Coenzyme Q10 and Silymarin Reduce CCl4-Induced Oxidative Stress and Liver and Kidney Injury in Ovariectomized Rats—Implications for Protective Therapy in Chronic Liver and Kidney Diseases
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Animals and Care
2.2. Study Design and Treatment
2.3. Biochemical Assays
2.3.1. Measurement of 17β-Estradiol
2.3.2. Estimation of Lipid Peroxidation as Malondialdehyde (MDA)
2.3.3. Estimation of Nitric Oxide (NO)
2.3.4. Determination of Advanced Protein Oxidation Products (APOP)
2.3.5. Determination of GSH
2.3.6. Determination of CAT
2.3.7. Determination of SOD Level
2.3.8. Assessment of Liver Toxicity
2.4. Histopathology Procedure
2.5. Statistical Analysis
3. Results
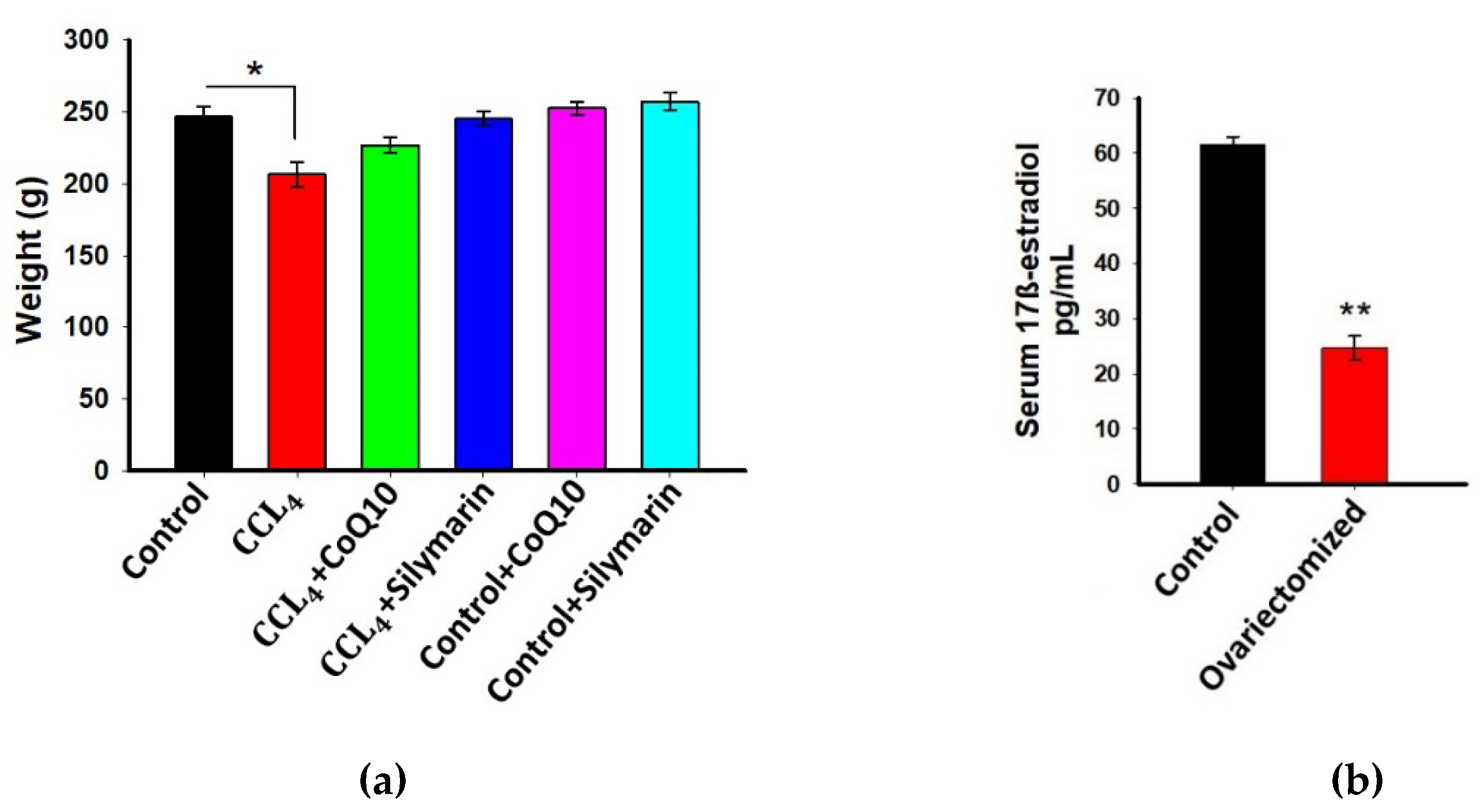
3.1. Effect of CoQ10 Supplementation on Body Weight of Ovariectomized Rats
3.2. Estimation of 17β-Estradiol in Plasma
3.3. Effect of CoQ10 Supplementation on Oxidative Stress Level in Kidney, Liver, and Plasma
3.4. Effect of CoQ10 and Silymarin Supplementation on Antioxidant Enzymes in Kidney, Liver, and Plasma of Ovariectomized Rats
3.5. Effect of CoQ10 and Silymarin Supplementation on ALP, AST, and ALT Levels in Plasma
3.6. Histopathological Observation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bae, J.H.A.; Lee, S.H.; Kim, H.J.; Lee, J.Y. The Role of Salicornia herbacea in Ovariectomy-Induced Oxidative Stress. Biol. Pharm. Bull. 2006, 29, 1305–1309. [Google Scholar]
- Jain, P.; Haque, A.; Islam, T.; Alam, M.A.; Reza, H.M. Comparative evaluation of Ziziphus mauritiana leaf extracts for phenolic content, antioxidant and antibacterial activities. J. Herbs Spices Med. Plants 2019, 25, 236–258. [Google Scholar] [CrossRef]
- Mills, K.T.; Xu, Y.; Zhang, W.; Bundy, J.D.; Chen, C.S.; Kelly, T.N. A systematic analysis of worldwide population-based data on the global burden of chronic kidney disease in 2010. Kidney Int. 2015, 88, 950–957. [Google Scholar] [CrossRef] [Green Version]
- Saha, P.; Talukdar, A.D.; Nath, R.; Sarker, S.D.; Nahar, L.; Sahu, J.; Choudhury, M.D. Role of Natural Phenolics in Hepatoprotection: A Mechanistic Review and Analysis of Regulatory Network of Associated Genes. Front. Pharmacol. 2019, 10, 509. [Google Scholar] [CrossRef]
- Adebayo, A.H.; Yakubu, O.F.; Balogun, T.M. Protective properties of Citrullus lanatus on carbon tetrachloride induced liver damage in rats. Eur. J. Med. Plants 2014, 4, 979–989. [Google Scholar] [CrossRef] [Green Version]
- Boll, M.; Lutz, W.; Becker, E.; Stampfl, A. Mechanism of carbon tetrachloride-induced hepatotoxicity. Hepatocellular damage by reactive carbon tetrachloride metabolites. Z. Naturforsch. C 2001, 56, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Arnal, J.F.; Clamens, S.; Pechet, C.; Negre-Salvayre, A.; Allera, C.; Girolami, J.P.; Salvayre, R.; Bayard, F. Ethinylestradiol does not enhance the expression of nitric oxide synthase in bovine endothelial cells but increases the release of bioactive nitric oxide by inhibiting superoxide anion production. Proc. Natl. Acad. Sci. USA 1996, 93, 4108–4113. [Google Scholar] [CrossRef] [Green Version]
- Brady, C.W. Liver disease in menopause. World J. Gastroenterol. 2015, 21, 7613–7620. [Google Scholar] [CrossRef]
- Lee, B.; Lin, Y.; Huang, Y.; Ko, Y.; Hsia, S.; Lin, P. The relationship between Coenzyme Q10, oxidative stress, and antioxidant enzymes activities and coronary artery disease. Sci. World J. 2012, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Martelli, A.; Testai, L.; Colletti, A.; Cicero, A.F. Coenzyme Q10: Clinical applications in cardiovascular diseases. Antioxidants 2020, 9, 341. [Google Scholar] [CrossRef] [Green Version]
- Farsi, F.; Heshmati, J.; Keshtkar, A.; Irandoost, P.; Alamdari, N.; Akbari, A. Can coenzyme Q10 supplementation effectively reduce human tumor necrosis factor-α and interleukin-6 levels in chronic inflammatory diseases? A systematic review and meta-analysis of randomized controlled trials. Pharmacol. Res. 2019, 148, 104290. [Google Scholar] [CrossRef]
- Razavi, B.M.; Karimi, G. Protective effect of silymarin against chemical induced cardiotoxicity. Iran J. Basic Med. Sci. 2016, 19, 916–923. [Google Scholar]
- Darvishi-Khezri, H.; Salehifar, E.; Kosaryan, M.; Karami, H.; Alipour, A.; Shaki, F.; Aliasgharian, A. The impact of silymarin on antioxidant and oxidative status in patients with β-thalassemia major: A crossover, randomized controlled trial. Complement Ther. Med. 2017, 35, 25–32. [Google Scholar] [CrossRef]
- Borah, A.; Paul, R.; Choudhury, S.; Choudhury, A.; Bhuyan, B.; Talukdar, A.D.; Choudhury, M.D.; Mohanakumar, K.P. Neuroprotective potential of Silymarin against CNS disorders: Insight into the pathways and molecular mechanisms of action. CNS Neurosci. Ther. 2013, 19, 847–853. [Google Scholar] [CrossRef]
- Vedder, H.; Anthes, N.; Stumm, G.; Werz, C.; Behl, C.; Kreig, J.C. Estrogen hormones reduce lipid peroxidation in cells and tissues of the central nervous system. J. Neurochem. 1999, 72, 2531–2538. [Google Scholar] [CrossRef]
- Ozacmak, V.H.; Sayan, H. The Effects of 17β Estradiol, 17α Estradiol and Progesterone on oxidative stress biomarkers in ovariectomized female rat brain subjected to global cerebral ischemia. Physiol. Res. 2009, 58, 909–912. [Google Scholar]
- Kii, N.; Adachi, N.; Liu, K.; Arai, T. Acute effects of 17 beta-estradiol on oxidative stress in ischemic rat striatum. J. Neurosurg. Anesthesiol. 2005, 17, 27–32. [Google Scholar]
- Ali, S. Protective Effect of L-Carnitine and Coenzyme Q10 on CCl4-induced liver injury in Rats. Sci. Pharm. 2010, 78, 881–896. [Google Scholar] [CrossRef] [Green Version]
- Mahli, A.; Koch, A.; Czech, B.; Peterburs, P.; Lechner, A.; Haunschild, J.; Müller, M.; Hellerbrand, C. Hepatoprotective effect of oral application of a silymarin extract in carbon tetrachloride-induced hepatotoxicity in rats. Clin. Phytoscience 2015, 1, 5. [Google Scholar] [CrossRef] [Green Version]
- Niehaus, W.; Samuelsson, B. Formation of malonaldehyde from phospholipid arachidonate during microsomal lipid per-oxidation. FEBS J. 1968, 6, 126–130. [Google Scholar]
- Hibbs, J.B., Jr.; Taintor, R.R.; Vavrin, Z.; Rachlin, E.M. Nitric oxide: A cytotoxic activated macrophage effector molecule. Biochem. Biophys. Res. Commun. 1988, 157, 87–94. [Google Scholar] [CrossRef]
- Witko-Sarsat, V.; Friedlander, M.; Capeillere-Blandin, C.; Nguyen-Khoa, T.; Nguyen, A.T.; Zingraff, J.; Jungers, P.; Descamps-Latscha, B. Advanced oxidation protein products as a novel marker of oxidative stress in uremia. Kidney Int. 1996, 49, 1304–1313. [Google Scholar] [CrossRef] [Green Version]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Khan, R.A. Protective effects of Sonchus asper (L.) Hill, (Asteraceae) against CCl4-induced oxidative stress in the thyroid tissue of rats. BMC Complement. Altern. Med. 2012, 12, 181. [Google Scholar] [CrossRef] [Green Version]
- Ma, L.; Liu, J.; Li, N.; Wang, J.; Duan, Y.; Yan, J.; Liu, H.; Wang, H.; Hong, F. Oxidative stress in the brain of mice caused by translocated nanoparticulate TiO2 delivered to the abdominal cavity. Biomaterials 2010, 31, 99–105. [Google Scholar] [CrossRef]
- Singal, P.K.; Beamish, R.E.; Dhalla, N.S. Potential oxidative pathways of catecholamines in the formation of lipid peroxides and genesis of heart disease. Adv. Exp. Med. Bio. 1983, 161, 91–401. [Google Scholar]
- Wattanapitayakul, S.K.; Bauer, J.A. Oxidative pathways in cardiovascular disease: Roles, mechanisms, and therapeutic implications. Pharmacol. Ther. 2001, 89, 187–206. [Google Scholar] [CrossRef]
- Popova, M.A.; Popove, C.S. Effect of chemical agents on some enzyme activities and on the stability of membrane structures. Bulg. J. Vet. Med. 2005, 8, 163–171. [Google Scholar]
- Saravanan, G.; Prakash, J. Effect of garlic (Allium sativum) on lipid peroxidation in experimental myocardial infarction in rats. J. Ethnopharmacol. 2004, 94, 155–158. [Google Scholar] [CrossRef]
- Pedraza-Chaverrí, J.; Maldonado, P.D.; Barrera, D.; Cerón, A.; Medina-Campos, O.N.; Hernández-Pando, R. Protective effect of diallyl sulfide on oxidative stress and nephrotoxicity induced by gentamicin in rats. Mol. Cell Biochem. 2003, 254, 125–130. [Google Scholar] [CrossRef]
- Lee, B.J.; Huang, Y.C.; Chen, S.J.; Lin, P.T. Coenzyme Q10 supplementation reduces oxidative stress and increases antioxidant enzyme activity in patients with coronary artery disease. Nutrition 2012, 28, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Sohet, F.M.; Neyrinck, A.M.; Pachikian, B.D.; de Backer, F.C.; Bindels, L.B.; Niklowitz, P.; Menke, T.; Cani, P.D.; Delzenne, N.M. Coenzyme Q10 supplementation lowers hepatic oxidative stress and inflammation associated with diet-induced obesity in mice. Biochem. Pharmacol. 2009, 78, 1391–1400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, R.; Cao, L.; Du, J.; Xu, P.; Jeney, G.; Yin, G. The protective effect of silymarin on the carbon tetrachloride (CCl4)-induced liver injury in common carp (Cyprinus carpio). In Vitr. Cell. Dev. Biol. Anim. 2013, 49, 155–161. [Google Scholar] [CrossRef]
- Yoshikawa, T.; Furukawa, Y.; Wakamatsu, Y.; Nishida, K.; Takemura, S.; Tanaka, H.; Kondo, M. The protection of coenzyme Q10 against carbon tetrachloride hepatotoxicity. Gastroenterol. Jpn. 1981, 16, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Tsai, J.H.; Liu, J.Y.; Wu, T.T.; Ho, P.C.; Huang, C.Y.; Shyu, J.C.; Hsieh, Y.S.; Tsai, C.C.; Liu, Y.C. Effects of silymarin on the resolution of liver fibrosis induced by carbon tetrachloride in rats. J. Viral Hepat. 2008, 15, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Clichici, S.; Olteanu, D.; Filip, A.; Nagy, A.L.; Oros, A.; Mircea, P.A. Beneficial Effects of Silymarin After the Discontinuation of CCl4-Induced Liver Fibrosis. J. Med. Food 2016, 19, 789–797. [Google Scholar] [CrossRef] [PubMed]
- Cave, M.; Falkner, K.C.; McClain, C.J. Zakim and Boyer’s Hepatology: A Textbook of Liver Disease; Elsevier Saunders: Philadelphia, PA, USA, 2011; pp. 476–492. [Google Scholar]
- Renner, H. The limited relevance of models used for testing human hepatic diseases and their prevention. In Mechanisms of Hepatocyte Injury and Death; Keppler, E., Popper, H., Bianchi, L., Reutter, W., Eds.; MTP Press Ltd.: Lancaster, PA, USA, 1985; pp. 311–320. [Google Scholar]
- Shah, G.H.; Patel, B.G.; Shah, G.B. Development of carbon tetrachloride-induced chronic hepatotoxicity model in rats and its application in evaluation of hepatoprotective activity of silymarin. Asian J. Pharm Clin. Res. 2017, 10, 274–278. [Google Scholar] [CrossRef] [Green Version]
- Bigoniya, P.; Singh, C.S.; Shukla, A. A comprehensive review of different liver toxicants used in experimental pharmacology. Int. J. Pharm. Sci. Drug Res. 2009, 1, 124–135. [Google Scholar]
- Carocho, M.; Ferreira, I.C. A review on antioxidants, prooxidants and related controversy: Natural and synthetic compounds, screening and analysis methodologies and future perspectives. Food Chem. Toxicol. 2013, 51, 15–25. [Google Scholar] [CrossRef]
- Muraki, A.; Miyashita, K.; Mitsuishi, M.; Tamaki, M.; Tanaka, K.; Itoh, H. Coenzyme Q10 reverses mitochondrial dysfunction in atorvastatin-treated mice and increases exercise endurance. J. Appl. Physiol. 2012, 13, 479–486. [Google Scholar] [CrossRef] [Green Version]
- Arsalane, K.; Dubois, C.M.; Muanza, T.; Begin, R.; Boudreau, F.; Asselin, C.; Cantin, A.M. Transforming growth factor-beta1 is a potent inhibitor of glutathione synthesis in the lung epithelial cell line A549: Transcriptional effect on the GSH rate-limiting enzyme gamma-glutamylcysteine synthetase. Am. J. Respir. Cell Mol. Biol. 1997, 17, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Kayanoki, Y.; Fujii, J.; Suzuki, K.; Kawata, S.; Matsuzawa, Y.; Taniguchi, N. Suppression of antioxidative enzyme expression by transforming growth factorbeta 1 in rat hepatocytes. J. Biol. Chem. 1994, 269, 15488–15492. [Google Scholar] [CrossRef]
- White, A.C.; Das, S.K.; Fanburg, B.L. Reduction of glutathione is associated with growth restriction and enlargement of bovine pulmonary artery endothelial cells produced by transforming growth factor-beta 1. Am. J. Respir. Cell Mol. Biol. 1992, 6, 364–368. [Google Scholar] [CrossRef]
- Bakin, A.V.; Stourman, N.V.; Sekhar, K.R.; Rinehart, C.; Yan, X.; Meredith, M.J.; Arteaga, C.L.; Freeman, M.L. Smad3-ATF3 signaling mediates TGF-beta suppression of genes encoding phase II detoxifying proteins. Free Radic. Biol. Med. 2005, 38, 375–387. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Pokharel, Y.R.; Lim, S.C.; Han, H.; Ryu, C.S.; Kim, S.K.; Kwak, M.K.; Kang, K.W. Inhibition of liver fibrosis by solubilized coenzyme Q10: Role of Nrf2 activation in inhibiting transforming growth factor-beta1 expression. Toxicol. Appl. Pharmacol. 2009, 240, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Li, C.C.; Hsiang, C.Y.; Wu, S.L.; Ho, T.Y. Identification of novel mechanisms of silymarin on the carbon tetrachloride-induced liver fibrosis in mice by nuclear factor-kappaB bioluminescent imaging-guided transcriptomic analysis. Food Chem. Toxicol. 2012, 50, 1568–1575. [Google Scholar] [CrossRef] [PubMed]
- Gharagozloo, M.; Velardi, E.; Bruscoli, S.; Agostini, M.; Di Sante, M.; Donato, V.; Amirghofran, Z.; Riccardi, C. Silymarin suppress CD4+ T cell activation and proliferation: Effects on NF-kappaB activity and IL-2 production. Pharmacol. Res. 2010, 61, 405–409. [Google Scholar] [CrossRef]
- Jeong, D.; Lee, G.; Jeong, W.; Do, S.; Yang, H.; Yuan, D.; Park, H.; Kim, K.; Jeong, K. Alterations of mast cells and TGF-β1 on the silymarin treatment for CCl4-induced hepatic fibrosis. World J. Gastroenterol. 2005, 28, 1141–1148. [Google Scholar] [CrossRef]
- Eraky, S.M.; El-Mesery, M.; El-Karef, A.; Eissa, L.A.; El-Gayar, A.M. Silymarin and caffeine combination ameliorates experimentally-induced hepatic fibrosis through down-regulation of LPAR1 expression. Biomed. Pharmacother. 2018, 101, 49–57. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lamia, S.S.; Emran, T.; Rikta, J.K.; Chowdhury, N.I.; Sarker, M.; Jain, P.; Islam, T.; Gias, Z.T.; Shill, M.C.; Reza, H.M. Coenzyme Q10 and Silymarin Reduce CCl4-Induced Oxidative Stress and Liver and Kidney Injury in Ovariectomized Rats—Implications for Protective Therapy in Chronic Liver and Kidney Diseases. Pathophysiology 2021, 28, 50-63. https://doi.org/10.3390/pathophysiology28010005
Lamia SS, Emran T, Rikta JK, Chowdhury NI, Sarker M, Jain P, Islam T, Gias ZT, Shill MC, Reza HM. Coenzyme Q10 and Silymarin Reduce CCl4-Induced Oxidative Stress and Liver and Kidney Injury in Ovariectomized Rats—Implications for Protective Therapy in Chronic Liver and Kidney Diseases. Pathophysiology. 2021; 28(1):50-63. https://doi.org/10.3390/pathophysiology28010005
Chicago/Turabian StyleLamia, Samanta Sifat, Tushar Emran, Jubaida Khatun Rikta, Nowreen Islam Chowdhury, Manoneeta Sarker, Preeti Jain, Tabinda Islam, Zarin Tasnim Gias, Manik Chandra Shill, and Hasan Mahmud Reza. 2021. "Coenzyme Q10 and Silymarin Reduce CCl4-Induced Oxidative Stress and Liver and Kidney Injury in Ovariectomized Rats—Implications for Protective Therapy in Chronic Liver and Kidney Diseases" Pathophysiology 28, no. 1: 50-63. https://doi.org/10.3390/pathophysiology28010005
APA StyleLamia, S. S., Emran, T., Rikta, J. K., Chowdhury, N. I., Sarker, M., Jain, P., Islam, T., Gias, Z. T., Shill, M. C., & Reza, H. M. (2021). Coenzyme Q10 and Silymarin Reduce CCl4-Induced Oxidative Stress and Liver and Kidney Injury in Ovariectomized Rats—Implications for Protective Therapy in Chronic Liver and Kidney Diseases. Pathophysiology, 28(1), 50-63. https://doi.org/10.3390/pathophysiology28010005








