Transthoracic Echocardiography-Based Prediction Model of Adverse Event Risk in Patients with COVID-19
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Population Profile
2.2. Echocardiographic Analysis
2.3. Reproducibility
2.4. Statistical Analysis
3. Results
3.1. Echocardiographic Analysis
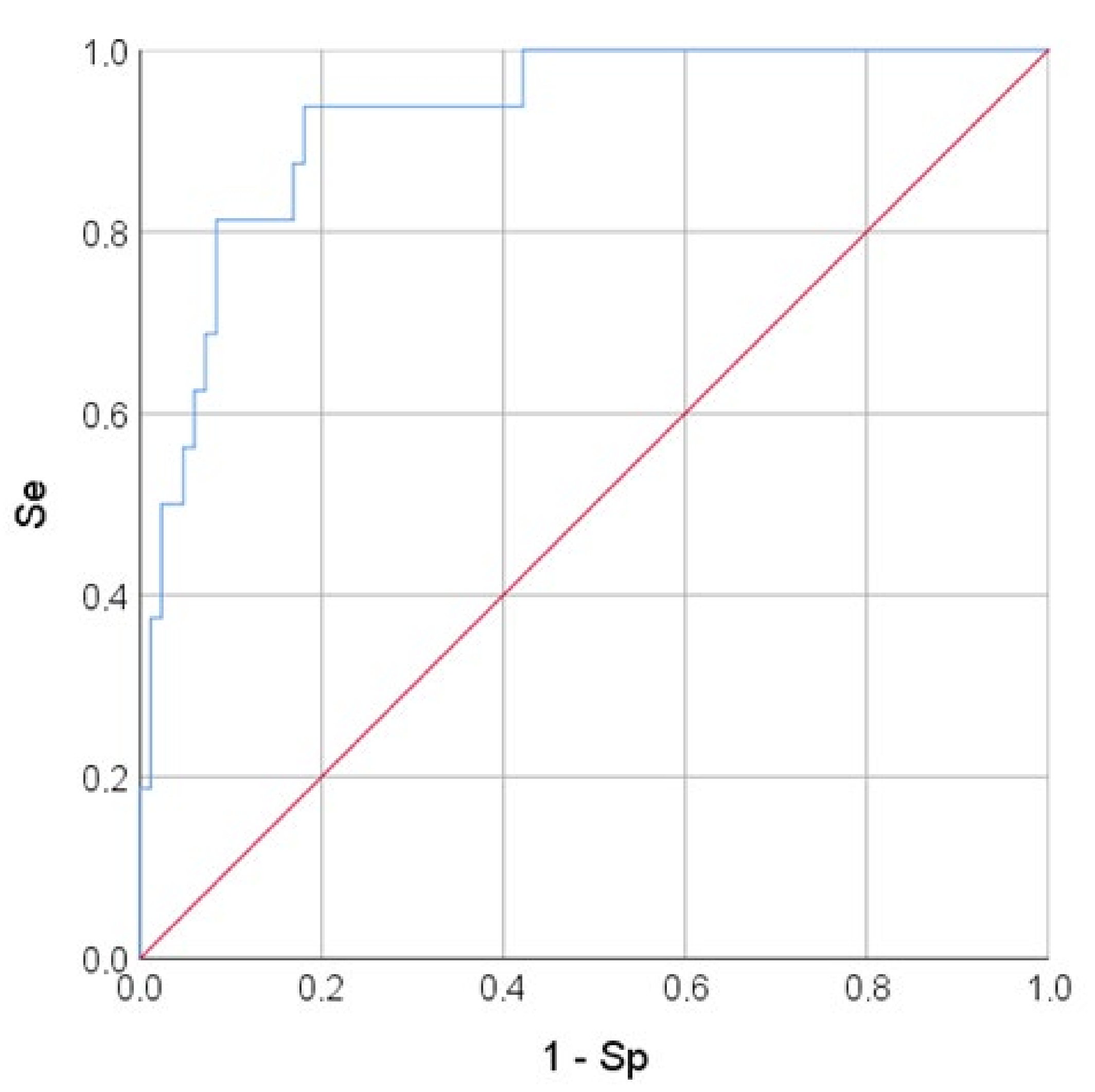
3.2. Logistic Regression Analysis and Prognostic Model Quality Assessment
3.3. Assessment of Reproducibility
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| TTE | transthoracic echocardiography |
| AUC | area under the curve |
| ROC | receiver operating characteristic |
| RV | right ventricle |
| LV | left ventricle |
| OR | odds ratio |
| sPAP | systolic pulmonary artery pressure |
| RV FW LS | right ventricle free wall longitudinal strain |
| 95% CI | 95% confidence interval |
| Se | sensitivity |
| Sp | specificity |
| ARDS | acute respiratory distress syndrome |
| CT | computed tomography |
| IQR | interquartile range |
| ICU | intensive care unit |
| COPD | chronic obstructive pulmonary disease |
| ASE | American Society of Echocardiography |
| EACVI | European Association of Cardiovascular Imaging |
| WHO | World Health Organization |
| TR | tricuspid regurgitation |
| LA | left atrium |
| Vol | volume |
| TDI | tissue Doppler imaging |
| FAC | fractional area change |
| EDA | end-diastolic area |
| ESA | end-systolic area |
| TAPSE | tricuspid annular plane systolic excursion |
| 2D STE | two-dimensional speckle-tracking echocardiography |
| PW | pulse-wave |
| RA | right atrium |
| MeanPAP | mean pulmonary artery pressure |
| BSA | body surface area |
| NEWS | patient severity rating scale |
| SpO2 | blood oxygen saturation |
| EDI | end-diastolic volume index |
| ESI | end-systolic volume index |
| SI | stroke index |
| EF | ejection fraction |
| CI | cardiac index |
| ICC | intra-class correlation coefficient |
| AIRVO | humidifier with integrated air flow generator |
| VV ECMO | veno-venous extracorporeal membrane oxygenation |
| PA | pulmonary artery |
| PH | pulmonary hypertension |
| 95% CI | 95% confidence interval |
References
- Capotosto, L.; Nguyen, B.L.; Ciardi, M.R.; Mastroianni, C.; Vitarelli, A. Heart, COVID-19, and echocardiography. Echocardiography 2020, 37, 1454–1464. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Mesa, J.E.; Galindo-Coral, S.; Montes, M.C.; Muñoz Martin, A.J. Thrombosis and Coagulopathy in COVID-19. Curr. Probl. Cardiol. 2021, 46, 100742. [Google Scholar] [CrossRef] [PubMed]
- Bourgonje, A.R.; Abdulle, A.E.; Timens, W.; Hillebrands, J.L.; Navis, G.J.; Gordijn, S.J.; Bolling, M.C.; Dijkstra, G.; Voors, A.A.; Osterhaus, A.D.; et al. Angiotensin-converting enzyme 2 (ACE2), SARS-CoV-2 and the pathophysiology of coronavirus disease 2019 (COVID-19). J. Pathol. 2020, 251, 228–248. [Google Scholar] [CrossRef] [PubMed]
- Clerkin, K.J.; Fried, J.A.; Raikhelkar, J.; Sayer, G.; Griffin, J.M.; Masoumi, A.; Jain, S.S.; Burkhoff, D.; Kumaraiah, D.; Rabbani, L.R.; et al. COVID-19 and Cardiovascular Disease. Circulation 2020, 141, 1648–1655. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.; Shi, L.; Wang, Y.; Zhang, J.; Huang, L.; Zhang, C.; Liu, S.; Zhao, P.; Liu, H.; Zhu, L.; et al. Pathological findings of COVID-19 associated with acute respiratory distress. Lancet Respir. Med. 2020, 8, 420–422. [Google Scholar] [CrossRef]
- Wu, C.; Chen, X.; Cai, Y.; Xia, J.; Zhou, X.; Xu, S.; Huang, H.; Zhang, L.; Zhou, X.; Du, C.; et al. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern. Med. 2020, 180, 934–943. [Google Scholar] [CrossRef] [Green Version]
- Inciardi, R.M.; Adamo, M.; Lupi, L.; Cani, D.S.; Di Pasquale, M.; Tomasoni, D.; Italia, L.; Zaccone, G.; Tedino, C.; Fabbricatore, D.; et al. Characteristics and outcomes of patients hospitalized for COVID-19 and cardiac disease in Northern Italy. Eur. Heart J. 2020, 41, 1821–1829. [Google Scholar] [CrossRef]
- Lairez, O.; Blanchard, V.; Houard, V.; Vardon-Bounes, F.; Lemasle, M.; Cariou, E.; Lavie-Badie, Y.; Ruiz, S.; Cazalbou, S.; Delmas, C.; et al. Cardiac imaging phenotype in patients with coronavirus disease 2019 (COVID-19): Results of the cocarde study. Int. J. Cardiovasc. Imaging 2021, 37, 449–457. [Google Scholar] [CrossRef]
- Chu, D.K.; Akl, E.A.; Duda, S.; Solo, K.; Yaacoub, S.; Schünemann, H.J.; El-harakeh, A.; Bognanni, A.; Lotfi, T.; Loeb, M.; et al. Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: A systematic review and meta-analysis. Lancet 2020, 395, 1973–1987. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–270. [Google Scholar] [CrossRef]
- McFee, R. COVID-19 medical management including World Health Organization (WHO) suggested management strategies. Dis. Mon. 2020, 66, 101068. [Google Scholar] [CrossRef] [PubMed]
- Kirkpatrick, J.N.; Mitchell, C.; Taub, C.; Kort, S.; Hung, J.; Swaminathan, M. ASE Statement on Protection of Patients and Echocardiography Service Providers During the 2019 Novel Coronavirus Outbreak: Endorsed by the American College of Cardiology. J. Am. Soc. Echocardiogr. 2020, 33, 648–653. [Google Scholar] [CrossRef] [PubMed]
- World Federation for Ultrasound in Medicine and Biology Safety Committee; Abramowicz, J.S.; Basseal, J.M. World Federation for Ultrasound in Medicine and Biology Position Statement: How to Perform a Safe Ultrasound Examination and Clean Equipment in the Context of COVID-19. Ultrasound Med. Biol. 2020, 46, 1821–1826. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, C.; Collins, K.; Hua, L.; McClanahan, C.; Shea, E.; Umland, M.; Wasserman, M. Specific Considerations for Sonographers When Performing Echocardiography during the 2019 Novel Coronavirus Outbreak: Supplement to the American Society of Echocardiography Statement. J. Am. Soc. Echocardiogr. 2020, 33, 654–657. [Google Scholar] [CrossRef] [PubMed]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F.; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2016, 29, 277–314. [Google Scholar] [CrossRef] [Green Version]
- Rudski, L.G.; Lai, W.W.; Afilalo, J.; Hua, L.; Handschumacher, M.D.; Chandrasekaran, K.; Solomon, S.D.; Louie, E.K.; Schiller, N.B. Guidelines for the echocardiographic assessment of the right heart in adults: A report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J. Am. Soc. Echocardiogr. 2010, 23, 685–788. [Google Scholar] [CrossRef]
- Abbas, A.E.; Fortuin, F.D.; Schiller, N.B.; Appleton, C.P.; Moreno, C.A.; Lester, S.J. Echocardiographic determination of mean pulmonary artery pressure. Am. J. Cardiol. 2003, 92, 1373–1376. [Google Scholar] [CrossRef]
- Elrobaa, I.H.; New, K.J. COVID-19: Pulmonary and Extra Pulmonary Manifestations. Front. Public Health 2021, 9, 711616. [Google Scholar] [CrossRef]
- Zaim, S.; Chong, J.H.; Sankaranarayanan, V.; Harky, A. COVID-19 and Multiorgan Response. Curr. Probl. Cardiol. 2020, 45, 100618. [Google Scholar] [CrossRef]
- Huang, D.; Yang, H.; Yu, H.; Wang, T.; Yao, R.; Liang, Z. A novel risk score to predict cardiovascular complications in patients with coronavirus disease 2019 (COVID-19): A retrospective, multicenter, observational study. Immun. Inflamm. Dis. 2020, 8, 638–649. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Zhao, M.; Wang, M.; Zhang, J.; Gu, J.; Zhang, P.; Xu, Y.; Ye, J.; Wang, Z.; Ye, D.; Pan, W.; et al. Comparison of clinical characteristics and outcomes of patients with coronavirus disease 2019 at different ages. Aging 2020, 12, 10070–10086. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, L. SARS-CoV-2: Virus dynamics and host response. Lancet Infect. Dis. 2020, 20, 515–516. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA 2020, 323, 1061–1069. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Koennecke, H.-C.; Belz, W.; Berfelde, D.; Endres, M.; Fitzek, S.; Hamilton, F.; Kreitsch, P.; Mackert, B.M.; Nabavi, D.G.; Nolte, C.; et al. Factors influencing in-hospital mortality and morbidity in patients treated on a stroke unit. Neurology 2011, 77, 965–972. [Google Scholar] [CrossRef] [PubMed]
- Bhatla, A.; Mayer, M.M.; Adusumalli, S.; Hyman, M.C.; Oh, E.; Tierney, A.; Moss, J.; Chahal, A.A.; Anesi, G.; Denduluri, S.; et al. COVID-19 and cardiac arrhythmias. Heart Rhythm. 2020, 17, 1439–1444. [Google Scholar] [CrossRef]
- Tajbakhsh, A.; Gheibi Hayat, S.M.; Taghizadeh, H.; Akbari, A.; Inabadi, M.; Savardashtaki, A.; Johnston, T.P.; Sahebkar, A. COVID-19 and cardiac injury: Clinical manifestations, biomarkers, mechanisms, diagnosis, treatment, and follow up. Expert. Rev. Anti Infect. Ther. 2021, 19, 345–357. [Google Scholar] [CrossRef]
- Cresti, A.; Barchitta, A.; Barbieri, A.; Monte, I.P.; Trocino, G.; Ciampi, Q.; Miceli, S.; Petrella, L.; Jaric, E.; Solari, M.; et al. Echocardiography and Multimodality Cardiac Imaging in COVID-19 Patients. J. Cardiovasc. Echogr. 2020, 30 (Suppl. 2), S18–S24. [Google Scholar] [CrossRef]
- Cameli, M.; Pastore, M.C.; Aboumarie, H.S.; Mandoli, G.E.; D’Ascenzi, F.; Cameli, P.; Bigio, E.; Franchi, F.; Mondillo, S.; Valente, S. Usefulness of echocardiography to detect cardiac involvement in COVID-19 patients. Echocardiography 2020, 37, 1278–1286. [Google Scholar] [CrossRef]
- Kim, J.; Volodarskiy, A.; Sultana, R.; Pollie, M.P.; Yum, B.; Nambiar, L.; Tafreshi, R.; Mitlak, H.W.; RoyChoudhury, A.; Horn, E.M.; et al. Prognostic Utility of Right Ventricular Remodeling Over Conventional Risk Stratification in Patients With COVID-19. J. Am. Coll. Cardiol. 2020, 76, 1965–1977. [Google Scholar] [CrossRef] [PubMed]
- Pagnesi, M.; Baldetti, L.; Beneduce, A.; Calvo, F.; Gramegna, M.; Pazzanese, V.; Ingallina, G.; Napolano, A.; Finazzi, R.; Ruggeri, A.; et al. Pulmonary hypertension and right ventricular involvement in hospitalised patients with COVID-19. Heart 2020, 106, 1324–1331. [Google Scholar] [CrossRef] [PubMed]
- Bleakley, C.; Singh, S.; Garfield, B.; Morosin, M.; Surkova, E.; Mandalia, M.S.; Dias, B.; Androulakis, E.; Price, L.C.; McCabe, C.; et al. Right ventricular dysfunction in critically ill COVID-19 ARDS. Int. J. Cardiol. 2021, 327, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Martha, J.W.; Pranata, R.; Wibowo, A.; Lim, M.A. Tricuspid annular plane systolic excursion (TAPSE) measured by echocardiography and mortality in COVID-19: A systematic review and meta-analysis. Int. J. Infect. Dis. 2021, 105, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Carluccio, E.; Biagioli, P.; Alunni, G.; Murrone, A.; Zuchi, C.; Coiro, S.; Riccini, C.; Mengoni, A.; D’Antonio, A.; Ambrosio, G. Prognostic Value of Right Ventricular Dysfunction in Heart Failure With Reduced Ejection Fraction: Superiority of Longitudinal Strain Over Tricuspid Annular Plane Systolic Excursion. Circ. Cardiovasc. Imaging 2018, 11, e006894. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Li, H.; Zhu, S.; Xie, Y.; Wang, B.; He, L.; Zhang, D.; Zhang, Y.; Yuan, H.; Wu, C.; et al. Prognostic Value of Right Ventricular Longitudinal Strain in Patients With COVID-19. JACC Cardiovasc. Imaging 2020, 13, 2287–2299. [Google Scholar] [CrossRef]
- Longobardo, L.; Suma, V.; Jain, R.; Carerj, S.; Zito, C.; Zwicke, D.L.; Khandheria, B.K. Role of Two-Dimensional Speckle-Tracking Echocardiography Strain in the Assessment of Right Ventricular Systolic Function and Comparison with Conventional Parameters. J. Am. Soc. Echocardiogr. 2017, 30, 937–946.e6. [Google Scholar] [CrossRef]
- Wibowo, A.; Pranata, R.; Astuti, A.; Tiksnadi, B.B.; Martanto, E.; Martha, J.W.; Purnomowati, A.; Akbar, M.R. Left and right ventricular longitudinal strains are associated with poor outcome in COVID-19: A systematic review and meta-analysis. J. Intensive Care 2021, 9, 9. [Google Scholar] [CrossRef]
- Prihadi, E.A.; van der Bijl, P.; Dietz, M.; Abou, R.; Vollema, E.M.; Marsan, N.A.; Delgado, V.; Bax, J.J. Prognostic Implications of Right Ventricular Free Wall Longitudinal Strain in Patients With Significant Functional Tricuspid Regurgitation. Circ. Cardiovasc. Imaging 2019, 12, e008666. [Google Scholar] [CrossRef] [Green Version]
- Argulian, E.; Sud, K.; Vogel, B.; Bohra, C.; Garg, V.P.; Talebi, S.; Lerakis, S.; Narula, J. Right Ventricular Dilation in Hospitalized Patients With COVID-19 Infection. JACC Cardiovasc. Imaging 2020, 13, 2459–2461. [Google Scholar] [CrossRef]
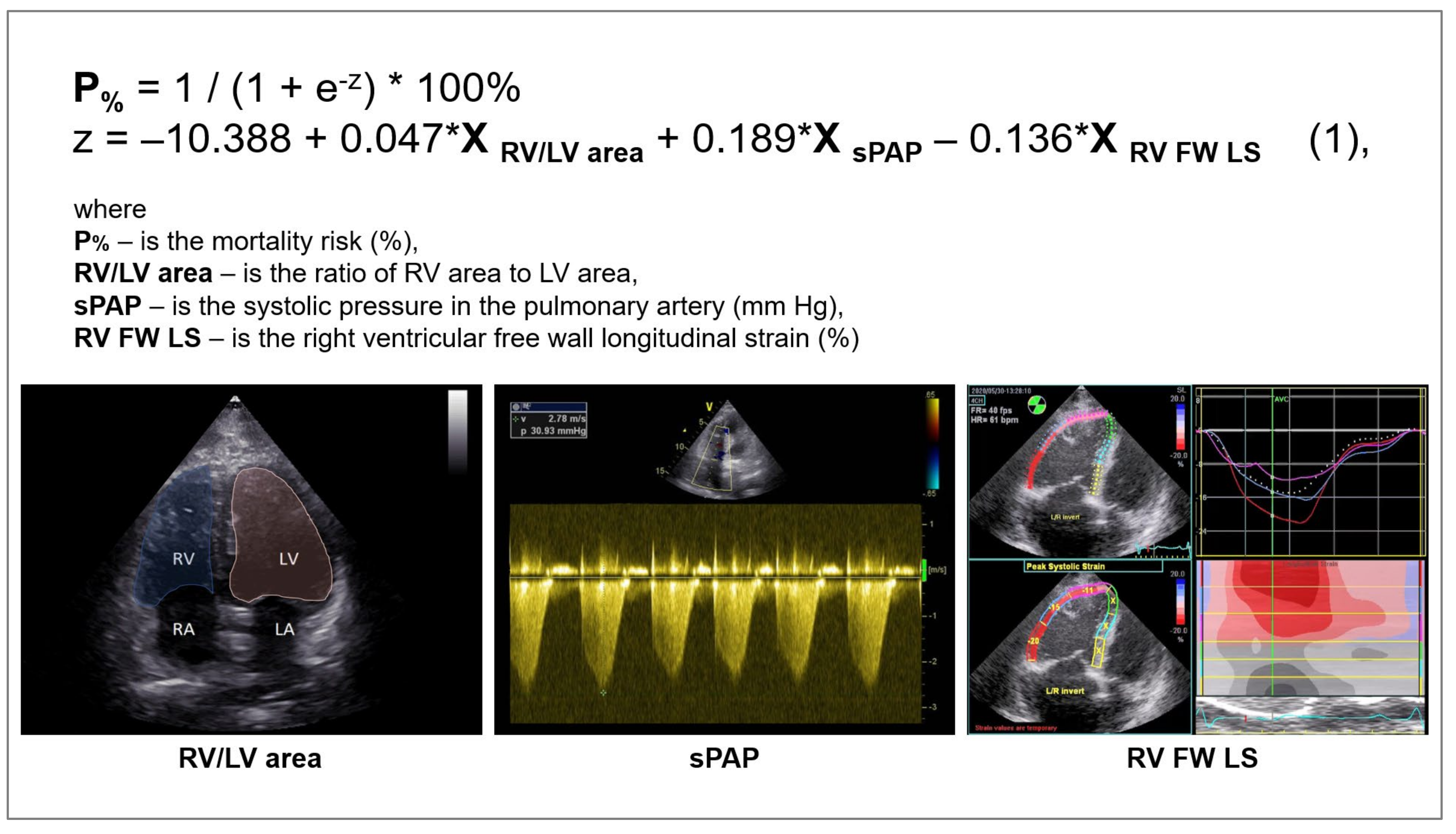
| Variables | Overall (n = 110) | |
|---|---|---|
| Age, years | 63 [51; 74] | |
| Male | 63 (57.3%) | |
| BSA, m/m2 | 2.01 [1.85; 2.13] | |
| Rhythm | Sinus | 89 (80.9%) |
| Atrial fibrillation | 18 (16.4%) | |
| Pacemaker (without atrial fibrillation) | 3 (2.7%) | |
| NEWS, scores | 6 [5; 7] | |
| SpO2 on admission to the COVID-19 hospital, % | 92 [91; 93] | |
| Comorbidities | ||
| Hypertension | 81 (73.6%) | |
| Diabetes mellitus | 20 (18.2%) | |
| Cancer | 18 (16.4%) | |
| Encephalopathy at admission | 15 (13.6%) | |
| Stroke or transient ischemic attack | 14 (12.7%) | |
| COPD | 12 (10.9%) | |
| Bronchial asthma | 9 (8.2%) | |
| Chronic kidney disease | 8 (7.3%) | |
| Rheumatoid arthritis | 3 (2.7%) | |
| Current smoking | 4 (3.6%) | |
| TTE data | ||
| Maximum LA Vol (i), mL/m2 | 22.3 [19.1; 28.8] | |
| LV EDI, mL/m2 | 49.6 [43.2; 58.1] | |
| LV ESI, mL/m2 | 17.1 [14.3; 22.3] | |
| LV SI, mL/m2 | 2.41 [2.00; 3.25] | |
| LV EF, % | 65 [60; 68] | |
| CI, L/min/m2 | 2.44 [2.01; 3.20] | |
| E/A | 1.00 [0.80; 1.28] | |
| E/e′ | 7.72 [6.15; 9.80] | |
| Maximum RA Vol (i), mL/m2 | 26.2 [19.9; 35.1] | |
| Basal RV diameter, mm | 40 [37; 43] | |
| Mid cavitary RV diameter, mm | 35 [30; 39] | |
| RV longitudinal dimension, mm | 60 [57; 66] | |
| RV/LV area | 0.64 [0.55; 0.74] | |
| RV FAC, % | 52.4 [45.1; 58.7] | |
| TAPSE, mm | 20 [18; 22] | |
| Tricuspid annular S′ wave, cm/s (PW) | 13 [11; 15] | |
| RV FW LS, % (2D STE) | 21.7 [16.2; 25.0] | |
| sPAP, mm Hg | 35 [30; 43] | |
| Variables | Survivors (n = 93) | Non-Survivors (n = 17) | p-Value |
|---|---|---|---|
| Age, years | 62 [50; 73] | 72 [60; 82] | 0.046 |
| Male | 53 (57.0%) | 10 (58.8%) | 1.000 |
| BSA, m/m2 | 1.99 [1.87; 2.10] | 2.03 [1.84; 2.18] | 0.738 |
| Sinus | 79 (84.9%) | 10 (58.8%) | 0.019 |
| Atrial fibrillation | 12 (12.9%) | 6 (35.3%) | 0.033 |
| Pacemaker | 2 (2.2%) | 1 (5.9%) | 0.399 |
| NEWS | 6 [5; 7] | 7 [6; 8] | 0.047 |
| SpO2 on admission to a COVID-19 hospital, % | 93 [92; 93] | 90 [86; 92] | 0.002 |
| Comorbidities | |||
| Hypertension | 66 (71.0%) | 15 (88.2%) | 0.230 |
| Diabetes mellitus | 16 (17.2%) | 4 (23.5%) | 0.508 |
| Cancer | 16 (17.2%) | 2 (11.8%) | 0.734 |
| Encephalopathy at admission | 10 (10.8%) | 5 (29.4%) | 0.055 |
| Stroke or transient ischemic attack | 8 (8.6%) | 6 (35.3%) | 0.008 |
| COPD | 9 (9.7%) | 3 (17.6%) | 0.393 |
| Bronchial asthma | 6 (6.5%) | 3 (17.6%) | 0.143 |
| Chronic kidney disease | 6 (6.5%) | 2 (11.8%) | 0.607 |
| Rheumatoid arthritis | 1 (1.1%) | 2 (11.8%) | 0.062 |
| Current smoking | 4 (4.3%) | 0 | 1.000 |
| Laboratory tests | |||
| Monocytes, ×109/L | 0.49 [0.34; 0.63] | 0.45 [0.35; 0.63] | 0.908 |
| Neutrophils, ×109/L | 4.70 [3.16; 6.63] | 6.07 [3.42; 8.47] | 0.270 |
| Lymphocytes, ×109/L | 1.15 [0.92; 1.54] | 0.89 [0.60; 1.13] | 0.011 |
| D-dimer, ng/L | 572 [342; 859] | 1614 [385; 3187] | 0.048 |
| Hemoglobin, g/L | 140.3 [131.0; 148.1] | 130.9 [116.1; 142.1] | 0.088 |
| Erythrocytes, ×1012/L | 4.83 [4.48; 5.09] | 4.63 [4.36; 5.06] | 0.335 |
| White blood cells, ×109/L | 6.6 [4.9; 9.2] | 9.5 [4.0; 11.8] | 0.169 |
| C-reactive protein, mg/L | 5.5 [2.4; 9.3] | 76.8 [35.3; 116.2] | <0.001 |
| Platelets, ×109/L | 213.5 [164.2; 260.4] | 148.8 [119.1; 198.1] | 0.004 |
| Lactate dehydrogenase, units/L | 288 [227; 387] | 422 [298; 665] | <0.001 |
| Lung injury and oxygen therapy | |||
| Lung tissue injury volume (CT data), % | 36 [28; 48] | 80 [64; 92] | <0.001 |
| Nasal cannula (O2 up to 15 L/min) | 81 (87.1%) | 0 | <0.001 |
| High-flow nasal oxygen (AIRVO) | 5 (5.4%) | 0 | 1.000 |
| Non-invasive ventilation | 5 (5.4%) | 0 | 1.000 |
| Invasive mechanical ventilation | 2 (2.2%) | 17 (100%) | <0.001 |
| VV ECMO | 0 | 4 (23.5%) | <0.001 |
| Efferent therapy methods | |||
| Plasmapheresis | 2 (2.2%) | 3 (17.6%) | 0.026 |
| Hemosorption | 2 (2.2%) | 3 (17.6%) | 0.026 |
| Complications | |||
| Acute respiratory distress syndrome | 1 (1.1%) | 13 (76.5%) | <0.001 |
| Systemic inflammatory response syndrome | 5 (5.4%) | 9 (52.9%) | <0.001 |
| Acute heart failure | 2 (2.2%) | 11 (41.2%) | <0.001 |
| Venous thrombosis | 9 (9.7%) | 2 (11.8%) | 0.678 |
| Multiple organ dysfunction | 1 (1.1%) | 9 (52.9%) | <0.001 |
| Acute kidney injury | 1 (1.1%) | 4 (23.5%) | 0.002 |
| Disseminated intravascular coagulation | 0 | 4 (23.5%) | <0.001 |
| Cerebral edema | 0 | 3 (17.6%) | 0.003 |
| Gastrointestinal bleeding | 0 | 1 (5.9%) | 0.155 |
| Haemorrhagic stroke | 1 (1.1%) | 0 | 1.000 |
| Characteristics of admission and hospital stay | |||
| Days from illness onset to hospital admission, days | 8 [6; 11] | 9 [6; 10] | 0.533 |
| ICU admission | 5 (5.4%) | 6 (35.3%) | 0.002 |
| In-hospital transfer to the ICU | 10 (10.8%) | 11 (64.7%) | <0.001 |
| In-hospital stay, days | 13 [12; 16] | 12 [8; 17] | 0.320 |
| Duration of ICU stay (only ICU patients), days | 1 [0; 3] | 9 [7; 13] | <0.001 |
| Variables | Survivors (n = 93) | Non-Survivors (n = 17) | p-Value |
|---|---|---|---|
| Minimum LA diameter, mm | 39 [36; 42] | 40 [38; 44] | 0.388 |
| Maximum LA diameter, mm | 52 [48; 56] | 60 [52; 61] | 0.014 |
| Maximum LA Vol (i), ml/m2 | 21.6 [19.1; 28.7] | 25.6 [21.9; 31.0] | 0.088 |
| LV EDI, mL/m2 | 49.3 [43.2; 56.6] | 51.4 [43.7; 68.3] | 0.370 |
| LV ESI, mL/m2 | 16.7 [14.2; 22.1] | 18.0 [15.2; 28.7] | 0.239 |
| LV SI, mL/m2 | 31.8 [27.2; 36.8] | 38.8 [30.2; 41.6] | 0.404 |
| LV EF, % | 65.0 [60.0; 69.0] | 63.0 [58.0; 65.0] | 0.116 |
| CI, l/min/m2 | 2.39 [2.00; 3.09] | 3.02 [2.19; 3.71] | 0.171 |
| E/A | 0.94 [0.79; 1.20] | 1.40 [1.02; 1.87] | 0.009 |
| E/e′ | 7.56 [6.00; 9.14] | 9.87 [8.40; 12.29] | 0.001 |
| LV septal wall thickness, mm | 13 [12; 16] | 15 [13; 17] | 0.215 |
| Characteristics of the right heart chambers | |||
| Minimum RA diameter, mm | 42 [37; 45] | 44 [43; 49] | 0.007 |
| Maximum RA diameter, mm | 51 [47; 55] | 56 [51; 61] | 0.004 |
| Maximum RA Vol (i), mL/m2 | 24.7 [19.6; 34.8] | 32.2 [26.4; 42.5] | 0.002 |
| Basal RV diameter, mm | 39 [37; 43] | 42 [40; 48] | 0.004 |
| Mid cavitary RV diameter, mm | 34 [30; 39] | 36 [33; 41] | 0.156 |
| RV longitudinal dimension, mm | 60 [57; 66] | 64 [61; 69] | 0.119 |
| RV/LV area | 0.64 [0.55; 0.73] | 0.70 [0.54; 1.05] | 0.228 |
| PA trunk diameter, mm | 25 [23; 27] | 25 [25; 30] | 0.396 |
| RV FAC, % | 53.4 [46.4; 60.2] | 49.0 [42.5; 53.1] | 0.007 |
| TAPSE, mm | 20 [19; 22] | 16 [16; 19] | <0.001 |
| Tricuspid annular S′ wave, cm/s (PW) | 13 [12; 15] | 12 [9; 13] | 0.016 |
| RV FW LS, % (2D STE) | 22.3 [17.7; 26.2] | 15.2 [11.7; 18.3] | 0.006 |
| RV wall thickness (subcostal), mm | 5 [4; 6] | 6 [5; 6] | 0.009 |
| Pulmonary hemodynamic parameters | |||
| sPAP, mm Hg | 35 [28; 39] | 47 [42; 55] | <0.001 |
| meanPAP, mm Hg | 15 [11; 21] | 23 [20; 31] | <0.001 |
| Inferior vena cava diameter, mm | 22 [19; 24] | 23 [20; 24] | 0.319 |
| TR moderate to severe | 14 (15.4%) | 7 (43.7%) | 0.015 |
| Prognostic Factor | B | OR (95% CI) | p-Value |
|---|---|---|---|
| Minimum diameter LA, mm | 0.072 | 1.074 (0.969–1.191) | 0.173 |
| Maximum diameter LA, mm | 0.093 | 1.097 (1.019–1.182) | 0.014 |
| Maximum LA Vol (i), mL/m2 | 0.068 | 1.070 (1.008–1.136) | 0.026 |
| LV EDI, mL/m2 | 0.022 | 1.022 (0.983–1.064) | 0.272 |
| LV ESI, mL/m2 | 0.059 | 1.06 (0.980–1.147) | 0.145 |
| LV SI, mL/m2 | 0.018 | 1.018 (0.955–1.085) | 0.585 |
| LV EF, % | −0.060 | 0.942 (0.870–1.021) | 0.144 |
| CI, L/min/m2 | 0.416 | 1.516 (0.775–2.964) | 0.224 |
| E/A | 1.099 | 3.001 (1.273–7.076) | 0.012 |
| E/e′ | 0.222 | 1.249 (1.079–1.445) | 0.003 |
| LV septal wall thickness, mm | 0.038 | 1.039 (0.872–1.237) | 0.670 |
| Minimum RA diameter, mm | 0.098 | 1.103 (1.016–1.198) | 0.019 |
| Maximum RA diameter, mm | 0.115 | 1.122 (1.039–1.211) | 0.003 |
| Maximum RA Vol (i), mL/m2 | 0.074 | 1.076 (1.029–1.125) | 0.001 |
| Basal RV diameter, mm | 0.129 | 1.138 (1.033–1.253) | 0.009 |
| Mid cavitary RV diameter, mm | 0.069 | 1.072 (0.993–1.157) | 0.075 |
| RV longitudinal dimension, mm | 0.046 | 1.048 (0.978–1.122) | 0.184 |
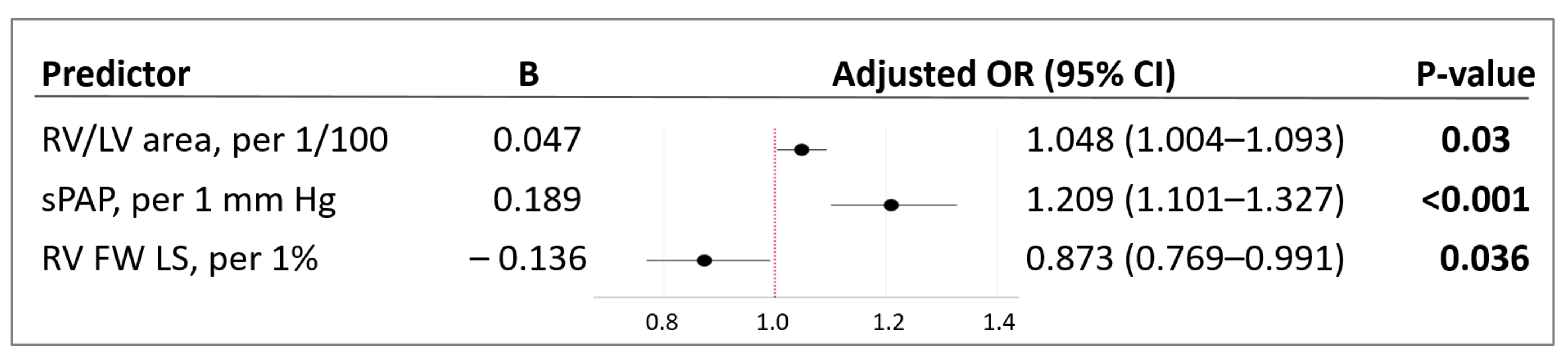
| RV/LV area, 1/100 | 0.041 | 1.041 (1.010–1.074) | 0.010 |
| PA trunk diameter, mm | 0.090 | 1.095 (0.937–1.279) | 0.256 |
| RV FAC, % | −0.069 | 0.933 (0.885–0.983) | 0.010 |
| TAPSE, mm | −0.558 | 0.572 (0.429–0.764) | <0.001 |
| Tricuspid annular S′ wave, cm/s (PW) | −0.230 | 0.794 (0.656–0.962) | 0.019 |
| RV FW LS, % (2D STE) | −0.122 | 0.885 (0.808–0.970) | 0.009 |
| RV wall thickness (subcostal), mm | 0.718 | 2.051 (1.123–3.745) | 0.019 |
| sPAP, mm Hg | 0.169 | 1.184 (1.093–1.282) | <0.001 |
| meanPAP, mm Hg | 0.168 | 1.183 (1.082–1.293) | <0.001 |
| Inferior vena cava diameter, mm | 0.099 | 1.104 (0.906–1.346) | 0.325 |
| TR, moderate to severe/mild | 1.575 | 4.831 (1.535–15.207) | 0.007 |
| Variable | ICC (95% Confidence Interval) | ||
|---|---|---|---|
| Intra-Observer | Inter-Observer | Test-Retest | |
| RV FW LS | 0.88 (0.68–0.96) | 0.84 (0.60–0.94) | 0.85 (0.63–0.95) |
| TR velocity | 0.96 (0.90–0.99) | 0.96 (0.89–0.98) | 0.95 (0.87–0.98) |
| RV area | 0.97 (0.92–0.99) | 0.93 (0.80–0.97) | 0.95 (0.86–0.98) |
| LV area | 0.98 (0.96–0.99) | 0.97 (0.92–0.99) | 0.98 (0.94–0.99) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Golukhova, E.Z.; Slivneva, I.V.; Mamalyga, M.L.; Marapov, D.I.; Alekhin, M.N.; Rybka, M.M.; Volkovskaya, I.V. Transthoracic Echocardiography-Based Prediction Model of Adverse Event Risk in Patients with COVID-19. Pathophysiology 2022, 29, 157-172. https://doi.org/10.3390/pathophysiology29020014
Golukhova EZ, Slivneva IV, Mamalyga ML, Marapov DI, Alekhin MN, Rybka MM, Volkovskaya IV. Transthoracic Echocardiography-Based Prediction Model of Adverse Event Risk in Patients with COVID-19. Pathophysiology. 2022; 29(2):157-172. https://doi.org/10.3390/pathophysiology29020014
Chicago/Turabian StyleGolukhova, Elena Zelikovna, Inessa Viktorovna Slivneva, Maxim Leonidovich Mamalyga, Damir Ildarovich Marapov, Mikhail Nikolaevich Alekhin, Mikhail Mikhailovich Rybka, and Irina Vasilevna Volkovskaya. 2022. "Transthoracic Echocardiography-Based Prediction Model of Adverse Event Risk in Patients with COVID-19" Pathophysiology 29, no. 2: 157-172. https://doi.org/10.3390/pathophysiology29020014






