Abstract
Caulerpa lentillifera is a type of green seaweed widely consumed as a fresh vegetable, specifically in Southeast Asia. Interestingly, this green seaweed has recently gained popularity in the food sector. Over the last two decades, many studies have reported that C. lentillifera is rich in polyunsaturated fatty acids, minerals, vitamins, and bioactive compounds that contribute many health benefits. On the other hand, there is currently hardly any article dedicated specifically to C. lentillifera regarding nutritional composition and recent advancements in its potential health benefits. Hence, this study will summarise the findings on the nutritional content of C. lentillifera and compile recently discovered beneficial properties throughout the past decade. From the data compiled in this review paper, it can be concluded that the nutrient and phytochemical profile of C. lentillifera differs from one region to another depending on various external factors. As a result, this paper will offer researchers the groundwork to develop food products based on C. lentillifera. The authors of this paper are hopeful that a more systematic review could be done in the future as currently, existing data is still scarce.
1. Introduction
In 2019, Asia contributed to 97.4 percent of global seaweed production (99.1 percent from cultivation), with seven of the top ten producing countries located in Eastern or South-eastern Asia [1]. This indicates a significant regional imbalance in seaweed production which is largely influenced by the fact that seaweeds are a regular part of human diets in East Asia compared to elsewhere [2]. Seaweeds have been a food source since the fourth century in Japan and the sixth century in China. According to historical sources, people gathered macroalgae for sustenance as early as 500 B.C. in China and a thousand years later in Europe. People who lived near coastal areas preferred to consume seaweeds as a main dish or in soup [3]. Europeans usually consume smaller amounts of seaweed than Asians due to European regulations and dietary habits [4].
Although macroalgae intake is not as prevalent in Europe as in Asia, microalgae have acquired popularity because of their physiologically active components, earning them the reputation of "new superfoods" [5]. Between 1950 and 2019, global seaweed cultivation and production increased by a thousand-fold, with mainly brown seaweed (from 3.1 million tonnes to 16.4 million tonnes) and red seaweed cultivation (from 1 million tonnes to 18.3 million tonnes) being the main contributors [1]. However, the world cultivation of green seaweed decreased from 31,000 tonnes to 17,000 tonnes during the same period [2]. The 16,696 tonnes of green seaweeds grown in 2019 represented only 0.05 percent of the total seaweed production in the same year. Among the 16,696 tonnes produced were Caulerpa spp., Monostroma nitidum, Enteromorpha [Ulva] prolifera, Capsosiphon fulvescens, and Codium fragile, all of which are included in FAO’s Aquatic Sciences and Fisheries Information System. Out of 100 known Caulerpa species, only seven are utilised for human consumption globally, with C. lentillifera and Caulerpa racemosa dominating in this aspect [6]. Table 1 shows the global seaweed production and comparison by region in 2019.

Table 1.
Global seaweed production and comparison by region in 2019 [2].
Due to their grape-like appearance, they are commonly known as sea grapes or sea caviars. They are also known by different names in certain countries; most names directly translating the term "sea grape" into their vernacular. For instance, “nama” in Fiji, “bulung boni” in Indonesia, “umi budo” (海ぶどう) or “kumejima” in Japan, “bada podo” (바다 포도) in Korea, “lato”, “lelato”, or “ararosip” in the Philippines, “latok” in Malaysia, and “rong nho” or “rong nho biển” in Vietnam [7,8,9,10,11]. They usually inhabit sandy or muddy shallow sea bottoms [12]. C. lentillifera J. Agardh was originally described from the Red Sea coast [13]. It has been reported to be widely distributed in subtropical and tropical locations, such as the South China Sea, Southeast Asia, Japan, Taiwan, and Oceania, where it is directly consumed as a snack, in salads, and sushi, or in its salt-preserved form [14]. It has been described to have a salty taste and succulent texture. Figure 1 illustrates fresh C. lentillifera.

Figure 1.
Fresh C. lentillifera.
C. lentillifera is an alternative food that can also be used therapeutically. Over the years, it has gained popularity owing to its nutritional value, potential pharmacological benefits, and sustainability [15,16]). Within the past five years, several publications have reviewed various aspects of Caulerpa spp., such as its consumption, nutritional value, and farming [6], bioactive components and biotechnological applications [17], metabolite roles in cancer treatments [18], as well as its position as a functional food [11]. Only two publications had focused on reviewing the green algae genus Caulerpa in chemical composition, diversity, ecology, farming, pharmacological and industrial potential [10,19]). However, the review did not critically evaluate C. lentillifera specifically. To the best of our knowledge, no publication has focused solely on C. lentillifera in terms of its nutrient content and recent advances in potential health benefits that would make it suitable for pharmaceutical and nutraceutical use. Therefore, this article aims to review relevant literature in the past decade from reliable sources regarding C. lentillifera based on its nutrient content and beneficial properties. This paper would provide a good foundation for future researchers to develop functional food products utilising C. lentillifera.
2. Nutritional Value of C. lentillifera
The proximate composition and the total dietary fibre content of C. lentillifera from different countries are shown in Table 2.

Table 2.
Proximate composition and fibre contents of C. lentillifera from different countries.
2.1. Carbohydrate and Fibres
The most abundant component in C. lentillifera are carbohydrates and dietary fibre. Dietary fibre is a complex mixture of carbohydrates and polymers in plants, including oligosaccharides and polysaccharides. Other non-carbohydrate components such as polyphenols, resistant proteins, saponins, and waxes may also be present [38]. However, these may vary even within its species [39]. For instance, although belonging to the same genus and family, C. lentillifera has higher carbohydrate content than C. racemosa [40]. C. lentillifera contains as low as 0.36% and as high as 72.9% carbohydrates in its dry matter (Table 2). Its dietary fibre content is approximately 17.5 to 36.7% in 100 g dried C. lentillifera, respectively. Water-soluble fibre content is approximately 2.45–17.21%. Water-soluble fibres are usually higher in red algae, around 15 to 22% in the dry matter, such as in Chondrus crispus (Irish moss) and Porphyra/Pyropia spp. (nori) [41,42].
In seaweed, soluble fibres can absorb water up to 20 times its volume [43]. This helps enhance the binding of water with food pellets in the gut and aids in stool bulking and shortening transit time in the colon; these act as positive factors that may prevent colon cancer [44]. In Caulerpa spp., soluble polysaccharides mostly consist of glucans and sulfated polysaccharides [19]. Sulfated polysaccharides from C. lentillifera have been reported to have physiological benefits, which will be discussed in the latter part of this review. Insoluble dietary fibres are generally not digested in the human gastrointestinal tract. Upon contact with water, they do not form gels but retain water in their structural matrix, increasing faecal bulk and accelerated intestinal transit [45]. Insoluble dietary fibres of C. lentillifera range from 15.75 to 28.98% (Table 2). However, C. lentillifera has lower dietary fibre content than other green seaweeds, such as C. racemosa and Ulva reticulata, at 65.7% and 64.9%, respectively [40,46,47]. In adults, high consumption of dietary fibre, particularly fermentable fibres, has been linked with increased short-chain fatty acid (SCFA) contents in the stool [44,48].
2.2. Protein and Amino Acids
With increased population growth and demand for protein, seaweeds are plausibly viable and sustainable protein sources due to their low environmental impact and fast-growing rate. Furthermore, the protein content of whole algae is very high compared to common food staples such as cereals, legumes, and nuts [49]. With its versatility and simplicity of usage, whole algal protein has the potential to be a tremendous whole-food protein source, as well as a great way to supplement protein-deficient diets [50]. When comparing the protein contents, the levels of proteins are higher in Rhodophyta (red), followed by Chlorophyta (green), and Ochrophyta (brown) [51,52]. The protein content of C. lentillifera ranged from 0.43 to 19.38% in various countries (Table 2). The wide difference and instability of the protein content could be affected by various external factors, such as water temperature, season, geography, weather, and other factors [46]. It was reported that protein content in seaweed was higher in winter than in autumn and summer [53,54].
The protein quality depends on the presence and quantity of essential amino acids. Amino acids are the building blocks that form proteins bound together via peptide bonds formed between the carboxyl group of an amino acid and the amino group of the next amino acid in line [55]. C. lentillifera are considered to have high-quality proteins as the essential amino acids present and were close to egg and soya protein content [54]. Except for tryptophan, almost all essential amino acids (EAA) are present. Their amino acid profile is dominated mainly by leucine, valine, aspartic acid, glutamic acid, and glycine. The major amino acids in seaweed proteins are aspartic and glutamic acid, which contribute to the umami flavour [56]. The amino acid profile of C. lentillifera is shown in Table 3.

Table 3.
Amino acid profile of C. lentillifera.
2.3. Minerals
Minerals absent from freshwater algae and terrestrial crops are mostly available in seaweeds [57]. Minerals are essential and required in certain amounts for the normal metabolic functioning of the human body [58]. The mineral element found present in C. lentillifera, including essential minerals and toxic minerals, are presented in Table 4. The mineral content varies due to the phylum or class of the seaweed and geographical origin, along with seasonal, environmental, and physiological variations [39].

Table 4.
Mineral element composition in C. lentillifera in different countries.
Essential minerals crucial for human wellbeing include Ca, Cu, Fe, Mg, Zn, K, Na, P, Se, Mn, Cr, and I [60,61]. On the other hand, toxic minerals such as Al, As, Cd, Hg, and Pb do not possess any benefits to humans but cause detrimental effects, which are present in C. lentillifera [62,63]. As stated in Table 4, Na, Mg, K, Ca, and Mn has a wide range of concentrations, among all mineral elements, with the highest concentration in Na (14.90–130,794 mg/100 g). For Mg, the highest concentration value was around 8126.59 mg/100 g (in China) to 10,663 mg/100 g (in Vietnam). The highest concentration value of K and Mn were found in C. lentillifera from China, 4967.34 mg/100 g and 1341.07 mg/100 g.
The calcium content in C. lentillifera is comparable to common foods such as milk products, meat, fish, poultry, and legumes. For instance, the highest concentration value found in C. lentillifera was 8137 mg/100 g (in Vietnam) which is 4 times higher than the calcium content in high calcium milk powder, 2000 mg/100 g [64,65]. Iodine and iron are important to the human diet, both of which can be found in high concentrations in seaweeds, including C. lentillifera [66]. Insufficiency and deficiency of iodine could lead to goiter and hypothyroidism [65]. Although the iodine content in C. lentillifera is relatively low compared to in other green seaweed such as Ulva clathrata [67], it can be considered a cheap and reasonable option to fulfil the minimum iodine required needed by the body [65,68].
The deficiency of iron is a major health problem worldwide. The root of this problem is caused by prolonged inadequate intake due to low bioavailability in the diet. Especially during the period of growth and chronic blood loss, the increase in iron requirement may also cause iron deficiency [65]. The consumption of C. lentillifera could be a potential iron supplement to combat iron deficiency. However, it is difficult to generalise or conclude whether the mineral contents in C. lentillifera is high or low, as different sampling region have greatly varied environmental conditions [19]. From the compiled data in Table 4, it can be concluded C. lentillifera are rich in minerals that meet the requirement of the human body. However, the Na/K ratios need careful consideration, as it has been reported to be higher than in other seaweeds such as Sargassum polycystum and Eucheuma cottonii [24]. If the Na/K ratio is too high, it is detrimental to the sodium to potassium balance in the human body, which can result in cardiovascular diseases. A simple desalting operation, such as soaking, is recommended before eating [32].
2.4. Lipids
C. lentillifera are significantly low in lipid content ranging from 0.05 to 14.0% in dry weight. Despite low lipid composition, C. lentillifera has raised interest due to a high content of long-chain polyunsaturated fatty acids (PUFAs) and carotenoids [19,41]. Compared to terrestrial vegetables, C. lentillifera contain significantly higher levels of polyunsaturated fatty acids, which act as strong antioxidants, such as ω-3 and ω-6 [69], which have various roles in the prevention of cardiovascular diseases, osteoarthritis, and diabetes [70]. The fatty acids profile of C. lentillifera are shown in Table 5. The ω-3 and ω-6 PUFAs, particularly linoleic acid (18:2ω6) and 𝛼-linolenic acid (18:3ω3), cannot be synthesised by most heterotrophic organisms and can only be obtained through dietary intake [32,71]. All these PUFAs can be found in C. lentillifera, with 𝛼-linolenic acid (18:3ω3) being the most abundant [71]. The fatty acid compositions of C. lentillifera are as tabulated in Table 5.

Table 5.
Fatty acids composition in C. lentillifera.
2.5. Vitamins
Seaweeds are known to be a good source of both water-soluble and fat-soluble vitamins. The requirements of vitamin A, B2, B12, and two-thirds of vitamin C in the human body could be fulfilled by consuming 100 g of seaweed [72,73]. Table 6 indicates the vitamin content found in C. lentillifera with its daily RNI and daily UL for comparison purposes.

Table 6.
Vitamin content in C. lentillifera, the daily recommended nutrient intake (RNI), and the tolerable upper intake level (U.L.) per day.
Water-soluble vitamin C is the most abundant in C. lentillifera, the major contributor to its antioxidant properties, with concentrations ranging from 0.028–274 mg/100 g. Among other seaweed groups, C. lentillifera is generally rich in B group vitamins [74]. Vitamin B1, B2, and B3 were present in C. lentillifera in trace amounts; however, the amount detected still exceeded the recommended daily intake. The total amount of vitamin B2 in C. lentillifera is considerably higher than in various legumes, including chickpeas, lentils, red and black grain, and soya beans, which contain relatively high riboflavin levels of around 0.2–0.5 mg/100 g [64]. Recent data on the riboflavin content of selected commercial rice, such as fragrant rice, basmati rice, and Siam rice, showed that all varieties contain 0.06 mg riboflavin per 100 g [75]. The amount of B3 in C. lentillifera, 1.9–200 mg/100 g, was also higher than that of Ulva fasciata, 1.02 mg/100 g, and E. flexuosa, 0.98 mg/100 g [76].
2.6. Pigments
The most abundant pigments in the Caulerpa species are chlorophylls, mostly composed of chlorophyll a and b [77]. Chlorophylls have an antioxidant property that makes them useful nutritional and a health supplement [78]. Chlorophylls available in our diet are obtained via the consumption of green vegetables. Several studies have demonstrated that chlorophylls and their degradation products have anti-proliferative and anticancer properties [41,79]. Carotenoids which are tetraterpenoid pigments are also found in C. lentillifera. Most carotenoids were present in seaweeds, such as α- and 𝛽-Carotene, lutein, and zeaxanthin, in which all except α-carotene were detected in C. lentillifera, as shown in Table 7.

Table 7.
The concentration of pigments found in Caulerpa lentillifera.
β-carotene is a precursor of vitamin A (retinol), an essential vitamin that promotes a healthy immune system, good skin, and eye health [78]. β-carotene also has antioxidant properties that protect the body from free radicals produced by oxidation of other molecules [81]. Carotenoids like lutein and zeaxanthin prevent the progress of age-related macular degeneration [56,82]. Caulerpin is a bis-indole alkaloid found in genus Caulerpa [83]. In C. lentillifera, it is found present at concentrations of 25.79–33.59 μg/g. This compound contributes to some of its reported therapeutic activities. For instance, caulerpin isolated from Caulerpa taxifolia showed anti-diabetic properties [84], whereas caulerpin sourced from other Caulerpa spp. demonstrated potential anti-inflammatory and anti-nociceptive properties [85]. The health benefits of caulerpin extracted from C. lentillifera will be covered in the next section of this review paper.
3. Health Benefits of Caulerpa lentillifera
C. lentillifera has been discovered to have health-related functionalities that could be used for medical treatment and prevention, as illustrated in Figure 2. Table 8 highlights the documented health benefits of C. lentillifera in the literature throughout the previous decade.
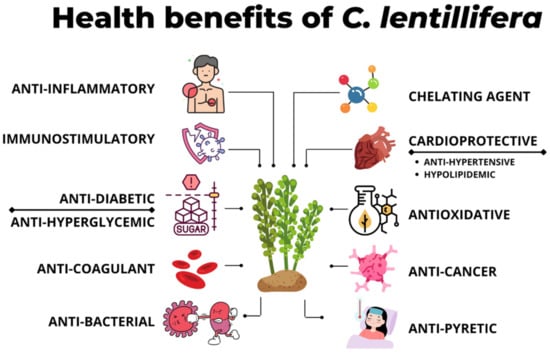
Figure 2.
Overview of health benefits reported of C. lentillifera.
3.1. Cardioprotective
3.1.1. Anti-Hypertensive
Cardiovascular disease (CVD) is one of the noncommunicable diseases that is the most probable cause of mortality globally, besides cancer, diabetes, and chronic respiratory diseases, among people between the age of 30 to 70 years old [86]. Hypertension, or an increase in arterial blood pressure, is a major risk factor for CVD, affecting 15% to 20% of the world population [87,88,89,90]. One of the most important therapeutic approaches in managing hypertension is the inhibition of the Angiotensin-converting enzyme (ACE), as demonstrated in many clinical trials [8,87]. ACE inhibitors block the conversion of angiotensin I to angiotensin II, resulting in blood vessel relaxation and decreased blood pressure [89]. Pharmaceutical manufacturers have commercialised many ACE inhibitors to lower angiotensin II concentrations for the treatment of hypertension; however, these drugs possess adverse side effects, emphasising the need for developing natural food-derived inhibitors with fewer undesirable side effects [91].
In an in vitro study, protein hydrolysates from C. lentillifera were obtained using four different enzymes: α-chymotrypsin; pepsin; thermolysin; trypsin [92]. All hydrolysates obtained have demonstrated ACE-inhibiting properties, with the thermolysin hydrolysate showing the highest inhibition with 90.64% inhibition at a dose concentration of 1 mg/mL [92]. From their investigation, they concluded that the bioactive components responsible for this inhibitory activity were oligopeptides, FDGIP (FP-5), and AIDPVRA (AA-7). Although this is the first reported study utilising protein peptides from C. lentillifera, there are many other similar studies sourcing protein peptides from different seaweed species such as Undaria pinnatifida, Saccharina japonica, Sargassum fusiforme, S. maclurei (Ochrophyta), Gracilariopsis lemaneiformis, Mazzaella japonica, Palmaria palmata, Pyropia/Porphyra spp., Bangia fusco-purpurea (Rhodophyta), Ulva rigida, U. chlatrata, and U. intestinalis (Chlorophyta) [93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108].

Table 8.
Health benefits reported in Caulerpa lentillifera.
Table 8.
Health benefits reported in Caulerpa lentillifera.
| Health Benefits Reported | Extract from C. lentillifera | Model of Study | Dosage | Reference |
|---|---|---|---|---|
| Anti-hypertensive | Dried C. lentillifera powder | α-chymotrypsin, pepsin, thermolysin, and trypsin | 1 mg/mL | [92] |
| Male Wistar rats (8–9 weeks old; 338 g) | 5% dw | [24] | ||
| Anti-hyperlipidaemic | Dried C. lentillifera | Male Sprague-Dawley rats (10 weeks old, 260–300 g) | 5 g/100 g | [24] |
| C. lentillifera extract | Male rabbits | 10, 158.5 and 39,810.70 mg/kg bwt | [109] | |
| Aqueous extract | Porcine pancreatin | 5 mg | [14] | |
| Anti-bacterial; anti-microbial | C. lentillifera extracts | E. coli, S. aureus, Streptococcus sp., Salmonella sp. | [26] | |
| Caulerpin | E. coli, S. aureus, Streptococcus sp., Salmonella sp. | |||
| Methanolic extract | Staphylococcus aureus, Streptococcus mutans | 25–250 mg/mL | [110] | |
| Methanolic extract | Methilin-resistant Staphylococcus aureus (MRSA), Escherichia coli K1 | 250 µg/mL | [111] | |
| Anti-tumour; anti-cancer; anti-proliferative; apoptotic | ß-1,3-Xylan | Human breast cancer cells, MCF-7 cells | 1–2 mg/mL | [112] |
| Ethanol-hexane Extract | A172 Human glioblastoma cells | 200–1000 µg/mL | [113] | |
| Anti-coagulant | ß-1,3-Xylan | Rabbit plasma | 1, 3, 5, 10 and 20 mg/mL | [114] |
| Aqueous extract | Male albino rabbits (4–6 months old, 1.0–1.25 kg) and canine blood samples | 3 mg/mL | [115] | |
| Anti-hyperglycaemic | Hydroethanolic Extract | Male albino mice | 10 and 50 mg/kg | [116] |
| Freeze-dried aqueous extract | Male BALB/c mice (6 weeks old) | 600 and 1000 mg/kg bwt | [34] | |
| Anti-diabetic | Ethanolic extract | Rat insulinoma cells (RIN), 3T3-L1 cells | 1000 µg/mL | [117] |
| 10–25 µg/mL | ||||
| L6 rat skeletal muscle cells | 250 µg/mL | [118] | ||
| 6-week-old db/db male mice | 250 and 500 mg/kg | |||
| Rat insulinoma (RIN)-m5F cells | 250, 500, and 1000 μg/mL | [119] | ||
| Anti-inflammatory | C. lentillifera extracts | Murine macrophage RAW 264.7 cells | 50 µg/mL | [26] |
| Caulerpin | Murine macrophage RAW 264.7 cells | 25, 50, 100 µg/mL | ||
| Sulphated polysaccharides | HT29 colonic carcinoma cells | 50, 100, 200, 300 and 400 µg/mL | [120] | |
| Antioxidative | Freeze-dried aqueous extract | Male BALB/c mice (6 weeks old) | 600 and 1000 mg/kg bwt | [24] |
| Anti-pyretic | Aqueous extract | Adult male mice (24–30 g) | 500 mg/kg bwt | [121] |
| Chelating agent | Aqueous extract | Male Sprague Dawley rats (4 weeks, 150–180 g) | 500 mg/kg bwt | [122] |
| Immunostimulatory | Sulphated polysaccharides | Murine macrophage RAW 264.7 cells | 1–5 µg/mL | [123] |
| Xylogalactomannnans | Murine macrophage RAW 264.7 cells | 50–800 µg/mL | [124] | |
| Polysaccharides | Mouse RAW264.7 cells | 6.25, 12.5, 25 and 50 μg/mL | [125] | |
| In vitro fermented culture | 60 cytoxan (CTX) induced immunosuppressed male BALB/c mice; 20 g | 25, 50, and 100 mg/kg bwt | [126] |
3.1.2. Anti-Hyperlipidaemic
Lipids are one of the important nutrients required by the human body. High intake of lipids, however, could lead to obesity and hyperlipidaemia [127]. Hyperlipidaemia is characterised by a rise in blood total cholesterol (TC), low-density lipoprotein (LDL), very low-density lipoprotein (VLDL), and a reduction in high-density lipoprotein (HDL) [128]. It is a major cardiac risk factor, and it has been linked to an increased risk of cardiovascular disease in these patients [129]. Although the current drugs used in medical practices are very effective in lowering LDL levels, these drugs do have side effects which cause patients to seek treatments using safe and naturally derived drugs. At present, much research has evaluated seaweed-polysaccharides effect in lowering blood lipid levels. The evaluations are mainly conducted based on in vivo and in vitro experiments. In an in vitro experiment, mice were fed a high cholesterol and high fat (HCF) diet to establish a hyperlipidaemic model study. Then, the mice were treated with seaweed polysaccharides in which their blood lipid-related factors, lipase inhibition rate, and bile salts binding capacity were determined.
In an in vivo study, treatment of HCF rats with 5% dried C. lentillifera for 16 weeks significantly lowered their body weight by 39.5%, increased HDL levels by 48.7%, reduced TC by 18.4%, LDL by 34.6%, and triglycerides levels by 33.7%, and lowered lipid peroxidation level by 9%, erythrocyte glutathione peroxidase level by 31.8% and catalase level by 3.14%, compared to the corresponding levels in HCF rats [24]. Similar findings were obtained in another in vivo study where a decrease in total cholesterol levels was observed among hypocholesterolaemia-induced male rabbits administered with crude C. lentillifera extract [109]. The anti-hyperlipidaemic effects of different polysaccharide fractions of C. lentillifera extract, i.e., WCLP25, WCLP40, WCLP55, WCLP70, and WCLP85, were assessed in a simulated bile acid-binding experiment. From the in vitro experiment conducted, they found out that WCLP-55 and WCLP-70 are potentially applicable for lowering blood lipids as these fractions have significantly higher binding capacities for cholic acid, deoxycholic acid, glycocholic acid, and taurocholic acid) [14]. Other polysaccharides sourced from different seaweed species demonstrated hypolipidemic properties, such as Sargassum polycystum, Enteromorpha prolifera, Monostroma nitidum, Sargassum fusiforme, and Ulva pertusa [130,131,132,133].
3.2. Antibacterial and Antimicrobial Activity
Antimicrobials are compounds that kill or hinder the growth of microbial pathogens, respectively, whereas antibiotics and antifungals are compounds that help kill them [130]. Antimicrobials primarily impact microbial cells, targeting the phospholipid bilayer of the cell membrane, destroying enzyme systems, and altering the bacteria’ genetic material [134]. Secondary metabolites from seaweeds such as polyphenols or other bioactive compounds can disrupt the permeability of the microbial cell, and interfere with membrane function, thus, consequently causing cell apoptosis [135]. In a study, the antibacterial potential of C. lentillifera extracts and caulerpin against four common food microbial pathogen strains, i.e., E. coli, Salmonella sp., Streptococcus sp., and Staphylococcus aureus, were evaluated [26]. The seaweed extract was found to demonstrate antimicrobial activities in all test organisms with the range of minimum inhibitory concentrations of 136.5, 125.25, 175.25, and 140.50 MIC/mg mL−1 in E. coli, Staphylococcus aureus, Streptococcus sp., and Salmonella sp. respectively. As for the caulerpin extract, it demonstrated antimicrobial activities in all test organisms with minimum inhibitory concentrations of 5.25 MIC/mg mL−1 in E. coli, S aureus, and Salmonella sp., and the lowest in Streptococcus sp., 15.50 MIC/mg mL−1.
3.3. Anti-Cancer
Existing anticancer medicines are often nonspecific, have side effects, or are exceedingly expensive; therefore, the search for improved therapeutics continues, with a particular focus on naturally occurring compounds. In an in-vitro study, it was discovered that ß-1,3-Xylan extracted from C. lentillifera inhibited the growth of MCF-7 human breast cancer cells and triggered chromatin condensation, degradation of poly ADP-ribose polymerase (PARP), and activation of caspase-3/7, indicating that it promoted death in these cells (MCF-7 cells) [123]. In other in vivo and in vitro experiments utilising bioactive compounds extracted from Sargassum wightii and E. cottonii, similar findings have been observed [136,137,138]. Despite similar outcomes observed in the previously mentioned experiments, different molecular mechanisms may occur as each compound, i.e., phloroglucinol, fucoxanthin, and fucoidan, have different action mechanisms, such as anti-angiogenic, antioxidative, anti-metastasis, anti-proliferative, and pro-apoptotic [139]. Recently, a fascinating discovery by Tanawoot and others [113] revealed that A147 glioblastoma cells treated with ethanol-hexane seaweed extracted from C. lentillifera demonstrated a drastic drop in cells viability and inhibited glioblastoma cell cycle progression in a high dose-dependent manner. The seaweed extracts also promoted the apoptosis of A147 cells.
3.4. Anti-Coagulant
Anti-coagulant is a key agent for preventing thrombosis, with heparin being the most often used commercial antithrombotic medication [140]. An in-vitro study using rabbit plasma treated with different concentrations of ß-1,3-Xylan, a polysaccharide compound extracted from C. lentillifera, has demonstrated prolonged activated partial thromboplastin time (aPTT). A similar result was observed in another study by Arenajo and colleagues, whereby an aqueous extracted from C. lentillifera, the concentration of 3 mg/mL tested in male albino rabbits and canine blood samples was able to prolong the clotting time dose-dependently [115].
3.5. Anti-Diabetic and Anti-Hyperglycaemic
Diabetes is a type of metabolic disorder considered a chronic health problem globally. This disorder occurs when the pancreas does not produce enough insulin, Type-1 Diabetes, or when the body cannot use the insulin effectively upon production, Type-2 Diabetes [86]. Seaweeds have been widely used for anti-diabetic treatments [34,141]. Ethanolic extracts of C. lentillifera have been assessed both in in vivo and in vitro experiments resulting in positive anti-diabetic effects. An in vitro experiment conducted by Sharma and others [117] in rat insulinoma cells (RIN), 3T3-L1 cells exhibited a decrease in dipeptidyl peptidase-IV and α-glucosidase enzyme activities at 1000 µg/mL dosage concentration, whereas, at a 10–25 µg/mL dose concentration, ethanolic C. lentillifera extract showed inhibited cell death and iNOS expression in interleukin- 1β and interferon-γ induced RIN cells. Enhanced insulin secretion in pancreatic β-cells and increased insulin sensitivity and glucose uptake in 3T3-L1 adipocytes were observed at 10–25 µg/mL dose concentration administered [117]. According to the American Diabetes Association, hyperglycaemia refers to a high blood glucose level where the blood glucose is greater than 125 mg/dL while fasting with greater than 180 mg/dL 2 h postprandial. When 10 mg/kg and 50 mg/kg of hydroethanolic extract from C. lentillifera were introduced to male albino mice, it induced significant antihyperglycemic effects in the fasting state and 2-h postprandial loading in a dose-dependent manner [116]. Similarly, 600 and 1000 mg/kg of freeze-dried aqueous extract from C. lentillifera were orally administered to male BALB/c mice, which showed improved plasma glucose, insulin, and homeostasis model assessment-insulin resistance (HOMA-IR) levels after 6 weeks [34].
3.6. Anti-Inflammatory
Inflammation is a natural defensive response to harmful stimuli such as irritants, pathogens, or damaged cells. Microbial infections, tissue stress, and some traumas are all examples of threats that trigger inflammation, which is frequently followed by symptoms such as fever, redness, swelling, and pain [142,143]. The inflammatory response is characterized by the overproduction of proinflammatory cytokines such as tumour necrosis factor-alpha (TNF-𝛼), interleukin (IL) (IL-6 and IL-1), prostaglandin E2 (PGE2), nitric oxide (NO), and increased production of reactive oxygen species (ROS) [144]. Increased inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) activity is often linked to increasing NO and PGE2 production [141]. Although C. lentillifera has been a research subject regarding its health benefits, not many reports are found pertaining to its anti-inflammatory activity except for an in-vitro study by Nagappan and Vairappan [26] and Sun and others [120].
Using murine macrophage RAW 264.7 cells as the model of study, Nagappan and Vairappan found that the C. lentillifera extracts and caulerpin, an active ingredient extracted from C. lentillifera, when subjected to the RAW 264.7, did not release lactate dehydrogenase (LDH) and suppress the NO production. They also found that the production of nitrite and proinflammatory cytokines, TNF- 𝛼 and IL-6, were lowered in a dose-dependent manner. Meanwhile, Sun and others studied the anti-inflammatory activity of C. lentillifera by treating HT29 colonic carcinoma cells that have been lipopolysaccharides (LPS) induced with four different fractions of sulphated polysaccharides extracts, i.e., CLGP1, CLGP2, CLGP3, and CLGP4 [120]. From their research, they concluded that LPS-stimulated HT29 cells treated with CLGP4, C. lentillifera polysaccharides demonstrated a powerful inhibition of the production of interleukin-1ß (IL-1ß) as well as the tumour necrosis factor (TNF-α), significantly reduced the mucin2 production in a dose-dependent manner. Among all sulphated saccharides, CLGP4 had the best anti-inflammatory effect in vitro.
3.7. Antioxidant
Antioxidant phytochemical compounds can scavenge reactive oxygen and nitrogen species (ROS and RNS) in the human body, slowing or preventing the onset of oxidative stress-related diseases such as cancers, cardiovascular diseases, delayed sexual development, kidney and liver diseases, neurological disease, respiratory diseases, and rheumatoid arthritis [145,146,147,148]. One of the most prominent health benefits of C. lentillifera is its antioxidant properties. Among all solvent extractions used, it can be observed that ethanolic and methanolic extracts showed antioxidant activity in various tests, as tabulated in Table 8. The different levels of activities exhibited in these antioxidant tests could be correlated to the polarity of the solvent extraction used. However, C. lentillifera extracted using water, i.e., the most polar organic solvent, showed a much lower antioxidant activity than when methanol and ethanol were used. This could be due to the dependency of the antioxidant activity on the synergistic effects of the extraction solvent used [149]. In a recent in vivo study, freeze-dried aqueous extract of C. lentillifera was observed to reduce antioxidative stress in diabetic mice and prevent male reproductive system dysfunction [34]. Overall, C. lentillifera can be seen to have high phenolics and flavonoid content, good scavenging and reducing properties, and high Trolox equivalent antioxidant capacity, as shown by the data tabulated in Table 9.

Table 9.
Antioxidant activities in Caulerpa lentillifera.
3.8. Anti-Pyretic
Anti-pyretic or analgesic helps prevent or alleviate fever. Fever is a common medical symptom characterised by a 37.2 °C fever induced by infection or inflammation. Three of the most common over-the-counter synthetic anti-pyretic drugs are aspirin, acetaminophen (paracetamol), and ibuprofen [152,153]. These drugs reduce fever by inhibiting the expression of cyclooxygenase (COX-2), which results in the production of prostaglandins [153,154]. However, these synthetic anti-pyretic drugs raise considerable concerns since they can lead to a few adverse pharmacological [155,156]. Hence, there is a global need for drugs produced from natural resources that have a minimal detrimental impact on human health. C. lentillifera has been evaluated in an in vivo study to determine whether it could potentially be used as an anti-pyretic agent [121]. 500 mg/kg per body weight of aqueous extracts from the seaweed were administered orally to adult male mice with body weights ranging around 24 to 30 g. From their experiment, they concluded that C. lentillifera showed a significant anti-pyretic effect as the rectal temperature of mice with fever decreased by 1.15 °C, 5 h after consumption of aqueous seaweed extract as compared to the control given 10 mg/kg of acetaminophen.
3.9. Anti-Chelating Agent
Heavy metals are divided into two categories based on their toxicity: essential heavy metals and non-essential heavy metals. Essential heavy metals are harmless or relatively less harmless at low concentrations, such as zinc, copper, iron, and cobalt. However, when the accumulation of the same elements is higher than the threshold, they can cause toxicity, whereas non-essential metals are highly toxic, even at a low concentration, such as cadmium, mercury, arsenic, and chromium [157]. Heavy metal accumulation in the human body severely damages different organs, including the respiratory, nervous, and reproductive systems, as well as the digestive tract [158,159]. Common chelating agents used as chelators are dimercaprol, dimercaptosuccinic acid, 2,3-Dimercaptopropane-1-sulfonic acid, sodium-calcium edetate, deferoxamine, and penicillamine [157]. However, some patients have deteriorating conditions from these chelating agents. Common side effects reported were fever, nausea, headache, vomiting, irregular blood pressure, gastrointestinal distress, sore muscles, pain at the injection site, and burning sensation.
In worst scenarios, it could also cause heart failure, breathing difficulties, respiratory failure, low blood pressure, irreversible kidney damage, convulsions, and low blood calcium [160,161,162]. Hence, researchers are searching for new antidotes sourced from natural sources with higher treatment efficacy with fewer side effects. A recent in vivo study by Daud and others tested an aqueous extract from C. lentillifera against lead accumulation in internal tissues of male rats [122]. With C. lentillifera being rich in antioxidants, they hypothesised that it might be a good candidate as a chelating agent, as administration of antioxidants has been reported to have protective effects against heavy metal-induced tissue damage. From their experiment, lead intoxicated rats treated with the seaweed extract had significantly higher body weight compared to lead-acetate treated rats, which indicated the capability of the extract to reduce the ill effects. The lead accumulation levels in the blood and internal organs among the intoxicated rats were also reduced.
3.10. Immunostimulatory
The innate immune system is one of the physiological defence mechanisms that identifies and eliminates foreign substances while maintaining immune homeostasis via mechanisms that compete with cell proliferation and death [163]. The immune system protects organs from pathogens and antigens, and the development of natural, non-toxic immunomodulators to enhance the immune regulatory system is more effective for long-term health care [125]. Recent studies showed that C. lentillifera possesses potential and potent immunomodulatory capabilities [123,124,125,126]. An in vitro experiment conducted by Maeda and colleagues, sulfated polysaccharides from C. lentillifera were found to activate and promote the growth of murine macrophages RAW 264.7 cells after 24 h of incubation, with a dosage concentration of 1–5 µg/mL. In addition, the data showed that the secretion of IL-6, TNF-α, and IL-1β was promoted after incubation with C. lentillifera polysaccharides, and the functions of CLP were like those of lipopolysaccharides [123].
Sun and others assessed the potential as a natural immunomodulator of four novel purified polysaccharides (CLGP1, CLGP2, CLGP3, CLGP4), suggesting a type of xyloglactomannan. All polysaccharides demonstrated immunostimulatory activity at concentrations of 50 to 800 µg/mL, which stimulated the viability of RAW264.7 cells, phagocytic activity, production of nitrite, and acid phosphatase signal enzyme. They concluded that CLGP4 showed the most potent immunostimulatory activities among all polysaccharides [124]. Similar outcomes were observed in another in vitro experiment using RAW 264.7 cells as the study model [125]. In another study, an in vivo experiment was conducted using polysaccharides from C. lentillifera that underwent in vitro fermentation using cytoxan (CTX)-induced immunosuppressed BALB/c mice. At concentrations of 25, 50, and 100 mg/kg of C. lentillifera polysaccharides, a significant increase in short-chain fatty acids concentrations and regulated the diversity and composition of gut microbiota were observed in immunocompromised BALB/c mice. This results in improved immunostimulatory effects against CTX-induced immunosuppression, including repairing body weight, colon length, and thymus/spleen indexes, and stimulating the production of IL-1, TNF-α, secretory immunoglobulin A, mucin2, and superoxidase dismutase. These findings indicate that C. lentillifera can act as microbiota regulators in the gut, potentially improving the immune system in immunocompromised mouse models.
4. Methodology
The information was electronically retrieved through various online databases (Scopus, ScienceDirect, Google Scholar, PubMed, etc.) from 2002 to 2022. Using the primary search phrase "Caulerpa lentillifera", a total of 138 records were found. Upon screening by applying other relevant keywords such as "nutrient content", “nutritional value”, “antioxidant”, and “health benefits” to obtain relevant journal articles with valuable data inputs, a total of 50 papers were selected. In this review, the data on nutrient composition and reported health benefits were obtained only from journal articles written in English, excluding review articles and conference papers. Data from organizations such as the World Health Organization, the Ministry of Health Malaysia, and the European Food Safety Authority were also adopted in this review paper.
5. Conclusions
In this review, the nutrient composition of C. lentillifera was compiled. This included carbohydrates and fibre, proteins and amino acids, lipids and fatty acids, minerals, vitamins, pigments, and antioxidant profiles. Health benefits contributed by C. lentillifera reported in past studies, such as cardioprotective properties (i. e., anti-hypertensive and hypolipidemic), antibacterial, anticancer, anti-coagulant, anti-hyperglycaemic, anti-diabetic, anti-inflammatory, antioxidative, anti-pyretic, chelating agent, and immunostimulatory, were also described and discussed. Despite the excellent nutrient profile of C. lentillifera, it is still underutilised and only wildly cultivated globally. In the future, we hope that more studies on functional food development and cultivation techniques concerning C. lentillifera will be conducted, as it could be a solution for food and nutrient security problems around the world. Furthermore, extensive studies on the isolates and extracts from C. lentillifera are extremely important. They are needed to understand its bioactivity and mechanisms of action while highlighting its commercialization potential, especially for nutraceutical and pharmaceutical uses.
Author Contributions
Conceptualization, N.S. and P.M.; Investigation, N.S.; writing—original draft preparation, N.S.; writing—review and editing, P.M., R.G., F.Y.C., W.P., S.M., N.A.W., F.M.F. and P.H.G.; visualization, N.S.; supervision, P.M. and R.G.; project administration, P.M. and R.G.; funding acquisition, P.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by a research collaborative grant between Universiti Malaysia Sabah and SCU Southeast Asia Sdn Bhd with the project code GKP0035-2021.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to express their appreciation to Universiti Malaysia Sabah and SCU Southeast Asia Sdn Bhd for the research collaborative grant with project code GKP0035-2021 and financial support for the publication fee funding from Research Management Centre, Universiti Malaysia Sabah.
Conflicts of Interest
Philip Huanqing Gu is affiliated with a company (Stemcell United Pte. Ltd). The company’s role in the research was to provide resources and analysis support for studying nutrient composition and development of food products from the green seaweed, Caulerpa lentillifera. The authors declare no conflict of interest.
References
- Cai, J.; Lovatelli, A.; Aguilar-Manjarrez, J.; Cornish, L.; Dabbadie, L.; Desrochers, A.; Diffey, S.; Garrido Gamarro, E.; Geehan, J.; Hurtado, A.; et al. Seaweeds and Microalgae: An Overview for Unlocking Their Potential in Global Aquaculture Development; FAO Fisheries and Aquaculture Circular No. 1229; FAO: Rome, Italy, 2021. [Google Scholar] [CrossRef]
- FAO. Fishery and Aquaculture Statistics. Global Aquaculture Production 1950–2019 (FishStatJ). Licence: CC BY-NC-SA 3.0. In FAO Fisheries Division; FAO: Rome, Italy, 2021; Available online: https://www.fao.org/3/cb5670en/cb5670en.pdf (accessed on 12 July 2022).
- Kilinc, B.; Cirik, S.; Turan, G.; Tekogul, H.; Koru, E. Seaweeds for food and industrial applications. In Food Industry; InTech: London, UK, 2013; pp. 735–748. [Google Scholar] [CrossRef]
- Sanjeewa, K.A.; Lee, W.; Jeon, Y.J. Nutrients, and bioactive potentials of edible green and red seaweed in Korea. Fish. Aquat. Sci. 2018, 21, 19. [Google Scholar] [CrossRef]
- Cofrades, S.; Serdaroğlu, M.; Jiménez-Colmenero, F. Design of healthier foods and beverages containing whole algae. In Functional Ingredients from Algae for Foods and Nutraceuticals; Woodhead Publishing: Sawston, UK, 2013; pp. 609–633. [Google Scholar] [CrossRef]
- De Gaillande, C.; Payri, C.; Remoissenet, G.; Zubia, M. Caulerpa consumption, nutritional value, and farming in the Indo-Pacific region. J. Appl. Phycol. 2017, 29, 2249–2266. [Google Scholar] [CrossRef]
- Mary, A.; Mary, V.; Lorella, A.; Matias, J.R. Rediscovery of naturally occurring seagrape Caulerpa lentillifera from the Gulf of Mannar and its mariculture. Curr. Sci. 2009, 97, 1418–1420. [Google Scholar]
- Nguyen, V.T.; Ueng, J.P.; Tsai, G.J. Proximate composition, total phenolic content, and antioxidant activity of seagrape (Caulerpa lentillifera). J. Food Sci. 2011, 76, 950–958. [Google Scholar] [CrossRef]
- Robledo, D.; Pellegrin, Y.F. Chemical and mineral composition of six potentially edible seaweed species of Yucatán. Bot. Mar. 1997, 40, 301–306. [Google Scholar] [CrossRef]
- Rushdi, M.I.; Abdel-Rahman, I.A.; Attia, E.Z.; Abdelraheem, W.M.; Saber, H.; Madkour, H.A.; Amin, E.; Hassan, H.M.; Abdelmohsen, U.R. A review on the diversity, chemical and pharmacological potential of the green algae genus Caulerpa. S. Afr. J. Bot. 2020, 132, 226–241. [Google Scholar] [CrossRef]
- Tapotubun, A.M.; Matrutty, T.E.; Riry, J.; Tapotubun, E.J.; Fransina, E.G.; Mailoa, M.N.; Riry, W.A.; Setha, B.; Rieuwpassa, F. Seaweed Caulerpa sp. position as functional food. In Proceedings of the 240th ECS Meeting, Orlando, FL, USA, 10–24 October 2019. [Google Scholar] [CrossRef]
- Horstmann, U. Cultivation of the green alga, Caulerpa racemosa, in tropical waters and some aspects of its physiological ecology. Aquaculture 1983, 32, 361–371. [Google Scholar] [CrossRef]
- Agardh, J.G. Novae species algarum, quas in itinere ad oras Maris Rubri collegit Eduardus Rüppell: Cum observationibus nonnullis in species rariores antea cognitas. Abh. Mus. Senck. 1837, 2, 169–174. [Google Scholar]
- Long, H.; Gu, X.; Zhou, N.; Zhu, Z.; Wang, C.; Liu, X.; Zhao, M. Physicochemical characterization and bile acid-binding capacity of water-extract polysaccharides fractionated by stepwise ethanol precipitation from Caulerpa lentillifera. Int. J. Biol. Macromol. 2020, 150, 654–661. [Google Scholar] [CrossRef]
- Leandro, A.; Pacheco, D.; Cotas, J.; Marques, J.C.; Pereira, L.; Gonçalves, A.M. Seaweed’s bioactive candidate compounds to food industry and global food security. Life 2020, 10, 140. [Google Scholar] [CrossRef]
- Ahern, M.; Thilsted, S.; Oenema, S.; Barange, M.; Cartmill, M.; Brandstrup, S.; Doumeizel, V.; Dyer, N.; Frøyland, L.; Garrido-Gamarro, E.; et al. The Role of Aquatic Foods in Sustainable Healthy Diets; UN Nutrition: Rome, Italy, 2021. [Google Scholar]
- Chen, X.; Sun, Y.; Liu, H.; Liu, S.; Qin, Y.; Li, P. Advances in cultivation, wastewater treatment application, bioactive components of Caulerpa lentillifera and their biotechnological applications. PeerJ 2019, 7, 6118. [Google Scholar] [CrossRef] [PubMed]
- Mehra, R.; Bhushan, S.; Bast, F.; Singh, S. Marine macroalga Caulerpa: Role of its metabolites in modulating cancer signaling. Mol. Biol. Rep. 2019, 46, 3545–3555. [Google Scholar] [CrossRef] [PubMed]
- Zubia, M.; Draisma, S.G.; Morrissey, K.L.; Varela-Álvarez, E.; De Clerck, O. Concise review of the genus Caulerpa JV Lamouroux. J. Appl. Phycol. 2020, 32, 23–39. [Google Scholar] [CrossRef]
- McDermid, K.J.; Stuercke, B. Nutritional composition of edible Hawaiian seaweeds. J. Appl. Phycol. 2003, 15, 513–524. [Google Scholar] [CrossRef]
- Paul, N.A.; Neveux, N.; Magnusson, M.; De Nys, R. Comparative production and nutritional value of “sea grapes”—The tropical green seaweeds Caulerpa lentillifera and C. racemosa. J. Appl. Phycol. 2014, 26, 1833–1844. [Google Scholar] [CrossRef]
- Chaiklahan, R.; Srinorasing, T.; Chirasuwan, N.; Tamtin, M.; Bunnag, B. The potential of polysaccharide extracts from Caulerpa lentillifera waste. Int. J. Biol. Macromol. 2020, 161, 1021–1028. [Google Scholar] [CrossRef]
- Salleh, A.; Wakid, S.A. Nutritional Composition of Macroalgae in Tanjung Tuan, Port Dickson, Malaysia. Malaysian J. Sci. 2008, 27, 19–26. [Google Scholar]
- Matanjun, P.; Mohamed, S.; Muhammad, K.; Mustapha, N.M. Comparison of cardiovascular protective effects of tropical seaweeds, Kappaphycus alvarezii, Caulerpa lentillifera, and Sargassum polycystum, on high-cholesterol/high-fat diet in rats. J. Med. Food 2010, 13, 792–800. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, F.; Sulaiman, M.R.; Saimon, W.; Yee, C.F.; Matanjun, P. Proximate compositions and total phenolic contents of selected edible seaweed from Semporna, Sabah, Malaysia. Borneo Sci. 2016, 31, 85–96. [Google Scholar]
- Nagappan, T.; Vairappan, C.S. Nutritional and bioactive properties of three edible species of green algae, genus Caulerpa (Caulerpaceae). J. Appl. Phycol. 2014, 26, 1019–1027. [Google Scholar] [CrossRef]
- Delan, G.G.; Legados, J.A.; Pepito, A.R.; Cunado, V.D.; Rica, R.L.; Abdon, H.C.; Ilano, A.S. The Influence of Habitat on the Quality Characteristics of the Green Macro Alga Caulerpa lentillifera Agardh (Caulerpaceae, Chlorophyta). Trop. Technol. J. 2015, 19, 1–7. [Google Scholar] [CrossRef]
- Nofiani, R.; Hertanto, S.; Zaharah, T.A.; Gafur, S. Proximate compositions, and biological activities of Caulerpa lentillifera. Molekul 2018, 13, 141–147. [Google Scholar] [CrossRef]
- Nurjanah, J.A.; Asmara, D.A.; Hidayat, T. Phenolic compound of fresh and boiled sea grapes (Caulerpa sp.) from Tual, Maluku. Food ScienTech J. 2019, 1, 31–39. [Google Scholar] [CrossRef]
- Long, H.; Gu, X.; Zhu, Z.; Wang, C.; Xia, X.; Zhou, N.; Liu, X.; Zhao, M. Effects of bottom sediment on the accumulation of nutrients in the edible green seaweed Caulerpa lentillifera (sea grapes). J. Appl. Phycol. 2020, 32, 705–716. [Google Scholar] [CrossRef]
- Jiang, F.Y.; Song, W.M.; Yang, N.; Huang, H. Analysis, and evaluation of nutrient content of Caulerpa lentillifera in Hainan. Sci. Technol. Food Ind. 2014, 35, 356–359. [Google Scholar] [CrossRef]
- Zhang, M.; Ma, Y.; Che, X.; Huang, Z.; Chen, P.; Xia, G.; Zhao, M. Comparative analysis of nutrient composition of Caulerpa lentillifera from different regions. J. Ocean Univ. China 2020, 19, 439–445. [Google Scholar] [CrossRef]
- Ratana-Arporn, P.; Chirapart, A. Nutritional evaluation of tropical green seaweeds Caulerpa lentillifera and Ulva reticulata. Agric. Nat. Resour. 2006, 40 (Suppl. S6), 75–83. [Google Scholar]
- Khairuddin, K.; Sudirman, S.; Huang, L.; Kong, Z.L. Caulerpa lentillifera polysaccharides-rich extract reduces oxidative stress and proinflammatory cytokines levels associated with male reproductive functions in diabetic mice. Appl. Sci. 2020, 10, 8768. [Google Scholar] [CrossRef]
- Alcantara, J.D.S.; Lazaro-Llanos, N. Mineral availability, dietary fiber contents, and short-chain fatty acid fermentation products of Caulerpa lentillifera and Kappaphycus alvarezii seaweeds. Kimika 2020, 31, 1–10. [Google Scholar] [CrossRef]
- Du Preez, R.; Majzoub, M.E.; Thomas, T.; Panchal, S.K.; Brown, L. Caulerpa lentillifera (sea grapes) improves cardiovascular and metabolic health of rats with diet-induced metabolic syndrome. Metabolites 2020, 10, 500. [Google Scholar] [CrossRef]
- Hoan, N.X.; Quan, D.H.; Dong, D.H.; Phuong, N.T.; Cuong, D.X.; Ha, H.T.; Van Thinh, P. Effect of Drying Methods on Sensory and Physical Characteristics, Nutrient and Phytochemistry Compositions, Vitamin, and Antioxidant Activity of Grapes Seaweed Caulerpa lentillifera Grown in Vietnam. J. Pharm. Sci. Res. 2020, 12, 624–630. [Google Scholar]
- Elleuch, M.; Bedigian, D.; Roiseux, O.; Besbes, S.; Blecker, C.; Attia, H. Dietary fibre and fibre-rich by-products of food processing: Characterisation, technological functionality, and commercial applications: A review. Food Chem. 2011, 124, 411–421. [Google Scholar] [CrossRef]
- Pereira, L. Nutritional composition of the main edible algae. In Therapeutic and Nutritional Uses of Algae; Pereira, L., Ed.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2018; pp. 65–127. [Google Scholar] [CrossRef]
- Pereira, L. Chapter 2: A review of the nutrient composition of selected edible seaweeds. In Seaweed: Ecology, Nutrient Composition and Medicinal Uses; Pomin, V.H., Ed.; Nova Science Publishers, Inc.: New York, NY, USA, 2011; pp. 15–47. [Google Scholar]
- Holdt, S.L.; Kraan, S. Bioactive compounds in seaweed: Functional food applications and legislation. J. Appl. Phycol. 2011, 23, 543–597. [Google Scholar] [CrossRef]
- Ruperez, P.; Saura-Calixto, F. Dietary fibre and physicochemical properties of edible Spanish seaweeds. Eur. Food Res. Technol. 2001, 212, 349–354. [Google Scholar] [CrossRef]
- Rajapakse, N.; Kim, S.K. Nutritional and digestive health benefits of seaweed. Adv. Food Nutr. Res. 2011, 64, 17–28. [Google Scholar] [PubMed]
- Gill, S.K.; Rossi, M.; Bajka, B.; Whelan, K. Dietary fibre in gastrointestinal health and disease. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 101–116. [Google Scholar] [CrossRef]
- Peñalver, R.; Lorenzo, J.M.; Ros, G.; Amarowicz, R.; Pateiro, M.; Nieto, G. Seaweeds as a functional ingredient for a healthy diet. Mar. Drugs 2020, 18, 301. [Google Scholar] [CrossRef]
- Kumar, M.; Gupta, V.; Kumari, P.; Reddy, C.R.K.; Jha, B. Assessment of nutrient composition and antioxidant potential of Caulerpaceae seaweeds. J. Food Compos. Anal. 2011, 24, 270–278. [Google Scholar] [CrossRef]
- Annian, S.; Chendur, P. Biochemical composition and fatty acid profile of the green alga Ulva reticulate. Asian J. Biochem. 2008, 3, 26–31. [Google Scholar] [CrossRef][Green Version]
- Cuervo, A.; Salazar, N.; Ruas-Madiedo, P.; Gueimonde, M.; Gonzalez, S. Fiber from a regular diet is directly associated with fecal short-chain fatty acid concentrations in the elderly. Nutr Res. 2013, 33, 811–816. [Google Scholar] [CrossRef]
- USDA National Nutrient Database for Standard Reference. Available online: https://data.nal.usda.gov/dataset/usda-national-nutrient-database-standard-reference-legacy-release (accessed on 23 February 2021).
- Klamczynska, B.; Mooney, W.D. Heterotrophic microalgae: A scalable and sustainable protein source. In Sustainable Protein Sources, 1st ed.; Nadathur, S.R., Wanasundara, J.P.D., Scanlin, L., Eds.; Academic Press: Oxford, UK, 2017; pp. 327–339. [Google Scholar]
- Mohamed, S.; Hashim, S.N.; Rahman, H.A. Seaweeds: A sustainable functional food for complementary and alternative therapy. Trends Food Sci. Technol. 2012, 23, 83–96. [Google Scholar] [CrossRef]
- O’Connor, K. Seaweed: A Global History; Reaktion Books: London, UK, 2017; pp. 12–15. [Google Scholar]
- Fleurence, J. Seaweed proteins: Biochemical, nutritional aspects and potential uses. Trends Food Sci. Technol. 1999, 10, 25–28. [Google Scholar] [CrossRef]
- Samarathunga, J.; Wijesekara, I.; Jayasinghe, M. Seaweed proteins as a novel protein alternative: Types, extractions, and functional food applications. Food Rev. Int. 2022, 1–26. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Dietary reference values for nutrients summary report. EFSA J. 2017, 14, 15121. [Google Scholar]
- Imchen, T. Nutritional value of seaweeds and their potential to serve as nutraceutical supplements. Phycologia 2021, 60, 534–546. [Google Scholar] [CrossRef]
- Drum, R. Sea Vegetables for Food and Medicine. 2021. Available online: http://www.ryandrum.com/seaxpan1.html (accessed on 12 February 2022).
- Karatela, S.; Paterson, J.; Ward, N.I. Domain specific effects of postnatal toenail methylmercury exposure on child behaviour. J. Trace Elem. Med. Biol. 2017, 41, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Ismail, M.F.; Ramaiya, S.D.; Zakaria, M.H.; Ikhsan, N.F.M.; Awang, M.A. Mineral content and phytochemical properties of selected Caulerpa species from Malaysia. Malaysian J. Sci. 2020, 39, 115–131. [Google Scholar] [CrossRef]
- Mann, J.; Truswell, A.S. (Eds.) Essentials of Human Nutrition, 5th ed.; Oxford University Press: Oxford, UK, 2017. [Google Scholar]
- Lozano Muñoz, I.; Díaz, N.F. Minerals in edible seaweed: Health benefits and food safety issues. Crit. Rev. Food Sci. Nutr. 2020, 62, 1592–1607. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.D. Lifestyle, minerals, and health. Med. Hypotheses 2001, 57, 521–531. [Google Scholar] [CrossRef] [PubMed]
- Lajçi, N.; Sadiku, M.; Lajçi, X.; Baruti, B.; Nikshiq, S. Assessment of major and trace elements of freshwater springs in village Pepaj, Rugova region, Kosova. J. Int. Environ. Appl. Sci. 2017, 12, 112–120. [Google Scholar]
- Tee, E.S.; Ismail, M.N.; Nasir, M.A.; Khatijah, I. Nutrient Composition of Malaysian Foods; Institute Medical Research: Kuala Lumpur, Malaysia, 1997.
- National Coordinating Committee on Food and Nutrition (NCCFSN), Ministry of Health, Malaysia. Recommended Nutrient Intakes for Malaysia: A Report of the Technical Group on Nutritional Guidelines; National Coordinating Committee on Food and Nutrition (NCCFSN), Ministry of Health: Putrajaya, Malaysia, 2017.
- Wells, M.L.; Potin, P.; Craigie, J.S.; Raven, J.A.; Merchant, S.S.; Helliwell, K.E.; Smith, A.G.; Camire, M.E.; Brawley, S.H. Algae as nutritional and functional food sources: Revisiting our understanding. J. Appl. Phycol. 2017, 29, 949–982. [Google Scholar] [CrossRef]
- MacArtain, P.; Gill, C.I.; Brooks, M.; Campbell, R.; Rowland, I.R. Nutritional value of edible seaweeds. Nutr. Rev. 2007, 65, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Mišurcová, L.; Machů, L.; Orsavová, J. Seaweed minerals as nutraceuticals. Adv. Food Nutr. Res. 2011, 64, 371–390. [Google Scholar] [CrossRef] [PubMed]
- Mendis, E.; Kim, S.K. Present and future prospects of seaweeds in developing functional foods. Adv. Food Nutr. Res. 2011, 64, 1–15. [Google Scholar]
- Fleurence, J.; Levine, I. (Eds.) Seaweed in Health and Disease Prevention; Academic Press: London, UK, 2016. [Google Scholar]
- Schmid, M.; Kraft, L.G.; Van der Loos, L.M.; Kraft, G.T.; Virtue, P.; Nichols, P.D.; Hurd, C.L. Southern Australian seaweeds: A promising resource for omega-3 fatty acids. Food Chem. 2018, 265, 70–77. [Google Scholar] [CrossRef]
- Ortiz, J.; Romero, N.; Robert, P.; Araya, J.; Lopez-Hernández, J.; Bozzo, C.; Navarrete, E.; Osorio, A.; Rios, A. Dietary fiber, amino acid, fatty acid and tocopherol contents of the edible seaweeds Ulva lactuca and Durvillaea antarctica. Food Chem. 2006, 99, 98–104. [Google Scholar] [CrossRef]
- Ortiz, J.; Uquiche, E.; Robert, P.; Romero, N.; Quitral, V.; Llantén, C. Functional and nutritional value of the Chilean seaweeds Codium fragile, Gracilaria chilensis and Macrocystis pyrifera. Eur. J. Lipid Sci. Technol. 2009, 111, 320–327. [Google Scholar] [CrossRef]
- Debbarma, J.; Viji, P.; Rao, B.M.; Ravishankar, C.N. Seaweeds: Potential Applications of the Aquatic Vegetables to Augment Nutritional Composition, Texture, and Health Benefits of Food and Food Products. In Sustainable Global Resources of Seaweeds; Ranga Rao, A., Ravishankar, G.A., Eds.; Springer: Cham, Switzerland, 2022; Volume 2, pp. 12–24. [Google Scholar]
- Fairulnizal, M.N.; Norhayati, M.K.; Zaiton, A.; Norliza, A.H.; Rusidah, S.; Aswir, A.R.; Suraiami, M.; Naeem, M.N.; Jo-Lyn, A.; Azerulazree, J.M.; et al. Nutrient content in selected commercial rice in Malaysia: An update of Malaysian food composition database. Int. Food Res. J. 2015, 2, 768. [Google Scholar]
- Ganesan, A.R.; Subramani, K.; Shanmugam, M.; Seedevi, P.; Park, S.; Alfarhan, A.H.; Rajagopal, R.; Balasubramanian, B. A comparison of nutritional value of underexploited edible seaweeds with recommended dietary allowances. J. King Saud Univ. Sci. 2020, 32, 1206–1211. [Google Scholar] [CrossRef]
- Balasubramaniam, V.; Chelyn, L.J.; Vimala, S.; Fairulnizal, M.M.; Brownlee, I.A.; Amin, I. Carotenoid composition and antioxidant potential of Eucheuma denticulatum, Sargassum polycystum and Caulerpa lentillifera. Heliyon 2020, 6, 4654. [Google Scholar] [CrossRef]
- Pérez-Gálvez, A.; Viera, I.; Roca, M. Carotenoids and chlorophylls antioxidants. Antioxidants 2020, 9, 505. [Google Scholar] [CrossRef] [PubMed]
- Vaňková, K.; Marková, I.; Jašprová, J.; Dvořák, A.; Subhanová, I.; Zelenka, J.; Novosádová, I.; Rasl, J.; Vomastek, T.; Sobotka, R.; et al. Chlorophyll-mediated changes in the redox status of pancreatic cancer cells are associated with its anticancer effects. Oxid. Med. Cell. Longev. 2018, 2018, 4069167. [Google Scholar] [CrossRef]
- Othman, R.; Md Amin, N.A.; Abu Bakar, A.E.; Ahmad Fadzillah, N.; Mahmad, N. Carotenoid Pigments of Red, Green and Brown Macroalgae Species as Potential Active Pharmaceutical Ingredients. J. Pharm. Nutr. Sci. 2019, 9, 14–19. [Google Scholar] [CrossRef]
- Corsetto, P.A.; Montorfano, G.; Zava, S.; Colombo, I.; Ingadottir, B.; Jonsdottir, R.; Sveinsdottir, K.; Rizzo, A.M. Characterization of antioxidant potential of seaweed extracts for enrichment of convenience food. Antioxidants 2020, 9, 249. [Google Scholar] [CrossRef]
- Buscemi, S.; Corleo, D.; Di Pace, F.; Petroni, M.L.; Satriano, A.; Marchesini, G. The effect of lutein on eye and extra-eye health. Nutrients 2018, 10, 1321. [Google Scholar] [CrossRef] [PubMed]
- Lunagariya, J.; Bhadja, P.; Zhong, S.; Vekariya, R.; Xu, S. Marine natural product bis-indole alkaloid caulerpin: Chemistry and biology. Mini Rev. Med. Chem. 2019, 19, 751–761. [Google Scholar] [CrossRef] [PubMed]
- Mao, S.C.; Guo, Y.W.; Shen, X. Two novel aromatic valerenane-type sesquiterpenes from the Chinese green alga Caulerpa taxifolia. Bioorg. Med. Chem. Lett. 2006, 16, 2947–2950. [Google Scholar] [CrossRef] [PubMed]
- De Souza, É.T.; Pereira de Lira, D.; Cavalcanti de Queiroz, A.; Costa da Silva, D.J.; Bezerra de Aquino, A.; Campessato Mella, E.A.; Prates Lorenzo, V.; De Miranda, G.E.C.; Araújo-Júnior, D.; Xavier, J.; et al. The antinociceptive and anti-inflammatory activities of caulerpin, a bisindole alkaloid isolated from seaweeds of the genus Caulerpa. Mar. Drugs 2009, 7, 689–704. [Google Scholar] [CrossRef]
- World Health Statistics 2022: Monitoring Health for the SDGs, Sustainable Development Goals; World Health Organization: Geneva, Switzerland, 2022.
- Wijesekara, I.; Kim, S.K. Angiotensin-I-converting enzyme (ACE) inhibitors from marine resources: Prospects in the pharmaceutical industry. Mar. Drugs 2010, 8, 1080–1093. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, C.; Aluko, R.E.; Hossain, M.; Rai, D.K.; Hayes, M. Potential of a renin inhibitory peptide from the red seaweed Palmaria palmata as a functional food ingredient following confirmation and characterization of a hypotensive effect in spontaneously hypertensive rats. J. Agric. Food Chem. 2014, 62, 8352–8356. [Google Scholar] [CrossRef]
- Cheung, R.C.F.; Ng, T.B.; Wong, J.H. Marine peptides: Bioactivities and applications. Mar. Drugs 2015, 13, 4006–4043. [Google Scholar] [CrossRef] [PubMed]
- Collins, P.; Webb, C.M.; De Villiers, T.J.; Stevenson, J.C.; Panay, N.; Baber, R.J. Cardiovascular risk assessment in women—An update. Climacteric 2016, 19, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Torruco-Uco, J.; Chel-Guerrero, L.; Martínez-Ayala, A.; Dávila-Ortíz, G.; Betancur-Ancona, D. Angiotensin-I converting enzyme inhibitory and antioxidant activities of protein hydrolysates from Phaseolus lunatus and Phaseolus vulgaris seeds. LWT Food Sci. Technol. 2009, 42, 1597–1604. [Google Scholar] [CrossRef]
- Joel, C.H.; Sutopo, C.C.; Prajitno, A.; Su, J.H.; Hsu, J.L. Screening of angiotensin-I converting enzyme inhibitory peptides derived from Caulerpa lentillifera. Molecules 2018, 23, 3005. [Google Scholar] [CrossRef]
- Sato, M.; Hosokawa, T.; Yamaguchi, T.; Nakano, T.; Muramoto, K.; Kahara, T.; Funayama, K.; Kobayashi, A.; Nakano, T. Angiotensin I-converting enzyme inhibitory peptides derived from wakame (Undaria pinnatifida) and their antihypertensive effect in spontaneously hypertensive rats. J. Agric. Food Chem. 2002, 50, 6245–6252. [Google Scholar] [CrossRef]
- Suetsuna, K.; Nakano, T. Identification of an antihypertensive peptide from peptic digest of wakame (Undaria pinnatifida). J. Nutr. Biochem. 2000, 11, 450–454. [Google Scholar] [CrossRef]
- Suetsuna, K.; Maekawa, K.; Chen, J.R. Antihypertensive effects of Undaria pinnatifida (wakame) peptide on blood pressure in spontaneously hypertensive rats. J. Nutr. Biochem. 2004, 15, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Liu, Y.; Wang, J.; Wu, S.; Geng, L.; Sui, Z.; Zhang, Q. Antihypertensive effects of two novel angiotensin I-converting enzyme (ACE) inhibitory peptides from Gracilariopsis lemaneiformis (Rhodophyta) in spontaneously hypertensive rats (SHRs). Mar. Drugs 2018, 16, 299. [Google Scholar] [CrossRef]
- Cao, D.; Lv, X.; Xu, X.; Yu, H.; Sun, X.; Xu, N. Purification and identification of a novel ACE inhibitory peptide from marine alga Gracilariopsis lemaneiformis protein hydrolysate. Eur. Food. Res. Technol. 2017, 243, 1829–1837. [Google Scholar] [CrossRef]
- Carrizzo, A.; Conte, G.M.; Sommella, E.; Damato, A.; Ambrosio, M.; Sala, M.; Scala, M.C.; Aquino, R.P.; Lucia, M.D.; Madonna, M.; et al. Novel potent decameric peptide of Spirulina platensis reduces blood pressure levels through a PI3K/AKT/eNOS-dependent mechanism. Hypertension 2019, 73, 449–457. [Google Scholar] [CrossRef]
- Kumagai, Y.; Kitade, Y.; Kobayashi, M.; Watanabe, K.; Kurita, H.; Takeda, H.; Yasui, H.; Kishimura, H. Identification of ACE inhibitory peptides from red alga Mazzaella japonica. Eur. Food Res. Technol. 2020, 246, 2225–2231. [Google Scholar] [CrossRef]
- Suetsuna, K. Purification and identification of angiotensin I converting enzyme inhibitors from the red alga Porphyra yezoensis. J. Mar. Biotechnol. 1998, 6, 163–167. [Google Scholar]
- Suetsuna, K. Separation and identification of angiotensin I converting enzyme inhibitory peptides from peptic digest of Hizikia fusiformis protein. Nippon Suisan Gakkaishi 1998, 64, 862–866. [Google Scholar] [CrossRef]
- Sun, S.; Xu, X.; Sung, X.; Zhang, X.; Chen, X.; Xu, N. Preparation and identification of ACE inhibitory peptides from the marine macroalga Ulva intestinalis. Mar. Drugs 2019, 17, 179. [Google Scholar] [CrossRef]
- Pan, S.; Wang, S.; Jing, L.; Yao, D. Purification and characterisation of a novel angiotensin-I converting enzyme (ACE)-inhibitory peptide derived from the enzymatic hydrolysate of Enteromorpha clathrata protein. Food Chem. 2016, 211, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, Y.; Toji, K.; Katsukura, S.; Morikawa, R.; Uji, T.; Yasui, H.; Shimizu, T.; Kishimura, H. Characterization of ACE inhibitory peptides prepared from Pyropia pseudolinearis protein. Mar. Drugs 2021, 19, 200. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.C.; Wang, J.; Zheng, B.-D.; Pang, J.; Chen, L.-J.; Lin, H.-T.; Guo, X. Simultaneous Determination of 8 Small Antihypertensive Peptides with Tyrosine at the C-Terminal in Laminaria japonica Hydrolysates by RP-HPLC Method. J. Food Process. Preserv. 2016, 40, 492–501. [Google Scholar] [CrossRef]
- Wu, Q.; Cai, Q.-F.; Yoshida, A.; Sun, L.-C.; Liu, Y.-X.; Liu, G.-M.; Su, W.-J.; Cao, M.-J. Purification and characterization of two novel angiotensin I-converting enzyme inhibitory peptides derived from r-phycoerythrin of red algae (Bangia fusco-purpurea). Eur. Food Res. Technol. 2017, 243, 779–789. [Google Scholar] [CrossRef]
- Admassu, H.; Gasmalla, M.A.A.; Yang, R.; Zhao, W. Identification of bioactive peptides with α-amylase inhibitory potential from enzymatic protein hydrolysates of red seaweed (Porphyra spp.). J. Agric. Food Chem. 2018, 66, 4872–4882. [Google Scholar] [CrossRef]
- Cermeño, M.; Stack, J.; Tobin, P.R.; O’Keeffe, M.B.; Harnedy, P.A.; Stengel, D.B.; FitzGerald, R.J. Peptide identification from a Porphyra dioica protein hydrolysate with antioxidant, angiotensin converting enzyme and dipeptidyl peptidase IV inhibitory activities. Food Funct. 2019, 10, 3421–3429. [Google Scholar] [CrossRef]
- Loquellano, M.C.S.; Rafael, D.V.K.N.; Bat-ao, M.; Bermudez, L.E.; Inting, D. Cholesterol Lowering Activity of Formulated Green Caviar (Caulerpa lentillifera J. Agardh Caulerpaceae) Seaweed Extract Tablet in Hypercholesterolemia-Induced Rabbits. Root Gatherers 2012, 1, 82–99. [Google Scholar]
- Sabirin, F.; Kazi, J.A.; Ibrahim, I.S.; Rashit, M.M.A. Screening of seaweeds potential against oral infections. J. Appl. Sci. Res. 2015, 11, 1–6. [Google Scholar]
- Yap, W.F.; Tay, V.; Tan, S.H.; Yow, Y.Y.; Chew, J. Decoding antioxidant and antibacterial potentials of Malaysian green seaweeds: Caulerpa racemosa and Caulerpa lentillifera. Antibiotics 2019, 8, 152. [Google Scholar] [CrossRef] [PubMed]
- Maeda, R.; Ida, T.; Ihara, H.; Sakamoto, T. Induction of apoptosis in MCF-7 cells by β-1,3-xylooligosaccharides prepared from Caulerpa lentillifera. Biosci. Biotechnol. Biochem. 2012, 76, 1032–1034. [Google Scholar] [CrossRef] [PubMed]
- Tanawoot, V.; Vivithanaporn, P.; Siangcham, T.; Meemon, K.; Niamnont, N.; Sobhon, P.; Tamtin, M.; Sangpairoj, K. Hexane Extract of Seaweed Caulerpa lentillifera Inhibits Cell Proliferation and Induces Apoptosis of Human Glioblastoma Cells. Sci. Technol. Asia 2021, 26, 128–137. [Google Scholar]
- Liang, W.S.; Liu, T.C.; Chang, C.J.; Pan, C.L. Bioactivity of β-1,3-xylan Extracted from Caulerpa lentillifera by Using Escherichia coli ClearColi BL21 (DE3)-β-1,3-xylanase XYLII. J. Food. Nutr. Res. 2015, 3, 437–444. [Google Scholar] [CrossRef]
- Arenajo, A.R.; Ybañez, A.P.; Ababan, M.M.P.; Villajuan, C.E.; Lasam, M.R.M.; Young, C.P.; Reyes, J.L.A. The potential anticoagulant property of Caulerpa lentillifera crude extract. Int. J. Health Sci. 2017, 11, 29–32. [Google Scholar]
- Abou Zid, S.F.; Ahmed, O.M.; Ahmed, R.R.; Mahmoud, A.; Abdella, E.; Ashour, M.B. Antihyperglycemic effect of crude extracts of some Egyptian plants and algae. J. Med. Food 2014, 17, 400–406. [Google Scholar] [CrossRef]
- Sharma, B.R.; Rhyu, D.Y. Anti-diabetic effects of Caulerpa lentillifera: Stimulation of insulin secretion in pancreatic β-cells and enhancement of glucose uptake in adipocytes. Asian Pac. J. Trop. Biomed. 2014, 4, 575–580. [Google Scholar] [CrossRef]
- Sharma, B.R.; Kim, H.J.; Rhyu, D.Y. Caulerpa lentillifera extract ameliorates insulin resistance and regulates glucose metabolism in C57BL/KsJ-db/db mice via PI3K/AKT signaling pathway in myocytes. J. Transl. Med. 2015, 13, 62. [Google Scholar] [CrossRef]
- Sharma, B.R.; Kim, H.J.; Rhyu, D.Y. Caulerpa lentillifera inhibits protein-tyrosine phosphatase 1B and protects pancreatic beta cell via its insulin mimetic effect. Food Sci. Biotechnol. 2017, 26, 495–499. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Liu, Z.; Song, S.; Zhu, B.; Zhao, L.; Jiang, J.; Liu, N.; Wang, J.; Chen, X. Anti-inflammatory activity and structural identification of a sulfated polysaccharide CLGP4 from Caulerpa lentillifera. Int. J. Biol. Macromol. 2020, 146, 931–938. [Google Scholar] [CrossRef]
- Daud, D.; Arsad, N.F.M.; Ismail, A.; Tawang, A. Anti-pyretic action of Caulerpa lentillifera, Hibiscus rosa-sinensis and Piper sarmentosum aqueous extract in mice. Asian J. Pharm. Clin. Res. 2016, 9, 9–11. [Google Scholar]
- Daud, D.; Zainal, A.N.; Nordin, M.N.; Tawang, A.; Ismail, A. Chelation activity and protective effect of Caulerpa lentillifera aqueous extract against lead acetate-induced toxicity in Sprague Dawley rats. J. Appl. Pharm. Sci. 2020, 10, 145–148. [Google Scholar] [CrossRef]
- Maeda, R.; Ida, T.; Ihara, H.; Sakamoto, T. Immunostimulatory activity of polysaccharides isolated from Caulerpa lentillifera on macrophage cells. Biosci. Biotechnol. Biochem. 2012, 76, 501–505. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Gong, G.; Guo, Y.; Wang, Z.; Song, S.; Zhu, B.; Zhao, L.; Jiang, J. Purification, structural features and immunostimulatory activity of novel polysaccharides from Caulerpa lentillifera. Int. J. Biol. Macromol. 2018, 108, 314–323. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhao, M.; Qing, Y.; Luo, Y.; Xia, G.; Li, Y. Study on immunostimulatory activity and extraction process optimization of polysaccharides from Caulerpa lentillifera. Int. J. Biol. Macromol. 2020, 143, 677–684. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, Y.; Ai, C.; Song, S.; Chen, X. Caulerpa lentillifera polysaccharides enhance the immunostimulatory activity in immunosuppressed mice in correlation with modulating gut microbiota. Food Funct. 2019, 10, 4315–4329. [Google Scholar] [CrossRef]
- Jing, Y.S.; Ma, Y.F.; Pan, F.B.; Li, M.S.; Zheng, Y.G.; Wu, L.F.; Zhang, D.S. An Insight into Antihyperlipidemic Effects of Polysaccharides from Natural Resources. Molecules 2022, 27, 1903. [Google Scholar] [CrossRef]
- Lee, Y.Y.; Choo, O.S.; Kim, Y.J.; Gila, E.S.; Jang, J.H.; Kang, Y.; Choung, Y.H. Atorvastatin prevents hearing impairment in the presence of hyperlipidemia. Biochim. Biophys. Acta Mol. Cell. Res. 2020, 1867, 11885. [Google Scholar] [CrossRef]
- Pratap, K.; Abdul, B.A.; Saikat, S.; Raja, C. A comprehensive review on polysaccharides with hypolipidemic activity: Occurrence, chemistry and molecular mechanism. Int. J. Biol. Macromol. 2022, 206, 681–698. [Google Scholar] [CrossRef]
- Raghavendran, H.R.B.; Sathivel, A.; Devaki, T. Effect of Sargassum polycystum (Phaeophyceae)-sulphated polysaccharide extract against acetaminophen-induced hyperlipidemia during toxic hepatitis in experimental rats. Mol. Cell Biochem. 2005, 276, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.H.; Gao, H.W.; Wang, S.; Wen, S.H.; Qin, S. Hypolipidemic and antioxidant properties of a polysaccharide fraction from Enteromorpha prolifera. Int. J. Biol. Macromol. 2013, 58, 186–189. [Google Scholar] [CrossRef] [PubMed]
- Hoang, M.H.; Kim, J.Y.; Lee, J.H.; You, S.G.; Lee, S.J. Antioxidative, hypolipidemic, and anti-inflammatory activities of sulfated polysaccharides from Monostroma nitidum. Food Sci. Biotechnol. 2015, 24, 199–205. [Google Scholar] [CrossRef]
- Jia, R.B.; Li, Z.R.; Wu, J.; Ou, Z.R.; Zhu, Q.Y.; Sun, B.G.; Lin, L.Z.; Zhao, M.M. Physicochemical properties of polysaccharide fractions from Sargassum fusiforme and their hypoglycemic and hypolipidemic activities in type 2 diabetic rats. Int. J. Biol. Macromol. 2020, 147, 428–438. [Google Scholar] [CrossRef] [PubMed]
- Kumar, Y.; Tarafdar, A.; Badgujar, P.C. Seaweed as a source of natural antioxidants: Therapeutic activity and food applications. J. Food Qual. 2021, 2021, 5753391. [Google Scholar] [CrossRef]
- Abu-Ghannam, N.; Rajauria, G. Functional Ingredients from Algae for Foods and Nutraceuticals; Woodhead Publishing: Sawston, UK, 2013. [Google Scholar]
- Namvar, F.; Mohamed, S.; Fard, S.G.; Behravan, J.; Mustapha, N.M.; Alitheen, N.B.M.; Othman, F. Polyphenol-rich seaweed (Eucheuma cottonii) extract suppresses breast tumour via hormone modulation and apoptosis induction. Food Chem. 2012, 130, 376–382. [Google Scholar] [CrossRef]
- Vaikundamoorthy, R.; Krishnamoorthy, V.; Vilwanathan, R.; Rajendran, R. Structural characterization and anticancer activity (MCF7 and MDA-MB-231) of polysaccharides fractionated from brown seaweed Sargassum wightii. Int. J. Biol. Macromol. 2018, 111, 1229–1237. [Google Scholar] [CrossRef]
- Xue, M.; Ji, X.; Xue, C.; Liang, H.; Ge, Y.; He, X.; Zhang, L.; Bian, K.; Zhang, L. Caspase-dependent and caspase-independent induction of apoptosis in breast cancer by fucoidan via the PI3K/AKT/GSK3β pathway in vivo and in vitro. Biomed. Pharmacother. 2017, 94, 898–908. [Google Scholar] [CrossRef]
- Pádua, D.; Rocha, E.; Gargiulo, D.; Ramos, A.A. Bioactive compounds from brown seaweeds: Phloroglucinol, fucoxanthin and fucoidan as promising therapeutic agents against breast cancer. Phytochem. Lett. 2015, 14, 91–98. [Google Scholar] [CrossRef]
- Otero, P.; Carpena, M.; Garcia-Oliveira, P.; Echave, J.; Soria-Lopez, A.; García-Pérez, P.; Fraga-Corral, M.; Cao, H.; Nie, S.; Xiao, J.; et al. Seaweed polysaccharides: Emerging extraction technologies, chemical modifications, and bioactive properties. Crit. Rev. Food Sci. Nutr. 2021, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Sharifuddin, Y.; Chin, Y.X.; Lim, P.E.; Phang, S.M. Potential bioactive compounds from seaweed for diabetes management. Mar. Drugs 2015, 13, 5447–5491. [Google Scholar] [CrossRef] [PubMed]
- Kotas, M.E.; Medzhitov, R. Homeostasis, inflammation, and disease susceptibility. Cell 2015, 160, 816–827. [Google Scholar] [CrossRef] [PubMed]
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef]
- Dray, A. Inflammatory mediators of pain. Br. J. Anaesth. 1995, 75, 125–131. [Google Scholar] [CrossRef]
- Hotamisligil, G.S. Inflammation and metabolic disorders. Nature 2006, 444, 860–867. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative stress: Harms and benefits for human health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Shah, M.D.; Seelan Sathiya Seelan, J.; Iqbal, M. Phytochemical investigation and antioxidant activities of methanol extract, methanol fractions and essential oil of Dillenia suffruticosa leaves. Arab J. Chem. 2020, 13, 7170–7182. [Google Scholar] [CrossRef]
- Shah, M.D.; Iqbal, M. Diazinon-induced oxidative stress and renal dysfunction in rats. Food Chem. Toxicol. 2010, 48, 3345–3353. [Google Scholar] [CrossRef]
- Chan, P.T.; Matanjun, P.; Yasir, S.M.; Tan, T.S. Antioxidant activities and polyphenolics of various solvent extracts of red seaweed, Gracilaria changii. J. Appl. Phycol. 2015, 27, 2377–2386. [Google Scholar] [CrossRef]
- Matanjun, P.; Mohamed, S.; Mustapha, N.M.; Muhammad, K. Nutrient content of tropical edible seaweeds, Eucheuma cottonii, Caulerpa lentillifera and Sargassum polycystum. J. Appl. Phycol. 2009, 21, 75–80. [Google Scholar] [CrossRef]
- Osotprasit, S.; Samrit, T.; Chaiwichien, A.; Changklungmoa, N.; Meemon, K.; Niamnont, N.; Manohong, P.; Noonong, K.; Tamtin, M.; Sobhon, P. Toxicity and anti-oxidation capacity of the extracts from Caulerpa lentillifera. Chiang Mai Univ. J. Nat. Sci. 2021, 20, 2021065. [Google Scholar] [CrossRef]
- Ooi, E.E.; Dhar, A.; Petruschke, R.; Locht, C.; Buchy, P.; Low, J.G.H. Use of analgesics/antipyretics in the management of symptoms associated with COVID-19 vaccination. NPJ Vaccines 2022, 7, 31. [Google Scholar] [CrossRef] [PubMed]
- Przybyła, G.W.; Szychowski, K.A.; Gmiński, J. Paracetamol—An old drug with new mechanisms of action. Clin. Exp. Pharmacol. 2021, 48, 3–19. [Google Scholar] [CrossRef]
- Sultana, S.; Asif, H.M.; Akhtar, N.; Ahmad, K. Medicinal plants with potential antipyretic activity: A review. Asian Pac. J. Trop. Dis. 2015, 5, 202–208. [Google Scholar] [CrossRef]
- Carnieto, A., Jr.; Dourado, P.M.M.; Luz, P.L.D.; Chagas, A.C.P. Selective cyclooxygenase-2 inhibition protects against myocardial damage in experimental acute ischemia. Clinics 2009, 64, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Ruan, C.H.; So, S.P.; Ruan, K.H. Inducible COX-2 dominates over COX-1 in prostacyclin biosynthesis: Mechanisms of COX-2 inhibitor risk to heart disease. Life Sci. 2011, 88, 24–30. [Google Scholar] [CrossRef]
- Kim, J.J.; Kim, Y.S.; Kumar, V. Heavy metal toxicity: An update of chelating therapeutic strategies. J. Trace Elem. Med. Biol. 2019, 54, 226–231. [Google Scholar] [CrossRef]
- Jan, A.T.; Azam, M.; Siddiqui, K.; Ali, A.; Choi, I.; Haq, Q.M. Heavy metals and human health: Mechanistic insight into toxicity and counter defense system of antioxidants. Int. J. Mol. Sci. 2015, 16, 29592–29630. [Google Scholar] [CrossRef]
- Huat, T.J.; Camats-Perna, J.; Newcombe, E.A.; Valmas, N.; Kitazawa, M.; Medeiros, R. Metal toxicity links to Alzheimer’s disease and neuroinflammation. J. Mol. Biol. 2019, 431, 1843–1868. [Google Scholar] [CrossRef]
- Cao, Y.; Skaug, M.A.; Andersen, O.; Aaseth, J. Chelation therapy in intoxications with mercury, lead and copper. J. Trace Elem. Med. Biol. 2015, 31, 188–192. [Google Scholar] [CrossRef] [PubMed]
- Pulicherla, K.K.; Verma, M.K. Targeting therapeutics across the blood brain barrier (BBB), prerequisite towards thrombolytic therapy for cerebrovascular disorders—An overview and advancements. AAPS PharmSciTech 2015, 16, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Bjørklund, G.; Mutter, J.; Aaseth, J. Metal chelators and neurotoxicity: Lead, mercury, and arsenic. Arch. Toxicol. 2017, 91, 3787–3797. [Google Scholar] [CrossRef]
- Janeway, C.A., Jr.; Medzhitov, R. Innate immune recognition. Annu. Rev. Immunol. 2002, 20, 197–216. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).