Chronic Fatigue Exhibits Heterogeneous Autoimmunity Characteristics Which Reflect Etiology
Abstract
:1. Introduction
2. Material and Methods
- Percentage gap compared to the results of the healthy donors’ pool sera (with a “+” sign (above the healthy pool value), or with a “-” sign (below the healthy pool value);
- The average autoimmune reactivity of an individual, calculated as the algebraic sum of all deviations from the control healthy donors’ pool for each type of AAb, divided by the number of measured autoantibodies;
- The profile of autoimmunity in an individual, representing the variation in the deviations of each AAb level, from the individual average autoimmune reactivity, taken as the isoline.
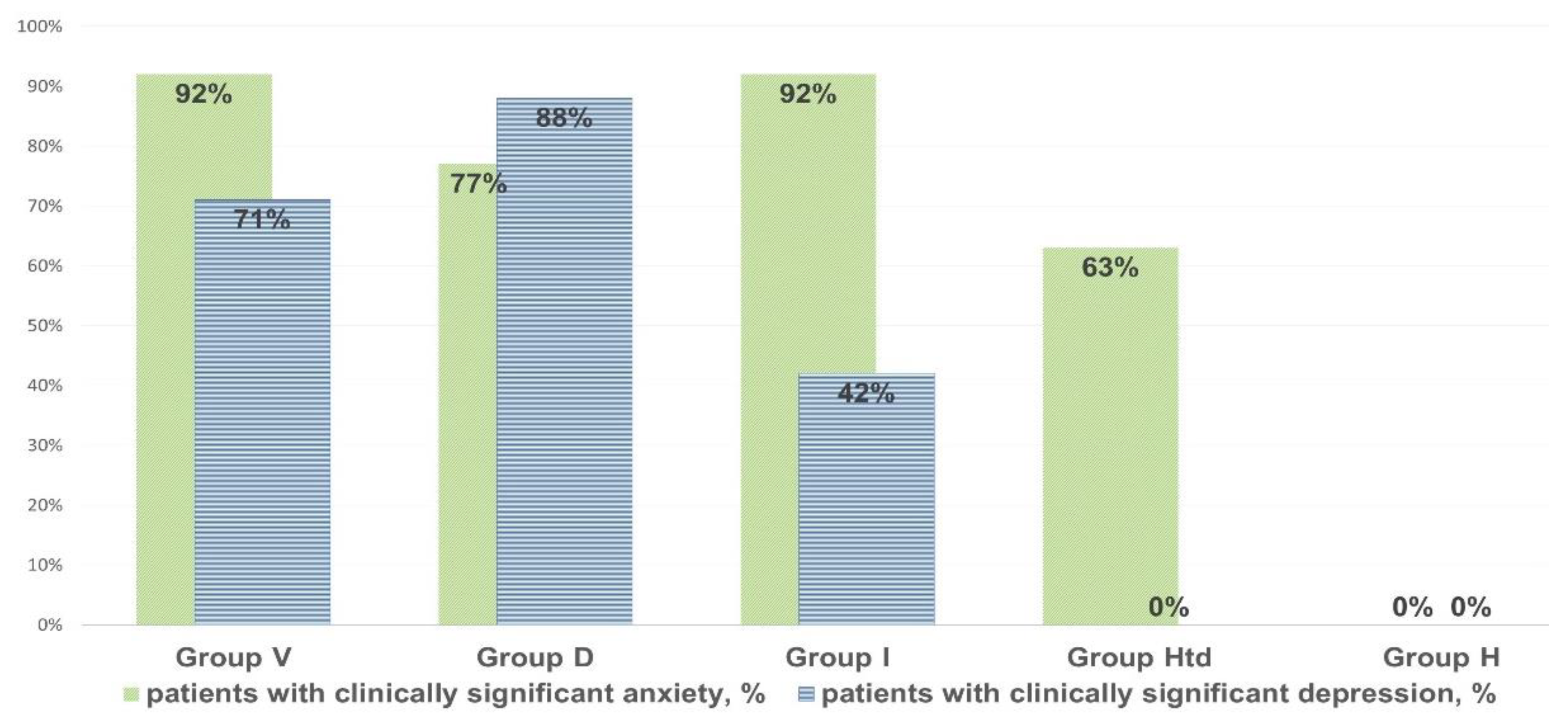
3. Results
- AAb to beta-2 glycoprotein-1 were increased in all cases of clinically significant chronic fatigue, but not in those individuals, who had complaints of non-CFS recurrent fatigue (“healthy but tired” group, HTd);
- Only post-viral asthenia (group V) is distinguished by a statistically significant increase in the level of AAb to voltage-dependent calcium channels, while the rise in the level of AAb to a number of autoantigens is the highest in post-viral chronic fatigue in comparison with other types of fatigue (p < 0.05);
- Only stress-related asthenia (group D) is characterized by a statistically significant increase in the level of autoantibodies to glutamate receptors;
- All types of fatigue, including acute recurrent subclinical fatigue, inappropriate to CFS/ME (group HTd), are characterized by an increase in the level of autoimmunity to the serotonin receptors and proteins GFAP and S-100, without significant differences between fatigue groups;
- No type of fatigue (neither positive, nor negative according to the CFS/ME criteria) is associated with an increase in autoimmune reactivity against the myelin basic protein.
4. Discussion
5. Conclusions
6. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sharif, K.; Watad, A.; Bragazzi, N.L.; Lichtbroun, M.; Martini, M.; Perricone, C.; Amital, H.; Shoenfeld, Y. On chronic fatigue syndrome and nosological categories. Clin. Rheumatol. 2018, 37, 1161–1170. [Google Scholar] [CrossRef]
- Institute of Medicine. Beyond Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Redefining the Illness; National Academies Press: Washington, DC, USA, 2015. [Google Scholar] [CrossRef]
- Bested, A.C.; Marshall, L.M. Review of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: An evidence-based approach to diagnosis and management by clinicians. Rev. Environ. Health 2015, 30, 223–249. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.; Barry, M.; Im, Y.; Brown, A.; Jason, L.J. An investigation of symptoms predating CFS onset. J. Prev. Interv. Community 2015, 43, 54–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, E.J.; Son, C.G. Review of case definitions for myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). J. Transl. Med. 2020, 18, 289. [Google Scholar] [CrossRef] [PubMed]
- Komaroff, A.L. Advances in understanding the pathophysiology of chronic fatigue syndrome. JAMA—J. Am. Med. Assoc. 2019, 322, 499–500. [Google Scholar] [CrossRef]
- Sotzny, F.; Blanco, J.; Capelli, E.; Castro-Marrero, J.; Steiner, S.; Murovska, M.; Scheibenbogen, C. European Network on ME/CFS (EUROMENE). Myalgic Encephalomyelitis/Chronic Fatigue Syndrome—Evidence for an autoimmune disease. Autoimmun Rev. 2018, 17, 601–609. [Google Scholar] [CrossRef]
- Blomberg, J.; Gottfries, C.G.; Elfaitouri, A.; Rizwan, M.; Rosén, A. Infection Elicited Autoimmunity and Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: An Explanatory Model. Front. Immunol. 2018, 9, 229–249. [Google Scholar] [CrossRef] [Green Version]
- Perez, M.; Jaundoo, R.; Hilton, K.; Alamo, A.D.; Gemayel, K.; Klimas, N.G.; Craddock, T.J.A.; Nathanson, L. Genetic Predisposition for Immune System, Hormone, and Metabolic Dysfunction in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: A Pilot Study. Front. Pediatr. 2019, 7, 206. [Google Scholar] [CrossRef] [Green Version]
- Castro-Marrero, J.; Faro, M.; Aliste, L.; Sáez-Francàs, N.; Calvo, N.; Martínez-Martínez, A.; Fernández de Sevilla, T.; Alegre, J. Comorbidity in Chronic Fatigue Syndrome/Myalgic Encephalomyelitis: A Nationwide Population-Based Cohort Study. Psychosomatics 2017, 58, 533–543. [Google Scholar] [CrossRef]
- Loebel, M.; Grabowski, P.; Heidecke, H.; Bauer, S.; Hanitsch, L.G.; Wittke, K.; Meisel, C.; Reinke, P.; Volk, H.D.; Fluge, Ø. Antibodies to β adrenergic and muscarinic cholinergic receptors in patients with Chronic Fatigue Syndrome. Brain Behav. Immun. 2016, 52, 32–39. [Google Scholar] [CrossRef] [Green Version]
- Scheibenbogen, C.; Loebel, M.; Freitag, H.; Krueger, A.; Bauer, S.; Antelmann, M.; Doehner, W.; Scherbakov, N.; Heidecke, H.; Reinke, P.; et al. Immunoadsorption to remove β2 adrenergic receptor antibodies in Chronic Fatigue Syndrome CFS/ME. PLoS ONE 2018, 13, e0193672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomas, C.; Newton, J.; Watson, S. A review of hypothalamic-pituitary-adrenal axis function in chronic fatigue syndrome. Int. Sch. Res. Not. 2013, 2013, 784520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morris, G.; Anderson, G.; Maes, M. Hypothalamic-Pituitary-Adrenal Hypofunction in Myalgic Encephalomyelitis (ME)/Chronic Fatigue Syndrome (CFS) as a Consequence of Activated Immune-Inflammatory and Oxidative and Nitrosative Pathways. Mol. Neurobiol. 2017, 54, 6806–6819. [Google Scholar] [CrossRef] [PubMed]
- Nakatomi, Y.; Mizuno, K.; Ishii, A.; Wada, Y.; Tanaka, M.; Tazawa, S.; Onoe, K.; Fukuda, S.; Kawabe, J.; Takahashi, K.; et al. Neuroinflammation in Patients with Chronic Fatigue Syndrome/Myalgic Encephalomyelitis: An 11C-(R)-PK11195 PET Study. J. Nucl. Med. 2014, 55, 945–950. [Google Scholar] [CrossRef] [Green Version]
- Zaichik, A.S.; Churilov, L.P. Fundamentals of General Pathology. Fundamentals of General Pathophysiology; ELBI Publishers: Saint Petersburg, Russia, 1999; Volume 1, p. 536. [Google Scholar]
- Hatziagelaki, E.; Adamaki, M.; Tsilioni, I.; Dimitriadis, G.; Theoharides, T.C. Myalgic Encephalomyelitis/Chronic Fatigue Syndrome—Metabolic Disease or Disturbed Homeostasis due to Focal Inflammation in the Hypothalamus? J. Pharmacol. Exp. Ther. 2018, 367, 155–167. [Google Scholar] [CrossRef]
- Noda, M.; Ifuku, M.; Hossain, M.S.; Katafuchi, T. Glial Activation and Expression of the Serotonin Transporter in Chronic Fatigue Syndrome. Front. Psychol. 2018, 9, 589–595. [Google Scholar] [CrossRef]
- Fomicheva, E.E.; Filatenkova, T.A.; Rybakina, E.G. Activity in the Hypothalamo-Hypophyseal-Adrenocortical System on Experimental Induction of Chronic Fatigue Syndrome. Neurosci. Behav. Physiol. 2010, 40, 245–250. [Google Scholar] [CrossRef]
- Giannoccaro, M.P.; Cossins, J.; Sørland, K.; Fluge, Ø.; Vincent, A. Searching for Serum Antibodies to Neuronal Proteins in Patients with Myalgic Encephalopathy/Chronic Fatigue Syndrome. Clin. Ther. 2019, 41, 836–847. [Google Scholar] [CrossRef] [Green Version]
- Churilov, L.P.; Danilenko, O.V. Immunoreactivity in chronic fatigue syndrome during remission. exacerbation and virus carriage. Clin. Pathophysiol. 2019, 25, 26–36. (In Russian) [Google Scholar]
- Hokama, Y.; Empey-Campora, C.; Hara, C.; Higa, N.; Siu, N.; Lau, R.; Kuribayashi, T.; Yabusaki, K. Acute phase phospholipids related to the cardiolipin of mitochondria in the sera of patients with chronic fatigue syndrome (CFS), chronic Ciguatera fish poisoning (CCFP), and other diseases attributed to chemicals, Gulf War, and marine toxins. J. Clin. Lab. Anal. 2008, 22, 99–105. [Google Scholar] [CrossRef]
- Hokama, Y.; Campora, C.E.; Hara, C.; Kuribayashi, T.; Le Huynh, D.; Yabusaki, K. Anticardiolipin antibodies in the sera of patients with diagnosed chronic fatigue syndrome. J Clin Lab. Anal. 2009, 23, 210–212. [Google Scholar] [CrossRef] [PubMed]
- Mackay, A.; Tate, W.P. A compromised paraventricular nucleus within a dysfunctional hypothalamus: A novel neuroinflammatory paradigm for ME/CFS. Int. J. Immunopathol. Pharmacol. 2018, 32, 2058738418812342. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, S.; Ouchi, Y.; Nakatsuka, D.; Tahara, T.; Mizuno, K.; Tajima, S.; Onoe, H.; Yoshikawa, E.; Tsukada, H.; Iwase, M.; et al. Reduction of [11C](+)3-MPB binding in brain of chronic fatigue syndrome with serum autoantibody against muscarinic cholinergic receptor. PLoS ONE 2012, 7, e51515. [Google Scholar] [CrossRef] [PubMed]
- Pashnina, I.A.; Krivolapova, I.M.; Fedotkina, T.V.; Ryabkova, V.A.; Chereshneva, M.V.; Churilov, L.P.; Chereshnev, V.A. Antinuclear Autoantibodies in Health: Autoimmunity Is Not a Synonym of Autoimmune Disease. Antibodies 2021, 10, 9. [Google Scholar] [CrossRef]
- WHO. ICD-11—Mortality and Morbidity Statistics 2020. Available online: https://icd.who.int/browse11/l-m/en#/http%3A%2F%2Fid.who.int%2Ficd%2Fentity%2F569175314 (accessed on 3 November 2020).
- Holmes, G.P.; Kaplan, J.E.; Gantz, N.M.; Komaroff, A.L.; Schonberger, L.B.; Straus, S.E.; Jones, J.F.; Dubois, R.E.; Cunningham-rundles, C.; Pahwa, S.; et al. Chronic fatigue syndrome: A working case definition. Ann. Intern. Med. 1988, 108, 387–389. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, K.; Straus, S.E.; Hickie, I.; Sharpe, M.C.; Dobbins, J.G.; Komaroff, A. The chronic fatigue syndrome: A comprehensive approach to its definition and study. Ann. Intern. Med. 1994, 121, 953–959. [Google Scholar] [CrossRef] [PubMed]
- Carruthers, B.M.; Jain, A.K.; De Meirleir, K.L.; Peterson, D.L.; Klimas, N.G.; Lerner, A.M.; Bested, A.C.; Flor-Henry, P.; Joshi, P.; Powles, A.C.P.; et al. Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. J. Chronic Fatigue Syndr. 2003, 11, 7–115. [Google Scholar] [CrossRef]
- Vorob’eva, O. Chronic fatigue syndrome: From symptom—to diagnosis. Tr. Patsient. 2010, 8, 16–21. [Google Scholar]
- Griffith, J.P.; Zarrouf, F.A. A systematic review of chronic fatigue syndrome: Don’t assume it’s depression. Prim. Care Companion J. Clin. Psychiatry 2008, 10, 120–128. [Google Scholar] [CrossRef]
- Zigmond, A.S.; Snaith, R.P. The hospital anxiety and depression scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef] [Green Version]
- Coetzee, N.; Maree, D.J.F.; Smit, B.N. The relationship between chronic fatigue syndrome. burnout. job satisfaction. social support and age among academics at a tertiary institution. Int. J. Occup Med. Environ. Health 2019, 32, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Poletaev, A.B.; Maltseva, L.I.; Zamaleeva, R.S.; Nukhnin, M.A.; Osipenko, L.G. Application of ELI-P Complex method in clinical obstetrics. Am. J. Reprod. Immunol. 2007, 57, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Poletaev, A.; Rizzo, C. New Approaches to Early Detection of Pathological Changes in the Human Body. ELI-Viscero-Test (Molecular Clinical Examination). Guidelines for Physicians; MIC Immunculus Publisher: Moscow, Russia, 2019; p. 84. [Google Scholar]
- Schreiber, K.; Sciascia, S.; De Groot, P.G.; Devreese, K.; Jacobsen, S.; Ruiz-Irastorza, G.; Shoenfeld, Y.; Shovman, O.; Hunt, B.J. Antiphospholipid syndrome. Nat. Rev. Dis. Primers 2018, 4, 18005. [Google Scholar] [CrossRef] [Green Version]
- Berg, D.; Berg, L.H.; Couvaras, J.; Harrison, H. Chronic fatigue syndrome and/or fibromyalgia as a variation of antiphospholipid antibody syndrome: An explanatory model and approach to laboratory diagnosis. Blood Coagul. Fibrinolysis 1999, 10, 435–438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shoenfeld, Y.; Ryabkova, V.A.; Scheibenbogen, C.; Brinth, L.; Martinez-Lavin, M.; Ikeda, S.; Heidecke, H.; Wated, A.; Bragazzi, N.L.; Chapman, J.; et al. Complex syndromes of chronic pain. fatigue and cognitive impairment linked to autoimmune dysautonomia and small fiber neuropathy. Clin. Immunol. 2020, 214, 108384. [Google Scholar] [CrossRef]
- Gaipl, U.S.; Munoz, L.E.; Grossmayer, G.; Lauber, K.; Kranz, S.; Sarter, K.; Voll, R.E.; Winkler, T.; Kuhn, A.; Kalden, J.; et al. Clearance deficiency and systemic lupus erythematosus (SLE). J. Autoimmun. 2007, 28, 114–121. [Google Scholar] [CrossRef]
- Vomero, M.; Manganelli, V.; Barbati, C.; Colasanti, T.; Capozzi, A.; Finucci, A.; Spinelli, F.R.; Ceccarelli, F.; Perricone, C.; Truglia, S.; et al. Reduction of autophagy and increase in apoptosis correlates with a favorable clinical outcome in patients with rheumatoid arthritis treated with anti-TNF drugs. Arthritis Res. Ther. 2019, 21, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feldmann, M.; Maini, R. TNF defined as a therapeutic target for rheumatoid arthritis and other autoimmune diseases. Nat. Med. 2003, 9, 1245–1250. [Google Scholar] [CrossRef] [PubMed]
- Smolen, J.; Aletaha, D. Rheumatoid arthritis therapy reappraisal: Strategies, opportunities and challenges. Nat. Rev. Rheumatol. 2015, 11, 276–289. [Google Scholar] [CrossRef]
- Mackay, A. A Paradigm for Post-COVID-19 Fatigue Syndrome Analogous to ME/CFS. Front. Neurol. 2021, 12, 701419. [Google Scholar] [CrossRef]
- Dotan, A.; Muller, S.; Kanduc, D.; David, P.; Halpert, G.; Shoenfeld, Y. The SARS-CoV-2 as an instrumental trigger of autoimmunity. Autoimmun. Rev. 2021, 20, 102792. [Google Scholar] [CrossRef] [PubMed]




| № | Antigen | Abbreviation |
|---|---|---|
| 1 | Double stranded deoxyribonucleic acid | ds-DNA |
| 2 | β2-glycoprotein-I | β2GPI |
| 3 | Fc-fragments of IgG | Fc-Ig |
| 4 | Membrane antigen of cardiomyocytes | CoM-0.2 |
| 5 | β1-adrenergic receptors of cardiomyocytes | β1AR |
| 6 | Platelet membrane antigen | TrM-03 |
| 7 | Cytoplasmic antigen of neutrophils | ANCA |
| 8 | Membrane antigen of renal glomerular cells | KiM-05 |
| 9 | Cytoplasmic antigen of renal glomerular cells | KiS-07 |
| 10 | Membrane antigen of pulmonary alveolocytes | LuM-02 |
| 11 | Cytoplasmic antigen of pulmonary alveolocytes with a molecular weight of ~80 kDa | LuS-06-80 |
| 12 | Cytoplasmic antigen of pulmonary alveolocytes with a molecular weight of ~300 kDa | LuS-300 |
| 13 | Collagen type IV | Collagen |
| 14 | Pulmonary elastin | Elastin |
| 15 | Membrane antigen of gastric wall cells | GaM-02 |
| 16 | Membrane antigen of cells of small intestine wall | ItM-07 |
| 17 | Cytoplasmic antigen of hepatocytes | HeS-08 |
| 18 | Membrane antigen of hepatocyte mitochondria | HMMP |
| 19 | Human insulin | Ins |
| 20 | Insulin receptors | Ins-R |
| 21 | Thyroglobulin | TG |
| 22 | Thyrotropin receptor | TSH-R |
| 23 | Membrane antigen of adrenal medulla cells | AdrM-D/C-0 |
| 24 | Membrane antigen of sperm and prostate cells | Spr-0.6 |
| 25 | γ-interferon | hamma-ifn/hamma-IFN |
| 26 | S100 protein | S100 |
| 27 | Glial fibrillary acidic protein | GFAP |
| 28 | Myelin basic protein | MBP |
| 29 | Voltage-dependent calcium channel | VDCh |
| 30 | N-cholinergic receptors | Hol-R |
| 31 | Serotonin receptors | Ser-R |
| 32 | γ-aminobutyric acid receptors | GABA-R |
| 33 | Dopamine receptors | Da-R |
| 34 | Glutamate receptors | Glu-R |
| 35 | Neurofilament protein 200 | NF-200 |
| Antibody | Correlation Values | p-Value |
|---|---|---|
| β2GPI | +0.792 (VS) | <0.001 |
| GFAP | +0.492 (S) | 0.006 |
| VDCh | +0.458 (S) | 0.011 |
| Hol-R | +0.385 (M) | 0.036 |
| Ser-R | +0.305 (M) | 0.119 |
| GABA-R | 0.187 (W) | 0.654 |
| MBP | Irrelated | 1 |
| Da-R | −0.201 (W) | 0.500 |
| S100 | −0.328 (M) | 0.081 |
| Glu-R | −0.377 (S) | 0.044 |
| NF-200 | −0.612 (VS) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Danilenko, O.V.; Gavrilova, N.Y.; Churilov, L.P. Chronic Fatigue Exhibits Heterogeneous Autoimmunity Characteristics Which Reflect Etiology. Pathophysiology 2022, 29, 187-199. https://doi.org/10.3390/pathophysiology29020016
Danilenko OV, Gavrilova NY, Churilov LP. Chronic Fatigue Exhibits Heterogeneous Autoimmunity Characteristics Which Reflect Etiology. Pathophysiology. 2022; 29(2):187-199. https://doi.org/10.3390/pathophysiology29020016
Chicago/Turabian StyleDanilenko, Olga V., Natalia Y. Gavrilova, and Leonid P. Churilov. 2022. "Chronic Fatigue Exhibits Heterogeneous Autoimmunity Characteristics Which Reflect Etiology" Pathophysiology 29, no. 2: 187-199. https://doi.org/10.3390/pathophysiology29020016






