Simulating Diffusion Induced Grain Boundary Migration in Binary Fe–Zn
Abstract
:1. Introduction
2. Phase-Field Model for DIGM in Fe–Zn
2.1. Governing Equations
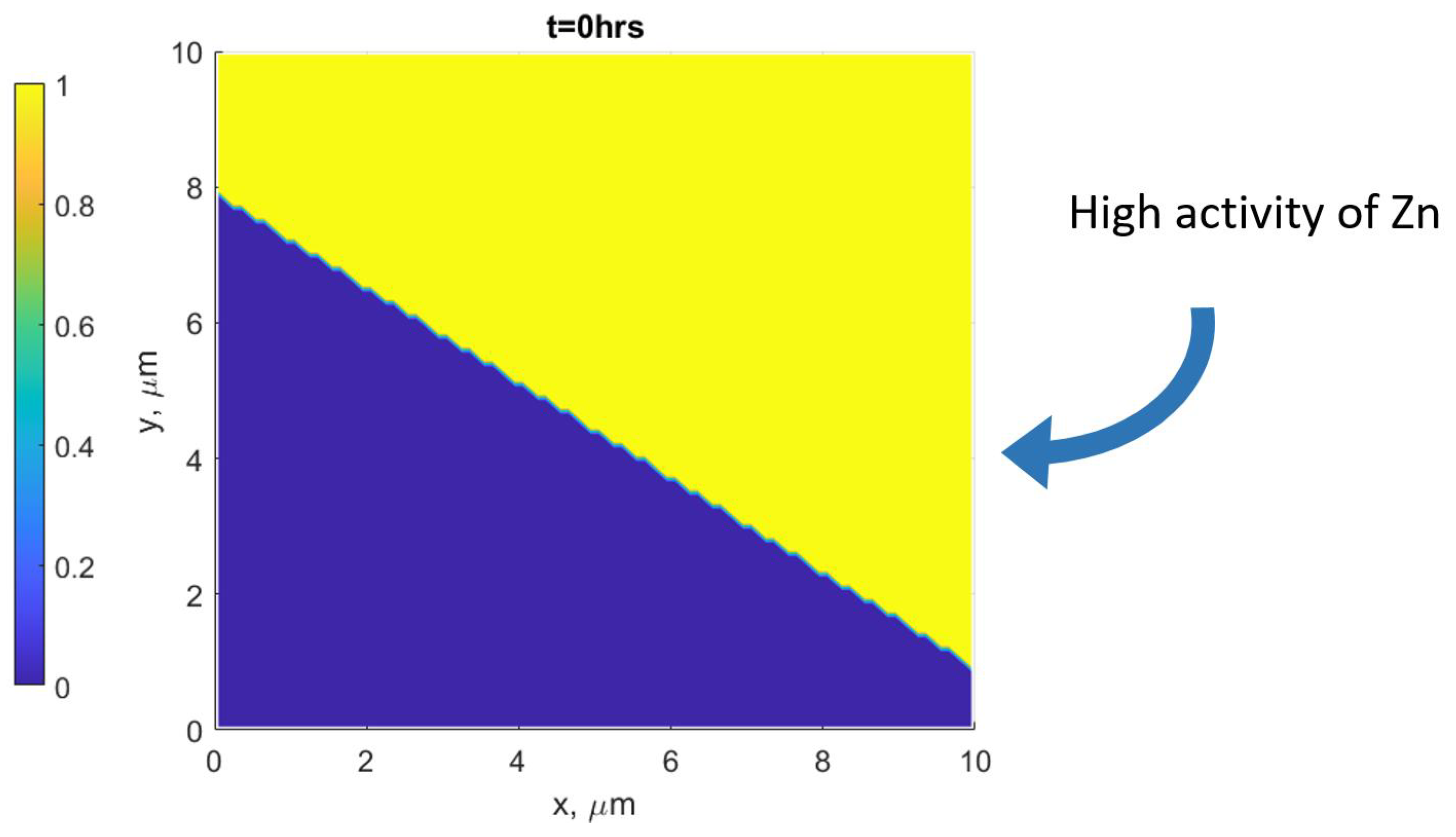
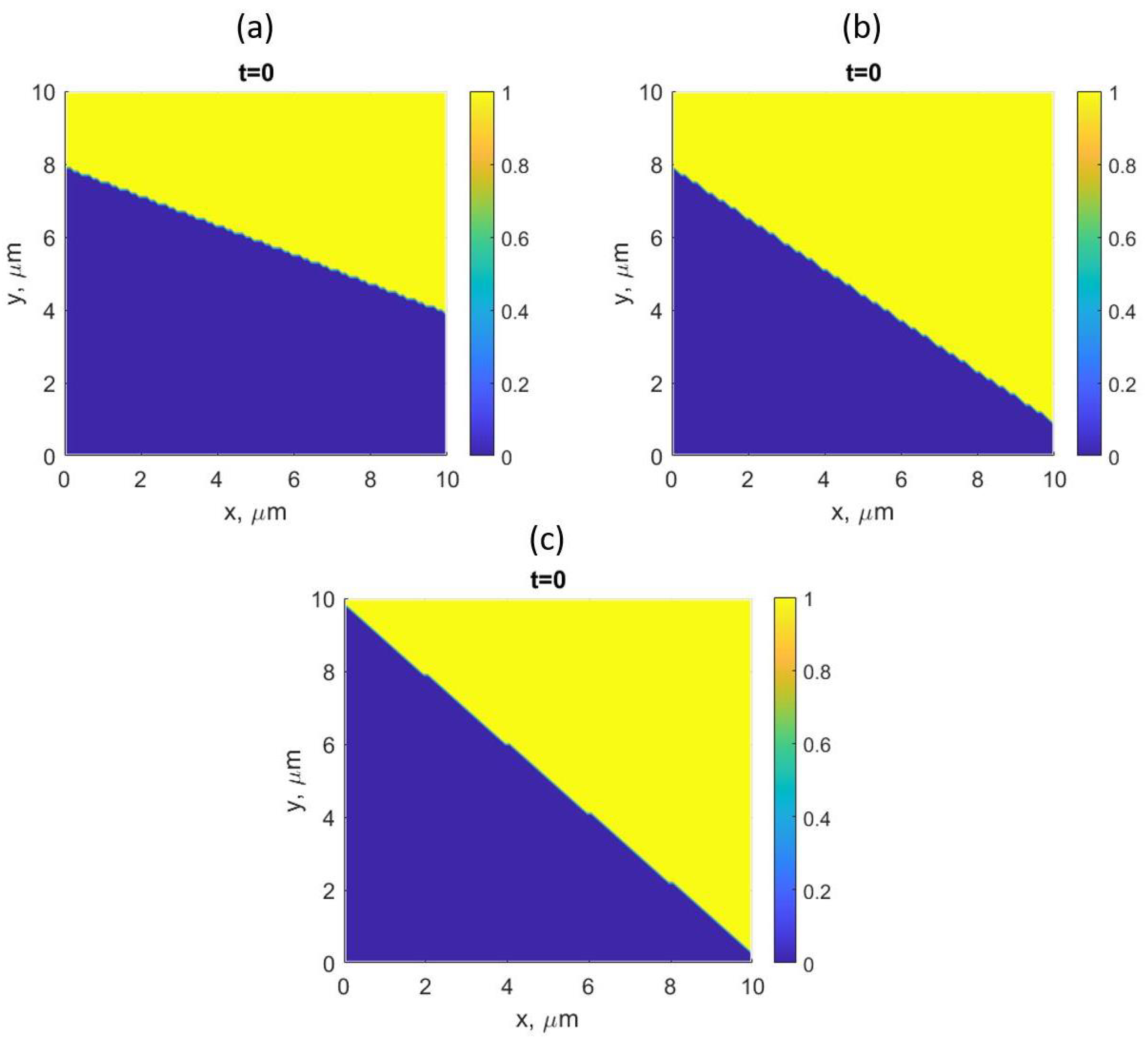
2.2. Simulation Setup
2.3. Numerical Details
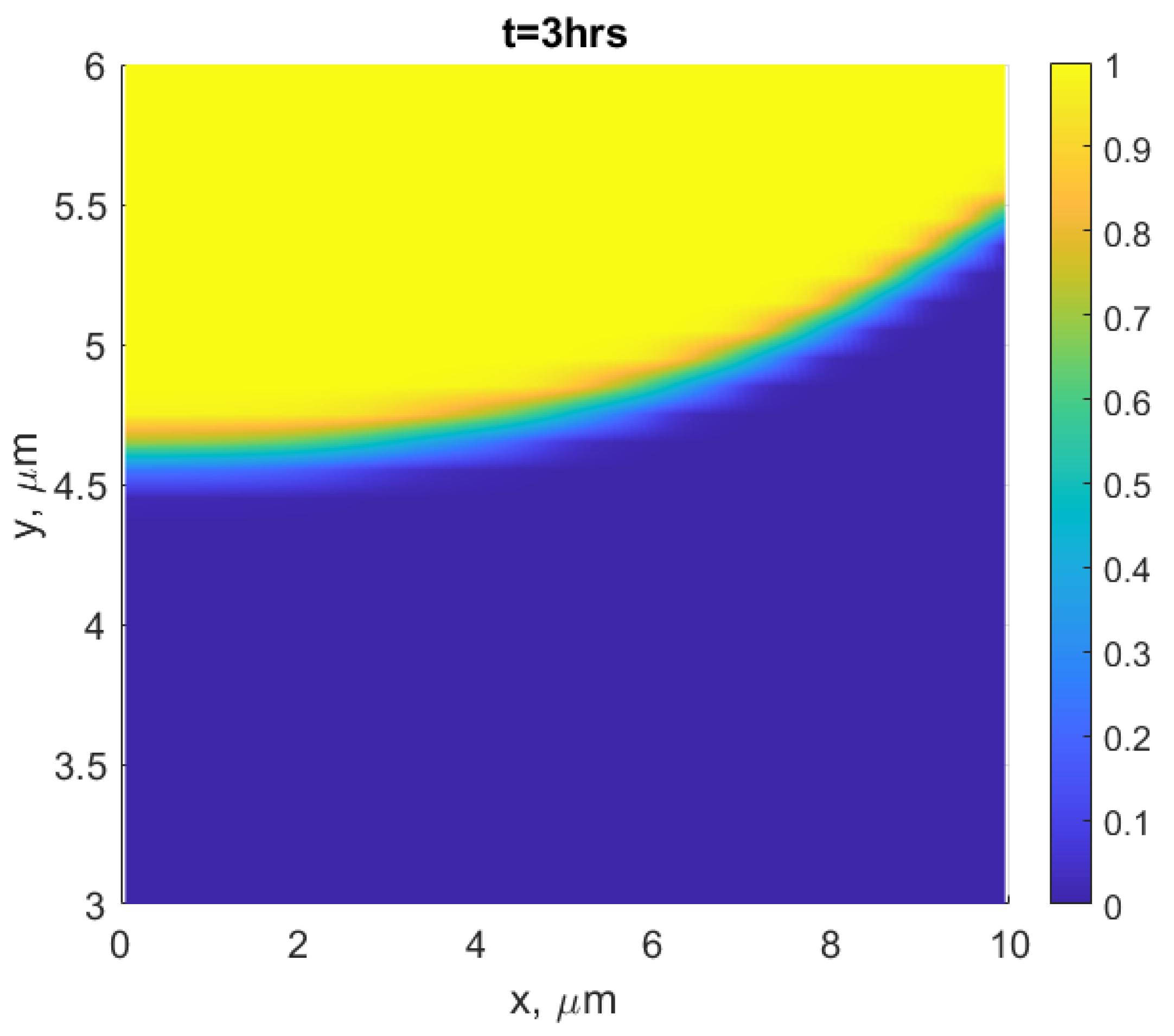
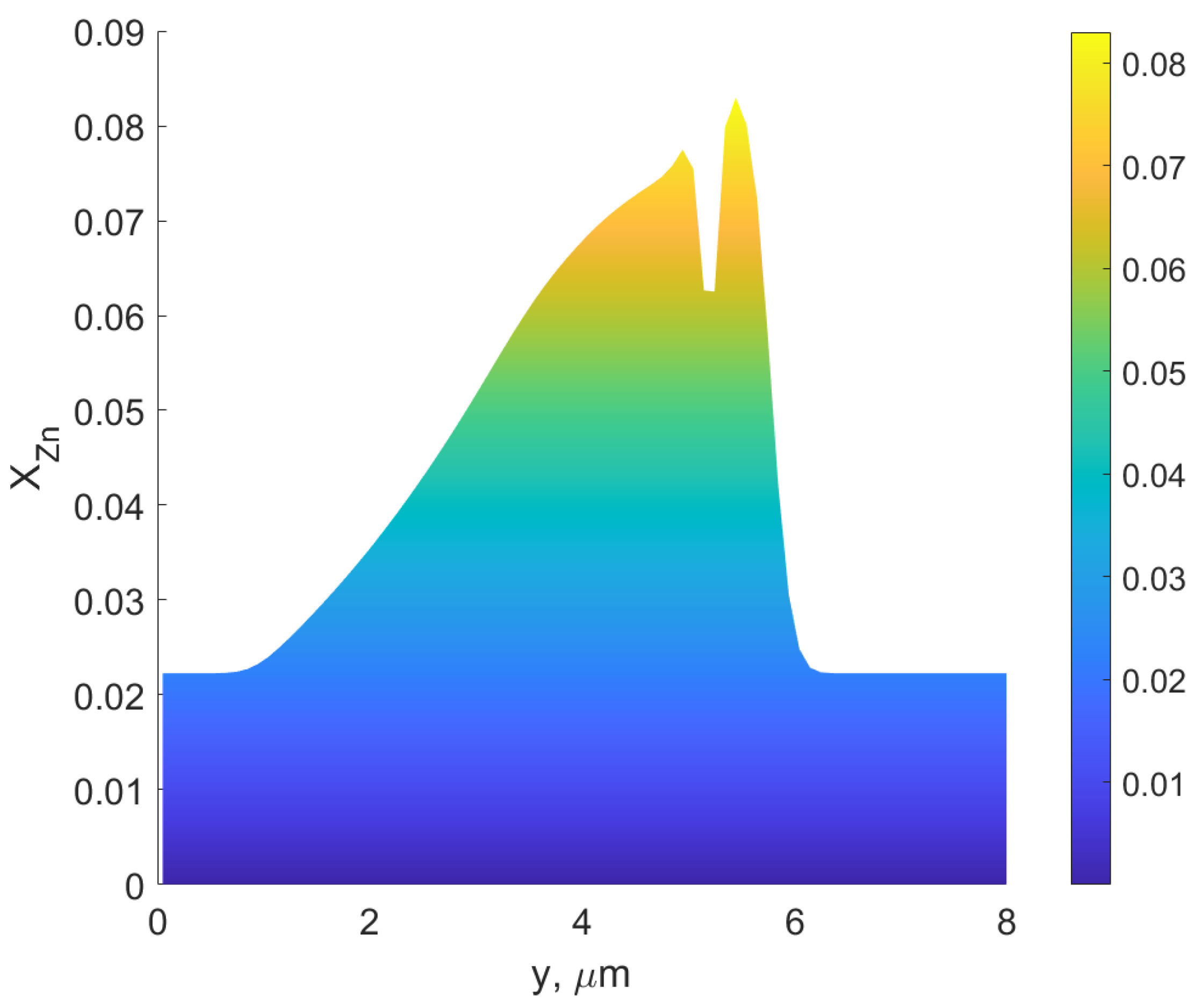
3. Results and Discussion
4. Conclusions
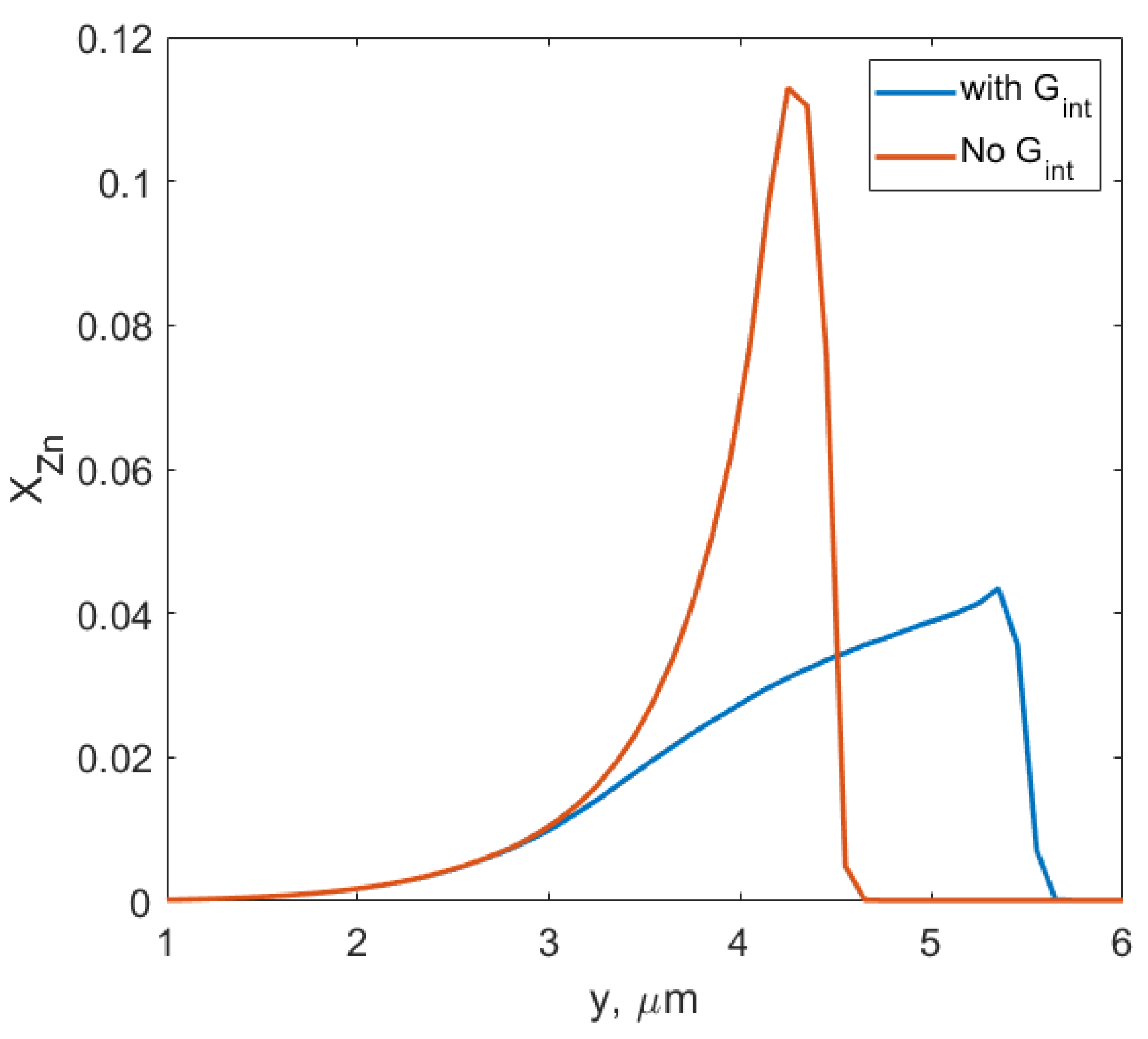
- The assumption that the driving force for DIGM comes from the coherency strain energy (), that is generated due to the concentration gradient across the interface, seems to be valid for Fe–Zn. This gradient was achieved by swift grain boundary diffusion that was controlled by the factor F, see Refs. [19,33].
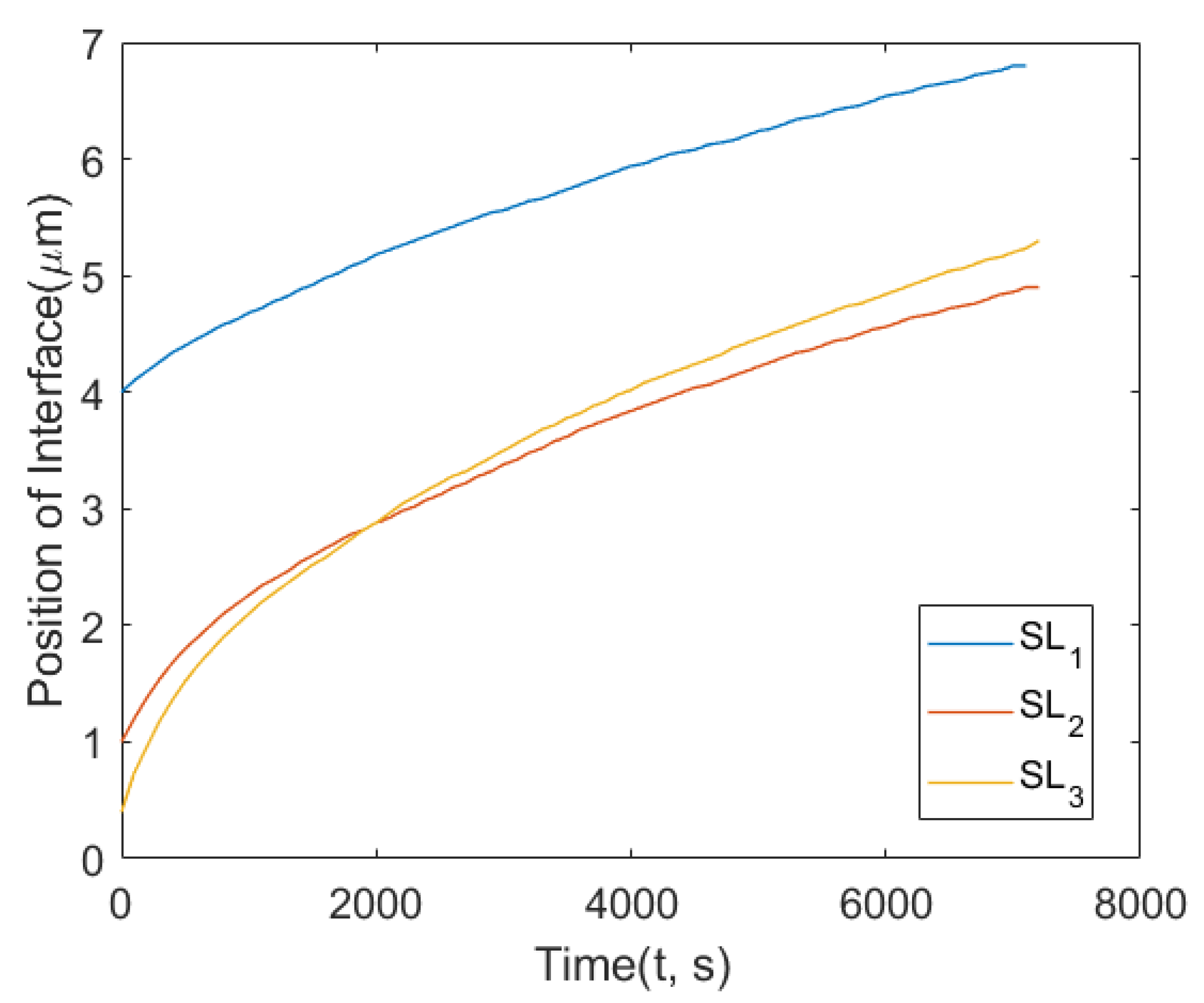
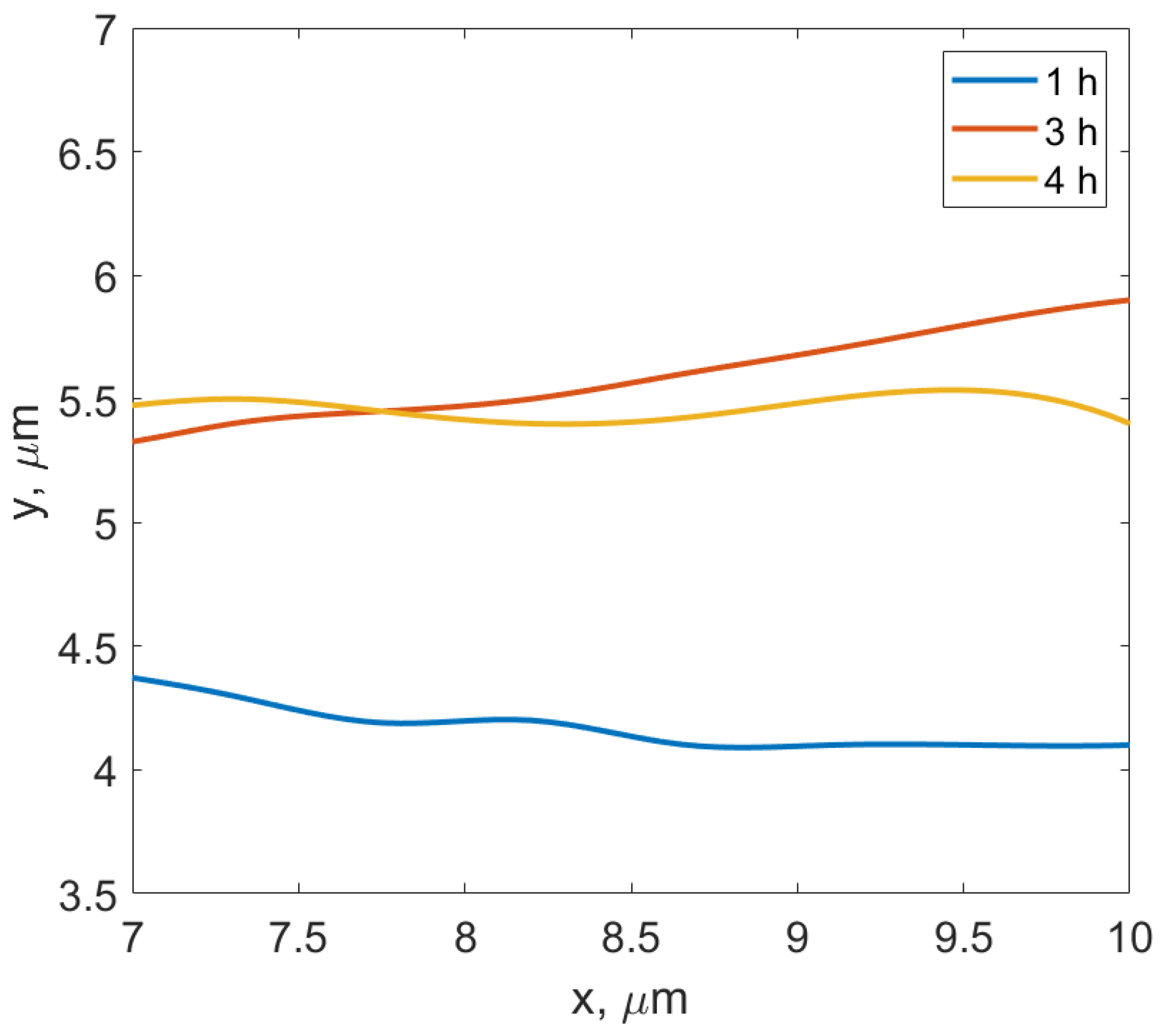
- The observed features from the experiments by Chongmo and Hillert [23], such as a distorted grain boundary at the junction of the boundary and the surface, and the oscillatory motion of the grain boundary at the surface agreed qualitatively well with the simulation results. The reason for the oscillatory motion is thought to be due to a competitive relationship between two forces, i.e., one due to the interaction term () and the other due to the curvature of the boundary. During the course of migration, these two forces either aligned, opposed, or balanced each other multiple times.
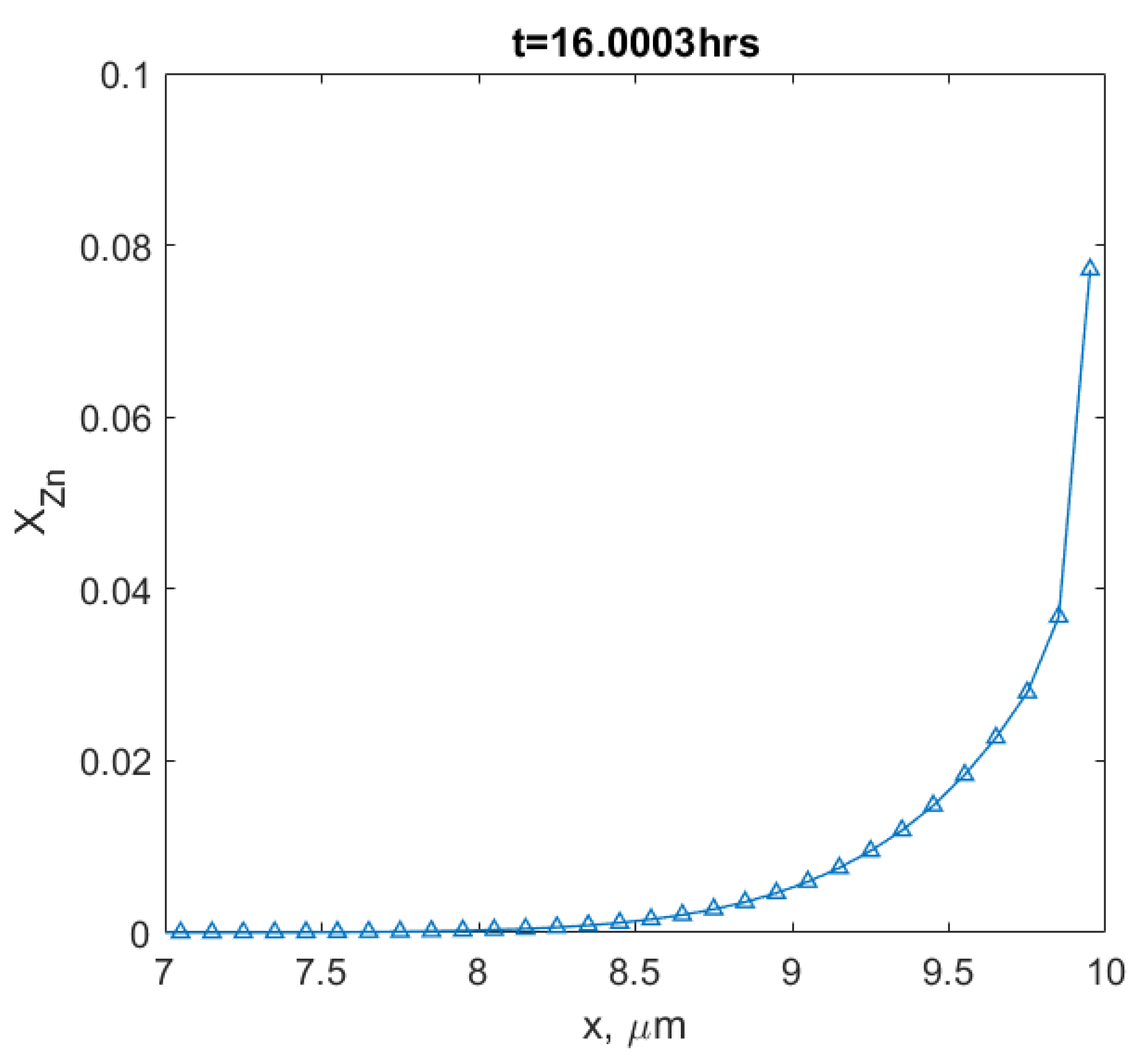
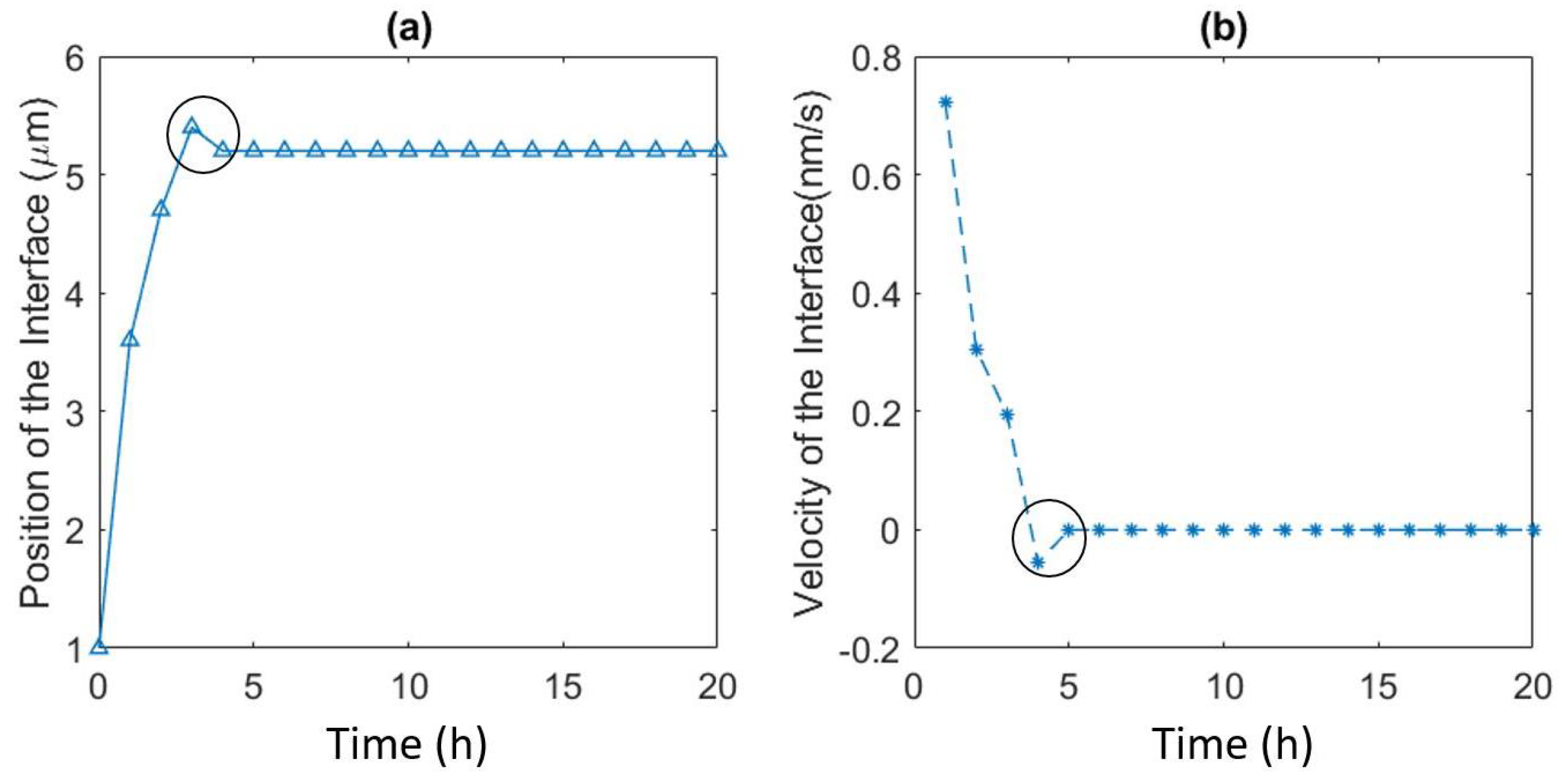
- The influence of the contact angle of a grain boundary with the surface on the velocity of the interface, and the mole fraction of Zn at the surface agreed quantitatively with the previous experiments [23]. The velocity of the interface from Chongmo and Hillert [23] was under the range of the values obtained from the simulation. It was also observed that the grain boundary with a higher angle of contact between the boundary and the surface (close to ) moved slower than a low-angled grain boundary. When evaluated, the velocity of the low-angled grain boundary was very high at the beginning of migration and then gradually dropped to follow a stop-and-go motion till it reached zero velocity.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DIGM | Diffusion Induced Grain Boundary Migration |
| DP | Discontinuous Precipitation |
References
- Smoluchowski, J.E.M.R.; Weyl, W.A. Precipitation from Solid Solutions of Metals. Phase Transformations in Solids; General Electric Company: Schenectady, NY, USA, 1951; pp. 387–545. [Google Scholar] [CrossRef]
- Turnbull, D. Theory of Cellular Precipitation. Acta 1955, 3, 55–63. [Google Scholar] [CrossRef]
- Cahn, J.W. The kinetics of cellular degregation reactions. Acta Metall. 1959, 7, 18–28. [Google Scholar] [CrossRef]
- Hillert, M. The role of interfaces in phase transformations. In The Mechanism of Phase Transformations in Crystalline Solids; Institute of Metals for the Metals & Metallurgy Trust: London, UK, 1969; Volume 33, pp. 231–247. [Google Scholar]
- Sulonen, M. On the driving force of discontinuous precipitation and dissolution. Acta Metall. 1964, 12, 749–753. [Google Scholar] [CrossRef]
- Fournelle, R.A.; Clark, J.B. The genesis of the cellular precipitation reaction. Metall. Trans. 1972, 3, 2757–2767. [Google Scholar] [CrossRef]
- Chronowski, M.; Straumal, B.; Baretzky, B.; Zieba, P. Jerky Motion of the Reaction Front during Dissolution in a Fe-13.5at.% Zn Alloy. Materials 2022, 15, 3525. [Google Scholar] [CrossRef]
- Opara, J.; Straumal, B.; Ziȩba, P. Cellular automata modelling of discontinuous precipitation. Materials 2021, 14, 4985. [Google Scholar] [CrossRef]
- Straumal, B.B.; Noskovich, O.I.; Semenov, V.N.; Shvindlerman, L.S.; Gust, W.; Predel, B. Premelting transition on 38°<100> tilt grain boundaries in (Fe-10at.% Si)-Zn alloys. Acta Metall. Et Mater. 1992, 40, 795–801. [Google Scholar] [CrossRef]
- Straumal, B.B.; Mazilkin, A.A.; Kogtenkova, O.A.; Protasova, S.G.; Baretzky, B. Grain boundary phase observed in Al-5 at.% Zn alloy by using HREM. Philos. Mag. Lett. 2007, 87, 423–430. [Google Scholar] [CrossRef]
- Hillert, M.; Purdy, G.R. Chemically Induced Grain Boundary Migration. Acta Metall. 1977, 26, 333–340. [Google Scholar] [CrossRef]
- Cahn, J.W.; Pan, J.D.; Balluffi, R.W. Diffusion induced grain boundary migration. Scr. Metall. 1979, 13, 503–509. [Google Scholar] [CrossRef]
- Hillert, M. On The Driving Force for Diffusion Induced Grain Boundary Migration. Scr. Metall. Mater. 1983, 17, 237–240. [Google Scholar] [CrossRef]
- Cahn, J.W.; Fife, P.; Penrose, O. A phase-field model for diffusion-induced grain-boundary motion. Acta Mater. 1997, 45, 4397–4413. [Google Scholar] [CrossRef]
- Penrose, O. On the elastic driving force in diffusion-induced grain boundary motion. Acta Mater. 2004, 52, 3901–3910. [Google Scholar] [CrossRef]
- King, A.H. Diffusion induced grain boundary migration in hexagonal materials. Int. Mater. Rev. 1987, 32, 173–189. [Google Scholar] [CrossRef]
- Chary, V.R.; Gupta, S.P. Diffusion induced grain boundary migration in the Ag-Zn system. Mater. Charact. 2009, 60, 1202–1213. [Google Scholar] [CrossRef]
- Bréchet, Y.J.M.; Purdy, G.R. On the possibility of DIGM-assisted abnormal grain growth. Scr. Mater. 2006, 54, 2009–2011. [Google Scholar] [CrossRef]
- Mukherjee, D.; Larsson, H.; Odqvist, J. Phase field modelling of diffusion induced grain boundary migration in binary alloys. Comput. Mater. Sci. 2020, 184, 1–9. [Google Scholar] [CrossRef]
- Cahn, J.W.; Hilliard, J.E. Free Energy of a Nonuniform System. I. Interfacial Free Energy. J. Chem. Phys. 1958, 28, 258–267. [Google Scholar] [CrossRef]
- Samuel, A.M.; Cahn, J.W. A microscopic theory for antiphase boundary motion and its application to antiphase domain coarsening. Acta Metall. 1978, 27, 1085–1095. [Google Scholar]
- Finel, A.; Le Bouar, Y.; Dabas, B.; Appolaire, B.; Yamada, Y.; Mohri, T. Sharp Phase Field Method. Phys. Rev. Lett. 2018, 121, 25501. [Google Scholar] [CrossRef] [Green Version]
- Chongmo, L.; Hillert, M. A metallographic study of diffusion-induced grain boundary migration in the Fe-Zn system. Acta Metall. 1981, 29, 1949–1960. [Google Scholar] [CrossRef]
- Feng, Z. The Lattice Parameter of Gamma Iron and Iron-Chromium Alloys. Ph.D. Thesis, Case Western Reserve University, Cleveland, OH, USA, 2015. [Google Scholar]
- Narkevicius, A.; Bucinskiene, D.; Rucinskiene, A.; Pakstas, V.; Bikulcius, G. Study on long term atmospheric corrosion of electrodeposited zinc and zinc alloys. Trans. Inst. Met. Finish. 2013, 91, 68–73. [Google Scholar] [CrossRef]
- Kaur, I.; Mishin, Y.; Gust, W. Fundamentals of Grain and Interphase Boundary Diffusion; John Wiley: Hoboken, NJ, USA, 1995; pp. 2–411. [Google Scholar]
- Loginova, I.; Odqvist, J.; Amberg, G.; Ågren, J. The phase-field approach and solute drag modeling of the transition to massive γ → α transformation in binary Fe-C alloys. Acta Mater. 2003, 51, 1327–1339. [Google Scholar] [CrossRef]
- Hillert, M.; Höglund, L. Mobility of α/γ phase interfaces in Fe alloys. Scr. Mater. 2006, 54, 1259–1263. [Google Scholar] [CrossRef]
- Thermo-Calc Software. TCFE Steels/Fe-Alloys Database, Version 12.0; Thermo-Calc Software, Inc.: McMurray, PA, USA, 2022. [Google Scholar]
- Thermo-Calc Software. TCFE Steels/Fe-Alloys Mobility, Version 7.0; Thermo-Calc Software, Inc.: McMurray, PA, USA, 2022. [Google Scholar]
- Larsson, H. The YAPFI phase-field implementation. arXiv 2021, arXiv:2104.11501. [Google Scholar]
- Wheeler, A.A.; Boettinger, W.J.; McFadden, G.B. Phase-field model for isothermal phase transitions in binary alloys. Phys. Rev. A 1992, 45, 7424–7440. [Google Scholar] [CrossRef]
- Fife, P.C.; Cahn, J.W.; Elliot, C.M. A free-boundary model for diffusion-induced grain boundary. Oxford 2001, 3, 291–336. [Google Scholar] [CrossRef] [Green Version]


| Parameters | Values |
|---|---|
| (m/J) | |
| (J/m) | |
| Mobility of Fe | |
| (mmol/J) | |
| Mobility of Zn | |
| (mmol/J) | |
| F | 200 |
| Y | 212 |
| (GPa) | |
| (m/mol) | |
| K | 10,323 |
| (J/mol) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mukherjee, D.; Larsson, H.; Odqvist, J. Simulating Diffusion Induced Grain Boundary Migration in Binary Fe–Zn. Metals 2022, 12, 1632. https://doi.org/10.3390/met12101632
Mukherjee D, Larsson H, Odqvist J. Simulating Diffusion Induced Grain Boundary Migration in Binary Fe–Zn. Metals. 2022; 12(10):1632. https://doi.org/10.3390/met12101632
Chicago/Turabian StyleMukherjee, Deepjyoti, Henrik Larsson, and Joakim Odqvist. 2022. "Simulating Diffusion Induced Grain Boundary Migration in Binary Fe–Zn" Metals 12, no. 10: 1632. https://doi.org/10.3390/met12101632
APA StyleMukherjee, D., Larsson, H., & Odqvist, J. (2022). Simulating Diffusion Induced Grain Boundary Migration in Binary Fe–Zn. Metals, 12(10), 1632. https://doi.org/10.3390/met12101632







