Abstract
The prevalence of cardiovascular disease, oxidative stress-related complications, and chronic age-related illnesses is gradually increasing worldwide. Several causes include the ineffectiveness of medicinal treatment therapies, their toxicity, their inability to provide radical solutions in some diseases, and the necessity of multiple drug therapy in certain chronic diseases. It is therefore necessary for alternative treatment methods to be sought. In this review, polyphenols were identified and classified according to their chemical structure, and the sources of these polyphenol molecules are indicated. The cardioprotective, ROS scavenging, anti-aging, anticancer properties of polyphenolic compounds have been demonstrated by the results of many studies, and these natural antioxidant molecules are potential alternative therapeutic agents.
1. Introduction
The emphasis on healthy diets and mindful eating patterns is gaining more importance among society. Until recently, concepts such as the Mediterranean-style diet, wine consumption habits, and dietary habits such as olive oil consumption were emphasized. Now, the word nutraceutical has begun to be added to these concepts. Nutraceuticals are natural substances such as vitamins, polyphenols, fiber, keratinoid, prebiotics, fatty acids, and bioactive peptides [1]. It is possible for these substances, which are defined as functional foods, to be included in foods. Polyphenols in this group, which are the secondary metabolites of plants, are found in high quantities in beverages such as tea, wine, grape juice, as well as in various fruits and vegetables [2,3]. Vegetable sources containing high levels of polyphenols include grape (Vitis vinifera) and red wine, mulberry (Morus rubra), peanut (Arachis hypogaea), and onion [4,5,6]. In addition, red pepper, eggplant, soybean, pear, garlic, carrot, and Ziziphus fruits are other foods that contain a significant amount of polyphenolic compounds [7,8,9]. Apart from plants, marine algae have been identified as important bioactive natural resources containing polyphenols, especially catechins, tannins and flavanol glycosides [10].
Environmental factors such as daylight, soil type, precipitation, and agricultural conditions have a major impact on the polyphenol content of natural products. For example, the biosynthesis of flavonoids as one of the most familiar members of polyphenols, is stimulated by light, and these polyphenols accumulate at different rates between the aerial tissues and other parts of the plants, even among fruits of the same tree [11,12]. In the extraction of plant-based polyphenols, methods such as maceration (by adding cold water to the plant and keeping it at room temperature for a period of a few hours to few days), percolation (passing the substances in the plants to the solvent by passing through a column), reflux, use of Soxhlet apparatus and boiling have been commonly used [13]. Depending on the employed methods, e.g., Soxhlet extraction, they may require the use of different solvents and/or high amounts of organic solvents, and therefore, the selectivity may be different for one polyphenolic component and may be high for other polyphenolic components. Therefore, novel extraction methods that are specific for certain polyphenolic need to be developed. Recently, supercritical fluid extraction, enzyme-assisted extraction, solid-phase extraction, or combinations of these similar techniques can afford some advantages [14].
Polyphenolic compounds can be in monomer, oligomer and polymer forms, or colored or colorless depending on pH. Although aglycone forms are not stable, polyphenolic compounds in plants are resistant to light, pH, or other conditions that can degrade them, due to glycosylation [7].
These natural substances have antioxidant, anti-inflammatory, anticancer, and antidiabetic properties and protective effects on cardiovascular and nervous system health [15,16]. It is thought that the consumption of foods containing polyphenolic components with a wide variety of protective and healing properties provides an alternative area that can be used for therapeutic purposes in many cases, including chronic diseases [17,18].
It is known that polyphenolics are closely related to the intestinal microbiota, which plays a major role in maintaining the healthy state of individuals or preventing diseases before they occur. Polyphenols can modulate the composition of the intestinal microbiota, thus affecting their own metabolism and bioavailability [19].
In this review, polyphenols are classified into four main groups according to their chemical structures. The mechanisms through which phenolic compounds act in chronic and acute diseases and the general pathology of cardiovascular disorders are explained. After the pathological processes are stated, the effects of polyphenols in each group are explained with various examples.
2. Classification of Polyphenols
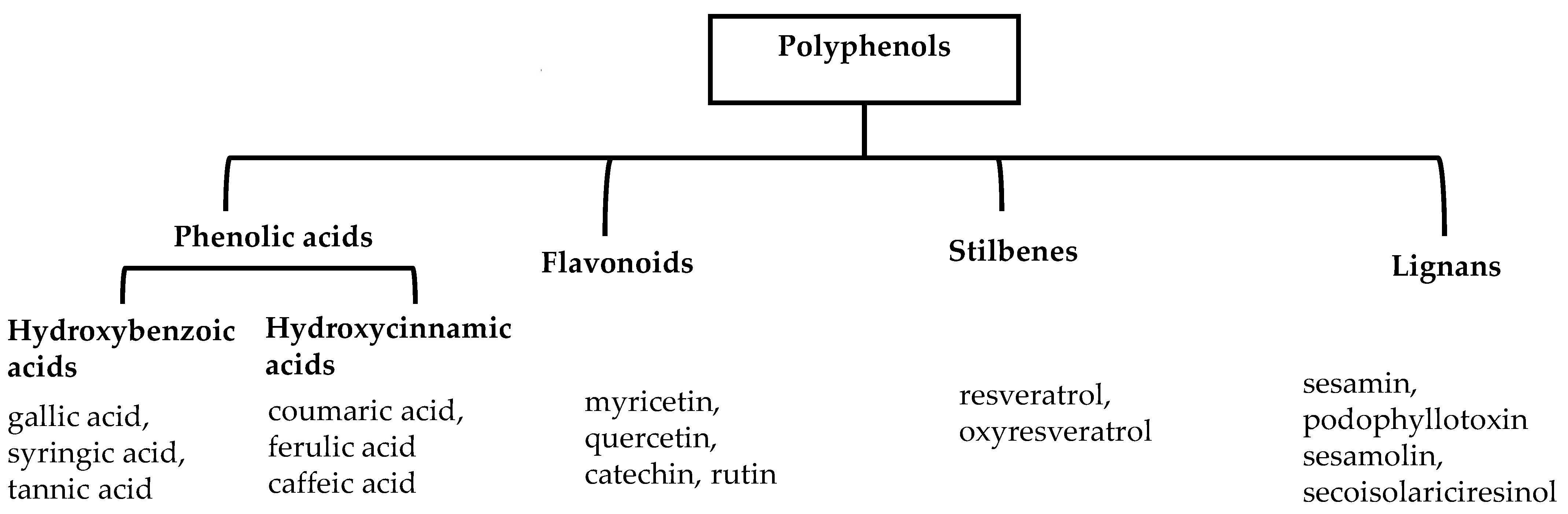
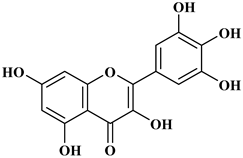
Polyphenols are a well-known group of phenolic systems characterized by at least two phenyl rings and one or more hydroxyl substituents [20]. Polyphenols can be found in all organs of plants. It is generally believed that flavonoids have weak resistance to diverse environmental stresses (heat, light, and oxidation). Polyphenols synthesized by plants can be simply divided into flavonoids and non-flavonoids. Polyphenols can be classified according to their chemical structure, whether they contain sugar or not, or by whether they are hydrolyzed or condensed. The sugar in polyphenol stabilizes the aglycone, increases its water solubility, and enhances its bioavailability and biocompatibility [21]. They can even be classified according to their resolution. Moreover, it can be classified based on its solubility or origin, for example, based on the plant or mammals that provide it [22]. As shown in Figure 1, polyphenols fall into four groups based on their chemical structure: phenolic acids, flavonoids, stilbenes, and lignans.
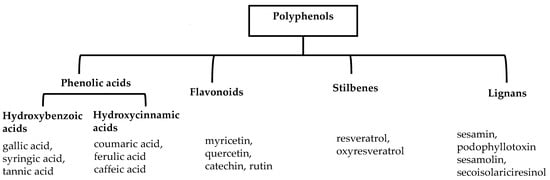
Figure 1.
Schematic presentation of classification of polyphenols.
2.1. Phenolic Acids
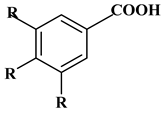
Phenolic acids contain an aromatic benzene ring and a carboxylic acid group as shown in Figure 1. Gallic acid, chlorogenic acid and coumaric acid are the most known and widely used phenolic acids. They provide organoleptic properties to foods [23]. Fruits, vegetables, and beverages are good sources of phenolic acid. Adding vegetables to people’s diet as a significant source of phenolic acids will probably bring about significant health benefits. [23]. Phenolic acids are of interest to pharmacologists as well as food engineers. They are recognized as components of nutraceutical products and are gaining more and more attention every day. They are divided into two types as hydroxybenzoic acid derivatives and hydroxycinnamic acid derivatives. Red fruits, onions, and black radish generally contain hydroxybenzoic acids [24]. Gallic acid, protocatechuic acid, syringic acid, and ellagic acid are examples of hydroxybenzoic acid derivatives.
Gallic acid (GA), 3,4,5-trihydroxy benzoic acid, is widely used as a reference substance in antioxidant tests [25,26]. GA is also a natural compound, and many fruits, nuts, and flowers (especially green tea and red wine) contain abundant amounts of GA [15]. GA has also been reported for its antioxidant, anticancer, neuroprotective capacity, anti-inflammatory properties, cardiovascular protective, hepatoprotective and gastroprotective activities [27]. GA has also been studied for anticancer properties [28] and for an antidiabetic effect [29]. Tannic acid is the most widely researched hydroxybenzoic acid. It is abundant in acorn and is inexpensive. Tannic acid hydrolyzes in acidic residue to form 10 molecules of gallic acid [30]. Due to its antimicrobial, antioxidant, anticancer properties, it is examined in biomedical materials [30]. In wound dressing materials, tannic acid is used as a coagulant because of its ability to clot blood [31].
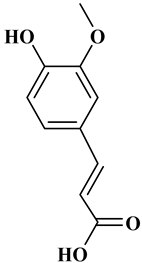
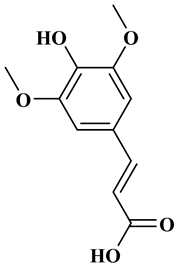
Ferulic acid, caffeic acid, chlorogenic acid, sinapic acid, rosmarinic acid, isoferulic acid, and coumaric acid are included in the hydroxycinnamic acid subgroup. Prune and coffee are the source of phenolic acids.
Rosmarinic acid (RA) is an example hydroxycinnamic acid. It is a known for its antioxidant and antidiabetic properties and is found in many plants including mint, rosemary, and basil [32]. There many research reports that detail the use of RA as a biomedical compound for treatment of organ injuries and in ocular delivery [33]. For type 2 diabetes mellitus, it has an effect on reducing the amount of glucose in the blood, as it inhibits the work of α-glucosidase and α-amylase enzymes that are involved in the breakdown of disaccharides [34]. RA also fights the complications of diabetes resulting in progressive organ damage via its anti-inflammatory and antioxidant activities [33]. The chemical structures and some of the subgroups of polyphenols are given in Table 1.

Table 1.
Chemical structure of polyphenols and some examples of subgroups of polyphenols.
2.2. Flavonoids
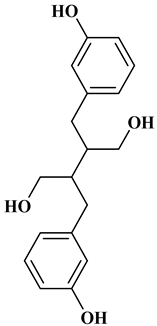
The 15-carbon skeleton is the structural basis of polyphenolic compounds called flavonoids [35]. Rutin, quercetin, naringin, myricetin, catechin hesperidin, naringenin, isosakuratenin, and heridictyol are the other examples of flavonoids [36].
Naringin (NR) is the form of naringenin that is substituted with two glucose moieties [21], and the chemical structure is given in Figure 1. The sugar serves to stabilize the aglyocone, increasing the biocompatibility [21]. NR is abundant in grapefruit and is one of the components that gives grapefruit a bitter taste. NR exhibits antioxidant, anti-inflammatory, anti-apoptotic, anticancer, antimutagenic, and redox protective properties. It also reduces cholesterol levels, relieves neurodegenerative disorders, improves patients with diabetes mellitus, reduces osteoporosis, and alleviates rheumatoid arthritis [37].
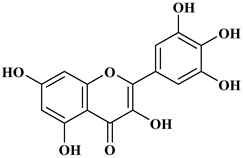
Myricetin, 3,5,7-Trihydroxy-2-(3,4,5-trihydroxyphenyl)-4H-chromen-4-one is a flavonol. It is found in vegetables such as tomatoes, fruits such as oranges, nuts, berries, tea, and red wine. Flavonoids are divided into subclasses such as anthocyanins, flavonols, flavones, and anthocyanidin [20].
Plants contain color flavonoids called anthocyanins, which are commonly found in flowers. In addition to pollination and seed dispersal, plants display pollination and seed dispersal in their fruits and flowers. These components are also involved in a host of defense mechanisms against pathogens and herbivores while also contributing to the photosynthesis process. Pelargonidin, cyanidin, peonidin, delphinidin, petunidin, and malvidin are the most common anthocyanidins found in plants [35]. It is commonly studied to determine the total anthyocin amount of plants or some composite fabricated by extraction from plants [38]. During storage, anthocyanins’ colors change depending on pH, protonation, and hydration reactions. Cyanidin, malvidin, and chalcones are well-known and studied antocyanin structures [38].
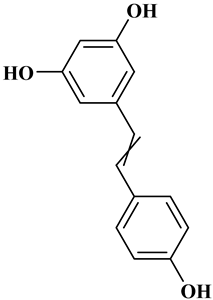
2.3. Stilbenes
Two aromatic rings are linked by an ethylene ring in the stilbene, which has a backbone of C6–C2–C6 [39]. Resveratrol and oxyresveratrol are some samples of the stilbenes [40]. Oxyresveratrol has been shown to have inhibitory activity on Trichophyton rubrum, which causes fungal infections such as tinea pedis, and it has been emphasized that it has the potential to reduce the use of antibiotics as adjuvant therapy with its antimicrobial activity [40]. The skin of grapes, blueberries, raspberries, mulberries, and peanuts is the source of stilbenes. Resveratrol is widely found in red wine and grape juice.
2.4. Lignans
Lignans represent a nonflavonoid class featuring two propylbenzenes. Lignans are found in legumes, grains, seeds, and vegetable oils; they are mainly found in their free forms. Flaxseeds are excellent sources of lignans. Lignans are structures that protect plants against herbivores. They are generally insoluble. Sesamin and sesamolin are kinds of lignin molecules found in sesame seeds. The other known plant sources of lignans are secoisolariciresinol, podophyllotoxin and hydroxymatairesinol. Hydroxymatairesinol is a lignan found in Norway spruce [41]. Hydroxymatairesinol has been observed in Parkinson’s disease, which is a chronic neurodegenerative disorder, to slow down its progression in rat models [41]. It has been determined that it has anticancer properties in prostate cancer [42]. Pinoresinol is another lignan molecule that has hepatoprotective effects [43].
3. Cardioprotective Effects of Polyphenolic Compounds
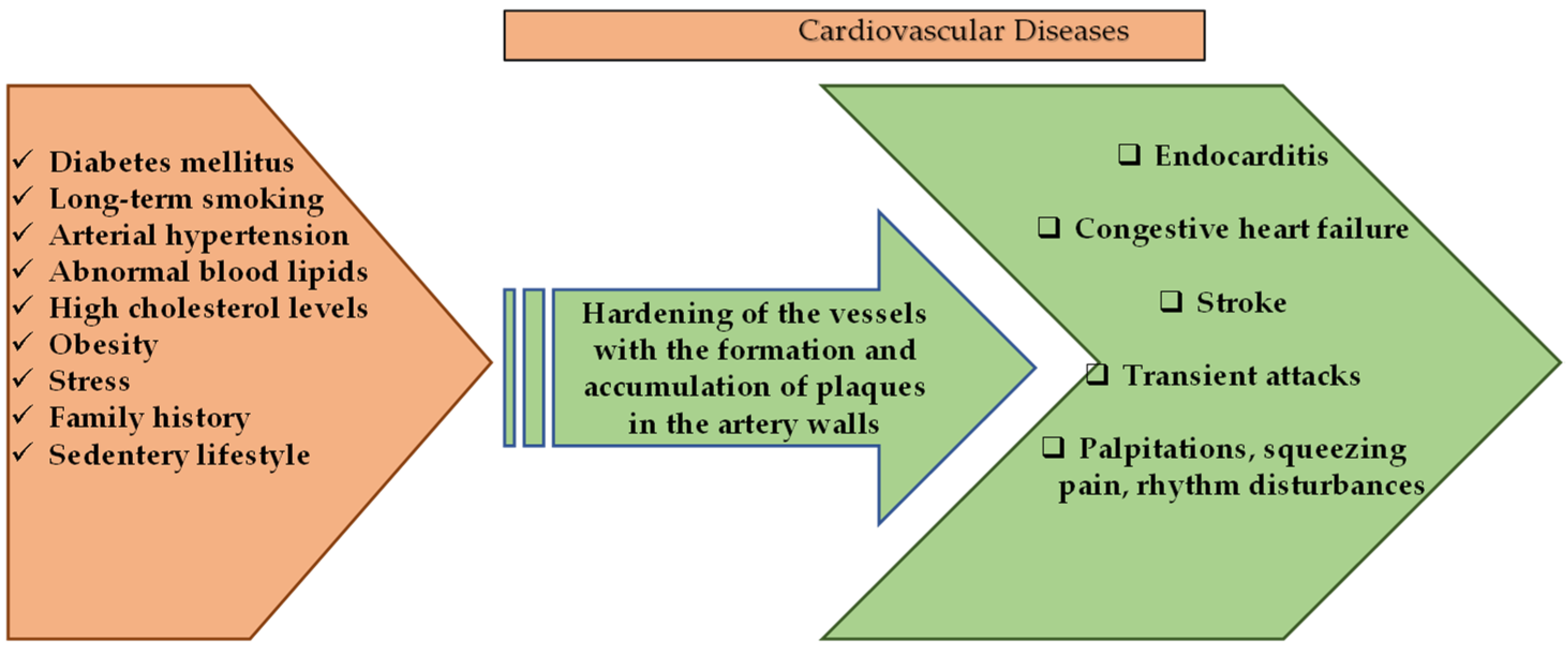
Cardiovascular diseases (CVD) are a group of disorders that are characterized by heart and blood vessels and include coronary heart disease, cerebrovascular disease, congestive heart failure, rheumatic and congenital heart diseases. transient ischemic attack, or stroke, and carry a mortality and morbidity rate [44]. According to the calculations of the World Health Organization in 2016, CVD constitutes approximately 30% of death causes worldwide [45] and is considered to be the first cause of chronic, non-communicable deaths in adults. Deaths due to heart attacks and stroke constitute a large part of this calculated percentage [44]. The dramatic changes in the frequency of symptoms of CVDs in different age groups, both genders, and all races have been alarming in recent years. Although mortality rates vary according to income distribution, demographic population, diet, and lifestyle, it is among the diseases with the highest prevalence and incidence all over the world [46,47]. Risk factors for the development of CVD include long-term smoking, diabetes mellitus, unhealthy diet, intense stress, presence of CVD in family history, arterial hypertension, abnormal blood lipids, sudden paralysis, high cholesterol, obesity, and sedentary life [44,48]. It is known that multiple factors affect pathogenesis, but it is generally caused by atherosclerosis, which is known to occur as a result of oxidative stress and chronic inflammatory conditions [48].
Atherosclerosis is a degenerative process of the arteries characterized by thickening and hardening of the innermost intimal layer of the arterial walls. It is the most common pathological factor involved in the development of CVD. The healthy endothelial cells release nitric oxide (NO) to induce vasodilation, and contain anticoagulants, antiplatelet receptors, and structures that prevent thrombus formation, thus preventing unwanted aggregation of platelets and regulating vascular tone [49]. Nitric oxide is known to prevent the oxidation of LDL cholesterol, and if there is endothelial dysfunction, decreasing production of NO and impaired NO activity leads to consequences promoting atherosclerosis and is present in conditions such as vasoconstriction, smooth muscle cell proliferation, and oxidative stress.
Atherosclerotic plaque develops with an “active biological environment” such as interaction of endothelial dysfunction, vascular smooth muscle cell migration, lipid deposition (LDL oxidation), calcification, matrix turnover, immune response and inflammatory responses [47,50,51]. Hypertension, or high blood pressure, is one of the leading causes of cardiovascular diseases [52]. Hypertension affects both the cardiovascular and cerebrovascular systems. It has been reported that the incidence of hypertension increases with age and, if not controlled, leads to the risk of heart attack and stroke in patients [53].
Cardiovascular diseases and general factors involved in their pathology are given in Figure 2.
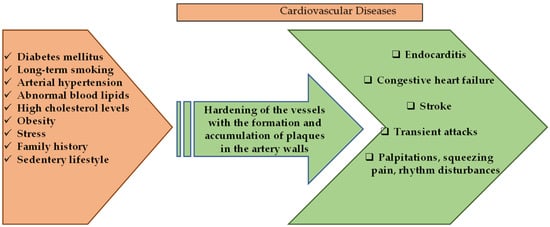
Figure 2.
Schematic presentation of cardiovascular diseases and general factors involved in their pathology.
Figure 2 demonstrates several genetic-based factors and many environmental factors involved in the pathological process that results in the emergence of cardiovascular system diseases. Many of these risk factors can be regulated by changes in the lifestyles of individuals [54,55]. Natural-based compounds are of interest in disease-prevention approaches and in the regulation of patients’ lifestyles, whose cardioprotective properties have already been proven or are currently being tested for effectiveness [55,56].
In addition to factors that cannot be changed, such as gender, genetic predisposition, and age, cardiovascular diseases also affect many external factors that can be controlled. The most important behavioral risk factors of cardiovascular diseases are unhealthy diet and physical inactivity. Among these, the way of nutrition and efforts to improve lifestyle habits sometimes provide a high protective effect against cardiovascular disease [54,55,57,58,59]. Alternative treatment methods are being developed for CVD, which has a high prevalence and incidence, and the consumption of functional foods and supplements has been on the agenda of clinical institutions in recent years [54].
As an example of the relationship between diet and CVD, excessive saturated fat diet increases the plasma LDL levels and subsequent risk of atherosclerosis [58]. Interest in new areas in the field of pharmaceutical and dietary strategies has started to minimize CVD risk as much as possible. Epidemiological studies have indicated that a high flavonoid content diet is positively related to lower incidence of coronary artery disease, hypertension, stroke, and other vascular diseases [60,61,62,63].
The protective effect of polyphenols on the cardiovascular system is due to their ability to delay the development and progression of early atherosclerotic lesions [54,57,58]. Another important mechanism of action is thought to be the regulation of NO release and antioxidant and anti-inflammatory properties of polyphenols [54,60].
The Mediterranean diet includes vegetables, fruits, bread, and other cereals, legumes and oilseeds, a moderate amount of alcohol consumption, and olive oil with a high content of polyphenolic compounds. It has been noted that this type of diet has a clear effect on reducing CVD risk factors and preventing cardiovascular diseases. The advantages of increased plasma HDL-C and decreasing LDL oxidation were seen through the controlled consumption of red wine [54,55,59]. In one of the studies evaluating the relationship between the Mediterranean diet and the risk of CV events, an inverse relationship was found between total polyphenol intake and CVD risk [64,65]. The effects of an herbal preparation consisting of procyanidin, daidzein, genistein, flavone, resveratrol on atherosclerosis in postmenopausal women without cardiovascular disease was investigated [66]. It was found that this preparation significantly suppressed the formation of atherosclerosis in individuals and could be a potential agent to slow the progression of existing plaques [66].
3.1. Phenolic Acids
An herbal combination with high polyphenol content composed of caffeic acid, chlorogenic acid, p-coumaric acid, and ferulic acid was administered to rabbits with myocardial cell damage, and it was emphasized that it could be used as an alternative treatment due to its synergistic cardio-protective potential [67]. In the study comparing the efficacy of administering a ferulic acid and aldose reductase inhibitor, which is the standard treatment, proved to alleviate insulin resistance and hypertension and mediated the restoration of endothelial-dependent relaxation by improving the bioavailability of NO [68]. Cardiac and kidney lesions, including oxidative stress, inflammation, vascular occlusion, and erosion of epithelial cells, were observed in rats that had been given the endocrine-disrupting chemical Bisphenol A (BPA). The addition of gallic acid into the BPA was found to mitigate the toxicity caused by the BPA [69].
The cardioprotective effects of tannic acid are thought to be mainly due to the reduction of myocardial oxidative stress, inhibition of inflammation, reduction of apoptosis, and increase in NO levels [70,71,72,73]. The potential cardioprotective properties of tannic acid were investigated in rabbits in a case of isoproterenol-induced myocardial ischemia [70]. Tannic acid has been proven to effectively heal ischemic damage. This therapeutic effect has been associated with inhibiting inflammation, providing endothelial protection, scavenging reactive oxygen species, reducing cell apoptosis and many other properties [70]. Tannic acid can reduce collagen accumulation on isoproterenol-induced myocardial tissue morphology, thereby reducing and healing myocardial mitochondrial damage [71]. It has been proven that tannic acid can target elastin stabilization in cardiovascular implants, rendering it enzymatically resistant [72]. Tannic acid at a concentration of 0.05% has been found to have minimal cytotoxic effect after 4 weeks of infarction, increasing the collagen content and significantly reducing infarction dilation. This treatment may attenuate adverse cardiac remodeling after acute myocardial infarction [74].
Another phenolic acid, caffeic acid, has cardioprotective properties, and relevant utilization and function from the literature are given in Table 2. During hypoxia or when exposed to uremic toxins, caffeic acid increased proliferation and angiogenesis as well as inhibited leukocyte adhesion and endothelial cell apoptosis [75].
3.2. Certain Cardioprotective Effects of Flavonoids
The most common flavonoids found in grapes are anthocyanins, flavonols, flavanols, dihydroflavanols, and proanthocyanidins. The study has shown that proanthocyanidins in grape seeds have the capacity to reduce or remove reactive oxygen radicals in the reperfused myocardium after ischemia, thereby leading to a recovery of the damage caused by reperfusion, a decrease in the incidence of arrhythmia, and a significant improvement in postischemic cardiac function [76]. Another study reported that the administration of grape polyphenol concentrate to rats with doxorubicin-induced cardiotoxicity resulted in a cardioprotective effect, possibly due to the increased level and activity of antioxidant enzymes [77]. In a study conducted in people at risk for type 2 diabetes, it was concluded that consumption of blueberry juice tends to improve systolic blood pressure through nitric oxide production [78].
The effect of a long-term quercetin diet on inflammation and physiological heart performance in dystrophic hearts were investigated [79]. According to the results, a quercetin-rich diet intake can be a potential treatment option for the complications of cardiac dystrophy. A medicinal plant, Thraatchathi Chooranam, containing various highly polyphenolic compounds such as gallic acid, quercetin, ellagic acid, galangin and naringenin provided a stabilizing, radical scavenging and protective effect on the cardiac membrane [80].
Epigallocatechin-3-gallate (EGCG), which is an important polyphenolic component also found in green tea, is thought to be cardioprotective against ischemia-reperfusion due to its antioxidant properties [81,82,83]. EGCG contributed to the heart’s adaptation to excessive pressure loading during the hypertrophy phase [84]. For this reason, it has been stated that EGCG can be a potential agent that can prevent heart failure due to cardiac hypertrophy [81]. EGCG can inhibit apoptosis by activating a certain pathway and shows a cardioprotective effect against ischemic damage [85]. Oral epigallocatechin-3-gallate pretreatment is thought to be an alternative cardioprotective application to prevent perioperative cardiac dysfunction during surgery [83].
The study examined increased cardiac toxicity associated with exposure to air pollution, and reported that feeding the rats that were exposed to air pollution for a long time dark chocolate containing high polyphenols downregulated myocardial inflammation genes and increased antioxidant and cardioprotective genes [86]. After consuming a fatty diet, drinking cocoa resulted in an increase in HDL and a decrease in LDL [87]. In addition, cocoa has been found to alleviate the effect of inflammation.
A dietary supplement containing the flavonoids of apigenin, kaempferol, and luteolin improved glucose tolerance and fat accumulation, as well as plasma triglyceride and total cholesterol levels, and reduced heart damage [88]. Limonin has been found to significantly lower the level of apolipoprotein B(Apo-B), a potential marker for coronary artery disease, and this effect is greater than that of other citrus flavonoids [89].
Myricetin contributes to the vitality of human vascular smooth muscle cells and the function of enzyme activity [90]. Furthermore, dihydromyricetin exerts an anti-inflammatory effect by reducing the release of interleukins, which are inflammatory agents, and prevents increases in the harmful effects of reactive oxygen species in the process of atherosclerosis [91,92].
3.3. Stilbenes
The effects of resveratrol and red wine in vivo (in hypercholesterolemic rabbits) and in vitro (on platelets isolated from normotensive healthy male volunteers) were investigated. It has been reported that resveratrol can inhibit platelet aggregation both in vitro and in vivo, possibly due to the cardioprotective effects of the polyphenol [93]. There was a significant increase in lipid levels in rabbits fed high-cholesterol diets, and they stated that hypercholesterolemia increases the susceptibility to platelet aggregation and has a risk of playing a role in myocardial infarction. The polyphenolic compounds found in red wine decreased intracellular cholesterol levels [94]. In an experimental study on hypertensive rats, a significant increase in HDL levels and a decrease in blood pressure were found [95].
Many studies show that resveratrol is a potential cardioprotective agent [96,97,98,99]. The effect of resveratrol on LDL oxidation and the free radical scavenging activity of the compounds on rat hearts was evaluated, and astringinin showed stronger cardioprotective activity than resveratrol in ischemia-reperfusion injury, and they thought that this was due to its high water solubility [97]. Ungvari et al. (2007) confirmed that resveratrol partially exerts antioxidant effects and plays a regulatory role in the vascular expression of some enzymes [96]. They stated that enzyme regulation shows antiapoptotic activity in the cardiovascular system in case of ischemia-reperfusion pathology. Resveratrol preserved cardiac function, suppressed the expression of inflammatory response-initiating genes, and had a significant ameliorating effect on myocarditis [98]. Resveratrol treatment was found to attenuate induced cardiomyocyte apoptosis and reduce toxicity on the heart by suppressing stress-induced overexpression [99]. In addition, resveratrol can protect the heart from doxorubicin damage with its antioxidant properties [100]. A significant reduction in gene conjugate required for autophagic expansion and termination in the group receiving resveratrol supplements was found. During myocardial stress, autophagy, an adaptive process in which damaged cellular components are removed or recycled, is cardioprotective. Resveratrol supplementation has been found to regulate autophagy and prevent endothelial dysfunction and cardiac remodeling in ischemic myocardium [101].
Resveratrol provides regulatory effects on free fatty acid oxidation, glucose homeostasis, glucose utilization, myocardial metabolic enzymes, and energy metabolism [102]. Resveratrol can exert a protective effect by inhibiting endoplasmic reticulum stress and reversing apoptotic pathway proteins’ expression in cardiomyocyte hypertrophy. Partially beneficial effects of resveratrol, which may be a new strategy for the treatment of cardiac hypertrophy, have also been reported in studies [103]. The curative effect of resveratrol in cardiac hypertrophy is through expression regulation as a result of breast cancer type 1 sensitivity protein inhibition [104].
In ischemia-reperfused rats, piceatannol slows the inactivation of I(Na) and inhibits arrhythmias [105]. Piceatannol can prevent cardiomyocyte cell damage caused by oxidative stress by activating superoxide dismutase, decreasing creatine kinase, and reducing lactate dehydrogenase production [106].
3.4. Lignans
Cardiotoxicity was induced in rabbits pre-treated with flaxseed, and the myocardium was examined histopathologically. A decrease in myocardial necrosis and inflammation as well as improvement in hemodynamic and biochemical markers were detected in the flaxseed-fed group. The antioxidant effect of flaxseed was found to contribute to its cardioprotective effect [107]. A similar result was obtained in the study, and it was suggested that the cardioprotective effect was due to the alpha-linolenic acid content [108]. It has been proven that lignan concentrate (flax lignan concentrate (500 mg/kg) and omega-3-fatty acid (1 mL/kg)) provide an antihyperlipidemic effect by normalizing lipid levels, and exerts an anti-inflammatory anti-apoptotic effect by affecting many parameters such as tumor necrosis factor-α level [109]. In another study, rats with experimental renal hypertension were divided into groups and administered angiotensin converting enzyme inhibitor (ACE inhibitor) or flax lignan concentrate. Flax lignan concentrate was observed to significantly reduce arterial blood pressure, similar to the standard ACE inhibitor. In addition, its antioxidant activity was shown to reduce histopathological damage in heart and kidney and to restore abnormal lipid profiles [110]. In addition, the consumption of lignan containing flaxseed and flax oil in moderate amounts causes a significant decrease in LDL cholesterol levels, which play a role in the pathogenesis of atherosclerosis [111]. Therefore, they may have an important role against ischemic heart disease. The protective effects of natural products containing polyphenolics on cardiovascular system health are given in Figure 2.
As can be seen in Figure 3, consumption of foods containing polyphenols has a boosting effect on cardiovascular health and plays a role in the regulation of heart rhythm and blood pressure against many factors such as aging.
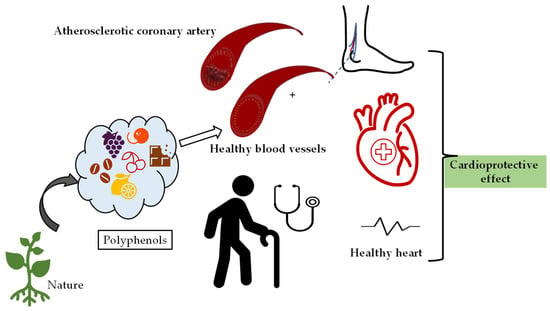
Figure 3.
Demonstration of the protective effects of natural products containing polyphenolics on cardiovascular system health.
Table 2 summarizes some of literature details on the application of some of polyphenols for cardiovascular diseases.

Table 2.
Some of the polyphenol efficacies, working concentration, and target of each substance on cardiovascular diseases.
Table 2.
Some of the polyphenol efficacies, working concentration, and target of each substance on cardiovascular diseases.
| Study Design | Outcomes | Ref. |
|---|---|---|
| Caffeic acid (100 nM and 1 μM) human umbilical vein-derived endothelial cells (HUVEC) | Caffeic acid increased proliferation and angiogenesis, inhibited leukocyte adhesion and endothelial cell apoptosis under hypoxia or by the uremic toxins’ conditions | [111] |
| Caffeic acid phenethyl ester (CAPE) 30 mg/kg/day administered by oral gavage for 6 weeks. Streptozotocin induced diabetes-induced atherosclerosis in rat model | CAPE abolished the diabetes-associated atherosclerotic changes by improving important functional and structural disorders in vessels. CAPE alleviated the elevation in systolic and diastolic BP | [112] |
| High fructose (HFCS) induced diabetic rats’ subacute CAPE administrations (50 μmol/kg/day intraperitoneally for 2 weeks | CAPE ameliorated the elevation in blood pressure, vascular damage, and it increased eNOS levels. CAPE lowered homocysteine and cholesterol levels | [113] |
| Atherogenic diet (Ath)-induced rat model administrations with caffeic acid 50 mg/kg, p.o. | CA ameliorated lipid profile and reduced the oxidative stress level. In aorta revealed reduction of the atherosclerotic lesions | [114] |
| Dietary 10% flaxseed content 1.37 mg/g SDG, (the lignan secoisolariciresinol diglucoside) LDL receptor-deficient mouse (LDLrKO) fed a cholesterol-supplemented diet and an increase in atherosclerotic plaque formation | Flaxseed lowered plasma cholesterol levels and saturated fatty acids, increased plasma ALA levels, and inhibited plaque formation in the aorta and reduced the inflammatory markers (IL-6, mac-3, and VCAM-1) | [115] |
| A 40 g/day of ground flaxseed administered Sixty-two men and post-menopausal women (LDL-C between 130 and 200) mg/dl 10 weeks | Flaxseed lowered LDL-C short lived and did not affect inflammation or oxidative stress | [116] |
| Low-density lipoprotein receptor knockout (LDLR−/−) mice with 170 g/kg sesame oil diet 3 months of feeding | Reduced atherosclerotic lesion formation, plasma cholesterol, triglyceride, and LDL cholesterol levels. Anti-inflammatory property (reduced inflammatory cytokines, such as MCP-1, RANTES, IL-1a, IL-6, and CXCL-16) | [117,118] |
| Sesamol 50 mg/kg orally for 6 weeks (DOCA)-salt-induced hypertensive rats | Decreased systolic and diastolic blood pressure and lipid peroxidation and enhanced the antioxidant activity. Hypertensive rats showed cardiac muscle fiber rupture and mononuclear infiltration, but Sesamol 50 mg/kg group heart showed to near-normal architecture | [119] |
| Isoproterenol treated myocardial infarcted rats, pretreated with gallic acid (15 mg/kg) daily for a period of 10 days | Prevented the changes in the activities of cardiac marker enzymes (CK-MB and LDH), reduced the levels of lipid peroxidation products (LPO), glutathione and lysosomal membrane damage | [120] |
| Rats were infused with AGEs (advanced glycation end products play a role development of cardiovascular disorders) and then treated with gallic acid (GA) by oral gavage daily at a dose of 25 mg/kg BW/day for 30 days | AGEs induced cardiac fibrosis and augmented oxidative stress in the heart tissues. GA prevented the upregulation of pro-fibrotic genes and ECM proteins (↓TNF-α, TGF-β, MMP-2 and -9 expression). GA treatment effectively prevented cardiac remodeling | [121] |
| NG-nitro-L-arginine methyl ester (L-NAME)-induced hypertensive mice treatment with gallic acid 100 mg/kg per day by daily intraperitoneal injections 3 or 8 weeks | GA attenuated cardiac fibrosis and remodeling, reduced the expression of histone deacetylase 1 (HDAC1) and 2 (HDAC2). GA lowered the elevated SBP | [122] |
4. Protective Effects of Polyphenolic Compounds on ROS-Induced Oxidative Stress
Reactive oxygen species (ROS) are molecular oxygen byproducts that occur as a result of normal metabolism (such as oxidation–reduction reactions) in mitochondria and other cell organelles [123,124]. Disruption of the balance between these radicals and antioxidant molecules (endogenous-exogenous) in biological systems describes the state of oxidative stress. After prolonged oxidative stress, physiological impairments such as peroxidation (oxidation of unsaturated molecules) of membrane lipids occur. In addition, highly active organic molecules change important structural proteins non-enzymatically. The increase in ROS in tissues causes changes in the functions of intracellular proteins, resulting in DNA structural damage and cell damage [123,124,125]. In addition to the fact that ROS can occur due to natural factors (such as aging), ischemia-reperfusion damage, exposure to ionizing radiation and chemical agents negatively affect this process [126,127].
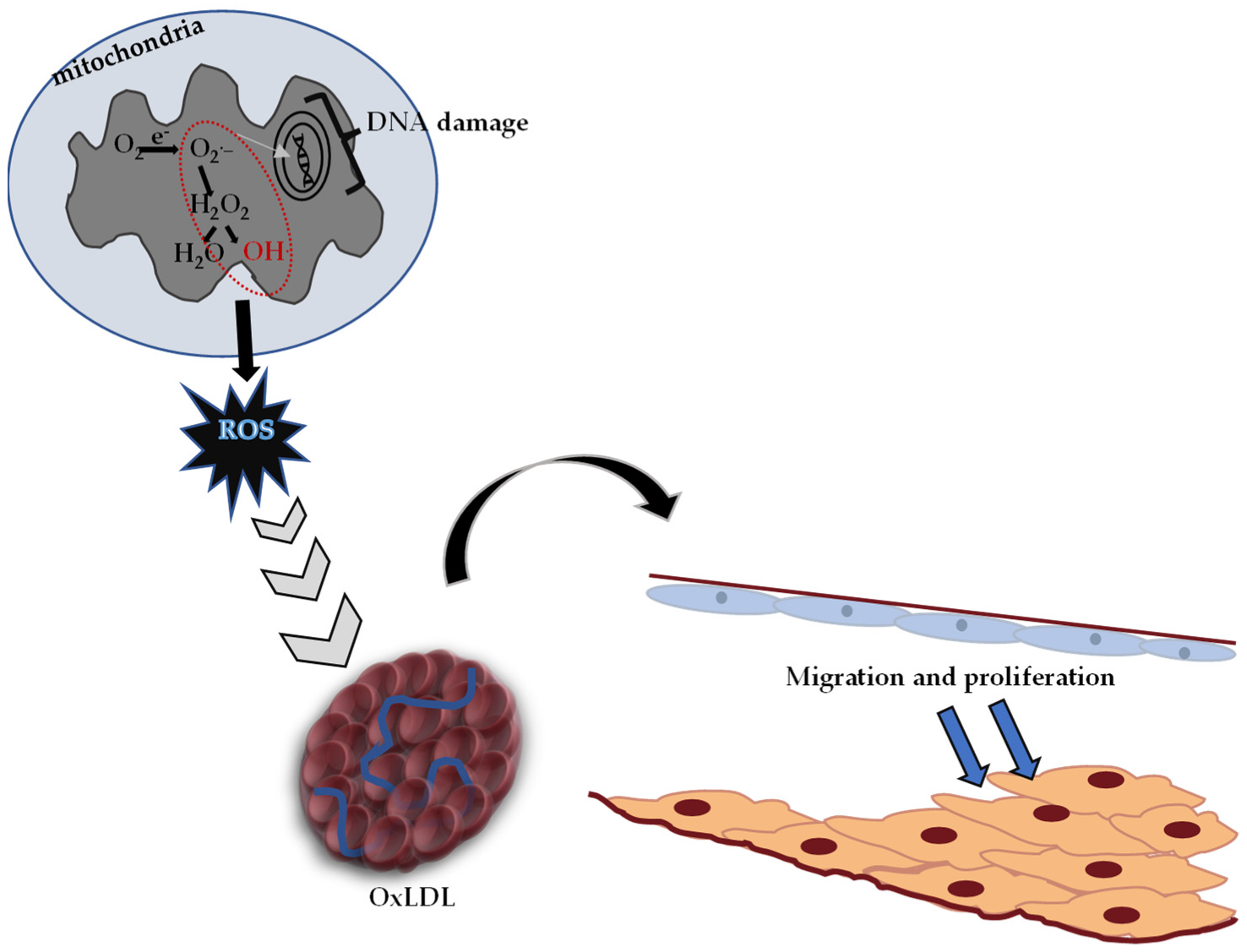
It is known that oxidative stress and the DNA damage caused by it play an important role in the formation process of diabetes, cancer, atherosclerosis, heart attack, stroke and inflammatory disorders [128]. Reactive oxygen species (ROS) causing oxidative stress, low-density lipoprotein (LDL) oxidation and atherosclerotic plaque formation are given in Figure 4.
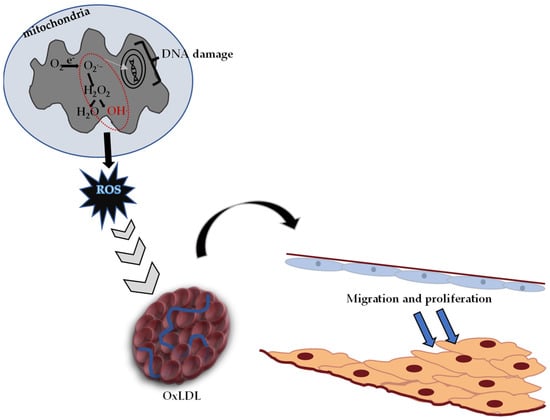
Figure 4.
Reactive oxygen species (ROS) causing oxidative stress, oxidized low-density lipoprotein (OxLDL) and atherosclerotic plaque formation (adapted from ref. [129]).
Toxic effects caused by free oxygen radicals, which are produced in significant amounts at the cellular level, are normally tried to be eliminated by the antioxidant defense system in the body. In cases where the antioxidant defense is insufficient, oxidative stress occurs, and Figure 4 shows the pathological process characterized by hardening in the arteries.
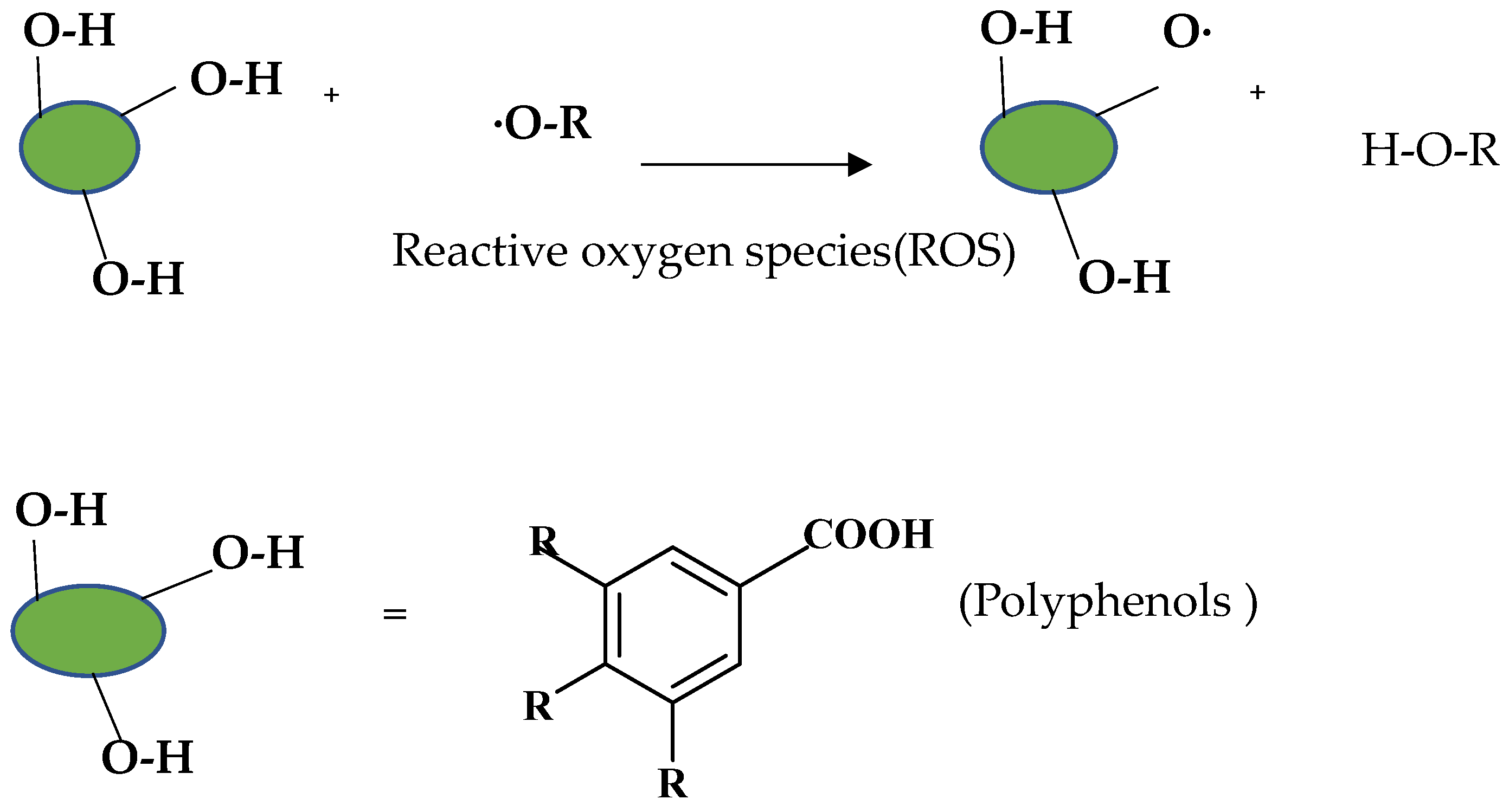
Diet has a great effect on the antioxidant balance of the body. Pathological conditions can occur when the body’s defense mechanisms are destroyed due to nutritional deficiencies [130]. The effects of a diet rich in polyphenolic compounds, which are natural antioxidants, have been examined in various studies, and it has been observed that it reduces oxidative DNA damage [131,132]. As shown in Figure 5, polyphenols react with ROS stoichiometrically to form stabilized radicals [133].
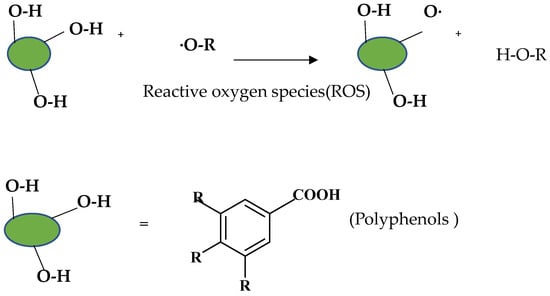
Figure 5.
Removal of hydrogen, extinction of ROS, and formation of stabilized polyphenol radicals.
Antioxidant activity depends on the existence of hydroxyl groups, which both provide removal of unstable hydrogen and augment the stability of the radical formed.
4.1. Phenolic Acids
Various in vivo and in vitro studies have shown that ferulic acid can reduce cell damage by reducing ROS production and regulating antioxidant enzyme activity, and can also regulate the activation of various cell protective genes against oxidative stress, improve lipid peroxidation and has cardioprotective effects [134]. Aldose reductase (AR) is nicotinamide adenine dinucleotide phosphate (NADPH) as a coenzyme. NADPH is known to play a critical role in the emergence and progression of various oxidative stress-related diseases. Ferulic acid has shown protective effects with its antioxidant and anti-inflammatory properties by acting as an AR inhibitor in the formation of cardiovascular and diabetic complications [68]. The protective effects of gallic acid against oxidative stress appear by regulating lipid peroxidation and NO production and increasing its antioxidant capacity [135]. Gallic acid may be a new generation agent that can be used to combat cellular toxicity caused by arsenic and its metabolite.
4.2. Flavonoids
Quercetin is attracting much attention for its potential to prevent cardiovascular, neoplastic and neurodegenerative diseases in which oxidative stress plays a role [136,137,138]. A study investigating the passage of quercetin through the blood–brain barrier (BBB) has shown that oral administration of quercetin to rats exposed to chronic forced swimming alleviates the increased oxidative stress in the brains of the rats through its antioxidant activity [139]. The antioxidative activities of quercetin and catechin were examined in cocoa liquor, and it was stated that quercetin offered higher antioxidant activity than quercetin glycosides [140]. Furthermore, clovamide showed more activity than other components due to the catechin group [140]. The antioxidant properties of coffee have been attributed to both the effect on plasma uric acid and direct absorption of coffee polyphenols [132]. The chlorogenic acid in the composition of coffee showed therapeutic properties by inducing selective anticancer effects in lung cancer cells and leukemia cells at certain concentrations. In contrast, this polyphenolic component has been reported to induce cellular DNA damage at a low millimolar range [141].
Delphinidin, an anthocyanidin, has been found to act as a topoisomerase inhibitor in human colon carcinoma cells [142]. In a different study, cyanidin-3-glucoside-rich blackberry extract (at a concentration of 76,000 µg/g) was reported to reduce the growth of HT29 (a human colon cancer cell line widely used in biological and cancer research) cells. In addition, it has been shown that by pre-treatment of HT29 cells with blackberry extract, the complex formation of topoisomerase poisons with DNA is suppressed [143]. Shih et al. (2007) did not detect any toxicity after 24 h of treatment with anthocyanin at 50 µM concentration [144]. Among the anthocyanin compounds they studied, malvidin, cyanidin, kuromanin, delphinidin compounds exhibited higher antioxidant activity. Achieving high expression of phase 2-detoxification enzymes and other antioxidant enzymes was effective in this activity.
4.3. Stilbenes
Regarding the cellular defense properties of resveratrol as a direct antioxidant agent, resveratrol has been demonstrated to be a very effective scavenger of a variety of oxidants; the inhibition of ROS/RNS production by intracellular systems exhibited inhibitory effects on the lipid peroxidation and protective effect against protein oxidation intracellular antioxidant defense systems. There are antioxidant defense mechanisms of resveratrol through regulation of signaling pathways. 5′adenosine monophosphate-activated kinase (AMPK) is regulated under metabolic stress and plays an important role in energy homeostasis. Resveratrol increases cell viability against ROS-induced oxidative damage on the myoblast cell line and shows cell protection by enabling AMPK activation [145]. In the case of oxidative stress, resveratrol treatment reduces the protein loss of myoblasts in a dose-dependent manner but cannot prevent the loss completely [146]. In addition, caspase-9 activity, which is a marker of the mitochondrial apoptotic pathway, ceased after 6 h of treatment with resveratrol [146]. Resveratrol exerts a therapeutic effect by activating the protein, an endogenous transcription factor called NRF-2 (the nuclear factor erythroid-derived 2-like), which is associated with antioxidant response [147]. As summarized in Table 3, resveratrol reduces SOD activity and inhibits ROS formation caused by ox-LDL [148].

Table 3.
Some of the information regarding polyphenols efficacies, working concentration, and target of each substance on ROS-induced oxidative stress.
4.4. Lignans
The effect of flaxseed oil and a flaxseed lignan glucoside derivative in metabolic syndrome disease (characterized by hypertension, increased triglyceride levels, and decreased HDL cholesterol levels) was examined, and it has been determined that oral administration of herbal compounds containing lignans reduces oxidative stress biomarkers such as lipid peroxidation and carbonyl levels [151]. It was stated that the heme oxygenase-1 enzyme, which plays a role in heme catabolism and which is known as a stress-response protein, is induced by the application of lignan-derived compounds, and the lignan derivatives have hepatoprotective and antioxidant properties [152]. Oxidative stress damage in the cerebral cortex recovered in a dose-dependent manner as a result of pretreatment with pinoresinol-4-O-β-D-glucopyranoside, a lignan glycoside has also been reported. It was also noted that this lignan derivative significantly reduced the oxidative stress marker malondialdehyde level and increased catalase activity by about 44% at the specified dose [150,153]. This lignan glycoside is thought to be a potential antioxidant agent in the treatment of hepatic damage, oxidative damage, and diabetes [150].
5. Polyphenols on DNA and Cancer
In cases where the density of free radicals increases in living cells, structural defects occur in important functional structures such as proteins and nucleic acids. It has been suggested that carcinogenesis, age-related degenerative diseases, and chronic diseases occur when antioxidant defense systems are not sufficiently effective against ROS, which is formed as a result of both biochemical reactions and external factors. In addition, strong findings indicate ROS in both the initiation and the increase in multistage carcinogenesis [154,155].
Oxidative stress causes DNA damage, which triggers multiple cellular responses. Mutations occur by the division of damaged cells that have not been repaired at all or repaired incorrectly. In case of mutations in vital genes such as oncogenes or tumor suppressor genes, cancer initiation and progression processes can be seen [155,156].
The DNA damage response involves the activation of multiple cellular activities that repair DNA lesions and have crucial importance in preventing tumor formation [157]. There is a potential loss of DNA damage repair abilities in the advanced stages of carcinogenesis [155,156]. Cancer cells usually have specific abnormalities in the DNA damage response system; however, chemotherapeutics also have toxicity and persistent side effects on normal cells. With the trial of chemotherapeutics in combination with polyphenols, recent therapeutic strategies such as chemoprevention are gaining importance [158].
Polyphenols are among the natural products that are on the agenda as anti-cancer and cancer-curative agents [159]. Dietary polyphenols can influence and modulate many different pathways and processes involved in carcinogenesis. Additionally, they can act as biological response modifiers that support the immune system and protect living cells from damage by free radicals. For example, polyphenolic natural products have anticarcinogenic properties such as modulating cell proliferation, tumor growth, angiogenesis, metastasis, inflammation and apoptosis [16,159]. Polyphenols are reported to protect the body against the effects of reactive oxygen species on DNA integrity but do so reliably only at low concentrations [160].
5.1. Phenolic Acids
Ferulic acid may exhibit anticancer properties against tumors that may occur as a result of conditions such as apoptosis mechanisms activated by various endogenous and exogenous pathways [161]. Ferulic acid can prevent oxidative stress from causing permanent damage to any tissue by downregulating related proteins in the mitochondrial apoptosis-promoting protein family [161].
Gallic acid protects against oxidative stress and acute toxicity that may occur in the heart in a study performed on Wistar rats [135]. Thus, it contributes to the protection of kidney and heart health, and it is promised that it can prevent possible cancer formation. Research has shown that gallic acid can inhibit the proliferation of tumor cells in cell cultures by causing apoptosis and/or cell cycle arrest [162]. It has also indicated that the GA induces apoptosis in human lung cancer cells, as well as suppressing DNA damage and provoking DNA repair in prostate cancer cells [163,164].
5.2. Flavonoids
In a study involving a large population (over 8000 individuals), high green tea consumption was shown to delay the onset of cancer in patients with premenopausal Stage I and II breast cancer [165]. It has also been found that a lower recurrence rate is observed in breast cancer patients [165]. Ellagitannins, flavonoids, ellagic acid and ellagic acid glycosides in the fruit content show anticancer activity in human colorectal carcinoma cells [166].
Epigallocatechin-3-gallate has been shown to provide anticancer activity through many mechanisms such as cell cycle arrest, regulatory role in carcinogenic metabolizing enzymes, inhibition of mitotic signal transduction, antioxidant effect and DNA inhibition. In addition, it regulates the p53 protein, which is one of the transcription factors that regulates the cell cycle [167,168].
Naringin’s anti-tumor effects were studied in thyroid cancer as given in Table 4. For 24, 48, or 72 h at 37 °C, Naringin (6, 12, and 25 g/mL) was applied to two cancer cells, TPC-1 and SW1736. In MTT assays, naringin inhibited TPC-1 and SW1736 cell proliferation in a dose- and time-dependent manner [169]. In another study, when Naringin was combined with atorvastatin, it synergistically inhibited prostate cancer cells, PC-3, and LNCaP cells [170].
Quercetin is effective in ovarian cancer by enhancing the antioxidant defense mechanism by suppressing ROS-induced damage [171,172] and in eye cancer by inhibiting the expression of signal proteins produced by hypoxia-induced angiogenesis-stimulating cells [171,172,173]. Furthermore, quercetin shows anticancer properties through different mechanisms in breast, gastric, bone, blood, lung, prostate, brain, skin, colon, thyroid and kidney cancers [174].
In the study of Schantz and coworkers (2010), intracellular ROS level and oxidative DNA damage significantly decreased after 24 h of application of anthocyanin-rich bilberry extract in colon tumor cells [175]. Anthocyanins such as cyanidin and delphinidin in purple tea offer antioxidant, anticancer, and immune system stimulating properties by regulating caspase-3/7 activity and expression levels of certain reductase enzymes [176]. Blueberry anthocyanins and anthocyanin–pyruvic acid adduct inhibited the invasion of matrix-degrading proteinases associated with tumor angiogenesis in breast cancer cell lines [177]. In addition, these two components prevented cell migration by creating a negative chemo-signal and showed an anticarcinogenic effect by preventing metastasis.
Another effective flavonoid, catechin, has anti-cancer properties according to MC38 colon cancer cells [178]. Cancer cell proliferation can be significantly inhibited by catechin concentrations of 250 to 1000 mg/mL, and there is a 50% inhibitory concentration at 250 mg/mL, which is the IC50 value, within an incubation period of 24 h, as given in Table 4. A cell’s death increases levels of triggered cancer cells sharply, and a 72 h incubation time was used with an IC50 value of 142 mg/mL.
5.3. Stilbenes
Resveratrol reduces the expression of matrix metalloproteinase, which destroys extracellular matrix components and is responsible for metastasis [179,180,181]. Resveratrol can exhibit both pro-oxidant and antioxidant properties depending on the dose and the cell type it affects [182]. Resveratrol can cause growth arrest and/or cell death in various cancer cells, but its anticancer mechanism of action is not fully known. The anticancer activity of resveratrol has been demonstrated in human colon cancer cells by increasing the level of intracellular ROS and inducing apoptosis through autophagy [183]. Resveratrol presents anticancer properties in lung cancer cells by selectively increasing the expression of a certain group of transmembrane enzymes (NADPH oxidase-5) and thus inducing ROS production [184].
5.4. Lignans
The effect of a lignan derivative, schisandrin B, on cardiotoxicity caused by doxorubicin, a cancer chemotherapeutic, was studied, and pretreatment with this compound significantly prevented doxorubicin-induced loss of cardiac function (based on results at 6 and 12 weeks) [185]. Arctigenin induces only inhibition in tumor cells, that is, selectivity between normal and cancerous cells [186]. Another study has concluded that lignan extract induces cytotoxicity, reduces tumor formation in estrogen receptor-positive breast cancer cells, has anti-estrogenic and antioxidant effects, especially at low doses, and may have antitumor components that could prevent chemically induced breast cancer in rats [187]. Some of the research details on the applications of polyphenols on DNA and cancer were presented Table 4.

Table 4.
Some of the information regarding polyphenols efficacies, working concentration, and target of each substance on DNA and cancer.
Table 4.
Some of the information regarding polyphenols efficacies, working concentration, and target of each substance on DNA and cancer.
| Study Design | Outcomes | Ref. |
|---|---|---|
| Naringin’s anti-tumor effects on thyroid cancer were studied. For 24, 48, or 72 h at 37 °C, Naringin (6, 12, and 25 g/mL) was applied to two cancer cells, TPC-1 and SW1736. | In MTT assays, naringin inhibited TPC-1 and SW1736 cell proliferation in a dose- and time-dependent manner. | [167] |
| Naringin was combined with the drug, atorvastatin. | The IC50 of naringin was determined as 196.2 μM in PC-3 and 117.2 μM in LNCaP cells. However, naringin and statin drug atorvastatin synergistically inhibited prostate cancer cells, PC-3, and LNCaP cells. | [168] |
| Catechin was studied on MC38 colon cancer cells. | The proliferation of cancer cells can be significantly inhibited by catechin concentrations between 250 and 1000 mg/mL, and the IC50 value is 250 mg/mL during a 24 h incubation period. Incubation time was 72 h, and IC50 was 142 mg/mL after triggering cancer cells. | [176] |
| An anticancer effect of gallic acid on non-small cell lung cancer cells A549. | The viability of A549 cells was determined by MTT assay after treatment with GA (0–52 g/mL) for 24 h. There was a dose-dependent decrease in viability after treatment with GA (0–52 g/mL). When compared with control cells, 12 g/mL GA significantly decreased cell viability. | [188] |
6. Polyphenols on Aging
ROS plays an important role in the aging process as well as being one of the underlying causes of many chronic diseases [189]. ROS are the normal byproducts of the metabolism of cells responsible for physiological and pathological functions, and considering the dual role of ROS in cells, an antioxidant defense system must be present to neutralize the overproduction of such reactive compounds. The cellular antioxidant defense system consists of endogenous antioxidant enzymes and peptides, as well as polyphenolic compounds, vitamins, and polyunsaturated fatty acids obtained from food sources. The antioxidant defense system gradually deteriorates in time, resulting in oxidative stress and the inability to cope with ROS production, which contributes to the aging process and the onset of age-related diseases. ROS is not only a direct mediator of aging, but also a regulator of the more fundamental aging process [190,191].
Under normal conditions, the skin has highly effective defense mechanisms such as antioxidant enzymes. However, it is known that these mechanisms are insufficient to sustain their effects due to the aging process [192].
In addition to being one of the underlying causes of many chronic diseases, ROS also plays an important role in the aging process [193]. Natural antioxidants are used in pharmaceutical and cosmetic formulations to delay the skin aging process and improve skin aesthetics [194].
Due to various physical factors (such as aging, prolonged exposure to UV radiation), a decrease in the number and activities of fibroblast cells is observed in the aging process. Decreased fibroblast activity is accompanied by decreased collagen synthesis. As a result, the activities of collagen proteins to protect the natural structure, moisture and elasticity of the skin are hampered. Wrinkles, roughnesses and hardness appear in the skin, which is seen as “aging” in the physical sense, and natural active ingredients that support fibroblast cell proliferation come to the fore in dermo-cosmetic areas [195,196].
Poplar bud extract containing many polyphenolic compounds such as pinocembrin, galangin, quercetin, kaempferol and apigenin has been studied by creating a special human fibroblast aging model. A two-fold increase in catalase gene expression was found in the group treated with this antioxidant herbal extract [192]. Mohammad et al. (2018) confirmed that the emulsion cream formulation, which also contains bark extract from a natural plant rich in flavonoids, has a skin-rejuvenating effect [197]. This extract also provided increased elasticity and hydration and enriched the skin nutritionally. The fruits of a plant containing polyphenolic components such as chlorogenic acid, catechin quercetin, gallic acid, and cinnamic acid were clinically evaluated, and the tested formulation reduced wrinkles and roughness of the skin by over 10% [198]. Green tea polyphenols were found to greatly support fibroblast cell proliferation and have high moisture retention ability [195].
6.1. Phenolic Acids
Directly, phenolic acids are antioxidants. Through indirect effects, they stimulate endogenous protective enzymes and signaling pathways. Phenolic acids show antioxidant properties due to the hydroxyl substitution in the aromatic ring and the reactivity of the phenol moiety [24]. In cases where oxidative damage causes tissue degeneration, ferulic acid shows cell proliferation and rapid wound healing properties due to its antioxidant and anti-inflammatory properties [68].
6.2. Flavonoids
The antioxidant capacity of anthocyanins varies depending on various modifications such as glycosylation and hydroxylation [199,200]. Among the anthocyanins, it obtained the highest antioxidant activity in the cyanidin-3-glucoside compound, which was even higher than a vitamin E analogue [200]. Auto-fluorescent pigment, an aging marker, was studied to evaluate anti-aging activity [201]. The results showed that plant extract, the main component of which is cyanidin-3-rutinoside, has a strong radical-scavenging effect, reducing the level of endogenous ROS and reducing oxidative stress. Anthocyanins have proven to be effective in the modulation of stress-response genes as well as decreasing ROS accumulation. Anthocyanins have proven to be involved in the modulation of stress-responsive genes, to reduce the accumulation of ROS, to delay aging-related markers, and to show anti-aging activities [201].
Aging is a risk factor for most neurodegenerative diseases that significantly affects quality of life and cognitive levels. The analyses show that anthocyanin might act as an aging suppressor. Anthocyanin was administered to the rats whose aging was accelerated experimentally in order to investigate the effects of anthocyanin on brain aging. When the cognitive and non-cognitive components of behavior were examined after 8 weeks, the use of anthocyanins was shown to reduce DNA damage levels and inflammation accumulation in the brain, prevent age-related cognitive decline and aging, and be a potential approach to maintain thinking and memory. Treatment with 30 mg/kg of anthocyanin improved brain–liver functions in aged model mice with significant deterioration of brain and liver indicators [202]. In addition, atrophy was prevented by preventing changes in brain and liver weight with this treatment.
To summarize, the anti-aging effects of anthocyanin and its derivatives are: (1) regulation of protein expression associated with DNA damage response; (2) stress-response-related gene expression; (3) stimulating the expression of longevity-related genes [200,201,202].
6.3. Stilbenes
It has been proven that resveratrol and pterostilbene provide antioxidant and anti-inflammatory effects by activating various signaling pathways and thus have important properties in modulating age-related and chronic diseases [203]. Resveratrol has skin whitening and anti-aging properties. Suppressing or inhibiting the activity of tyrosinase, a limiting enzyme to control melanin production, and ROS scavenging mechanisms play a role in delaying the complications of aging [204]. In a study on flies, the application of resveratrol-rich food alone did not have a lifespan prolonging effect, but it provided improvement in aging-related dysfunctions such as eye degeneration and locomotor disorders [205]. Increased ROS leads to the formation of toxic peroxynitrite and the release of nitric oxide radical, which causes vascular oxidative stress. It was found that increased nitric oxide concentration in cardiomyocytes due to the aging process was significantly reduced even 2 months after resveratrol treatment [206]. Buonocore et al. (2012) applied a nutraceutical 60-day treatment containing phenolic compounds such as resveratrol and procyanidin on 50 healthy middle-aged individuals (who have signs of aging in their skin) [207]. As a result, the antioxidant capacity of the stratum corneum, which is a very important layer of the epidermis, has increased, and skin hardness has been improved [207]. Endothelial dysfunction due to aging is an important risk factor for the development of cardiovascular disease, and alternative treatments are needed. Decreased bioavailability of nitric oxide (NO), vascular damage, autophagy, poor repair processes, and oxidative stress present positive feedback mechanisms. The addition of resveratrol to the treatment in the elderly is reported to be likely to improve vascular functions by causing the regulation of oxidative balance in the endothelium [208].
6.4. Lignans
The study with an experimental aging model with rats has concluded that the state of metabolic dysfunction can be regulated with amino acid, lipid and energy metabolism when lignan extract is administered, and that metabolic dysfunction is partially ameliorated by downregulation of some transcription factors. It has been stated that Lignan extract can be used in the prevention and treatment of aging [209].
Superoxide dismutase activity, which is produced as a byproduct of oxygen metabolism and causes cell damage if not regulated, significantly improved after lignan extract treatment. Therefore, it was concluded that lignans can be used clinically as an agent that will exhibit an antiaging function by the regulation of oxygen metabolism and protein expression [210].
Therefore, gel and other kinds of formulations containing the extract of a plant rich in polyphenols can be a promising, anti-aging natural source in the cosmetic field.
7. Conclusions and Future Respects
The prevalence of cardiovascular diseases, oxidative stress-related complications and chronic aging-related ailments is very high and is increasingly common worldwide. These ailments make the lives of patients difficult and in some cases require the use of multiple drugs, and sometimes they cannot be fully treated. It is a fact that when preparing treatment schemes for certain diseases, alternative therapeutic agents with fewer side effects, low toxicity and high patient compliance are needed. By providing appropriate diets, a positive response is obtained against most diseases before the disease is caught or in the early period of the disease. At this point, polyphenolic compounds with antioxidant, anticancer, antidiabetic, anti-inflammatory, and cardiovascular health-protective properties have been the subject of the agenda. Many experimental studies support the view that polyphenols and their sub-group flavonoids slow down the development of atherosclerosis and protect the cardiovascular system from oxidative damage that may result from LDL oxidation. In addition, due to the long-term consumption of foods and beverages with rich polyphenolic content, the formation of cardiovascular diseases is delayed.
The antioxidant properties of polyphenols are important besides their protective and life-enhancing properties on the heart. There are many studies in the literature that these natural antioxidant compounds reduce the oxidative stress caused by ROS and thereby reduce the risk of cancer formation. In addition, it has been discovered that they can delay the emergence of some diseases by slowing down the aging process that will occur due to many factors such as oxidative stress. Therefore, the importance of consuming foods containing appropriate amounts of polyphenolic compounds should not be underestimated.
In addition to these, the mechanism of action of many polyphenolic bioactive components in the body or which metabolites of these components affect the pathways have not been fully elucidated. Natural antioxidant polyphenols will appear more frequently in the new period experimental studies under the name of nutraceuticals and will take their place in the field as candidates for alternative therapeutic agents.
The polyphenolic contents and amounts for the same compound are different depending on the source. In this review, we examined the effect of certain polyphenols as therapeutic or supportive agents in the treatment of certain diseases. In addition, it was attempted to draw attention to the nutraceutical use of polyphenols by considering their disease-protective effect. However, the positive effects of many polyphenolic structures that have not been researched or entered the literature are waiting to be investigated. One type of feeding should be avoided as much as possible, and diets containing polyphenols should be given more priority. The contents of polyphenolic structures of local plants, which are not well known, should be investigated, and their effects on diseases should be investigated.
Antioxidant, anti-inflammatory, hypotensive, glucose- and lipid-lowering activities and cardioprotective effects of polyphenols have been demonstrated in many in vivo and in vitro studies. However, some of these observations are also supported by the results of existing clinical trials. One of the reasons for this inconsistency is that polyphenols are extensively metabolized in the human body, affecting their bioavailability. In this regard, it is important to make more modifications and develop new carrier and/or delivery systems to increase the bioavailability of polyphenols. Longer-term clinical studies with rational design and research are needed to examine the therapeutic use of these polyphenols in cardiovascular diseases and to evaluate the safety profile.
Author Contributions
Conceptualization, N.S.; methodology, A.S.Y. and M.S.; software, A.S.Y. and M.S.; validation, B.G., Y.A., A.S.Y. and M.S.; formal analysis, B.G.; investigation, M.S., A.S.Y., B.G. and N.S.; resources, N.S.; data curation, A.S.Y. and M.S.; B.G.; writing—original draft preparation, M.S., A.S.Y., B.G. and Y.A.; writing—review and editing, M.S., B.G. and N.S.; visualization, N.S.; supervision, N.S.; project administration, N.S.; funding acquisition, N.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rashidi, L. Different nano-delivery systems for delivery of nutraceuticals. Food Biosci. 2021, 43, 101258. [Google Scholar] [CrossRef]
- Lyu, X.; Lee, J.; Chen, W.N. Potential Natural Food Preservatives and Their Sustainable Production in Yeast: Terpenoids and Polyphenols. J. Agric. Food Chem. 2019, 67, 4397–4417. [Google Scholar] [CrossRef] [PubMed]
- Xie, G.; Zhao, A.; Zhao, L.; Chen, T.; Chen, H.; Qi, X.; Zheng, X.; Ni, Y.; Cheng, Y.; Lan, K.; et al. Metabolic Fate of Tea Polyphenols in Humans. J. Proteome Res. 2012, 11, 3449–3457. [Google Scholar] [CrossRef] [PubMed]
- Burns, J.; Yokota, T.; Ashihara, H.; Lean, M.E.J.; Crozier, A. Plant Foods and Herbal Sources of Resveratrol. J. Agric. Food Chem. 2002, 50, 3337–3340. [Google Scholar] [CrossRef] [PubMed]
- Tzanova, M.; Peeva, P. Rapid HPLC Method for Simultaneous Quantification of trans-Resveratrol and Quercetin in the Skin of Red Grapes. Food Anal. Methods 2018, 11, 514–521. [Google Scholar] [CrossRef]
- Sobolev, V.S.; Cole, R.J. trans-Resveratrol Content in Commercial Peanuts and Peanut Products. J. Agric. Food Chem. 1999, 47, 1435–1439. [Google Scholar] [CrossRef] [PubMed]
- Shrikanta, A.; Kumar, A.; Govindaswamy, V. Resveratrol content and antioxidant properties of underutilized fruits. J. Food Sci. Technol. 2015, 52, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.-P.; Li, Y.; Meng, X.; Zhou, T.; Zhou, Y.; Zheng, J.; Zhang, J.-J.; Li, H.-B. Natural Antioxidants in Foods and Medicinal Plants: Extraction, Assessment and Resources. Int. J. Mol. Sci. 2017, 18, 96. [Google Scholar] [CrossRef] [PubMed]
- Uddin, N.; Muhammad, N.; Nisar, M.; Aisha; Ali, N.; Ullah, R.; Ali, E.A.; Khan, A.A.; Rahman, I.U.; Khan, A.; et al. Distribution of polyphenolic compounds, antioxidant potential, and free amino acids in Ziziphus fruits extract; a study for determining the influence of wider geography. Food Sci. Nutr. 2022, 10, 1414–1430. [Google Scholar] [CrossRef]
- Besednova, N.N.; Andryukov, B.G.; Zaporozhets, T.S.; Kryzhanovsky, S.P.; Kuznetsova, T.A.; Fedyanina, L.N.; Makarenkova, I.D.; Zvyagintseva, T.N. Algae Polyphenolic Compounds and Modern Antibacterial Strategies: Current Achievements and Immediate Prospects. Biomedicines 2020, 8, 342. [Google Scholar] [CrossRef]
- Staszowska-Karkut, M.; Materska, M. Phenolic Composition, Mineral Content, and Beneficial Bioactivities of Leaf Extracts from Black Currant (Ribes nigrum L.), Raspberry (Rubus idaeus), and Aronia (Aronia melanocarpa). Nutrients 2020, 12, 463. [Google Scholar] [CrossRef] [PubMed]
- Oracz, J.; Zyzelewicz, D.; Nebesny, E. The Content of Polyphenolic Compounds in Cocoa Beans (Theobroma cacao L.), Depending on Variety, Growing Region, and Processing Operations: A Review. Crit. Rev. Food Sci. Nutr. 2015, 55, 1176–1192. [Google Scholar] [CrossRef] [PubMed]
- Alara, O.R.; Abdurahman, N.H.; Abdul Mudalip, S.K.; Olalere, O.A. Microwave-assisted extraction of Vernonia amygdalina leaf for optimal recovery of total phenolic content. J. Appl. Res. Med. Aromat. Plants 2018, 10, 16–24. [Google Scholar] [CrossRef]
- Pashazadeh, B.; Elhamirad, A.H.; Hajnajari, H.; Sharayei, P.; Armin, M. Optimization of the pulsed electric field-assisted extraction of functional compounds from cinnamon. Biocatal. Agric. Biotechnol. 2020, 23, 101461. [Google Scholar] [CrossRef]
- Sun, C.; Zhao, C.; Guven, E.C.; Paoli, P.; Simal-Gandara, J.; Ramkumar, K.M.; Wang, S.; Buleu, F.; Pah, A.; Turi, V.; et al. Dietary polyphenols as antidiabetic agents: Advances and opportunities. Food Front. 2020, 1, 18–44. [Google Scholar] [CrossRef]
- Niedzwiecki, A.; Roomi, M.; Kalinovsky, T.; Rath, M. Anticancer Efficacy of Polyphenols and Their Combinations. Nutrients 2016, 8, 552. [Google Scholar] [CrossRef]
- Xing, L.; Zhang, H.; Qi, R.; Tsao, R.; Mine, Y. Recent Advances in the Understanding of the Health Benefits and Molecular Mechanisms Associated with Green Tea Polyphenols. J. Agric. Food Chem. 2019, 67, 1029–1043. [Google Scholar] [CrossRef]
- Etxeberria, U.; Fernández-Quintela, A.; Milagro, F.I.; Aguirre, L.; Martínez, J.A.; Portillo, M.P. Impact of Polyphenols and Polyphenol-Rich Dietary Sources on Gut Microbiota Composition. J. Agric. Food Chem. 2013, 61, 9517–9533. [Google Scholar] [CrossRef]
- Duda-Chodak, A.; Tarko, T.; Satora, P.; Sroka, P. Interaction of dietary compounds, especially polyphenols, with the intestinal microbiota: A review. Eur. J. Nutr. 2015, 54, 325–341. [Google Scholar] [CrossRef]
- Singla, R.K.; Dubey, A.K.; Garg, A.; Sharma, R.K.; Fiorino, M.; Ameen, S.M.; Haddad, M.A.; Al-Hiary, M. Natural Polyphenols: Chemical Classification, Definition of Classes, Subcategories, and Structures. J. AOAC Int. 2019, 102, 1397–1400. [Google Scholar] [CrossRef]
- Sahiner, M.; Sahiner, N.; Sagbas, S.; Fullerton, M.L.; Blake, D.A. Fabrication of Biodegradable Poly(naringin) Particles with Antioxidant Activity and Low Toxicity. ACS Omega 2018, 3, 17359–17367. [Google Scholar] [CrossRef]
- Kumar, V.; Sharma, A.; Kohli, S.K.; Bali, S.; Sharma, M.; Kumar, R.; Bhardwaj, R.; Thukral, A.K. Differential distribution of polyphenols in plants using multivariate techniques. Biotechnol. Res. Innov. 2019, 3, 1–21. [Google Scholar] [CrossRef]
- Rashmi, H.B.; Negi, P.S. Phenolic acids from vegetables: A review on processing stability and health benefits. Food Res. Int. 2020, 136, 109298. [Google Scholar] [CrossRef]
- Kumar, N.; Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef]
- Sahiner, N.; Sagbas, S.; Sahiner, M.; Blake, D.A.; Reed, W.F. Polydopamine particles as nontoxic, blood compatible, antioxidant and drug delivery materials. Colloids Surf. B Biointerfaces 2018, 172, 618–626. [Google Scholar] [CrossRef]
- Nakamura, K.; Yamada, Y.; Ikai, H.; Kanno, T.; Sasaki, K.; Niwano, Y. Bactericidal Action of Photoirradiated Gallic Acid via Reactive Oxygen Species Formation. J. Agric. Food Chem. 2012, 60, 10048–10054. [Google Scholar] [CrossRef]
- De Cristo Soares Alves, A.; Mainardes, R.M.; Khalil, N.M. Nanoencapsulation of gallic acid and evaluation of its cytotoxicity and antioxidant activity. Mater. Sci. Eng. C 2016, 60, 126–134. [Google Scholar] [CrossRef]
- Santos, E.M.S.; da Rocha, R.G.; Santos, H.O.; Guimarães, T.A.; de Carvalho Fraga, C.A.; da Silveira, L.H.; Batista, P.R.; de Oliveira, P.S.L.; Melo, G.A.; Santos, S.H.; et al. Gallic acid modulates phenotypic behavior and gene expression in oral squamous cell carcinoma cells by interfering with leptin pathway. Pathol.-Res. Pract. 2018, 214, 30–37. [Google Scholar] [CrossRef]
- Garud, M.S.; Kulkarni, Y.A. Gallic acid attenuates type I diabetic nephropathy in rats. Chem. Biol. Interact. 2018, 282, 69–76. [Google Scholar] [CrossRef]
- Sahiner, N.; Sagbas, S.; Sahiner, M.; Silan, C. P(TA) macro-, micro-, nanoparticle-embedded super porous p(HEMA) cryogels as wound dressing material. Mater. Sci. Eng. C 2017, 70, 317–326. [Google Scholar] [CrossRef]
- Sahiner, M.; Kurt, S.B.; Sahiner, N. Biodiverse Properties of Tannic Acid-Based Fibers. Fibers Polym. 2021, 22, 2986–2994. [Google Scholar] [CrossRef]
- Sahiner, M.; Blake, D.A.; Fullerton, M.L.; Suner, S.S.; Sunol, A.K.; Sahiner, N. Enhancement of biocompatibility and carbohydrate absorption control potential of rosmarinic acid through crosslinking into microparticles. Int. J. Biol. Macromol. 2019, 137, 836–843. [Google Scholar] [CrossRef]
- Noor, S.; Mohammad, T.; Rub, M.A.; Raza, A.; Azum, N.; Yadav, D.K.; Hassan, M.I.; Asiri, A.M. Biomedical features and therapeutic potential of rosmarinic acid. Arch. Pharm. Res. 2022, 45, 205–228. [Google Scholar] [CrossRef]
- Sahiner, M. Hydrolytic nondegradable bioactive rosmarinic acid particles. Polym. Adv. Technol. 2021, 32, 4891–4901. [Google Scholar] [CrossRef]
- Friedman, M.; Levin, C.E. Analysis and Biological Activities of Potato Glycoalkaloids, Calystegine Alkaloids, Phenolic Compounds, and Anthocyanins. In Advances in Potato Chemistry and Technology; Elsevier: Amsterdam, The Netherlands, 2009; pp. 127–161. [Google Scholar]
- Sahiner, N.; Sagbas, S.; Sahiner, M.; Aktas, N. Degradable Natural Phenolic Based Particles with Micro- and Nano-Size Range. Recent Pat. Mater. Sci. 2018, 11, 33–40. [Google Scholar] [CrossRef]
- Chen, R.; Qi, Q.-L.; Wang, M.-T.; Li, Q.-Y. Therapeutic potential of naringin: An overview. Pharm. Biol. 2016, 54, 3203–3210. [Google Scholar] [CrossRef]
- Alpaslan, D.; Dudu, T.E.; Şahiner, N.; Aktas, N. Synthesis and preparation of responsive poly(Dimethyl acrylamide/gelatin and pomegranate extract) as a novel food packaging material. Mater. Sci. Eng. C 2020, 108, 110339. [Google Scholar] [CrossRef]
- El Khawand, T.; Courtois, A.; Valls, J.; Richard, T.; Krisa, S. A review of dietary stilbenes: Sources and bioavailability. Phytochem. Rev. 2018, 17, 1007–1029. [Google Scholar] [CrossRef]
- Lu, H.-P.; Jia, Y.-N.; Peng, Y.-L.; Yu, Y.; Sun, S.-L.; Yue, M.-T.; Pan, M.-H.; Zeng, L.-S.; Xu, L. Oxyresveratrol, a Stilbene Compound from Morus alba L. Twig Extract Active against Trichophyton rubrum. Phyther. Res. 2017, 31, 1842–1848. [Google Scholar] [CrossRef]
- Giuliano, C.; Siani, F.; Mus, L.; Ghezzi, C.; Cerri, S.; Pacchetti, B.; Bigogno, C.; Blandini, F. Neuroprotective effects of lignan 7-hydroxymatairesinol (HMR/lignan) in a rodent model of Parkinson’s disease. Nutrition 2020, 69, 110494. [Google Scholar] [CrossRef] [PubMed]
- Bylund, A.; Saarinen, N.; Zhang, J.; Bergh, A.; Widmark, A.; Johansson, A.; Lundin, E.; Adlercreutz, H.; Hallmans, G.; Stattin, P.; et al. Anticancer Effects of a Plant Lignan 7-Hydroxymatairesinol on a Prostate Cancer Model In Vivo. Exp. Biol. Med. 2005, 230, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-Y.; Kim, J.-K.; Choi, J.-H.; Jung, J.-Y.; Oh, W.-Y.; Kim, D.C.; Lee, H.S.; Kim, Y.S.; Kang, S.S.; Lee, S.-H.; et al. Hepatoprotective Effect of Pinoresinol on Carbon Tetrachloride–Induced Hepatic Damage in Mice. J. Pharmacol. Sci. 2010, 112, 105–112. [Google Scholar] [CrossRef]
- Zheng, J.; Zhou, Y.; Li, S.; Zhang, P.; Zhou, T.; Xu, D.-P.; Li, H.-B. Effects and Mechanisms of Fruit and Vegetable Juices on Cardiovascular Diseases. Int. J. Mol. Sci. 2017, 18, 555. [Google Scholar] [CrossRef] [PubMed]
- Cardiovascular Diseases. Available online: https://www.who.int/health-topics/cardiovascular-diseases#tab=tab_2 (accessed on 16 April 2021).
- Jamee Shahwan, A.; Abed, Y.; Desormais, I.; Magne, J.; Preux, P.M.; Aboyans, V.; Lacroix, P. Epidemiology of coronary artery disease and stroke and associated risk factors in Gaza community–Palestine. PLoS ONE 2019, 14, e0211131. [Google Scholar] [CrossRef]
- Braunwald, E. Cardiovascular Medicine at the Turn of the Millennium: Triumphs, Concerns, and Opportunities. N. Engl. J. Med. 1997, 337, 1360–1369. [Google Scholar] [CrossRef]
- Chiva-Blanch, G.; Arranz, S.; Lamuela-Raventos, R.M.; Estruch, R. Effects of Wine, Alcohol and Polyphenols on Cardiovascular Disease Risk Factors: Evidences from Human Studies. Alcohol Alcohol. 2013, 48, 270–277. [Google Scholar] [CrossRef]
- Jneid, H.; Bhatt, D.L.; Corti, R.; Badimon, J.J.; Fuster, V.; Francis, G.S. Aspirin and Clopidogrel in Acute Coronary Syndromes. Arch. Intern. Med. 2003, 163, 1145. [Google Scholar] [CrossRef]
- Frei, B. Natural Antioxidants in Human Health and Disease; Elsevier: Amsterdam, The Netherlands, 1994; ISBN 9780080571683. [Google Scholar]
- Davignon, J. Role of Endothelial Dysfunction in Atherosclerosis. Circulation 2004, 109, III-27–III-32. [Google Scholar] [CrossRef]
- Papadogiannis, D.E.; Protogerou, A.D. Blood pressure variability: A confounder and a cardiovascular risk factor. Hypertens. Res. 2011, 34, 162–163. [Google Scholar] [CrossRef]
- Polonikov, A.V.; Vialykh, E.K.; Churnosov, M.I.; Illig, T.; Freidin, M.B.; Vasil’eva, O.V.; Bushueva, O.Y.; Ryzhaeva, V.N.; Bulgakova, I.V.; Solodilova, M.A. The C718T polymorphism in the 3′-untranslated region of glutathione peroxidase-4 gene is a predictor of cerebral stroke in patients with essential hypertension. Hypertens. Res. 2012, 35, 507–512. [Google Scholar] [CrossRef]
- Leifert, W.R.; Abeywardena, M.Y. Cardioprotective actions of grape polyphenols. Nutr. Res. 2008, 28, 729–737. [Google Scholar] [CrossRef] [PubMed]
- Rasines-Perea, Z.; Teissedre, P.-L. Grape Polyphenols’ Effects in Human Cardiovascular Diseases and Diabetes. Molecules 2017, 22, 68. [Google Scholar] [CrossRef]
- Bozkurt, B.; Aguilar, D.; Deswal, A.; Dunbar, S.B.; Francis, G.S.; Horwich, T.; Jessup, M.; Kosiborod, M.; Pritchett, A.M.; Ramasubbu, K.; et al. Contributory Risk and Management of Comorbidities of Hypertension, Obesity, Diabetes Mellitus, Hyperlipidemia, and Metabolic Syndrome in Chronic Heart Failure: A Scientific Statement From the American Heart Association. Circulation 2016, 134, e535–e578. [Google Scholar] [CrossRef]
- Pirola, L.; Fröjdö, S. Resveratrol: One molecule, many targets. IUBMB Life 2008, 60, 323–332. [Google Scholar] [CrossRef]
- Xia, E.-Q.; Deng, G.-F.; Guo, Y.-J.; Li, H.-B. Biological Activities of Polyphenols from Grapes. Int. J. Mol. Sci. 2010, 11, 622–646. [Google Scholar] [CrossRef]
- Georgiev, V.; Ananga, A.; Tsolova, V. Recent Advances and Uses of Grape Flavonoids as Nutraceuticals. Nutrients 2014, 6, 391–415. [Google Scholar] [CrossRef]
- Pagliaro, B.; Santolamazza, C.; Simonelli, F.; Rubattu, S. Phytochemical Compounds and Protection from Cardiovascular Diseases: A State of the Art. BioMed Res. Int. 2015, 2015, 918069. [Google Scholar] [CrossRef]
- Loffredo, L.; Perri, L.; Nocella, C.; Violi, F. Antioxidant and antiplatelet activity by polyphenol-rich nutrients: Focus on extra virgin olive oil and cocoa. Br. J. Clin. Pharmacol. 2017, 83, 96–102. [Google Scholar] [CrossRef]
- Akinwumi, B.; Bordun, K.-A.; Anderson, H. Biological Activities of Stilbenoids. Int. J. Mol. Sci. 2018, 19, 792. [Google Scholar] [CrossRef]
- Romani, A.; Ieri, F.; Urciuoli, S.; Noce, A.; Marrone, G.; Nediani, C.; Bernini, R. Health Effects of Phenolic Compounds Found in Extra-Virgin Olive Oil, by-Products, and Leaf of Olea europaea L. Nutrients 2019, 11, 1776. [Google Scholar] [CrossRef]
- Tresserra-Rimbau, A.; Rimm, E.B.; Medina-Remón, A.; Martínez-González, M.A.; de la Torre, R.; Corella, D.; Salas-Salvadó, J.; Gómez-Gracia, E.; Lapetra, J.; Arós, F.; et al. Inverse association between habitual polyphenol intake and incidence of cardiovascular events in the PREDIMED study. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Castaldo, L.; Narváez, A.; Izzo, L.; Graziani, G.; Gaspari, A.; Di Minno, G.; Ritieni, A. Red Wine Consumption and Cardiovascular Health. Molecules 2019, 24, 3626. [Google Scholar] [CrossRef] [PubMed]
- Myasoedova, V.; Kirichenko, T.; Melnichenko, A.; Orekhova, V.; Ravani, A.; Poggio, P.; Sobenin, I.; Bobryshev, Y.; Orekhov, A. Anti-Atherosclerotic Effects of a Phytoestrogen-Rich Herbal Preparation in Postmenopausal Women. Int. J. Mol. Sci. 2016, 17, 1318. [Google Scholar] [CrossRef] [PubMed]
- Zafar, F.; Jahan, N.; Khalil-Ur-Rahman; Khan, A.; Akram, W. Cardioprotective Potential of Polyphenolic Rich Green Combination in Catecholamine Induced Myocardial Necrosis in Rabbits. Evid.-Based Complement. Altern. Med. 2015, 2015, 734903. [Google Scholar] [CrossRef]
- El-Bassossy, H.; Badawy, D.; Neamatallah, T.; Fahmy, A. Ferulic acid, a natural polyphenol, alleviates insulin resistance and hypertension in fructose fed rats: Effect on endothelial-dependent relaxation. Chem. Biol. Interact. 2016, 254, 191–197. [Google Scholar] [CrossRef]
- Ola-Davies, O.E.; Olukole, S.G. Gallic acid protects against bisphenol A-induced alterations in the cardio-renal system of Wistar rats through the antioxidant defense mechanism. Biomed. Pharmacother. 2018, 107, 1786–1794. [Google Scholar] [CrossRef]
- Hu, X.; Wang, H.; Lv, X.; Chu, L.; Liu, Z.; Wei, X.; Chen, Q.; Zhu, L.; Cui, W. Cardioprotective Effects of Tannic Acid on Isoproterenol-Induced Myocardial Injury in Rats: Further Insight into ‘French Paradox’. Phyther. Res. 2015, 29, 1295–1303. [Google Scholar] [CrossRef]
- Ma, D.; Zheng, B.; Du, H.; Han, X.; Zhang, X.; Zhang, J.; Gao, Y.; Sun, S.; Chu, L. The Mechanism Underlying the Protective Effects of Tannic Acid against Isoproterenol-Induced Myocardial Fibrosis in Mice. Front. Pharmacol. 2020, 11, 716. [Google Scholar] [CrossRef]
- Isenburg, J.C.; Simionescu, D.T.; Vyavahare, N.R. Elastin stabilization in cardiovascular implants: Improved resistance to enzymatic degradation by treatment with tannic acid. Biomaterials 2004, 25, 3293–3302. [Google Scholar] [CrossRef]
- Calixto, J.; Nicolau, M.; Rae, G. Pharmacological Actions of Tannic Acid. I. Effects on Isolated Smooth and Cardiac Muscles and on Blood Pressure. Planta Med. 1986, 52, 32–35. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, S.; Wang, D.; Wei, Y.; Hu, S.-S. Intramyocardial injection of tannic acid attenuates postinfarction remodeling: A novel approach to stabilize the breaking extracellular matrix. J. Thorac. Cardiovasc. Surg. 2009, 137, 216–222.e2. [Google Scholar] [CrossRef] [PubMed]
- Migliori, M.; Cantaluppi, V.; Mannari, C.; Bertelli, A.A.E.; Medica, D.; Quercia, A.D.; Navarro, V.; Scatena, A.; Giovannini, L.; Biancone, L.; et al. Caffeic Acid, a Phenol Found in White Wine, Modulates Endothelial Nitric Oxide Production and Protects from Oxidative Stress-Associated Endothelial Cell Injury. PLoS ONE 2015, 10, e0117530. [Google Scholar] [CrossRef] [PubMed]
- Pataki, T.; Bak, I.; Kovacs, P.; Bagchi, D.; Das, D.K.; Tosaki, A. Grape seed proanthocyanidins improved cardiac recovery during reperfusion after ischemia in isolated rat hearts. Am. J. Clin. Nutr. 2002, 75, 894–899. [Google Scholar] [CrossRef]
- Sergazy, S.; Shulgau, Z.; Fedotovskikh, G.; Chulenbayeva, L.; Nurgozhina, A.; Nurgaziyev, M.; Krivyh, E.; Kamyshanskiy, Y.; Kushugulova, A.; Gulyayev, A.; et al. Cardioprotective effect of grape polyphenol extract against doxorubicin induced cardiotoxicity. Sci. Rep. 2020, 10, 14720. [Google Scholar] [CrossRef]
- Stote, K.S.; Sweeney, M.I.; Kean, T.; Baer, D.J.; Novotny, J.A.; Shakerley, N.L.; Chandrasekaran, A.; Carrico, P.M.; Melendez, J.A.; Gottschall-Pass, K.T. The effects of 100% wild blueberry (Vaccinium angustifolium) juice consumption on cardiometablic biomarkers: A randomized, placebo-controlled, crossover trial in adults with increased risk for type 2 diabetes. BMC Nutr. 2017, 3, 45. [Google Scholar] [CrossRef]
- Ballmann, C.; Denney, T.; Beyers, R.J.; Quindry, T.; Romero, M.; Selsby, J.T.; Quindry, J.C. Long-term dietary quercetin enrichment as a cardioprotective countermeasure in mdx mice. Exp. Physiol. 2017, 102, 635–649. [Google Scholar] [CrossRef]
- Ganapathy, R.; Ramachandran, A.; Shivalingaiah, S.B.; Bishir, M.; Bhojaraj, S.; Sridhar, S.; Mohan, S.K.; Veeraraghavan, V.P.; Chidambaram, S.B.; Essa, M.M.; et al. Cardioprotective potential of polyphenols rich Thraatchathi Chooranam against isoproterenol induced myocardial necrosis in experimental rats. BMC Complement. Med. Ther. 2020, 20, 356. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Rodrigues, C.F.; Sharopov, F.; Docea, A.O.; Can Karaca, A.; Sharifi-Rad, M.; Kahveci Karıncaoglu, D.; Gülseren, G.; Şenol, E.; Demircan, E.; et al. Diet, Lifestyle and Cardiovascular Diseases: Linking Pathophysiology to Cardioprotective Effects of Natural Bioactive Compounds. Int. J. Environ. Res. Public Health 2020, 17, 2326. [Google Scholar] [CrossRef]
- Yochum, L.; Kushi, L.H.; Meyer, K.; Folsom, A.R. Dietary flavonoid intake and risk of cardiovascular disease in postmenopausal women. Climacteric 1999, 2, 237–238. [Google Scholar] [CrossRef]
- Qin, Y.; Shu, F.; Zeng, Y.; Meng, X.; Wang, B.; Diao, L.; Wang, L.; Wan, J.; Zhu, J.; Wang, J.; et al. Daidzein Supplementation Decreases Serum Triglyceride and Uric Acid Concentrations in Hypercholesterolemic Adults with the Effect on Triglycerides Being Greater in Those with the GA Compared with the GG Genotype of ESR-β RsaI. J. Nutr. 2014, 144, 49–54. [Google Scholar] [CrossRef]
- Hao, J.; Kim, C.-H.; Ha, T.-S.; Ahn, H.-Y. Epigallocatechin-3 gallate prevents cardiac hypertrophy induced by pressure overload in rats. J. Vet. Sci. 2007, 8, 121. [Google Scholar] [CrossRef] [PubMed]
- Xuan, F.; Jian, J. Epigallocatechin gallate exerts protective effects against myocardial ischemia/reperfusion injury through the PI3K/Akt pathway-mediated inhibition of apoptosis and the restoration of the autophagic flux. Int. J. Mol. Med. 2016, 38, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Villarreal-Calderon, R.; Reed, W.; Palacios-Moreno, J.; Keefe, S.; Herritt, L.; Brooks, D.; Torres-Jardón, R.; Calderón-Garcidueñas, L. Urban air pollution produces up-regulation of myocardial inflammatory genes and dark chocolate provides cardioprotection. Exp. Toxicol. Pathol. 2012, 64, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Davis, D.W.; Tallent, R.; Navalta, J.W.; Salazar, A.; Lyons, T.J.; Basu, A. Effects of Acute Cocoa Supplementation on Postprandial Apolipoproteins, Lipoprotein Subclasses, and Inflammatory Biomarkers in Adults with Type 2 Diabetes after a High-Fat Meal. Nutrients 2020, 12, 1902. [Google Scholar] [CrossRef]
- Poudyal, H.; Campbell, F.; Brown, L. Olive Leaf Extract Attenuates Cardiac, Hepatic, and Metabolic Changes in High Carbohydrate–, High Fat–Fed Rats. J. Nutr. 2010, 140, 946–953. [Google Scholar] [CrossRef]
- Kurowska, E.M.; Banh, C.; Hasegawa, S.; Manners, G.D. Regulation of Apo B Production in HepG2 Cells by Citrus Limonoids. In Citrus Limonoids—Functional Chemicals in Agriculture and Food; ACS Publications: Washington, DC, USA, 2000; pp. 175–184. [Google Scholar]
- Negrão, M.R.; Keating, E.; Faria, A.; Azevedo, I.; Martins, M.J. Acute Effect of Tea, Wine, Beer, and Polyphenols on ecto-Alkaline Phosphatase Activity in Human Vascular Smooth Muscle Cells. J. Agric. Food Chem. 2006, 54, 4982–4988. [Google Scholar] [CrossRef]
- Hu, Q.; Zhang, T.; Yi, L.; Zhou, X.; Mi, M. Dihydromyricetin inhibits NLRP3 inflammasome-dependent pyroptosis by activating the Nrf2 signaling pathway in vascular endothelial cells. BioFactors 2018, 44, 123–136. [Google Scholar] [CrossRef]
- Zeng, Y.; Peng, Y.; Tang, K.; Wang, Y.Q.; Zhao, Z.Y.; Wei, X.Y.; Xu, X. Le Dihydromyricetin ameliorates foam cell formation via LXRα-ABCA1/ABCG1-dependent cholesterol efflux in macrophages. Biomed. Pharmacother. 2018, 101, 543–552. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, Y.; Zou, J.; Cao, K.; Xu, Y.; Wu, J.M. Effects of red wine and wine polyphenol resveratrol on platelet aggregation in vivo and in vitro. Int. J. Mol. Med. 2002, 9, 77–79. [Google Scholar] [CrossRef]
- Pal, S.; Ho, N.; Santos, C.; Dubois, P.; Mamo, J.; Croft, K.; Allister, E. Red Wine Polyphenolics Increase LDL Receptor Expression and Activity and Suppress the Secretion of ApoB100 from Human HepG2 Cells. J. Nutr. 2003, 133, 700–706. [Google Scholar] [CrossRef]
- Soares Filho, P.R.; Castro, I.; Stahlschmidt, A. Efeito do vinho tinto associado ao exercício físico no sistema cardiovascular de ratos espontaneamente hipertensos. Arq. Bras. Cardiol. 2011, 96, 277–283. [Google Scholar] [CrossRef]
- Ungvari, Z.; Orosz, Z.; Rivera, A.; Labinskyy, N.; Xiangmin, Z.; Olson, S.; Podlutsky, A.; Csiszar, A. Resveratrol increases vascular oxidative stress resistance. Am. J. Physiol. Circ. Physiol. 2007, 292, H2417–H2424. [Google Scholar] [CrossRef]
- Hung, L.-M.; Su, M.-J.; Chu, W.-K.; Chiao, C.-W.; Chan, W.-F.; Chen, J.-K. The protective effect of resveratrols on ischaemia-reperfusion injuries of rat hearts is correlated with antioxidant efficacy. Br. J. Pharmacol. 2002, 135, 1627–1633. [Google Scholar] [CrossRef]
- Yoshida, Y.; Shioi, T.; Izumi, T. Resveratrol Ameliorates Experimental Autoimmune Myocarditis. Circ. J. 2007, 71, 397–404. [Google Scholar] [CrossRef]
- Lou, Y.; Wang, Z.; Xu, Y.; Zhou, P.; Cao, J.; Li, Y.; Chen, Y.; Sun, J.; Fu, L. Resveratrol prevents doxorubicin-induced cardiotoxicity in H9c2 cells through the inhibition of endoplasmic reticulum stress and the activation of the Sirt1 pathway. Int. J. Mol. Med. 2015, 36, 873–880. [Google Scholar] [CrossRef]
- Al-Harthi, S.E.; Alarabi, O.M.; Ramadan, W.S.; Alaama, M.N.; Al-Kreathy, H.M.; Damanhouri, Z.A.; Khan, L.M.; Osman, A.-M.M. Amelioration of doxorubicin-induced cardiotoxicity by resveratrol. Mol. Med. Rep. 2014, 10, 1455–1460. [Google Scholar] [CrossRef]
- Sabe, A.A.; Elmadhun, N.Y.; Dalal, R.S.; Robich, M.P.; Sellke, F.W. Resveratrol regulates autophagy signaling in chronically ischemic myocardium. J. Thorac. Cardiovasc. Surg. 2014, 147, 792–799. [Google Scholar] [CrossRef]
- Carolo dos Santos, K.; Pereira Braga, C.; Octavio Barbanera, P.; Rodrigues Ferreira Seiva, F.; Fernandes Junior, A.; Fernandes, A.A.H. Cardiac Energy Metabolism and Oxidative Stress Biomarkers in Diabetic Rat Treated with Resveratrol. PLoS ONE 2014, 9, e102775. [Google Scholar] [CrossRef]
- Akinwumi, B.; Raj, P.; Lee, D.; Acosta, C.; Yu, L.; Thomas, S.; Nagabhushanam, K.; Majeed, M.; Davies, N.; Netticadan, T.; et al. Disparate Effects of Stilbenoid Polyphenols on Hypertrophic Cardiomyocytes In Vitro vs. in the Spontaneously Hypertensive Heart Failure Rat. Molecules 2017, 22, 204. [Google Scholar] [CrossRef]
- Fan, Y.; Liu, L.; Fang, K.; Huang, T.; Wan, L.; Liu, Y.; Zhang, S.; Yan, D.; Li, G.; Gao, Y.; et al. Resveratrol Ameliorates Cardiac Hypertrophy by Down-regulation of miR-155 Through Activation of Breast Cancer Type 1 Susceptibility Protein. J. Am. Heart Assoc. 2016, 5, e002648. [Google Scholar] [CrossRef]
- Chen, W.-P.; Hung, L.-M.; Hsueh, C.-H.; Lai, L.-P.; Su, M.-J. Piceatannol, a derivative of resveratrol, moderately slows INa inactivation and exerts antiarrhythmic action in ischaemia-reperfused rat hearts. Br. J. Pharmacol. 2009, 157, 381–391. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, Y.; Zhang, C.; Gao, L.; Li, J. Piceatannol pretreatment alleviates acute cardiac injury via regulating PI3K-Akt-eNOS signaling in H9c2 cells. Biomed. Pharmacother. 2019, 109, 886–891. [Google Scholar] [CrossRef]
- Zanwar, A.; Hegde, M.; Bodhankar, S. Cardioprotective activity of flax lignan concentrate extracted from seeds of Linum usitatissimum in isoprenalin induced myocardial necrosis in rats. Interdiscip. Toxicol. 2011, 4, 90–97. [Google Scholar] [CrossRef]
- Parikh, M.; Kura, B.; O’Hara, K.A.; Dibrov, E.; Netticadan, T.; Slezak, J.; Pierce, G.N. Cardioprotective Effects of Dietary Flaxseed Post-Infarction Are Associated with Changes in MicroRNA Expression. Biomolecules 2020, 10, 1297. [Google Scholar] [CrossRef]
- Zanwar, A.A.; Hegde, M.V.; Bodhankar, S.L. Protective role of concomitant administration of flax lignan concentrate and omega-3-fatty acid on myocardial damage in doxorubicin-induced cardiotoxicity. Food Sci. Hum. Wellness 2013, 2, 29–38. [Google Scholar] [CrossRef][Green Version]
- Sawant, S.H.; Bodhankar, S.L. Flax lignan concentrate reverses alterations in blood pressure, left ventricular functions, lipid profile and antioxidant status in DOCA-salt induced renal hypertension in rats. Ren. Fail. 2016, 38, 411–423. [Google Scholar] [CrossRef]
- Bassett, C.M.C.; Rodriguez-Leyva, D.; Pierce, G.N. Experimental and clinical research findings on the cardiovascular benefits of consuming flaxseed. Appl. Physiol. Nutr. Metab. 2009, 34, 965–974. [Google Scholar] [CrossRef]
- Hassan, N.A.; El-Bassossy, H.M.; Mahmoud, M.F.; Fahmy, A. Caffeic acid phenethyl ester, a 5-lipoxygenase enzyme inhibitor, alleviates diabetic atherosclerotic manifestations: Effect on vascular reactivity and stiffness. Chem. Biol. Interact. 2014, 213, 28–36. [Google Scholar] [CrossRef]
- Gun, A.; Ozer, M.K.; Bilgic, S.; Kocaman, N.; Ozan, G. Effect of Caffeic Acid Phenethyl Ester on Vascular Damage Caused by Consumption of High Fructose Corn Syrup in Rats. Oxid. Med. Cell. Longev. 2016, 2016, 3419479. [Google Scholar] [CrossRef]
- Wang, Y.; Kaur, G.; Kumar, M.; Kushwah, A.S.; Kabra, A.; Kainth, R. Caffeic Acid Prevents Vascular Oxidative Stress and Atherosclerosis against Atherosclerogenic Diet in Rats. Evid.-Based Complement. Altern. Med. 2022, 2022, 8913926. [Google Scholar] [CrossRef]
- Dupasquier, C.M.C.; Dibrov, E.; Kneesh, A.L.; Cheung, P.K.M.; Lee, K.G.Y.; Alexander, H.K.; Yeganeh, B.K.; Moghadasian, M.H.; Pierce, G.N. Dietary flaxseed inhibits atherosclerosis in the LDL receptor-deficient mouse in part through antiproliferative and anti-inflammatory actions. Am. J. Physiol. Circ. Physiol. 2007, 293, H2394–H2402. [Google Scholar] [CrossRef] [PubMed]
- Bloedon, L.T.; Balikai, S.; Chittams, J.; Cunnane, S.C.; Berlin, J.A.; Rader, D.J.; Szapary, P.O. Flaxseed and Cardiovascular Risk Factors: Results from a Double Blind, Randomized, Controlled Clinical Trial. J. Am. Coll. Nutr. 2008, 27, 65–74. [Google Scholar] [CrossRef]
- Narasimhulu, C.A.; Selvarajan, K.; Litvinov, D.; Parthasarathy, S. Anti-Atherosclerotic and Anti-Inflammatory Actions of Sesame Oil. J. Med. Food 2015, 18, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Bhaskaran, S.; Santanam, N.; Penumetcha, M.; Parthasarathy, S. Inhibition of Atherosclerosis in Low-Density Lipoprotein Receptor-Negative Mice by Sesame Oil. J. Med. Food 2006, 9, 487–490. [Google Scholar] [CrossRef] [PubMed]
- Hemalatha, G.; Pugalendi, K.V.; Saravanan, R. Modulatory effect of sesamol on DOCA-salt-induced oxidative stress in uninephrectomized hypertensive rats. Mol. Cell. Biochem. 2013, 379, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Stanely Mainzen Prince, P.; Priscilla, H.; Devika, P.T. Gallic acid prevents lysosomal damage in isoproterenol induced cardiotoxicity in Wistar rats. Eur. J. Pharmacol. 2009, 615, 139–143. [Google Scholar] [CrossRef]
- Umadevi, S.; Gopi, V.; Elangovan, V. Regulatory mechanism of gallic acid against advanced glycation end products induced cardiac remodeling in experimental rats. Chem. Biol. Interact. 2014, 208, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Lin, M.Q.; Piao, Z.H.; Cho, J.Y.; Kim, G.R.; Choi, S.Y.; Ryu, Y.; Sun, S.; Kee, H.J.; Jeong, M.H. Gallic acid attenuates hypertension, cardiac remodeling, and fibrosis in mice with N G-nitro-L-arginine methyl ester-induced hypertension via regulation of histone deacetylase 1 or histone deacetylase 2. J. Hypertens. 2017, 35, 1502–1512. [Google Scholar] [CrossRef]
- Sies, H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef]
- Zhao, X.; Wu, Y. Correlations of Silent Information Regulator of Transcription 1 (SIRT1) Expression, Inflammatory Factors, and Oxidative Stress with Pulmonary Function in Patients with Acute Exacerbation of Chronic Obstructive Pulmonary Disease (AECOPD). Med. Sci. Monit. 2021, 27, e929046. [Google Scholar] [CrossRef]
- Kirkham, P.A.; Barnes, P.J. Oxidative Stress in COPD. Chest 2013, 144, 266–273. [Google Scholar] [CrossRef]
- Rivero, D.; Pérez-Magariño, S.; González-Sanjosé, M.L.; Valls-Belles, V.; Codoñer, P.; Muñiz, P. Inhibition of Induced DNA Oxidative Damage by Beers: Correlation with the Content of Polyphenols and Melanoidins. J. Agric. Food Chem. 2005, 53, 3637–3642. [Google Scholar] [CrossRef] [PubMed]
- Perron, N.R.; Hodges, J.N.; Jenkins, M.; Brumaghim, J.L. Predicting How Polyphenol Antioxidants Prevent DNA Damage by Binding to Iron. Inorg. Chem. 2008, 47, 6153–6161. [Google Scholar] [CrossRef]
- Bellion, P.; Digles, J.; Will, F.; Dietrich, H.; Baum, M.; Eisenbrand, G.; Janzowski, C. Polyphenolic Apple Extracts: Effects of Raw Material and Production Method on Antioxidant Effectiveness and Reduction of DNA Damage in Caco-2 Cells. J. Agric. Food Chem. 2010, 58, 6636–6642. [Google Scholar] [CrossRef]
- Loeb, L.A.; Wallace, D.C.; Martin, G.M. The mitochondrial theory of aging and its relationship to reactive oxygen species damage and somatic mtDNA mutations. Proc. Natl. Acad. Sci. USA 2005, 102, 18769–18770. [Google Scholar] [CrossRef]
- Barrero, A.F.; Herrador, M.M.; Arteaga, P.; Rosas-Romero, A.; Arteaga, J.F. Antioxidant Activity of Diterpenes and Polyphenols from Ophryosporus heptanthus. J. Agric. Food Chem. 2006, 54, 2537–2542. [Google Scholar] [CrossRef]
- Dulebohn, R.V.; Yi, W.; Srivastava, A.; Akoh, C.C.; Krewer, G.; Fischer, J.G. Effects of Blueberry (Vaccinium ashei) on DNA Damage, Lipid Peroxidation, and Phase II Enzyme Activities in Rats. J. Agric. Food Chem. 2008, 56, 11700–11706. [Google Scholar] [CrossRef]
- Natella, F.; Nardini, M.; Giannetti, I.; Dattilo, C.; Scaccini, C. Coffee Drinking Influences Plasma Antioxidant Capacity in Humans. J. Agric. Food Chem. 2002, 50, 6211–6216. [Google Scholar] [CrossRef]
- Rolt, A.; Cox, L.S. Structural basis of the anti-ageing effects of polyphenolics: Mitigation of oxidative stress. BMC Chem. 2020, 14, 50. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Rui, Y.; Guo, S.; Luan, F.; Liu, R.; Zeng, N. Ferulic acid: A review of its pharmacology, pharmacokinetics and derivatives. Life Sci. 2021, 284, 119921. [Google Scholar] [CrossRef]
- Hosseinzadeh, A.; Houshmand, G.; Goudarzi, M.; Sezavar, S.H.; Mehrzadi, S.; Mansouri, E.; Kalantar, M. Ameliorative effect of gallic acid on sodium arsenite-induced spleno-, cardio- and hemato-toxicity in rats. Life Sci. 2019, 217, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Murakami, A.; Ashida, H.; Terao, J. Multitargeted cancer prevention by quercetin. Cancer Lett. 2008, 269, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Kamada, C.; da Silva, E.L.; Ohnishi-Kameyama, M.; Moon, J.-H.; Terao, J. Attenuation of lipid peroxidation and hyperlipidemia by quercetin glucoside in the aorta of high cholesterol-fed rabbit. Free Radic. Res. 2005, 39, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Youdim, K.A.; Shukitt-Hale, B.; Joseph, J.A. Flavonoids and the brain: Interactions at the blood–brain barrier and their physiological effects on the central nervous system. Free Radic. Biol. Med. 2004, 37, 1683–1693. [Google Scholar] [CrossRef]
- Ishisaka, A.; Ichikawa, S.; Sakakibara, H.; Piskula, M.K.; Nakamura, T.; Kato, Y.; Ito, M.; Miyamoto, K.; Tsuji, A.; Kawai, Y.; et al. Accumulation of orally administered quercetin in brain tissue and its antioxidative effects in rats. Free Radic. Biol. Med. 2011, 51, 1329–1336. [Google Scholar] [CrossRef]
- Sanbongi, C.; Osakabe, N.; Natsume, M.; Takizawa, T.; Gomi, S.; Osawa, T. Antioxidative Polyphenols Isolated from Theobroma cacao. J. Agric. Food Chem. 1998, 46, 454–457. [Google Scholar] [CrossRef]
- Burgos-Morón, E.; Calderón-Montaño, J.M.; Orta, M.L.; Pastor, N.; Pérez-Guerrero, C.; Austin, C.; Mateos, S.; López-Lázaro, M. The Coffee Constituent Chlorogenic Acid Induces Cellular DNA Damage and Formation of Topoisomerase I– and II–DNA Complexes in Cells. J. Agric. Food Chem. 2012, 60, 7384–7391. [Google Scholar] [CrossRef]
- Esselen, M.; Fritz, J.; Hutter, M.; Marko, D. Delphinidin Modulates the DNA-Damaging Properties of Topoisomerase II Poisons. Chem. Res. Toxicol. 2009, 22, 554–564. [Google Scholar] [CrossRef]
- Esselen, M.; Boettler, U.; Teller, N.; Bächler, S.; Hutter, M.; Rüfer, C.E.; Skrbek, S.; Marko, D. Anthocyanin-Rich Blackberry Extract Suppresses the DNA-Damaging Properties of Topoisomerase I and II Poisons in Colon Carcinoma Cells. J. Agric. Food Chem. 2011, 59, 6966–6973. [Google Scholar] [CrossRef]
- Shih, P.-H.; Yeh, C.-T.; Yen, G.-C. Anthocyanins Induce the Activation of Phase II Enzymes through the Antioxidant Response Element Pathway against Oxidative Stress-Induced Apoptosis. J. Agric. Food Chem. 2007, 55, 9427–9435. [Google Scholar] [CrossRef]
- Hwang, J.-T.; Kwon, D.Y.; Park, O.J.; Kim, M.S. Resveratrol protects ROS-induced cell death by activating AMPK in H9c2 cardiac muscle cells. Genes Nutr. 2008, 2, 323–326. [Google Scholar] [CrossRef]
- Haramizu, S.; Asano, S.; Butler, D.C.; Stanton, D.A.; Hajira, A.; Mohamed, J.S.; Alway, S.E. Dietary resveratrol confers apoptotic resistance to oxidative stress in myoblasts. J. Nutr. Biochem. 2017, 50, 103–115. [Google Scholar] [CrossRef]
- Du, L.; Wang, L.; Wang, B.; Wang, J.; Hao, M.; Chen, Y.; Li, X.; Li, Y.; Jiang, Y.; Li, C.; et al. A novel compound AB38b attenuates oxidative stress and ECM protein accumulation in kidneys of diabetic mice through modulation of Keap1/Nrf2 signaling. Acta Pharmacol. Sin. 2020, 41, 358–372. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, X.; Li, J. Resveratrol protects against oxidized low-density lipoprotein-induced human umbilical vein endothelial cell apoptosis via inhibition of mitochondrial-derived oxidative stress. Mol. Med. Rep. 2017, 15, 2457–2464. [Google Scholar] [CrossRef] [PubMed]
- Soylemez, S.; Sepici, A.; Akar, F. Resveratrol Supplementation Gender Independently Improves Endothelial Reactivity and Suppresses Superoxide Production in Healthy Rats. Cardiovasc. Drugs Ther. 2009, 23, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Youssef, F.S.; Ashour, M.L.; El-Beshbishy, H.A.; Ahmed Hamza, A.; Singab, A.N.B.; Wink, M. Pinoresinol-4-O-β-D-glucopyranoside: A lignan from prunes (Prunus domestica) attenuates oxidative stress, hyperglycaemia and hepatic toxicity in vitro and in vivo. J. Pharm. Pharmacol. 2020, 72, 1830–1839. [Google Scholar] [CrossRef]
- Pilar, B.; Güllich, A.; Oliveira, P.; Ströher, D.; Piccoli, J.; Manfredini, V. Protective Role of Flaxseed Oil and Flaxseed Lignan Secoisolariciresinol Diglucoside against Oxidative Stress in Rats with Metabolic Syndrome. J. Food Sci. 2017, 82, 3029–3036. [Google Scholar] [CrossRef]
- Kim, J.W.; Yang, H.; Kim, H.W.; Kim, H.P.; Sung, S.H. Lignans from Opuntia ficus-indica seeds protect rat primary hepatocytes and HepG2 cells against ethanol-induced oxidative stress. Biosci. Biotechnol. Biochem. 2017, 81, 181–183. [Google Scholar] [CrossRef]
- Youssef, F.S.; Menze, E.T.; Ashour, M.L. A Potent Lignan from Prunes Alleviates Inflammation and Oxidative Stress in Lithium/Pilocarpine-Induced Epileptic Seizures in Rats. Antioxidants 2020, 9, 575. [Google Scholar] [CrossRef]
- Halazonetis, T.D.; Gorgoulis, V.G.; Bartek, J. An Oncogene-Induced DNA Damage Model for Cancer Development. Science 2008, 319, 1352–1355. [Google Scholar] [CrossRef]
- O’Connor, M.J. Targeting the DNA Damage Response in Cancer. Mol. Cell 2015, 60, 547–560. [Google Scholar] [CrossRef]
- Roos, W.P.; Thomas, A.D.; Kaina, B. DNA damage and the balance between survival and death in cancer biology. Nat. Rev. Cancer 2016, 16, 20–33. [Google Scholar] [CrossRef]
- Ali, R.; Rakha, E.A.; Madhusudan, S.; Bryant, H.E. DNA damage repair in breast cancer and its therapeutic implications. Pathology 2017, 49, 156–165. [Google Scholar] [CrossRef]
- Hosoya, N.; Miyagawa, K. Targeting DNA damage response in cancer therapy. Cancer Sci. 2014, 105, 370–388. [Google Scholar] [CrossRef]
- Panahi, Y.; Saadat, A.; Beiraghdar, F.; Sahebkar, A. Adjuvant Therapy with Bioavailability-Boosted Curcuminoids Suppresses Systemic Inflammation and Improves Quality of Life in Patients with Solid Tumors: A Randomized Double-Blind Placebo-Controlled Trial. Phyther. Res. 2014, 28, 1461–1467. [Google Scholar] [CrossRef]
- Azqueta, A.; Collins, A. Polyphenols and DNA Damage: A Mixed Blessing. Nutrients 2016, 8, 785. [Google Scholar] [CrossRef]
- Shukla, D.; Nandi, N.K.; Singh, B.; Singh, A.; Kumar, B.; Narang, R.K.; Singh, C. Ferulic acid-loaded drug delivery systems for biomedical applications. J. Drug Deliv. Sci. Technol. 2022, 75, 103621. [Google Scholar] [CrossRef]
- Agarwal, C.; Tyagi, A.; Agarwal, R. Gallic acid causes inactivating phosphorylation of cdc25A/cdc25C-cdc2 via ATM-Chk2 activation, leading to cell cycle arrest, and induces apoptosis in human prostate carcinoma DU145 cells. Mol. Cancer Ther. 2006, 5, 3294–3302. [Google Scholar] [CrossRef]
- Ji, B.-C.; Hsu, W.-H.; Yang, J.-S.; Hsia, T.-C.; Lu, C.-C.; Chiang, J.-H.; Yang, J.-L.; Lin, C.-H.; Lin, J.-J.; Suen, L.-J.W.; et al. Gallic Acid Induces Apoptosis via Caspase-3 and Mitochondrion-Dependent Pathways in Vitro and Suppresses Lung Xenograft Tumor Growth In Vivo. J. Agric. Food Chem. 2009, 57, 7596–7604. [Google Scholar] [CrossRef]
- Liu, K.-C.; Ho, H.-C.; Huang, A.-C.; Ji, B.-C.; Lin, H.-Y.; Chueh, F.-S.; Yang, J.-S.; Lu, C.-C.; Chiang, J.-H.; Meng, M.; et al. Gallic acid provokes DNA damage and suppresses DNA repair gene expression in human prostate cancer PC-3 cells. Environ. Toxicol. 2013, 28, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Fujiki, H.; Suganuma, M.; Okabe, S.; Sueoka, E.; Suga, K.; Imai, K.; Nakachi, K.; Kimura, S. Mechanistic Findings of Green Tea as Cancer Preventive for Humans. Exp. Biol. Med. 1999, 220, 225–228. [Google Scholar] [CrossRef]
- Mertens-Talcott, S.U.; Lee, J.-H.; Percival, S.S.; Talcott, S.T. Induction of Cell Death in Caco-2 Human Colon Carcinoma Cells by Ellagic Acid Rich Fractions from Muscadine Grapes (Vitis rotundifolia). J. Agric. Food Chem. 2006, 54, 5336–5343. [Google Scholar] [CrossRef]
- Luo, K.L.; Luo, J.-H.; Yu, Y.P. (−)-Epigallocatechin-3-gallate induces Du145 prostate cancer cell death via downregulation of inhibitor of DNA binding 2, a dominant negative helix-loop-helix protein. Cancer Sci. 2010, 101, 707–712. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Mukhtar, H. Modulation of signaling pathways in prostate cancer by green tea polyphenols. Biochem. Pharmacol. 2013, 85, 667–672. [Google Scholar] [CrossRef]
- Zhou, J.; Xia, L.; Zhang, Y. Naringin inhibits thyroid cancer cell proliferation and induces cell apoptosis through repressing PI3K/AKT pathway. Pathol.-Res. Pract. 2019, 215, 152707. [Google Scholar] [CrossRef]
- Wu, X.; Huang, Z.; Liu, J.; Chen, Y.; Huang, H.; He, Y.; Li, D.; Zhang, L.; Du, Z.; Zhang, K.; et al. Effects and mechanism of inhibition of naringin in combination with atorvastatin on prostate cancer cells in vitro and in vivo. Phytochem. Lett. 2019, 32, 168–176. [Google Scholar] [CrossRef]
- Li, N.; Sun, C.; Zhou, B.; Xing, H.; Ma, D.; Chen, G.; Weng, D. Low Concentration of Quercetin Antagonizes the Cytotoxic Effects of Anti-Neoplastic Drugs in Ovarian Cancer. PLoS ONE 2014, 9, e100314. [Google Scholar] [CrossRef]
- Parvaresh, A.; Razavi, R.; Rafie, N.; Ghiasvand, R.; Pourmasoumi, M.; Miraghajani, M. Quercetin and ovarian cancer: An evaluation based on a systematic review. J. Res. Med. Sci. 2016, 21, 34. [Google Scholar] [CrossRef]
- Chen, R.; Hollborn, M.; Grosche, A.; Reichenbach, A.; Wiedemann, P.; Bringmann, A.; Kohen, L. Effects of the vegetable polyphenols epigallocatechin-3-gallate, luteolin, apigenin, myricetin, quercetin, and cyanidin in primary cultures of human retinal pigment epithelial cells. Mol. Vis. 2014, 20, 242–258. [Google Scholar]
- Rauf, A.; Imran, M.; Khan, I.A.; Ur-Rehman, M.; Gilani, S.A.; Mehmood, Z.; Mubarak, M.S. Anticancer potential of quercetin: A comprehensive review. Phyther. Res. 2018, 32, 2109–2130. [Google Scholar] [CrossRef]
- Schantz, M.; Mohn, C.; Baum, M.; Richling, E. Antioxidative efficiency of an anthocyanin rich bilberry extract in the human colon tumor cell lines Caco-2 and HT-29. J. Berry Res. 2010, 1, 25–33. [Google Scholar] [CrossRef]
- Joshi, R.; Rana, A.; Kumar, V.; Kumar, D.; Padwad, Y.S.; Yadav, S.K.; Gulati, A. Anthocyanins enriched purple tea exhibits antioxidant, immunostimulatory and anticancer activities. J. Food Sci. Technol. 2017, 54, 1953–1963. [Google Scholar] [CrossRef] [PubMed]
- Faria, A.; Pestana, D.; Teixeira, D.; de Freitas, V.; Mateus, N.; Calhau, C. Blueberry anthocyanins and pyruvic acid adducts: Anticancer properties in breast cancer cell lines. Phyther. Res. 2010, 24, 1862–1869. [Google Scholar] [CrossRef] [PubMed]
- Suner, S.S.; Sahiner, M.; Mohapatra, S.; Ayyala, R.S.; Bhethanabotla, V.R.; Sahiner, N. Degradable poly(catechin) nanoparticles as a versatile therapeutic agent. Int. J. Polym. Mater. Polym. Biomater. 2022, 71, 1104–1115. [Google Scholar] [CrossRef]
- Igura, K.; Ohta, T.; Kuroda, Y.; Kaji, K. Resveratrol and quercetin inhibit angiogenesis in vitro. Cancer Lett. 2001, 171, 11–16. [Google Scholar] [CrossRef]
- Banerjee, S.; Bueso-Ramos, C.; Aggarwal, B.B. Suppression of 7,12-dimethylbenz(a)anthracene-induced mammary carcinogenesis in rats by resveratrol: Role of nuclear factor-kappaB, cyclooxygenase 2, and matrix metalloprotease 9. Cancer Res. 2002, 62, 4945–4954. [Google Scholar]
- Cao, Y.; Fu, Z.-D.; Wang, F.; Liu, H.-Y.; Han, R. Anti-angiogenic activity of resveratrol, a natural compound from medicinal plants. J. Asian Nat. Prod. Res. 2005, 7, 205–213. [Google Scholar] [CrossRef]
- Kim, T.; Park, J.; Woo, J. Resveratrol induces cell death through ROS-dependent downregulation of Notch1/PTEN/Akt signaling in ovarian cancer cells. Mol. Med. Rep. 2019, 19, 3353–3360. [Google Scholar] [CrossRef]
- Miki, H.; Uehara, N.; Kimura, A.; Sasaki, T.; Yuri, T.; Yoshizawa, K.; Tsubura, A. Resveratrol induces apoptosis via ROS-triggered autophagy in human colon cancer cells. Int. J. Oncol. 2012, 40, 1020–1028. [Google Scholar] [CrossRef]
- Luo, H.; Yang, A.; Schulte, B.A.; Wargovich, M.J.; Wang, G.Y. Resveratrol Induces Premature Senescence in Lung Cancer Cells via ROS-Mediated DNA Damage. PLoS ONE 2013, 8, e60065. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, Z.; Sun, J.; Pan, Q.; Sun, F.; Yan, Z.; Hu, X. Schisandrin B Prevents Doxorubicin-Induced Chronic Cardiotoxicity and Enhances Its Anticancer Activity In Vivo. PLoS ONE 2011, 6, e28335. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.-J.; Kuo, P.-L.; Hsu, Y.-C.; Huang, Y.-F.; Tsai, E.-M.; Hsu, Y.-L. Arctigenin, a dietary phytoestrogen, induces apoptosis of estrogen receptor-negative breast cancer cells through the ROS/p38 MAPK pathway and epigenetic regulation. Free Radic. Biol. Med. 2014, 67, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Zingue, S.; Cisilotto, J.; Tueche, A.B.; Bishayee, A.; Mefegue, F.A.; Sandjo, L.P.; Magne Nde, C.B.; Winter, E.; Michel, T.; Ndinteh, D.T.; et al. Crateva adansonii DC, an African ethnomedicinal plant, exerts cytotoxicity in vitro and prevents experimental mammary tumorigenesis in vivo. J. Ethnopharmacol. 2016, 190, 183–199. [Google Scholar] [CrossRef]
- Zhang, T.; Ma, L.; Wu, P.; Li, W.; Li, T.; Gu, R.; Dan, X.; Li, Z.; Fan, X.; Xiao, Z. Gallic acid has anticancer activity and enhances the anticancer effects of cisplatin in non-small cell lung cancer A549 cells via the JAK/STAT3 signaling pathway. Oncol. Rep. 2019, 41, 1779–1788. [Google Scholar] [CrossRef]
- De Camargo, A.C.; Regitano-d’Arce, M.A.B.; Biasoto, A.C.T.; Shahidi, F. Low Molecular Weight Phenolics of Grape Juice and Winemaking Byproducts: Antioxidant Activities and Inhibition of Oxidation of Human Low-Density Lipoprotein Cholesterol and DNA Strand Breakage. J. Agric. Food Chem. 2014, 62, 12159–12171. [Google Scholar] [CrossRef] [PubMed]
- Pham-Huy, L.A.; He, H.; Pham-Huy, C. Free radicals, antioxidants in disease and health. Int. J. Biomed. Sci. 2008, 4, 89–96. [Google Scholar] [PubMed]
- Raha, S.; Robinson, B.H. Mitochondria, oxygen free radicals, disease and ageing. Trends Biochem. Sci. 2000, 25, 502–508. [Google Scholar] [CrossRef]
- Dudonné, S.; Poupard, P.; Coutière, P.; Woillez, M.; Richard, T.; Mérillon, J.-M.; Vitrac, X. Phenolic Composition and Antioxidant Properties of Poplar Bud (Populus nigra) Extract: Individual Antioxidant Contribution of Phenolics and Transcriptional Effect on Skin Aging. J. Agric. Food Chem. 2011, 59, 4527–4536. [Google Scholar] [CrossRef]
- De Camargo, A.C.; Vidal, C.M.M.; Canniatti-Brazaca, S.G.; Shahidi, F. Fortification of Cookies with Peanut Skins: Effects on the Composition, Polyphenols, Antioxidant Properties, and Sensory Quality. J. Agric. Food Chem. 2014, 62, 11228–11235. [Google Scholar] [CrossRef]
- Cherubim, D.J.; Martins, C.V.; Fariña, L.; Lucca, R.A. Polyphenols as natural antioxidants in cosmetics applications. J. Cosmet. Dermatol. 2020, 19, 33–37. [Google Scholar] [CrossRef]
- Wei, X.; Liu, Y.; Xiao, J.; Wang, Y. Protective Effects of Tea Polysaccharides and Polyphenols on Skin. J. Agric. Food Chem. 2009, 57, 7757–7762. [Google Scholar] [CrossRef]
- Russo, G.L.; Spagnuolo, C.; Russo, M.; Tedesco, I.; Moccia, S.; Cervellera, C. Mechanisms of aging and potential role of selected polyphenols in extending healthspan. Biochem. Pharmacol. 2020, 173, 113719. [Google Scholar] [CrossRef]
- Mohammad, I.S.; Naveed, M.; Ijaz, S.; Shumzaid, M.; Hassan, S.; Muhammad, K.S.; Rasool, F.; Akhtar, N.; Ishaq, H.M.; Khan, H.M.S. Phytocosmeceutical formulation development, characterization and its in-vivo investigations. Biomed. Pharmacother. 2018, 107, 806–817. [Google Scholar] [CrossRef]
- Kashif, M.; Akhtar, N. Dermocosmetic emulgels for anti-aging effects: Evidence from chromatographic and non-invasive biophysical techniques. Pak. J. Pharm. Sci. 2019, 32, 845–852. [Google Scholar]
- Chen, W.; Müller, D.; Richling, E.; Wink, M. Anthocyanin-rich Purple Wheat Prolongs the Life Span of Caenorhabditis elegans Probably by Activating the DAF-16/FOXO Transcription Factor. J. Agric. Food Chem. 2013, 61, 3047–3053. [Google Scholar] [CrossRef]
- Wang, H.; Cao, G.; Prior, R.L. Oxygen Radical Absorbing Capacity of Anthocyanins. J. Agric. Food Chem. 1997, 45, 304–309. [Google Scholar] [CrossRef]
- Peixoto, H.; Roxo, M.; Krstin, S.; Röhrig, T.; Richling, E.; Wink, M. An Anthocyanin-Rich Extract of Acai (Euterpe precatoria Mart.) Increases Stress Resistance and Retards Aging-Related Markers in Caenorhabditis elegans. J. Agric. Food Chem. 2016, 64, 1283–1290. [Google Scholar] [CrossRef]
- Wei, J.; Zhang, G.; Zhang, X.; Xu, D.; Gao, J.; Fan, J.; Zhou, Z. Anthocyanins from Black Chokeberry (Aroniamelanocarpa Elliot) Delayed Aging-Related Degenerative Changes of Brain. J. Agric. Food Chem. 2017, 65, 5973–5984. [Google Scholar] [CrossRef]
- Li, Y.-R.; Li, S.; Lin, C.-C. Effect of resveratrol and pterostilbene on aging and longevity. Biofactors 2018, 44, 69–82. [Google Scholar] [CrossRef]
- Boo, Y.C. Human Skin Lightening Efficacy of Resveratrol and Its Analogs: From in Vitro Studies to Cosmetic Applications. Antioxidants 2019, 8, 332. [Google Scholar] [CrossRef]
- Khan, M.; Park, S.; Kim, H.-J.; Lee, K.-J.; Kim, D.-H.; Baek, S.-H.; Hong, S.-T. The Resveratrol Rice DJ526 Callus Significantly Increases the Lifespan of Drosophila (Resveratrol Rice DJ526 Callus for Longevity). Nutrients 2019, 11, 983. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Alonso, P.; Vera-López, O.; Brambila-Colombres, E.; Segura-Badilla, O.; Avalos-López, R.; Lazcano-Hernández, M.; Navarro-Cruz, A.R. Evaluation of Oxidative Stress in Cardiomyocytes during the Aging Process in Rats Treated with Resveratrol. Oxid. Med. Cell. Longev. 2018, 2018, 1390483. [Google Scholar] [CrossRef] [PubMed]
- Buonocore, D.; Lazzeretti, A.; Tocabens, P.; Nobile, V.; Cestone, E.; Santin, G.; Bottone, M.G.; Marzatico, F. Resveratrol-procyanidin blend: Nutraceutical and antiaging efficacy evaluated in a placebo-controlled, double-blind study. Clin. Cosmet. Investig. Dermatol. 2012, 5, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Diaz, M.; Degens, H.; Vanhees, L.; Austin, C.; Azzawi, M. The effects of resveratrol on aging vessels. Exp. Gerontol. 2016, 85, 41–47. [Google Scholar] [CrossRef]
- Sun, J.; Jing, S.; Jiang, R.; Wang, C.; Zhang, C.; Chen, J.; Li, H. Metabolomics study of the therapeutic mechanism of Schisandra chinensis lignans on aging rats induced by D-galactose. Clin. Interv. Aging 2018, 13, 829–841. [Google Scholar] [CrossRef]
- Lili, Z.; Jing, T.; Ying, C.; Ligang, J. Effects of Schisandra chinensis Extract on the Learning and Memory Ability of Mice with Learning and Memory Disorders. Nat. Prod. Commun. 2020, 15, 1–6. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).