Electrophysiological Brain Response to Error in Solving Mathematical Tasks
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
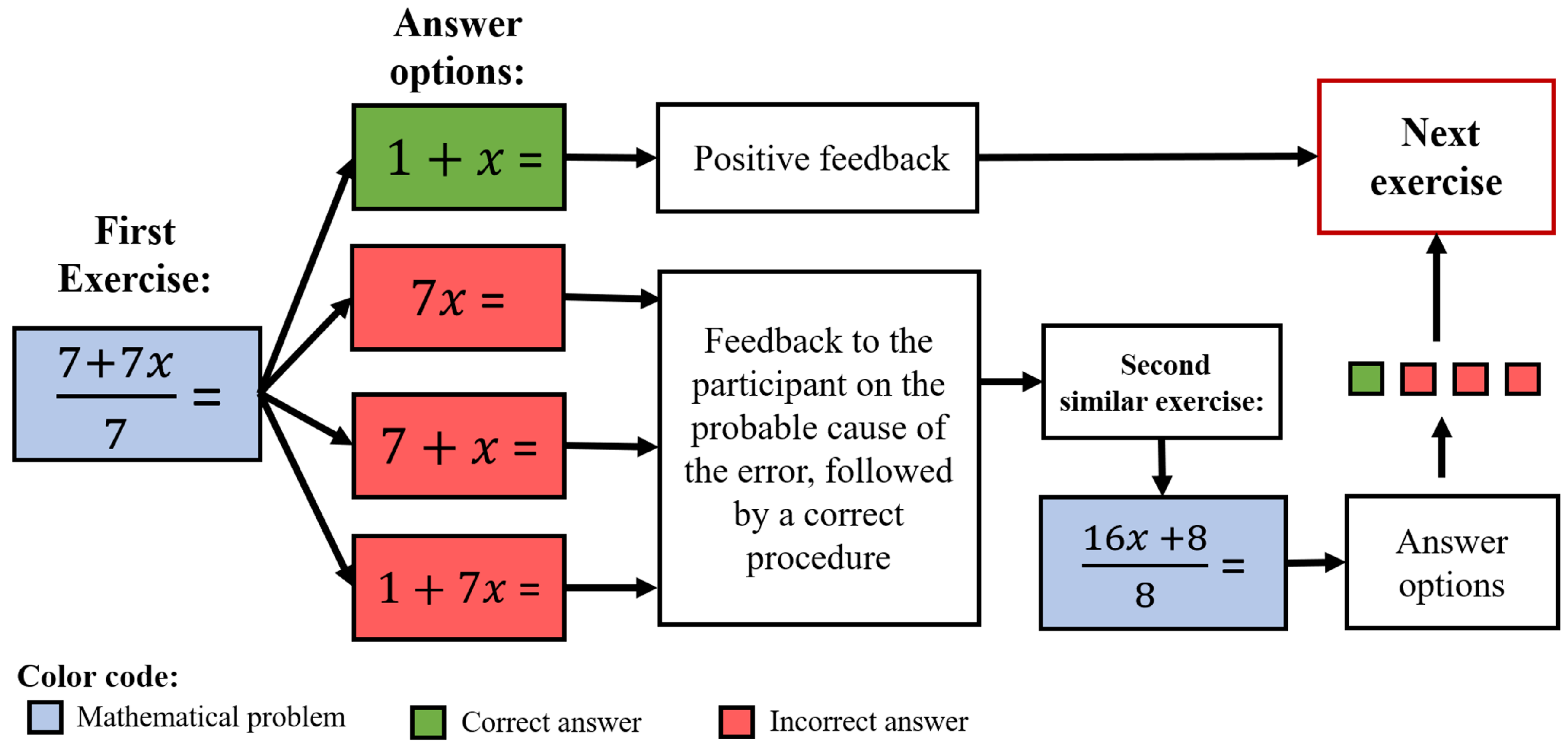
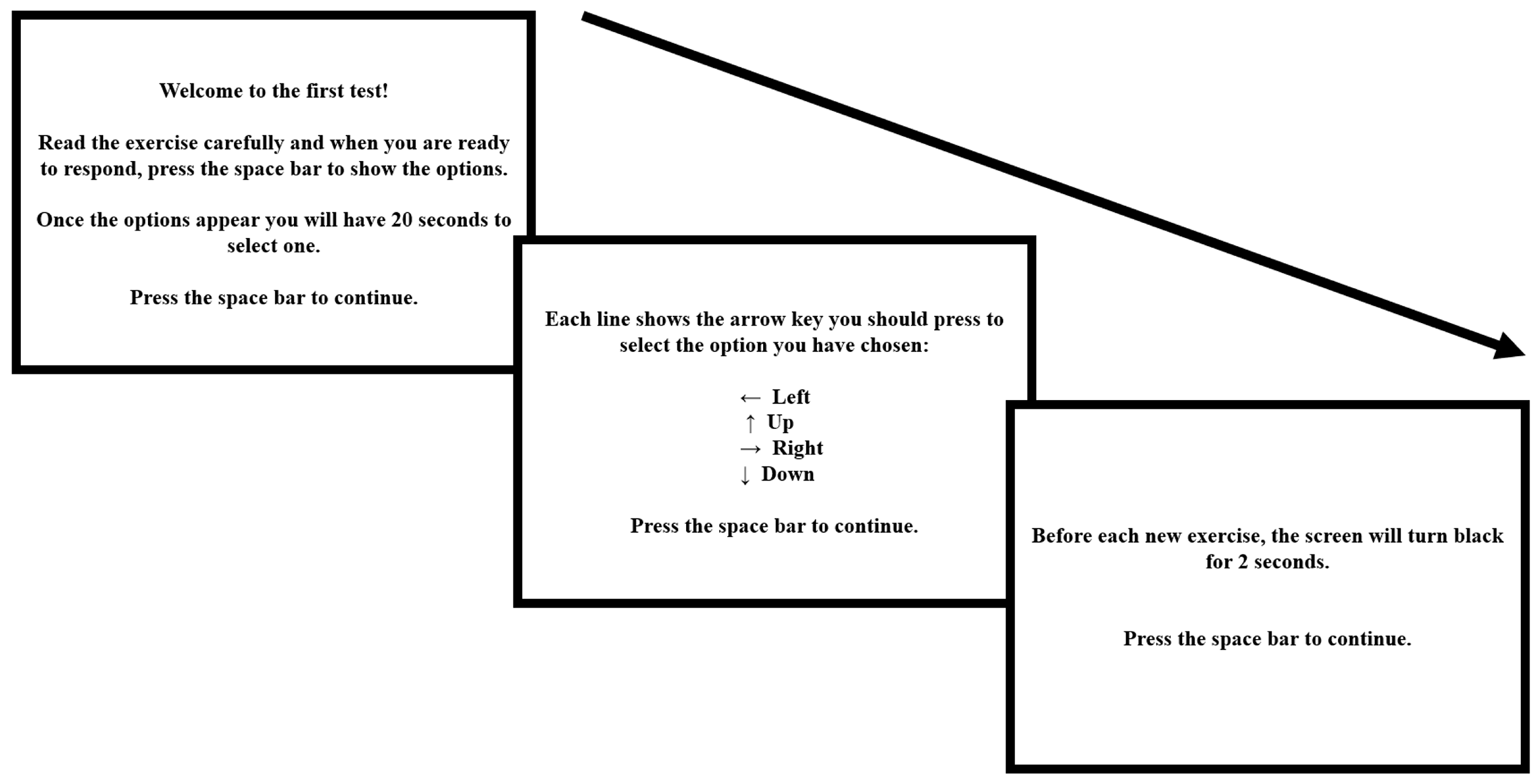
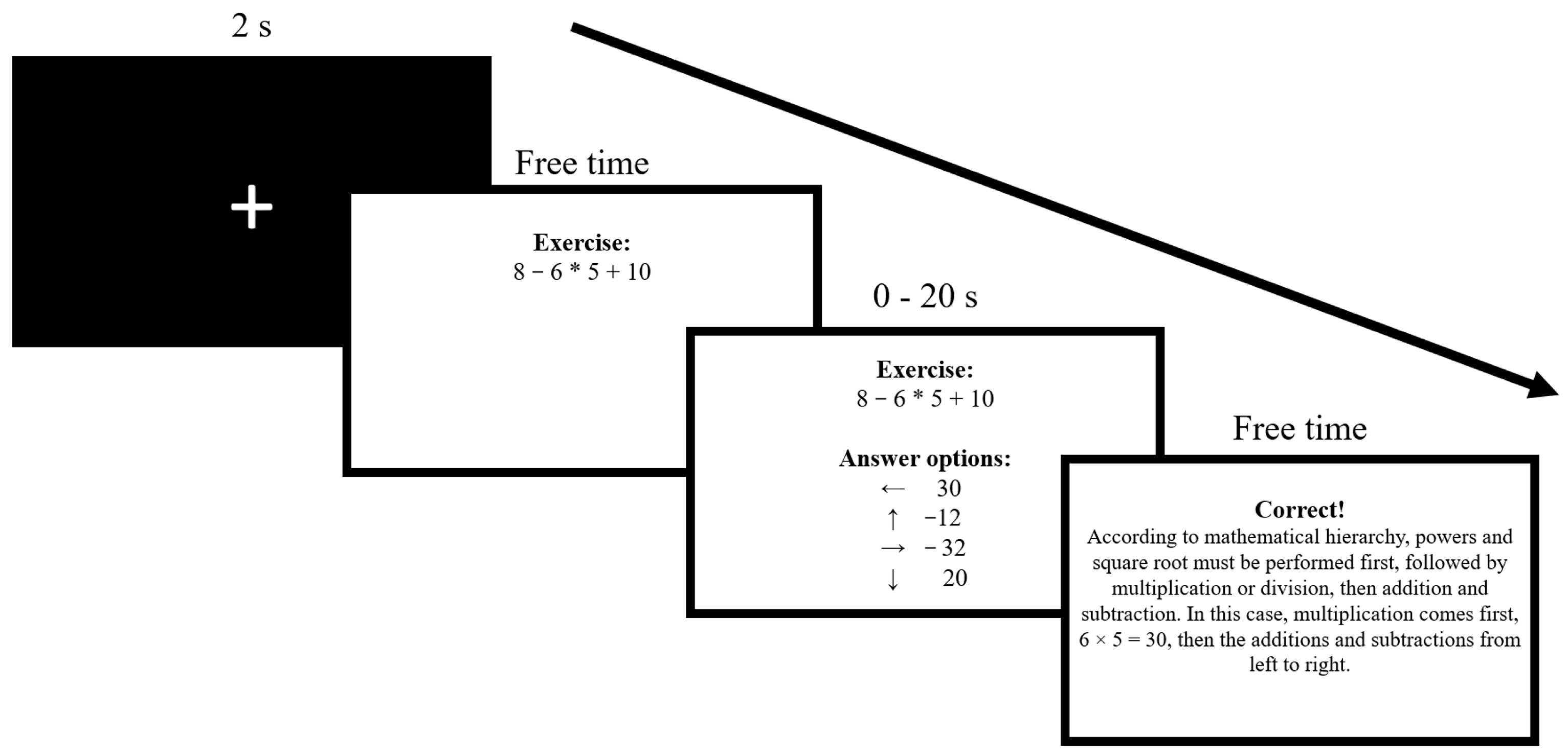
2.2. Experimental Task
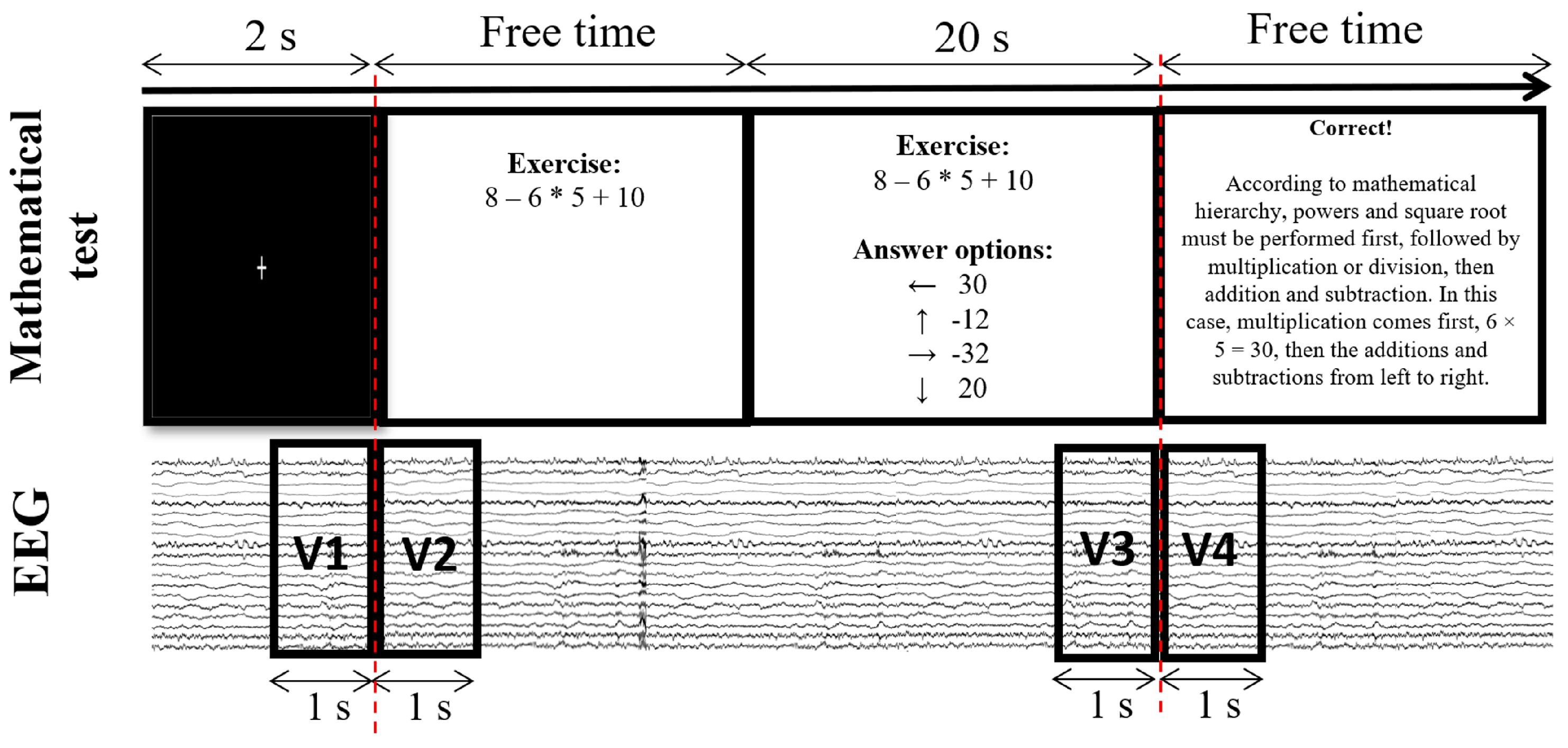
2.3. Recording Cerebral Activity
2.4. Behavioral Analysis
2.5. Electrophysiological Analysis
3. Results
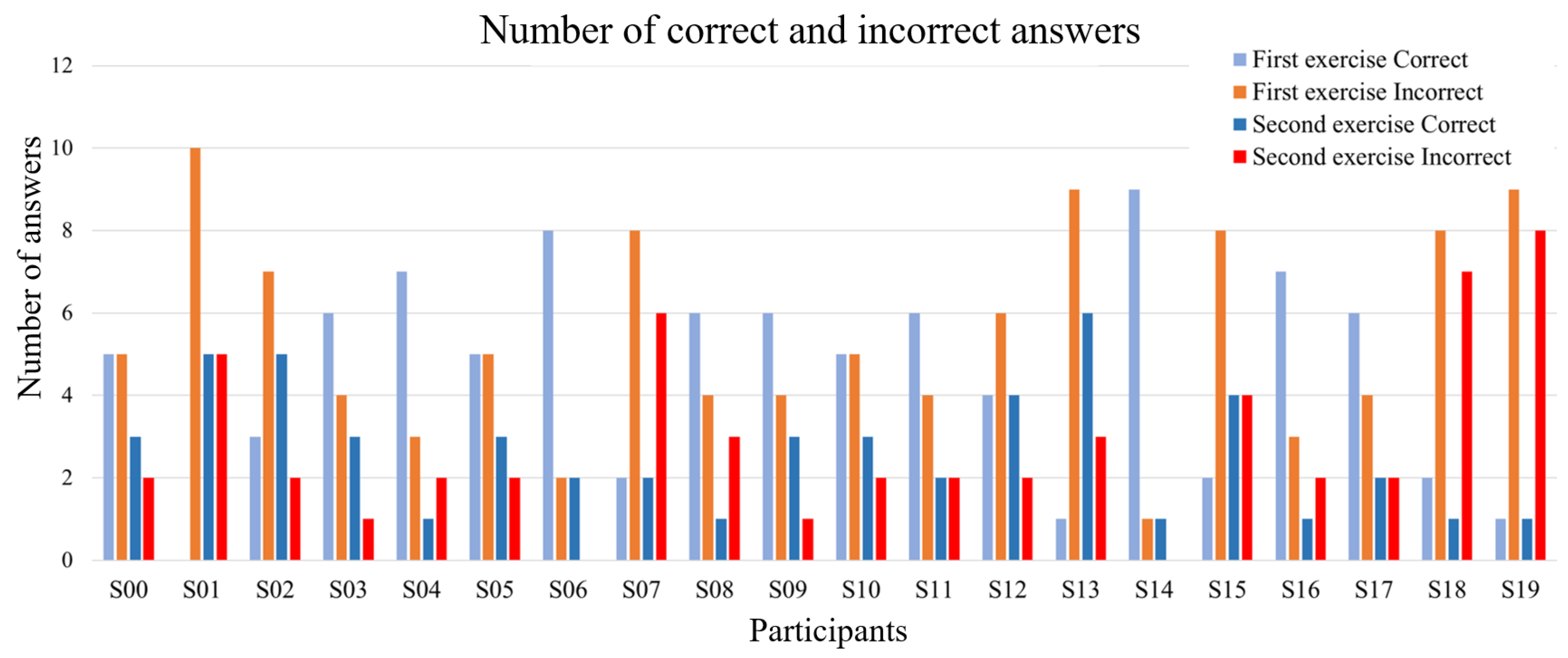
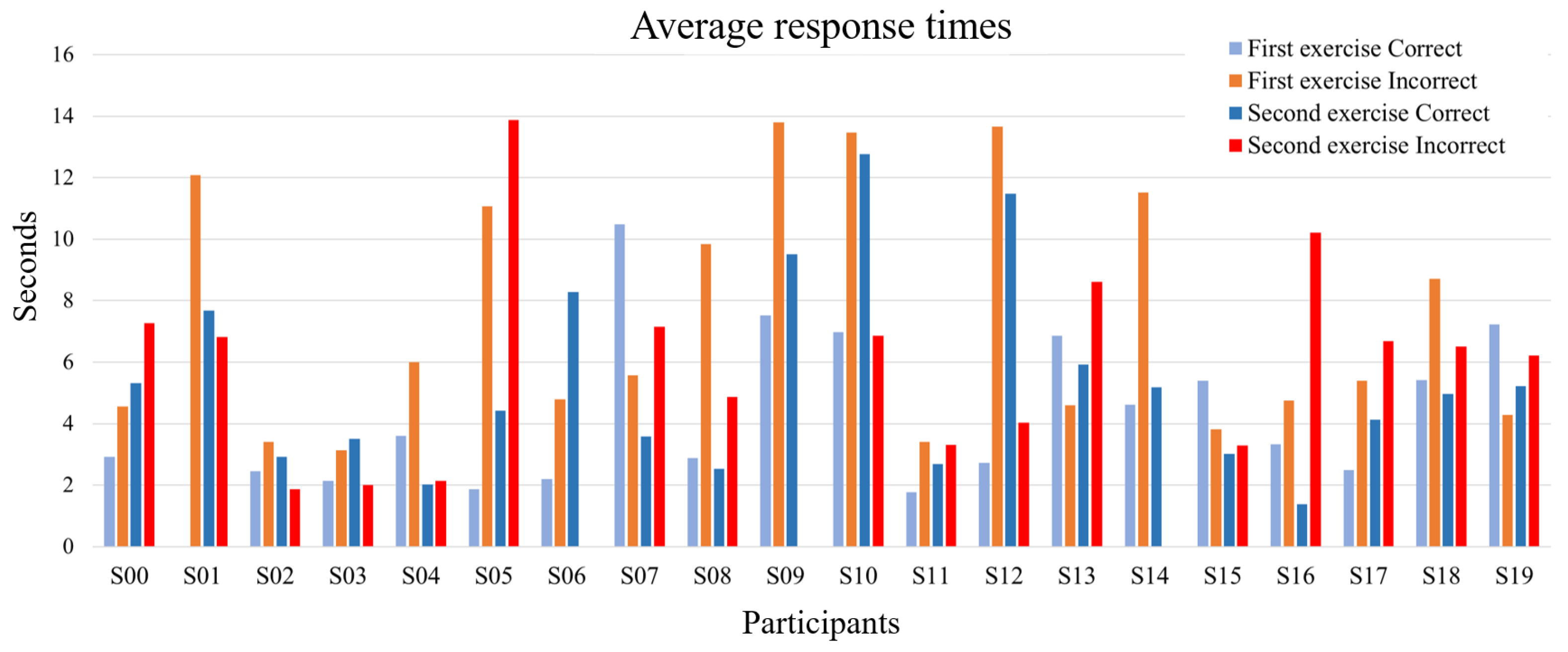
3.1. Behavioral Results
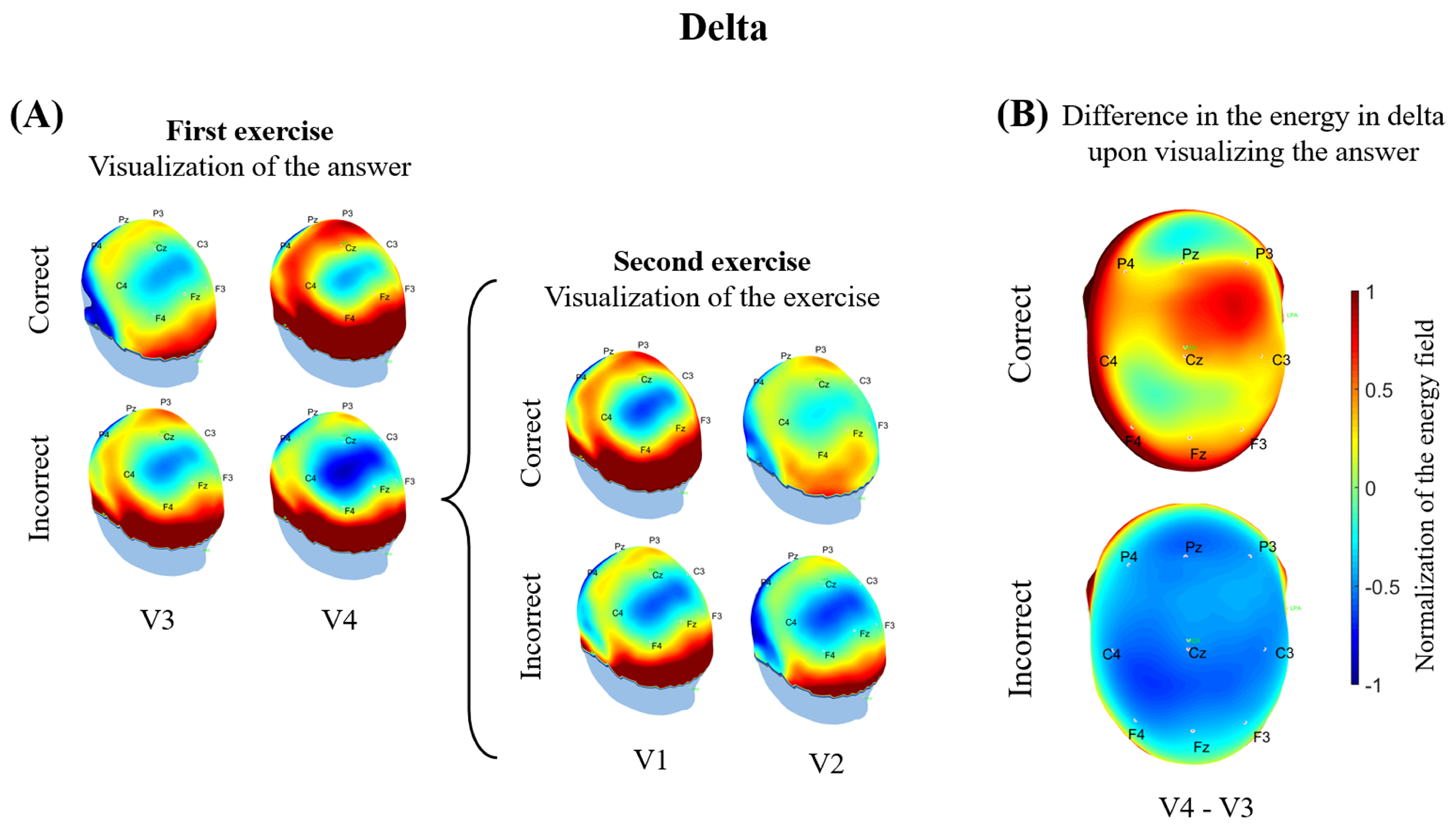
3.2. Electrophysiological Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
- Two items to test the concepts of mathematical hierarchy or order of operations, where common mistakes include calculating from left-to-right (item 1) and considering the sign as part of the quantity to be squared (item 2).
- Three items to evaluate the simplification of rational algebraic expressions that do not require factorization. The main error expected was a strikeout scheme.
- Two items tested simplifying rational algebraic expressions that required factorization, one with a notable product, the other with compound common factor factorization. In one item, simplifying the algebraic fraction required factorizing a perfect square trinomial. Expected errors were a “simplification” by means of a crossed-out scheme of the quadratic terms in the numerator and denominator or incorrect factorization. In the other, factorization was by a common factor that required identifying a compound common term.
- In this item, the numerator was not factorable, so only the expression in the numerator could be reduced.
- The challenge in this item was that factorization required knowledge of the laws of exponents. A common error consists of “simplifying” one of the numerator terms with the denominator term (i.e., wrong strikeout scheme).
- The last item was designed to generate a linearization error [40]; that is, the most common answers involve a conceptual error called concatenation.
References
- Lin, F.R.; Kao, C.M. Mental effort detection using EEG data in E-learning contexts. Comput. Educ. 2018, 122, 63–79. [Google Scholar] [CrossRef]
- Galán, F.C.; Beal, C.R. EEG estimates of engagement and cognitive workload predict math problem solving outcomes. In International Conference on User Modeling, Adaptation, and Personalization; Springer: Berlin/Heidelberg, Germany, 2012; pp. 51–62. [Google Scholar]
- Sakkalis, V. Review of advanced techniques for the estimation of brain connectivity measured with EEG/MEG. Comput. Biol. Med. 2011, 4, 1110–1117. [Google Scholar] [CrossRef]
- Khedher, A.B.; Jraidi, I.; Frasson, C. Tracking students’ mental engagement using EEG signals during an interaction with a virtual learning environment. J. Intell. Learn. Syst. Appl. 2019, 11, 89991. [Google Scholar] [CrossRef]
- Lubar, J.F.; Bianchini, K.J.; Calhoun, W.H.; Lambert, E.W.; Brody, Z.H.; Shabsin, H.S. Spectral analysis of EEG differences between children with and without learning disabilities. J. Learn. Disabil. 1985, 18, 403–408. [Google Scholar] [CrossRef]
- Dutta, S.; Hazra, S.; Nandy, A. Human cognitive state classification through ambulatory eeg signal analysis. In International Conference on Artificial Intelligence and Soft Computing; Springer: Cham, Switzerland, 2019; pp. 169–181. [Google Scholar]
- Yeh, M.K.C.; Gopstein, D.; Yan, Y.; Zhuang, Y. Detecting and comparing brain activity in short program comprehension using EEG. In Proceedings of the 2017 IEEE Frontiers in Education Conference (FIE), Indianapolis, IA, USA, 18–21 October 2017; pp. 1–5. [Google Scholar]
- Choi-Koh, S.S.; Ryoo, B.G. Differences of math anxiety groups based on two measurements, MASS and EEG. Educ. Psychol. 2019, 39, 659–677. [Google Scholar] [CrossRef]
- Garavalia, L.; Ray, M.; Murdoch, T.; Gredler, M. A comparative analysis of achievement calibration accuracy in developmental and nondevelopmental college students. Res. Teach. Dev. Educ. 2004, 21, 29–39. [Google Scholar]
- Macbeth, G.; Cortada de Kohan, N.; Kohan Cortada, A. Efecto de la experticia matemática en el sesgo de sobreconfianza. Perspect. Psicol. 2007, 4, 13–18. [Google Scholar]
- Kruger, J.; Dunning, D. Unskilled and unaware of it: How difficulties in recognizing one’s own incompetence lead to inflated self-assessments. J. Personal. Soc. Psychol. 1999, 77, 1121. [Google Scholar] [CrossRef]
- Moore, D.A.; Schatz, D. Overprecision increases subsequent surprise. PLoS ONE 2020, 15, e0227084. [Google Scholar] [CrossRef]
- Weinberg, A.; Meyer, A.; Hale-Rude, E.; Perlman, G.; Kotov, R.; Klein, D.N.; Hajcak, G. Error-related negativity (ERN) and sustained threat: Conceptual framework and empirical evaluation in an adolescent sample. Psychophysiology 2016, 53, 372–385. [Google Scholar] [CrossRef]
- Briceño, M.T. El uso del error en los ambientes de aprendizaje: Una visión transdisciplinaria. Rev. Teor. Didáct. Cienc. Soc. 2009, 14, 9–28. [Google Scholar]
- Wang, Y.; Cheung, H.; Yee, L.T.S.; Tse, C.Y. Feedback-related negativity (FRN) and theta oscillations: Different feedback signals for non-conform and conform decisions. Biol. Psychol. 2020, 153, 107880. [Google Scholar] [CrossRef]
- Ortiz, E. How the Brain’s Performance during Mathematics and Reading Fluency Tests Compare. Am. Educ. Res. Assoc. 2011. Online Submission. [Google Scholar]
- Zan, R.; Brown, L.; Evans, J.; Hannula, M.S. Affect in mathematics education: An introduction. Educ. Stud. Math. 2006, 63, 113–121. [Google Scholar] [CrossRef] [Green Version]
- Gehring, W.J.; Coles, M.G.; Meyer, D.E.; Donchin, E. A brain potential manifestation of error-related processing. Electroencephalogr. Clin. Neurophysiol.-Suppl. Only 1995, 44, 261–272. [Google Scholar]
- Trujillo, L.T.; Allen, J.J. Theta EEG dynamics of the error-related negativity. Clin. Neurophysiol. 2007, 118, 645–668. [Google Scholar] [CrossRef] [PubMed]
- Stenberg, G. Personality and the EEG: Arousal and emotional arousability. Personal. Individ. Differ. 1992, 13, 1097–1113. [Google Scholar] [CrossRef]
- Nakashima, K.; Sato, H. The effects of various mental tasks on appearance of frontal midline theta activity in EEG. J. Hum. Ergol. 1992, 21, 201–206. [Google Scholar]
- Asada, H.; Fukuda, Y.; Tsunoda, S.; Yamaguchi, M.; Tonoike, M. Frontal midline theta rhythms reflect alternative activation of prefrontal cortex and anterior cingulate cortex in humans. Neurosci. Lett. 1999, 274, 29–32. [Google Scholar] [CrossRef]
- Kubota, Y.; Sato, W.; Toichi, M.; Murai, T.; Okada, T.; Hayashi, A.; Sengoku, A. Frontal midline theta rhythm is correlated with cardiac autonomic activities during the performance of an attention demanding meditation procedure. Cogn. Brain Res. 2001, 11, 281–287. [Google Scholar] [CrossRef]
- Maurer, U.; Brem, S.; Liechti, M.; Maurizio, S.; Michels, L.; Brandeis, D. Frontal midline theta reflects individual task performance in a working memory task. Brain Topogr. 2015, 28, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Harmony, T. The functional significance of delta oscillations in cognitive processing. Front. Integr. Neurosci. 2013, 7, 83. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, A.Y. The problem of segmental description of human electroencephalogram. Hum. Physiol. Fiziol. Cheloveka 1999, 25, 107–114. [Google Scholar]
- Fingelkurts, A.A.; Fingelkurts, A.A. EEG oscillatory states: Temporal and spatial microstructure. Int. J. Psychophysiol. 2008, 3, 150. [Google Scholar] [CrossRef]
- Gonen, F.F.; Tcheslavski, G.V. Techniques to assess stationarity and gaussianity of EEG: An overview. Int. J. Bioautom. 2012, 16, 135. [Google Scholar]
- Vázquez, R.R.; Velez-Perez, H.; Ranta, R.; Dorr, V.L.; Maquin, D.; Maillard, L. Blind source separation, wavelet denoising and discriminant analysis for EEG artefacts and noise cancelling. Biomed. Signal Process. Control 2012, 7, 389–400. [Google Scholar] [CrossRef]
- Dolce, G.; Waldeier, H. Spectral and multivariate analysis of EEG changes during mental activity in man. Electroencephalogr. Clin. Neurophysiol. 1974, 36, 577–584. [Google Scholar] [CrossRef]
- Fernández, T.; Harmony, T.; Rodríguez, M.; Bernal, J.; Silva, J.; Reyes, A.; Marosi, E. EEG activation patterns during the performance of tasks involving different components of mental calculation. Electroencephalogr. Clin. Neurophysiol. 1995, 94, 175–182. [Google Scholar] [CrossRef]
- Vogel, W.; Broverman, D.M.; Klaiber, E.L. EEG and mental abilities. Electroencephalogr. Clin. Neurophysiol. 1968, 24, 166–175. [Google Scholar] [CrossRef]
- Harmony, T.; Fernández, T.; Silva, J.; Bernal, J.; Díaz-Comas, L.; Reyes, A.; Erzsébet, M.; Mario, R.; Rodríguez, M. EEG delta activity: An indicator of attention to internal processing during performance of mental tasks. Int. J. Psychophysiol. 1996, 24, 161–171. [Google Scholar] [CrossRef]
- Hinault, T.; Lemaire, P. What does EEG tell us about arithmetic strategies? A review. Int. J. Psychophysiol. 2016, 106, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Knyazev, G.G. EEG delta oscillations as a correlate of basic homeostatic and motivational processes. Neurosci. Biobehav. Rev. 2012, 36, 677–695. [Google Scholar] [CrossRef] [PubMed]
- Klimesch, W.; Doppelmayr, M. Encoding of new. Neuroreport 1996, 7, 1235–1240. [Google Scholar] [CrossRef] [PubMed]
- Polanía, R.; Nitsche, M.A.; Korman, C.; Batsikadze, G.; Paulus, W. The importance of timing in segregated theta phase-coupling for cognitive performance. Curr. Biol. 2012, 22, 1314–1318. [Google Scholar] [CrossRef]
- Grabner, R.H.; De Smedt, B. Oscillatory EEG correlates of arithmetic strategies: A training study. Front. Psychol. 2012, 3, 428. [Google Scholar] [CrossRef]
- Eccius-Wellmann, C. Mathematikdidaktische Fehleranalysen zur Schulalgebra: Schülerwissen und Lehrerprofessionswissen; VDM Verlag Dr. Müller: Riga, Latvia, 2008. [Google Scholar]
- Bagni, G.T. ‘Simple’Rules and General Rules in Some High School Students’ Mistakes. J. Math.-Didakt. 2000, 21, 124–138. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alvarado-Rodríguez, F.J.; Ibarra-González, K.P.; Eccius-Wellmann, C.; Vélez-Pérez, H.; Romo-Vázquez, R. Electrophysiological Brain Response to Error in Solving Mathematical Tasks. Mathematics 2022, 10, 3294. https://doi.org/10.3390/math10183294
Alvarado-Rodríguez FJ, Ibarra-González KP, Eccius-Wellmann C, Vélez-Pérez H, Romo-Vázquez R. Electrophysiological Brain Response to Error in Solving Mathematical Tasks. Mathematics. 2022; 10(18):3294. https://doi.org/10.3390/math10183294
Chicago/Turabian StyleAlvarado-Rodríguez, Francisco J., Karla P. Ibarra-González, Cristina Eccius-Wellmann, Hugo Vélez-Pérez, and Rebeca Romo-Vázquez. 2022. "Electrophysiological Brain Response to Error in Solving Mathematical Tasks" Mathematics 10, no. 18: 3294. https://doi.org/10.3390/math10183294





