, A.J.; Gilani, A.H. Blood pressure lowering and vasomodulator effects of piperine. J. Cardiovasc. Pharmacol. 2008, 52, 452–458. [Google Scholar] [CrossRef]
- Yu, S.; Liu, X.; Yu, D.C.E.; Yang, J. Piperine protects LPS-induced mastitis by inhibiting inflammatory response. Int. Immunopharmacol. 2020, 87, 106804. [Google Scholar] [CrossRef] [PubMed]
- Bawazeer, S.; Khan, I.; Rauf, A.; Aljohani, A.S.M.; Alhumaydhi, F.A.; Khalil, A.A.; Qureshi, M.N.; Ahmad, L.; Khan, S.A. Black pepper (Piper nigrum) fruit-based gold nanoparticles (BP-AuNPs): Synthesis, characterization, biological activities, and catalytic applications—A green approach. Green Process. Synth. 2022, 11, 11–28. [Google Scholar] [CrossRef]
- Chen, P.; Sun, J.; Ford, P. Differentiation of the Four Major Species of Cinnamons (C. burmannii, C. verum, C. cassia, and C. loureiroi) Using a Flow Injection Mass Spectrometric (FIMS) Fingerprinting Method. J. Agric. Food Chem. 2014, 62, 2516–2521. [Google Scholar] [CrossRef] [PubMed]
- Daigham, G.E.; Mahfouz, A.Y. Assessment of Antibacterial, Antifungal, Antioxidant and Antiviral Activities of Black pepper Aqueous Seed Extract. Al-Azhar Univ. J. Res. Stud. 2020, 2, 1–12. [Google Scholar]
- Hien, L.T.M.; Dao, D.T.A. Antibacterial Activity of Black Pepper Essential Oil Nanoemulsion Formulated by Emulsion Phase Inversion Method. Curr. Res. Nutr. Food Sci. 2022, 10, 311–320. [Google Scholar] [CrossRef]
- Martinelli, L.; Rosa, J.M.; Fereira, C.S.B.; Nascimento, G.M.L.; Freitas, M.S.; Pizato, L.C.; Santos, W.O.; Pires, R.F.; Okura, M.H.; Malpass, G.R.P.; et al. Antimicrobial activity and chemical constituents of essential oils and oleoresins extracted from eight pepper species. Ciênc. Rural 2017, 47, e20160899. [Google Scholar] [CrossRef]
- Zhang, J.; Ye, K.-P.; Zhang, X.; Pan, D.-D.; Sun, Y.-Y.; Cao, Y.-X. Antibacterial Activity and Mechanism of Action of Black Pepper Essential Oil on Meat-Borne Escherichia coli. Front. Microbiol. 2017, 7, 2094. [Google Scholar] [CrossRef]
- Zou, L.; Hu, Y.-Y.; Chen, W.-X. Antibacterial mechanism and activities of black pepper chloroform extract. J. Food Sci. Technol. 2015, 52, 8196–8203. [Google Scholar] [CrossRef] [PubMed]
- Chopathompikunrt, P.; Wattanathorn, J.; Muchimapura, S. Piperine, the main alkaloid of Thai black pepper, protects against neurodegeneration and cognitive impairment in animal model of cognitive deficit like condition of Alzheimer’s disease. Food Chem. Toxicol. 2010, 48, 798–802. [Google Scholar] [CrossRef] [PubMed]
- Elnaggar, Y.S.R.; Etman, S.M.; Abdelmonsif, D.A.; Abdallah, O.Y. Novel piperine-loaded Tween-integrated monoolein cubosomes as brain-targeted oral nanomedicine in Alzheimer’s disease: Pharmaceutical, biological, and toxicological studies. Int. J. Nanomed. 2015, 10, 5459–5473. [Google Scholar] [CrossRef] [PubMed]
- Etman, S.M.; Elnaggar, Y.S.R.; Abdelmonsif, D.A.; Abdallah, O.Y. Oral brain-targeted microemulsion for enhanced piperine delivery in Alzheimer’s disease therapy: In vitro appraisal, in vivo activity, and nanotoxicity. AAPS PharmSciTech 2018, 19, 3698–3711. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Chen, Y.; Fu, X.; Gu, J.; Sun, Y.; Zhang, Z.; Xu, J.; Qin, L. Effects of piperine on lipid metabolism in high-fat diet induced obese mice. J. Funct. Foods 2020, 71, 104011. [Google Scholar] [CrossRef]
- Lailiyah, N.N.; Ibrahim, M.D.; Fitriani, C.A.; Hermanto, F.E. Anti-Obesity Properties of Black Pepper (Piper nigrum L.): Completing Puzzle using Computational Analysis. J. Smart Bioprospect. Technol. 2021, 2, 101–106. [Google Scholar] [CrossRef]
- Meriga, B.; Parim, B.; Chunduri, V.R.; Naik, R.R.; Nemani, H.; Suresh, P.; Ganapathy, S.; Wu, S.B. Antiobesity potential of Piperonal: Promising modulation of body composition, lipid profiles and obesogenic marker expression in HFD-induced obese rats. Nutr. Metab. 2017, 14, 72. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.S.; Shah, G.B.; Singh, S.D.; Gohil, P.V.; Chauhan, K.; Shah, K.A.; Chorawala, M. Effect of piperine in the regulation of obesity-induced dyslipidemia in high-fat diet rats. Indian J. Pharmacol. 2011, 43, 296–299. [Google Scholar] [CrossRef]
- Srinivasan, K. Black Pepper and its Pungent Principle-Piperine: A Review of Diverse Physiological Effects. Crit. Rev. Food Sci. Nutr. 2007, 47, 735–748. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumar, R.S.; Nalini, N. Efficacy of piperine, an alkaloidal constituent from Piper nigrum on erythrocyte antioxidant status in high fat diet and antithyroid drug induced hyperlipidemic rats. Cell Biochem. Funct. 2006, 24, 491–498. [Google Scholar] [CrossRef]
- Dutta, M.; Ghosh, A.K.; Mishra, P.; Jain, G.; Rangari, V.; Chattopadhyay, A.; Das, T.; Bhowmick, D.; Bandyopadhyay, D. Protective effects of piperine against copper ascorbate induced toxic injury to goat cardiac mitochondria in vitro. Food Funct. 2014, 5, 2252. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhang, L.; Huang, J.; Himabindu, K.; Tewari, D.; Horbańczuk, J.O.; Xu, S.; Chen, Z.; Atanasov, A.G. Cardiovascular protective effect of black pepper (Piper nigrum L.) and its major bioactive constituent piperine. Trends Food Sci. Technol. 2021, 117, 34–45. [Google Scholar] [CrossRef]
- Mehmood, M.H.; Gilani, A.H. Pharmacological Basis for the Medicinal Use of Balck Pepper and Piperine in Gastrointestinal Disorders. J. Med. Food 2010, 13, 1086–1096. [Google Scholar] [CrossRef]
- Shamkuwar, P.B.; Shahi, S.R.; Jadhav, S.T. Evaluation of antidiarrhoeal effect of Black pepper (Piper nigrum L.). Asian J. Plant Sci. Res. 2012, 2, 48–53. [Google Scholar]
- Shamkuwar, P.B. Mechanisms of Antidiarrhoeal Effect of Piper nigrum. Int. J. PharmTech Res. 2013, 5, 1138–1141. [Google Scholar]
- Bae, G.-S.; Kim, M.-S.; Jeong, J.; Lee, H.-Y.; Park, K.-C.; Koo, B.C.; Kim, B.-J.; Kim, T.-H.; Lee, S.H.; Hwang, S.-Y.; et al. Piperine ameliorates the severity of cerulein-induced acute pancrea-titis by inhibiting the activation of mitogen activated protein kinases. Biochem. Biophys. Res. Commun. 2011, 410, 382–388. [Google Scholar] [CrossRef]
- Christina, A.J.M.; Saraswathy, G.R.; Heison, R.S.J.; Kothai, R.; Chidambaranatha, N.; Nalini, G.; Therasal, R.L. Inhibition of CCl4-induced liver fibrosis by Piper longum. Phytomedicine 2006, 13, 196–198. [Google Scholar] [CrossRef] [PubMed]
- Gurumurthy, P.; Vijayalatha, S.; Sumathy, A.; Asokan, M.; Naseema, M. Hepatoprotective effect of aqueous extract of Piper longum and piperine when administered with anti-tubercular drugs. Bioscan 2012, 7, 661–663. [Google Scholar]
- Matsuda, H.; Ninomiya, K.; Morikawa, T.; Yasuda, D.; Yamaguchi, I. Protective effects of amide constit-uents from the fruit of Piper chaba on D-galactosamine/TNF-alpha-induced cell death in mouse hepatocytes. Bioorg. Med. Chem. Lett. 2008, 18, 2038–2042. [Google Scholar] [CrossRef] [PubMed]
- Nirwane, A.M.; Bapat, A.R. Effect of methanolic extract of Piper nigrum fruits in Ethanol-CCl4 induced hepatotoxicity in Wistar rats. Pharm. Lett. 2012, 4, 795–802. [Google Scholar]
- Weerasekera, A.C.; Samarasinghe, K.; Sameera de Zoysa, H.K.; Bamunuarachchige, T.C.; Waisundara, V.Y. Cinnamomum zeylanicum: Morphology. In Antioxidant Properties and Bioactive Compounds: Antioxidants; Intech Open: London, UK, 2021. [Google Scholar]
- Al-Dhubiab, B.E. Pharmaceutical applications and phytochemical profile of Cinnamomum burmannii. Pharmacogn. Rev. 2012, 6, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.; Talreja, S. Importance of Cinnamomum Tamala in the Treatment of Various Diseases. Pharmacogn. J. 2020, 12, 1792–1796. [Google Scholar] [CrossRef]
- Kumar, S.; Kumari, R.; Mishra, S. Pharmacological properties and their medicinal uses of Cinnamomum: A review. J. Pharm. Pharmacol. 2019, 71, 1735–1761. [Google Scholar] [CrossRef]
- Anonymous. Information Cassia and Cinnamon. Available online: https://citeseerx.ist.psu.edu/viewdoc/similar?doi=10.1.1.409.6671&type=ab (accessed on 4 May 2022).
- Suriyagoda, L.; Mohotti, A.J.; Vidanarachchi, J.K.; Kodithuwakku, S.P.; Chathurika, M.; Bandaranayake, P.C.G.; Hetherington, A.M.; Beneragama, C.K. Ceylon cinnamon: Much more than just a spice. Plants People Planet 2021, 3, 319–336. [Google Scholar] [CrossRef]
- Gunia-Krzyżak, A.; Słoczyńska, K.; Popiół, J.; Marona, H.; Pękala, E. Cinnamic acid derivatives in cosmetics: Current use and future prospects. Int. J. Cosmet. Sci. 2018, 40, 356–366. [Google Scholar] [CrossRef]
- De Silva, D.A.M.; Jeewanthi, R.K.C.; Rajapaksha, R.H.N.; Weddagala, W.M.T.B.; Hirotsu, N.; Shimizu, B.-I.; Munasinghe, M.A.J.P. Clean vs. dirty labels: Transparency and authenticity of the labels of Ceylon cinnamon. PLoS ONE 2021, 16, e0260474. [Google Scholar] [CrossRef] [PubMed]
- Avula, B.; Smillie, T.J.; Wang, J.-H.; Zweigenbaum, J.; Khan, I.A. Authentication of true cinnamon (Cinnamon verum) utilising direct analysis in real time (DART)-QToF-MS. Food Addit. Contam. Part A 2015, 32, 1–8. [Google Scholar] [CrossRef]
- Rana, P.; Liaw, S.-Y.; Lee, M.-S.; Sheu, S.-C. Discrimination of Four Cinnamomum Species with Physico-Functional Properties and Chemometric Techniques: Application of PCA and MDA Models. Foods 2021, 10, 2871. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Liang, Z.; Li, P.; Zhao, Z.; Chen, J. Tissue-specific chemical profiling and quantitative analysis of bioactive components of Cinnamomum cassia by combining laser-microdissection with UPLC-Q/TOF–MS. Chem. Cent. J. 2018, 12, 71. [Google Scholar] [CrossRef] [PubMed]
- Lixourgioti, P.; Goggin, K.A.; Zhao, X.; Murphy, D.J.; Van Ruth, S.; Koidis, A. Authentication of cinnamon spice samples using FT-IR spectroscopy and chemometric classification. LWT-Food Sci. Technol. 2022, 154, 112760. [Google Scholar] [CrossRef]
- Lacy, A.; O’Kennedy, R. Studies on Coumarins and Coumarin-Related Compounds to Determine their Therapeutic Role in the Treatment of Cancer. Curr. Pharm. Des. 2004, 10, 3797–3811. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority (EFSA). Scientific Opinion of the Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food on a Request from the European Commission on Coumarinin Flavourings and Other Food Ingredients with Flavouring Properties. EFSA J. 2008, 793, 1–15. [Google Scholar]
- Ribeiro-Santos, R.; Andrade, M.; Madella, D.; Martinazzo, A.P.; Moura, L.A.G.; Melo, N.R.; Sanches-Silva, A. Revisiting an ancient spice with medicinal purposes: Cinnamon. Trends Food Sci. Technol. 2017, 62, 154–169. [Google Scholar] [CrossRef]
- Pandey, M.; Chandra, D.R. Evaluation of Ethanol and Aqueous extracts of Cinnamomum verum Leaf Galls for Potential Antioxidant and Analgesic activity. Indian J. Pharm. Sci. 2015, 77, 243–247. [Google Scholar]
- Dutta, A.; Chakraborty, A. Cinnamon in Anticancer Armamentarium: A Molecular Approach. J. Toxicol. 2018, 2018, 8978731. [Google Scholar] [CrossRef]
- Thompson, M.; Schmelz, E.M.; Bickford, L. Anti-Cancer Properties of Cinnamon Oil and its Active Component, TransCinnamaldehyde. J. Nutr. Food Sci. 2019, 9, 1000750. [Google Scholar]
- Anderson, R.A.; Zhan, Z.; Luo, R.; Guo, X.; Guo, Q.; Zhou, J.; Kong, J.; Davis, P.A.; Stoecker, B.J. Cinnamon extract lowers glucose, insulin and cholesterol in people with elevated serum glucose. J. Tradit. Complement. Med. 2015, 6, 332–336. [Google Scholar] [CrossRef]
- Zare, R.; Nadjarzadech, A.; Zarshenas, M.M.; Shams, M.; Heydari, M. Efficacy of cinnamon in patients with type II diabetes mellitus: A randomized controlled clinical trial. Clin. Nutr. 2019, 38, 549–556. [Google Scholar] [CrossRef]
- Shinjyo, N.; Waddell, G.; Green, J. A tale of two cinnamons: A comparative review of the clinical evidence of Cinnamomum verum and C. cassia as diabetes interventions. J. Herb. Med. 2020, 21, 00342. [Google Scholar] [CrossRef] [Green Version]
- Han, X.; Parker, T.L. Antiinflammatory Activity of Cinnamon (Cinnamomum zeylanicum) Bark Essential Oil in a Human Skin Disease Model. Phytother. Res. 2017, 31, 1034–1038. [Google Scholar] [CrossRef]
- Schink, A.; Naumoska, K.; Kitanovski, Z.; Kampf, C.J.; Nowoisky, J.F.; Thines, F.; Pöschl, U.; Schuppan, D.; Lucas, K. Anti-inflammatory effects of cinnamon extract and identification of active compounds influencing the TLR2 and TLR4 signaling pathways. Food Funct. 2018, 9, 5950–5964. [Google Scholar] [CrossRef] [PubMed]
- Shishehbor, F.; Safar, M.R.; Rajaei, E.; Haghighizadeh, M.H. Cinnamon Consumption Improves Clinical Symptoms and Inflammatory Markers in Women with Rheumatoid Arthritis. J. Am. Coll. Nutr. 2018, 37, 685–690. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Hack, M.E.; Alagawany, M.; Abdel-Moneim, A.-M.E.; Mohammed, N.G.; Khafaga, A.F.; Bin-Jumah, M.; Othman, S.I.; Allam, A.A.; Elnesr, S.S. Cinnamon (Cinnamomum zeylanicum) Oil as a Potential Alternative to Antibiotics in Poultry. Antibiotics 2020, 9, 210. [Google Scholar] [CrossRef]
- Arancibia, M.; Giménez, B.; López-Caballero, M.E.; Gómez-Guillén, M.C.; Montero, P. Release of Cinnamon Essential Oil from Polysaccharide Bilayer Films and Its Use for Microbial Growth Inhibition in Chilled Shrimps. LWT-Food Sci. Technol. 2014, 59, 989–995. [Google Scholar] [CrossRef]
- Atki, Y.E.; Aouam, I.; Kamari, F.E.; Taroq, A.; Nayme, K.; Timinouni, M.; Lyoussi, B.; Abdellaoui, A. Antibacterial activity of cinnamon essential oils and their synergistic potential with antibiotics. J. Adv. Pharm. Technol. Res. 2019, 10, 63–67. [Google Scholar] [CrossRef]
- Mnafgui, K.; Derbali, A.; Sayadi, S.; Gharsallah, N.; Alfeki, A.; Allouche, N. Anti-obesity and cardioprotective effects of cinnamic acid in high fat diet- induced obese rats. J. Food Sci. Technol. 2015, 52, 4369–4377. [Google Scholar] [CrossRef]
- Gulcin, I.; Kaya, R.; Goren, A.C.; Akincioglu, H.; Topal, M.; Bingol, Z.; Çakmak, K.C.; Sarikaya, S.B.O.; Durmaz, L.; Alwasel, S. Anticholinergic, antidiabetic and antioxidant activities of cinnamon (cinnamomum verum) bark extracts: Polyphenol contents analysis by LC-MS/MS. Int. J. Food Prop. 2019, 22, 1511–1526. [Google Scholar] [CrossRef]
- Jain, S.G.; Puri, S.; Misra, A.; Gulati, S.; Mani, K. Effect of oral cinnamon intervention on metabolic profile and body composition of Asian Indians with metabolic syndrome: A randomized double -blind control trial. Lipids Health Dis. 2017, 16, 113. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, M.S.; Karimi, E.; Hajishafiee, M.; Milajerdi, A.; Amini, M.R.; Esmaillzadeh, A. Anti-hypertensive effects of cinnamon supplementation in adults: A systematic review and dose-response Meta-analysis of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2020, 60, 3144–3154. [Google Scholar] [CrossRef]
- Tarkhan, M.M.; Balamsh, K.S.; El-Bassossy, H.M. Cinnamaldehyde protects from methylglyoxal-induced vascular damage: Effect on nitric oxide and advanced glycation end products. J. Food Biochem. 2019, 43, e12907. [Google Scholar] [CrossRef]
- Khan, A.U.; Khan, A.U.; Khanal, S.; Gyawali, S. Insect pests and diseases of cinnamon (Cinnamomum verum Presi.) and their management in agroforestry system: A review. Acta Entomol. Zool. 2020, 1, 51–59. [Google Scholar] [CrossRef]
- Attia, R.G.; Rizk, S.A.; Hussein, M.A.; Fatah, H.M.A.; Khalil, M.M.A.; Ma’moun, S.A.M. Effect of cinnamon oil encapsulated with silica nanoparticles on some biological and biochemical aspects of the rice moth, Corcyra cephalonica (Staint.) (Lepidoptera: Pyralidae). Ann. Agric. Sci. 2020, 65, 1–5. [Google Scholar] [CrossRef]
- Kang, Y.J.; Seo, D.G.; Park, S.Y. Phenylpropanoids from cinnamon bark reduced beta-amyloid production by the inhibition of beta-secretase in Chinese hamster ovarian cells stably expressing amyloid precursor protein. Nutr. Res. 2016, 36, 1277–1284. [Google Scholar] [CrossRef] [PubMed]
- Khasnavis, S.; Pahan, K. Cinnamon treatment upregulates neuroprotective proteins Parkin and DJ-1 and protects dopaminergic neurons in a mouse model of Parkinson’s disease. J. Neuroimmune Pharmacol. 2014, 9, 569–581. [Google Scholar] [CrossRef]
- PN-ISO 948; Spices Sampling. Polish Committee for Standardization: Warsaw, Poland, 1980.
- PN-ISO 2825; Herbs and Spices, Preparation of the Ground Sample for Analysis. Polish Committee for Standardization: Warsaw, Poland, 1981.
- Amin, I.; Norazaidah, Y.; Hainida, E.K.I. Antioxidant activity and phenolic content of raw and blanched Amaranthus species. Food Chem. 2006, 94, 47–52. [Google Scholar] [CrossRef]
- PN-A-86965:1997; Herbal Spices—Black Pepper. Polish Committee for Standardization: Warsaw, Poland, 1997.
- PN-EN ISO 6571:2009/A1; Spices and Herbs. Determination of Essential Oil Content (Hydrodistillation Method). Polish Committee for Standardization: Warsaw, Poland, 2009.
- Misharina, T.A.; Terenina, M.B.; Krikunova, N.I. Antioxidant properties of essential oils. Appl. Biochem. Microbiol. 2009, 45, 642–647. [Google Scholar] [CrossRef]
- Gülçin, I. The antioxidant and radical scavenging activities of black pepper (Piper nigrum) seeds. Int. J. Food Sci. Nutr. 2005, 56, 491–499. [Google Scholar] [CrossRef]
- Andradea, K.S.; Ferreira, K.S. Antioxidant activity of black pepper (Piper nigrum L.) oil obtained by supercritical CO2. In Proceedings of the III Iberoamerican Conference on Supercritical Fluids, Cartagena de Indias, Colombia, 1–5 April 2013. [Google Scholar]
- Ahmad, A.; Husain, A.; Mujeeb, M.; Khan, S.A.; Alhadrami, H.A.A.; Bhandari, A. Quantification of total phenol, flavonoid content and pharmacognostical evaluation including HPTLC fingerprinting for the standardization of Piper nigrum Linn fruits. Asian Pac. J. Trop. Biomed. 2015, 5, 101–107. [Google Scholar] [CrossRef]
- Nagy, M.; Socaci, S.A.; Tofana, M.; Pop, C.; Muresan, C.; Pop, A.V.; Salanta, L.; Rotar, A.M. Determination of total phenolics, antioxidant capacity and antimicrobial activity of selected aromatic spices. Bull. UASVM Food Sci. Technol. 2015, 72, 82–85. [Google Scholar] [CrossRef] [Green Version]
- Nahak, G.; Sahu, R.K. Phytochemical evaluation and antioxidant activity of Piper cubeba and Piper nigrum. J. Appl. Pharm. Sci. 2011, 1, 153–157. [Google Scholar]
- Gupta, R.K.; Chawla, P.; Tripathi, M.; Shukla, A.K.; Pandey, A. Synergistic antioxidant activity of tea with ginger, black pepper and tulusi. Int. J. Pharm. Pharm. Sci. 2014, 6, 477–479. [Google Scholar]
- Sruthi, D.; Zachariah, T.J. In vitro antioxidant activity and cytotoxicity of sequential extracts from selected black pepper (Piper nigrum L.) varieties and Piper species. Int. Food Res. J. 2017, 24, 75–85. [Google Scholar]
- Lee, J.-G.; Chae, Y.; Shin, Y.; Kim, Y.-J. Chemical composition and antioxidant capacity of black pepper pericarp. Appl. Biol. Chem. 2020, 63, 35. [Google Scholar] [CrossRef]
- Mallick, M.; Bose, A.; Mukhi, S. Comparative Evaluation of the Antioxidant Activity of Some commonly used Spices. Int. J. PharmTech Res. 2016, 9, 1–8. [Google Scholar]
- Trifan, A.; Zengin, G.; Brebu, M.; Skalicka-Woźniak, K.; Luca, S.V. Phytochemical Characteriza-tion and Evaluation of the Antioxidant and Anti-Enzymatic Activity of Five Common Spices: Focus on Their Essential Oils and Spent Material Extractives. Plants 2021, 210, 2692. [Google Scholar] [CrossRef]
- Suhaj, M.; Rácová, J.; Polovka, M.; Brezová, V. Effect of c-irradiation on antioxidant activity of black pepper (Piper nigrum L.). Food Chem. 2006, 97, 696–704. [Google Scholar] [CrossRef]
- Codex Alimentarius Standard for Black, White and Green Peppers. CXS 326-2017. Available online: https://www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCXS%2B326-2017%252FCXS_326e.pdf (accessed on 4 May 2022).
- European Spice Association (ESA). Quality Minima Document; European Spice Association (ESA): Bonn, Germany, 2015. [Google Scholar]
- Ravindran, P.N. Black Pepper, Piper nigrum, Medicinal and Aromatic Plants—Industrial Profiles. Phytochemistry 2001, 58, 827–829. [Google Scholar]
- Hamrapurkar, P.D.; Jadhav, K.; Zine, S. Quantitative Estimation of Piperine in Piper nigrum and Piper longum Using High Performance Thin Layer Chromatography. J. Appl. Pharm. Sci. 2011, 1, 117–120. [Google Scholar]
- Rajopadhye, A.A.; Upadhye, A.S.; Mujumdar, A.M. HPTLC Method for Analysis of Piperine in Fruits of Piper Species. J. Planar Chromatogr. 2011, 24, 57–59. [Google Scholar] [CrossRef]
- Zachariah, T.J.; Mathew, P.A.; Gobinath, P. Chemical quality of berries from black pepper varieties grafted on Piper colubrinum. J. Med. Aromat. Plant Sci. 2005, 27, 39–42. [Google Scholar]
- Sruthi, D.; Zachariah, T.J.; Leela, N.K.; Jayarajan, K. Correlation between chemical profiles of black pepper (Piper nigrum L.) var. Panniyur-1 collected from different locations. J. Med. Plants Res. 2013, 7, 2349–2357. [Google Scholar]
- Rezvanian, M.; Ooi, Z.T.; Jamal, J.A.; Husain, K.; Jalil, J.; Yaacob, Z.; Ghani, A.A. Pharmacognostical and Chromatographic Analysis of Malaysian Piper nigrum Linn. Fruits. Indian J. Pharm. Sci. 2016, 78, 334–343. [Google Scholar]
- Jansz, E.R.; Pathirana, I.C.; Packiyasothy, E.V. Determination of piperine in pepper (Piper nigrum L.). J. Natl. Sci. Counc. Sri Lanka 1983, 11, 129–138. [Google Scholar] [CrossRef]
- Shango, A.J.; Mkojera, B.T.; Majubwa, R.O.; Mamiro, D.P.; Maerere, A.P. Pre- and postharvest factors affecting quality and safety of Pepper (Piper nigrum L.). CABI Rev. 2021, 16, 031. [Google Scholar] [CrossRef]
- Ajmal, H. Isolation, identification and quantitative analysis of piperine from Piper nigrum Linn. of various regions of kerala by rp-hplc method. World J. Pharm. Pharm. Sci. 2018, 7, 1023–1049. [Google Scholar]
- Shrestha, S.; Chaudhary, N.; Sah, R.; Malakar, N. Analysis of Piperine in Black Pepper by High Performance Liquid Chromatography. J. Nepal Chem. Soc. 2020, 41, 80–86. [Google Scholar] [CrossRef]
- Nikolić, M.; Stojković, D.; Glamočlija, J.; Ćirić, A.; Marković, T.; Smiljković, M.; Soković, M. Could essential oils of green and black pepper be used as food preservatives? J. Food Sci. Technol. 2015, 52, 6565–6573. [Google Scholar] [CrossRef]
- Codex Alimentarius. CODEX STAN 192-1995—Codex General Standard for Food Additives. Adopted in 1995; Revision 1997, 1999, 2001, 2003, 2004, 2005, 2006, 2007, 2008, 2009, 2010, 2011, 2012, 2013; FAO: Rome, Italy; WHO: Geneva, Switzerland, 1995. [Google Scholar]
- Rouatbi, M.; Duquenoy, A.; Giampaoli, P. Extraction of the essential oil of thyme and black pepper by superheated steam. J. Food Eng. 2007, 78, 708–714. [Google Scholar] [CrossRef]
- Pino, J.; Rodriguez-Feo, G.; Borges, P.; Rosado, A. Chemical and sensory properties of black pepper oil. Nahrung 1990, 34, 555–560. [Google Scholar] [CrossRef]
- Zachariah, T.J.; Safeer, A.L.; Jayarajan, K.; Leela, N.K. Correlation of metabolites in the leaf and berries of selected black pepper varieties. Sci. Hortic. 2010, 123, 418–422. [Google Scholar] [CrossRef]
- Kurian, P.S.; Backiyarani, S.; Josephrajkumar, A.; Murugan, M. Varietal evaluation of black pepper (Piper nigrum L.) for yield, quality and anthracnose disease resistance in Idukki District, Kerala. J. Spices Aromat. Crops 2002, 11, 22–24. [Google Scholar]
- Rmili, R.; Ramdani, M.; Ghazi, Z.; Saidi, N.; El Mahi, B. Composition comparison of essential oils extracted by hydrodistillation and microwave-assisted hydrodistillation from Piper nigrum L. J. Mater. Environ. Sci. 2014, 5, 1560–1567. [Google Scholar]
- Hussain, M.D.S.; Hegde, L.; Goudar, S.A.; Hegde, N.K.; Shantappa, T.; Gurumurthy, S.B.; Manju, M.J.; Shivakumar, K.M. Evaluation of local black pepper (Piper nigrum L.) genotypes for yield and quality under arecanut based cropping system. Int. J. Pure Appl. Biosci. 2017, 5, 1396–1400. [Google Scholar] [CrossRef]
- Abraham, K. Cinnamon and coumarin—Clarification from the scientific and administrative angle. Dtsch. Lebensm. Rundsch. 2007, 103, 480–487. [Google Scholar]
- Yang, C.C.H.; Li, R.X.; Chuang, L.Y. Antioxidant activity of various parts of Cinnamomum cassia extracted with different extraction methods. Molecules 2012, 17, 7294–7304. [Google Scholar] [CrossRef] [PubMed]
- Mathew, S.; Abraham, T. Studies on the antioxidant activities of cinnamon (Cinnamomum verum) bark extracts, through various in vitro models. Food Chem. 2006, 94, 520–528. [Google Scholar] [CrossRef]
- Dragland, S.; Senoo, H.; Wake, K.; Holte, K.; Blomhoff, R. Several culinary and medicinal herbs are important sources of dietary antioxidants. J. Nutr. 2003, 133, 1286–1290. [Google Scholar] [CrossRef]
- Foti, M.; Piattelli, M.; Baratta, M.T.; Ruberto, G. Flavonoids, coumarins, and cinnamic acids as antioxidants in a micellar system structure-activity relationship. J. Agric. Food Chem. 1996, 44, 497–501. [Google Scholar] [CrossRef]
- Lin, C.C.; Wu, S.J.; Chang, C.H.; Ng, L.K. Antioxidant activity of Cinnamomum cassia. Phytother. Res. 2003, 17, 726–730. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Kim, B.S.; Kim, M.K. Suppression effect of Cinnamomum barks-derived component on nitric oxide synthase. J. Agric. Food Chem. 2002, 50, 7700–7703. [Google Scholar] [CrossRef] [PubMed]
- Prasad, K.N.; Yang, B.; Dong, X.; Jiang, G.; Zhang, H.; Xie, H.; Jiang, Y. Flavonoid contents and antioxidant activities from Cinnamomum species. Innov. Food Sci. Emerg. Technol. 2009, 10, 627–632. [Google Scholar] [CrossRef]
- Shobana, S.; Naidu, K.A. Antioxidant activity of selected Indian spices. Prostaglandins Leukot. Essent. Fat. Acids 2000, 62, 107–110. [Google Scholar] [CrossRef]
- Murcia, M.A.; Egea, I.; Romojaro, F.; Parras, P.; Jimenez, A.M.; Martinez-Tom, M. Antioxidant evaluation in dessert spices compared with common food additives. Influence of irradiation procedure. J. Agric. Food Chem. 2004, 52, 1872–1881. [Google Scholar] [CrossRef] [PubMed]
- Prakash, D.; Suri, S.; Upadhyay, G.; Singh, B.N. Total phenol, antioxidant and free radical scavenging activities of some medicinal plants. Int. J. Food Sci. Nutr. 2007, 58, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.C.; Yu, C.; Wu, S.J.; Yih, K. DPPH free-radical scavenging activity, total phenolic contents and chemical composition analysis of forty-two kinds of essential oils. J. Food Drug Anal. 2009, 17, 386–395. [Google Scholar] [CrossRef]
- Lee, R.; Balic, M.J. Sweet wood—Cinnamon and its importance as a spice and medicine. Ethnomedicine 2005, 1, 61–64. [Google Scholar] [CrossRef]
- Jayatilaka, A.; Poole, S.K.; Poole, C.F.; Chichila, T.M.P. Simultaneous micro steam distillation/solvent extraction for the isolation of semivolatile flavor compounds from cinnamon and their separation by series coupled-column gas chromatography. Anal. Chim. Acta 1995, 302, 147–162. [Google Scholar] [CrossRef]
- Sproll, C.; Ruge, W.; Andlauer, C.; Godelmann, R.; Lachenmeier, D.W. HPLC analysis and safety assessment of coumarin in foods. Food Chem. 2008, 109, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Blahova, J.; Svobodova, Z. Assessment of coumarin levels in ground cinnamon available in the Czech retail market. Sci. World J. 2012, 2012, 263851. [Google Scholar] [CrossRef] [PubMed]
- Woehrlin, F.; Fry, H.; Abraham, K.; Preiss-Weigert, A. Quantificion of Flavoring Constituents in Cinnamon High Variation of Coumarin in Cassia Bark from the German Retail Market and in Authentic Samples from Indonesia. J. Agric. Food Chem. 2010, 58, 10568–10575. [Google Scholar] [CrossRef] [PubMed]
- He, Z.D.; Qiao, C.F.; Han, Q.B.; Cheng, C.L.; Xu, H.X.; Jiang, R.W.; But, P.P.H.; Shaw, P.C. Authentication and quantitative analysis on the chemical profile of cassia bark (cortex. cinnamomum) by high pressure liquid chromatography. J. Agric. Food Chem. 2005, 53, 2424–2428. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.-C.; Hang, K.-S.; Hang, P.-W. Inhibition of neuroinflammation by cinnamon and its main componenets. Food Chem. 2013, 138, 2275–2282. [Google Scholar] [CrossRef] [PubMed]
- Lungarini, S.; Aureli, F.; Coni, E. Coumarin and cinnamaldehyde in cinnamon marketed in Italy: A natural chemical hazard? Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2008, 25, 1297–1305. [Google Scholar] [CrossRef] [PubMed]
- Ananthakrishnan, R.; Chandra, P.; Kumar, B.; Rameshkumar, K.B. Quantification of coumarin and related phenolics in cinnamon samples from south India using UHPLC-ESI-QqQLIT-MS/MS method. Int. J. Food Prop. 2018, 21, 50–57. [Google Scholar] [CrossRef]
- Raters, M.; Matissek, R. Analysis of coumarin in various foods using liquid chromatography with tandem mass spectrometric detection. Eur. Food Res. Technol. J. 2008, 227, 637–642. [Google Scholar] [CrossRef]
- Lee, C.J.; Chen, L.G.; Chang, T.L.; Ke, W.M.; Lo, Y.F.; Wang, C.C. The correlation between skin-care effects and phytochemical contents in Lamiaceae plants. Food Chem. 2011, 124, 833–841. [Google Scholar] [CrossRef]
- Regulation of the European Parliament and of the Council (EC) No. 1334/2008 of 16 December 2008 on Flavourings and Certain Food Ingredients with Flavouring Properties for Use in and on Foods and Amending Council Regulation (EEC) no 1601/91, Regulations (EC) no 2232/96 and (EC) no 110/2008 and Directive 2000/13/EC. Off. J. Eur. Communities 2008, 12, 34–50.
- French Agency for Food, Environmental and Occupational Health & Safety. Opinion of the French Agency for Food, Environmental and Occupational Health & Safety—Assessment of the Risk of Hepatotoxicity Associated with the Coumarin Content of Certain Plants That Can Be Consumed in Food Supplements or in Other Foodstuffs; ANSES Opinion Request No. 2018-SA-0180; ANSES: Maisons-Alfort, France, 2021. [Google Scholar]
- Balin, N.Z.; Sørensen, A.T. Coumarin content in cinnamon containing food products on the Danish market. Food Control 2014, 38, 198–203. [Google Scholar] [CrossRef]
- Steffensen, I.L.; Alexander, J.; Binderup, M.-L.; Helkåas, K.; Dahl Granum, B.; Hetland, R.B.; Husøy, T.; Paulsen, J.E.; Sanner, T.; Thrane, V. Risk Assessment of Coumarin Intake in the Norwegian Population. Eur. J. Nutr. Food Saf. 2019, 11, 72–75. [Google Scholar] [CrossRef]
- Schifferstein, H.N.J.; Kudrowitz, B.M.; Breuer, C. Food Perception and Aesthetics—Linking Sensory Science to Culinary Practice. J. Culin. Sci. Technol. 2020, 20, 293–335. [Google Scholar] [CrossRef]
- Embuscado, M.E. Bioactives from culinary spices and herbs: A review. J. Food Bioact. 2019, 6, 68–99. [Google Scholar] [CrossRef]
- Almatroodi, S.A.; Alashi, M.A.; Almatroodi, A.; Anwar, S.; Verma, A.K.; Dev, K.; Rahmani, A.H. Cinnamon and its active compounds: A potential candidate in disease and tumour management through modulating various genes activity. Gene Rep. 2020, 21, 100966. [Google Scholar] [CrossRef]

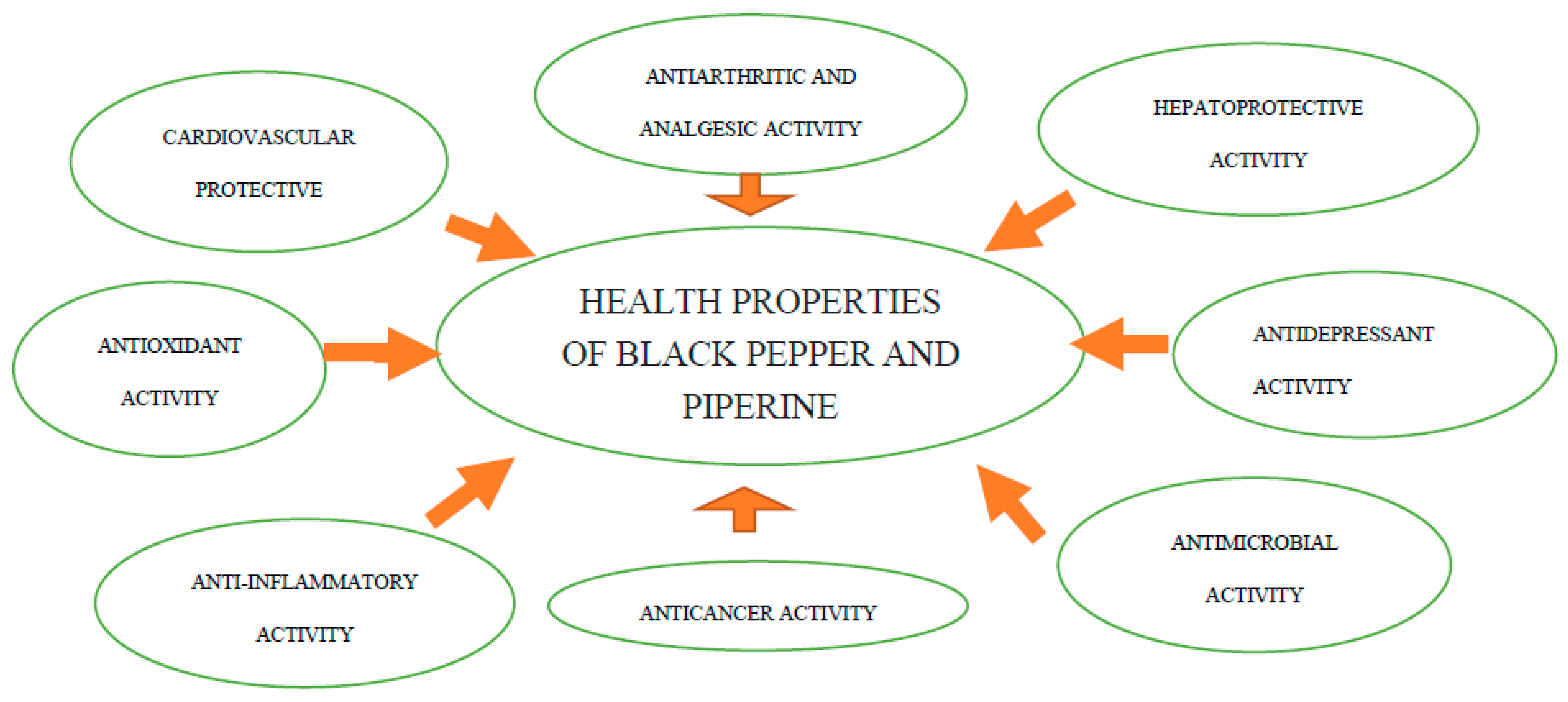


| Health Effects | Source |
|---|---|
| Antiallergic effect | Aswar et al., 2015; Kim & Lee 2009 [27,28] |
| Analgetic activity | Bukhari et al., 2013; Jeena et al., 2014; Tasleem et al., 2014 [29,30,31] |
| Antiarthritic activity | Bang et al., 2009; Umar et al., 2013 [32,33] |
| Anticancer activity | Banerjee et al., 2021; Greenshields et al., 2015; Morsy & Abd El-Salam 2017; Prashant et al., 2017 [34,35,36,37] |
| Antidiabetic activity | Atal et al., 2016; Kharbanda et al., 2016; Oboh et al., 2013; Sarfraz et al., 2021 [38,39,40,41] |
| Antidepressant activity | Emon et al., 2020; Ghosh et al., 2021; Hritcu et al., 2015; Mao et al., 2014 [42,43,44,45] |
| Antihypertensive activity | Hlavačková et al., 2011; Lee et al., 2015; Taqvi et al., 2008; [46,47,48] |
| Antiinflammatory activity | Bang et al., 2009; Jeena et al., 2014; Tasleem et al., 2014; Yu et al., 2020 [30,31,32,49] |
| Antimicrobial activity | Bawazeer et al., 2022; Chen et al., 2019; Daigham & Mahfouz 2020; Hien & Dao 2022; Martinelli et al., 2017; Morsy & Abd El-Salam 2017; Zhang et al., 2017; Zou et al., 2015 [36,50,51,52,53,54,55,56] |
| Antineurodegenerative activity | Chonpathompikunlert 2010; Elnaggar et al., 2015; Etman et al., 2018 [57,58,59] |
| Antiobesity activity | Du et al., 2020; Lailiyah et al., 2021; Meriga et al., 2017; Shah et al., 2011 [60,61,62,63] |
| Antioxidant activity | Jeena et al., 2014; Srinivasan 2007; Vijayakumar & Nalini 2006 [30,64,65] |
| Cardiovascular protection | Dutta et al., 2014; Taqvi et al., 2008; Wang et al., 2021 [48,66,67] |
| Gastrointestinal and antidiarrheal activity | Mehmood & Gilani 2010; Srinivasan 2007; Shamkuwar et al., 2012; Shamkuwar 2013 [64,68,69,70] |
| Hepatoprotective and pancreatitis activity | Bae et al., 2011; Christina et al., 2006; Gurumurthy et al., 2012; Matsuda et al., 2008; Nirwane & Bapat 2011 [71,72,73,74,75] |
| Systematic Term | Synonym | Common Term | Occurrence | Source |
|---|---|---|---|---|
| Cinnamomum verum J. Presl | Cinnamomum zeylanicum Blume | Ceylon cinnamon True cinnamon | Sri Lanca | Weerasekera et al., 2021 [76] |
| Cinnamomum cassia (L.) J.Presl | Cinnamomum aromaticum Ness | Chinese cinnamon | China, North-east asia | Zhang et al., 2017 [55] |
| Cinnamomum burmannii (Nees & T.Nees) Blume | — | Indonesian cinnamon Java cinnamon | Indonesia, Wietnam, Filipines | Al-Dhubiab 2012 [77] |
| Cinnamomum tamala (Buch.-Ham.) T.Ness & C.H. Eberm. | Cinnamomum albiflorum Ness Cinnamomum lindleyi Lukman Laurus tamala Buch.-Ham. | Indian cassia Indian bay leaf Tejpatta | India, Nepal, Bhutan, China | Tiwari & Talreja 2020 [78] |
| Cinnamomum loureiroi Ness | — | Saigon cinnamon Vietnamese cinnamon | Wietnam | Kumar et al., 2019 [79] |
| Cinnamon | Composition |
|---|---|
| Cinnamomum verum | cinnamaldehyde 1.99% (1.49–3.20%), cinnamylacetate, cinnamon alcohol 0.043% (nd–0.083%), eugenol, coumarin (nd–0.004%) |
| Cinnamomum cassia | cinnamaldehyde (0.005–9.383%), cinnamic acid (0.001–0.191%), cinnamyl alcohol (0.001–0.177%) cinnamamyl acetate, cinnamon alcohol, eugenol, coumarin 0.001–1.218% (up to 5%) |
| Health Properties | Source |
|---|---|
| Analgetic activity | Pandey & Chandra 2015 [91] |
| Anticancer activity | Dutta & Chakraborty 2018; Thompson et al., 2019 [92,93] |
| Antidiabetes activity | Anderson et al., 2015; Zare et al., 2019; Shinjyo et al., 2020 [94,95,96] |
| Antiinflammatory activity | Han & Parker 2017; Schink et al., 2018; Shishehbor et al., 2018 [97,98,99] |
| Antimicrobial activity | Abd El-Hack et al., 2020; Arancibia et al., 2014; Atki et al., 2019 [100,101,102] |
| Antiobesity activity | Mnafgui et al., 2015 [103] |
| Antioxidant activity | Gulcin et al., 2019; Weerasekera et al., 2021 [76,104] |
| Cardiovascular protection | Jain et al., 2017; Mnafgui et al., 2015; Mousavi et al., 2020; Tarkhan et al., 2019 [103,105,106,107] |
| Insecticidal effect | Khan et al., 2020; Attia et al., 2020 [108,109] |
| Neurological disorders | Kang et al., 2016; Khasnavis & Pahan 2014 [110,111] |
| n | TP M ± SD [mg GAE/g] | AADPPH M [%] | Piperine M ± SD [%] | Essential Oil M ± SD [mL/100 g d.m.] | |
|---|---|---|---|---|---|
| 1 | 3 | 13.02 ± 1.56 | 84.42 ± 2.32 | 6.49 ± 0.07 | 1.09 ± 0.09 |
| 2 | 3 | 32.13 ± 0.24 | 84.51 ± 3.92 | 5.26 ± 0.03 | 1.29 ± 0.01 |
| 3 | 3 | 11.52 ± 0.30 | 83.93 ± 1.29 | 7.11 ± 0.05 | 1.77 ± 0.08 |
| 4 | 3 | 13.18 ± 0.35 | 85.42 ± 2.34 | 9.23 ± 0.05 | 2.05 ± 0.17 |
| 5 | 3 | 11.72 ± 0.85 | 84.86 ± 3.79 | 6.19 ± 0.05 | 1.12 ± 0.01 |
| 6 | 3 | 11.90 ± 0.29 | 82.67 ± 3.99 | 5.77 ± 0.04 | 1.48 ± 0.08 |
| 7 | 3 | 10.67 ± 1.30 | 85.32 ± 4.71 | 6.40 ± 0.05 | 1.31 ± 0.08 |
| 8 | 3 | 14.41 ± 0.73 | 81.49 ± 2.22 | 5.91 ± 0.00 | 2.08 ± 0.08 |
| 9 | 3 | 11.37 ± 0.18 | 82.01 ± 6.48 | 6.17 ± 0.04 | 1.43 ± 0.00 |
| 10 | 3 | 11.80 ± 1.13 | 80.64 ± 3.21 | 6.61 ± 0.02 | 1.03 ± 0.16 |
| 11 | 3 | 12.29 ± 0.78 | 84.27 ± 4.43 | 7.19 ± 0.05 | 2.18 ± 0.08 |
| 12 | 3 | 11.18 ± 0.06 | 80.85 ± 3.84 | 5.77 ± 0.04 | 1.75 ± 0.08 |
| 13 | 3 | 10.88 ± 0.37 | 85.13 ± 2.93 | 7.19 ± 0.05 | 1.55 ± 0.14 |
| 14 | 3 | 13.31 ± 0.12 | 81.73 ± 5.11 | 5.90 ± 0.06 | 1.32 ± 0.17 |
| 15 | 3 | 12.38 ± 0.54 | 78.07 ± 3.27 | 6.44 ± 0.03 | 1.86 ± 0.14 |
| 16 | 3 | 12.85 ± 1.02 | 84.45 ± 4.12 | 3.92 ± 0.35 | 1.61 ± 0.16 |
| 17 | 3 | 11.59 ± 0.27 | 71.29 ± 3.84 | 4.87 ± 0.02 | 1.48 ± 0.08 |
| 18 | 3 | 12.03 ± 0.32 | 81.83 ± 4.01 | 5.55 ± 0.02 | 1.42 ± 0.07 |
| 19 | 3 | 11.65 ± 0.70 | 84.60 ± 5.82 | 3.98 ± 0.06 | 2.17 ± 0.14 |
| 20 | 3 | 14.02 ± 1.08 | 85.34 ± 4.17 | 5.78 ± 0.64 | 1.47 ± 0.08 |
| 21 | 3 | 12.85 ± 1.02 | 84.43 ± 2.22 | 4.61 ± 0.12 | 1.76 ± 0.08 |
| 22 | 3 | 11.24 ± 0.53 | 84.59 ± 2.81 | 4.95 ± 0.17 | 1.28 ± 0.14 |
| 23 | 3 | 9.75 ± 0.96 | 73.88 ± 3.23 | 4.87 ± 0.02 | 0.89 ± 0.08 |
| 24 | 3 | 14.53 ± 0.61 | 81.48 ± 1.11 | 5.58 ± 0.01 | 1.48 ± 0.08 |
| 25 | 3 | 11.28 ± 0.74 | 63.93 ± 0.86 | 4.51 ± 0.22 | 2.19 ± 0.15 |
| n | TP M ± SD [mgGAE/g] | AADPPH M [%] | Coumarine M ± SD [mg/kg] | |
|---|---|---|---|---|
| 1 | 3 | 94.71 ± 3.34 | 90.48 ± 4.13 | 1027.67 ± 50.36 |
| 2 | 3 | 92.32 ± 2.01 | 69.39 ± 2.88 | 3120.67 ± 81.25 |
| 3 | 3 | 85.77± 1.29 | 88.285 ± 1.49 | 2107.33 ± 103.24 |
| 4 | 3 | 74.58 ± 5.71 | 87.57 ± 2.11 | 3157.67 ± 59.77 |
| 5 | 3 | 73.38 ± 3.82 | 55.52 ± 7.56 | 2240.67 ± 57.01 |
| 6 | 3 | 72.33 ± 5.53 | 85.50 ± 2.65 | 3383.67 ± 57.07 |
| 7 | 3 | 71.22 ± 1.16 | 82.53 ± 1.99 | 2368.33 ± 37.58 |
| 8 | 3 | 77.575 ± 1.59 | 72.03 ± 3.47 | 3111.12 ± 34.28 |
| 9 | 3 | 52.345 ± 0.96 | 68.19 ± 3.47 | 3564.67 ± 42.12 |
| 10 | 3 | 69.16 ± 1.24 | 76.12 ± 2.81 | 3066.33 ± 60.12 |
| 11 | 3 | 97.17 ± 2.18 | 91.87 ± 2.93 | 2725.32 ± 41.29 |
| 12 | 3 | 94.64 ± 2.36 | 72.64 ± 3.02 | 4012.00 ± 79.57 |
| 13 | 3 | 64.38 ± 1.67 | 90.48 ± 3.33 | 3198.71 ± 81.82 |
| 14 | 3 | 88.27 ± 1.11 | 79.94 ± 4.61 | 3798.00 ± 90.04 |
| 15 | 3 | 79.25 ± 2.13 | 91.11 ± 2,63 | 3812.24 ± 61.29 |
| 16 | 3 | 69.91 ± 1.18 | 87.51 ± 1.85 | 2603.77 ± 83.64 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Newerli-Guz, J.; Śmiechowska, M. Health Benefits and Risks of Consuming Spices on the Example of Black Pepper and Cinnamon. Foods 2022, 11, 2746. https://doi.org/10.3390/foods11182746
Newerli-Guz J, Śmiechowska M. Health Benefits and Risks of Consuming Spices on the Example of Black Pepper and Cinnamon. Foods. 2022; 11(18):2746. https://doi.org/10.3390/foods11182746
Chicago/Turabian StyleNewerli-Guz, Joanna, and Maria Śmiechowska. 2022. "Health Benefits and Risks of Consuming Spices on the Example of Black Pepper and Cinnamon" Foods 11, no. 18: 2746. https://doi.org/10.3390/foods11182746
APA StyleNewerli-Guz, J., & Śmiechowska, M. (2022). Health Benefits and Risks of Consuming Spices on the Example of Black Pepper and Cinnamon. Foods, 11(18), 2746. https://doi.org/10.3390/foods11182746






