Pathophysiology of Methicillin-Resistant Staphylococcus aureus Superinfection in COVID-19 Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. RNA Extraction and RT-qPCR
2.2. Data Analysis
2.3. Ethical Approval
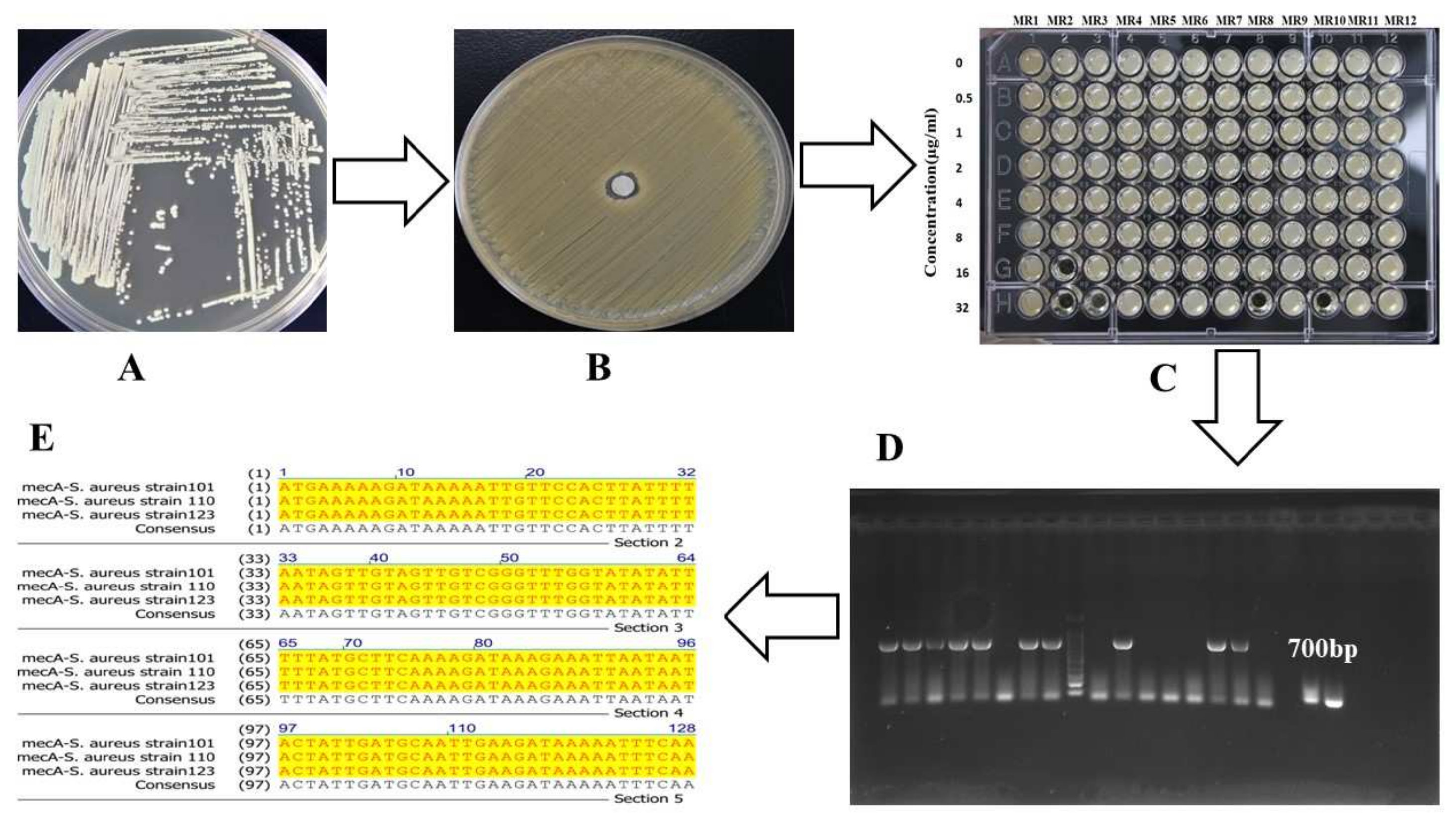
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Garcia-Vidal, C.; Sanjuan, G.; Moreno-García, E.; Puerta-Alcalde, P.; Garcia-Pouton, N.; Chumbita, M.; Fernandez-Pittol, M.; Pitart, C.; Inciarte, A.; Bodro, M.; et al. Incidence of co-infections and superinfections in hospitalized patients with COVID-19: A retrospective cohort study. Clin. Microbiol. Infect. 2020, 27, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Feldman, C.; Anderson, R. The role of co-infections and secondary infections in patients with COVID-19. Pneumonia 2021, 13, 5. [Google Scholar] [CrossRef] [PubMed]
- Kwon, W.J.; Li, G.; Zheng, M.; Kaur, H.; Magbual, N.; Dalai, S. Superinfections and coinfections in COVID-19–separating the signal from the noise. Medpage Today April 2020, 28. [Google Scholar]
- Ukuhor, H.O. The interrelationships between antimicrobial resistance, COVID-19, past, and future pandemics. J. Infect. Public Health 2020, 14, 53–60. [Google Scholar] [CrossRef]
- Clancy, C.J.; Schwartz, I.S.; Kula, B.; Nguyen, M.H. (Eds.) Bacterial Superinfections among Persons with Coronavirus Disease 2019: A Comprehensive Review of Data from Postmortem Studies. In Open Forum Infectious Diseases; Oxford University Press: Oxford, UK, 2021. [Google Scholar]
- Microbiology, N. Antimicrobial resistance in the age of COVID-19. Nat. Microbiol. 2020, 5, 779. [Google Scholar]
- Habib, G.; Zhu, Q.; Sun, B. Bioinformatics and functional assessment of toxin-antitoxin systems in Staphylococcus aureus. Toxins 2018, 10, 473. [Google Scholar] [CrossRef] [Green Version]
- Habib, G.; Zhu, J.; Sun, B. A novel type I toxin-antitoxin system modulates persister cell formation in Staphylococcus aureus. Int. J. Med. Microbiol. 2020, 310, 151400. [Google Scholar] [CrossRef]
- Zhao, D.; Yao, F.; Wang, L.; Zheng, L.; Gao, Y.; Ye, J.; Guo, F.; Zhao, H.; Gao, R. A Comparative Study on the Clinical Features of Coronavirus 2019 (COVID-19) Pneumonia With Other Pneumonias. Clin. Infect. Dis. 2020, 71, 756–761. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Bayer, A.; Cosgrove, S.E.; Daum, R.S.; Fridkin, S.K.; Gorwitz, R.J.; Kaplan, S.L.; Karchmer, A.W.; Levine, D.P.; Murray, B.E.; et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin. Infect. Dis. 2011, 52, e18–e55. [Google Scholar] [CrossRef] [Green Version]
- Lardaro, T.; Wang, A.Z.; Bucca, A.; Croft, A.; Glober, N.; Holt, D.B.; Musey, P.I., Jr.; Peterson, K.D.; Trigonis, R.A.; Schaffer, J.T.; et al. Characteristics of COVID-19 patients with bacterial coinfection admitted to the hospital from the emergency department in a large regional healthcare system. J. Med. Virol. 2021, 93, 2883–2889. [Google Scholar] [CrossRef]
- Coenen, S.; Buis, D.T.; Meijboom, L.J.; Schade, R.P.; Visser, C.E.; van Hest, R.; Kuijvenhoven, M.; Prins, J.M.; Nijman, S.F.; Sieswerda, E.; et al. Low frequency of community-acquired bacterial co-infection in patients hospitalized for COVID-19 based on clinical, radiological and microbiological criteria: A retrospective cohort study. Antimicrob. Resist. Infect. Control. 2021, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Liu, W.; Jiang, M.; Huang, P.; Xiang, Z.; Deng, D.; Chen, P.; Xie, L. Clinical characteristics of COVID-19 patients with clinically diagnosed bacterial co-infection: A multi-center study. PLoS ONE 2021, 16, e0249668. [Google Scholar] [CrossRef]
- Alqahtani, A.; Alamer, E.; Mir, M.; Alasmari, A.; Alshahrani, M.M.; Asiri, M.; Ahmad, I.; Alhazmi, A.; Algaissi, A. Bacterial Coinfections Increase Mortality of Severely Ill COVID-19 Patients in Saudi Arabia. Int. J. Environ. Res. Public Health 2022, 19, 2424. [Google Scholar] [CrossRef] [PubMed]
- Grinbaum, R.S.; Kiffer, C.R.V. Bacterial infections in COVID-19 patients: A review. Rev. Da Assoc. Méd. Bras. 2021, 67, 1863–1868. [Google Scholar] [CrossRef]
- Soltani, S.; Faramarzi, S.; Zandi, M.; Shahbahrami, R.; Jafarpour, A.; Rezayat, S.A.; Pakzad, I.; Abdi, F.; Malekifar, P.; Pakzad, R. Bacterial coinfection among coronavirus disease 2019 patient groups: An updated systematic review and meta-analysis. New Microbes New Infect. 2021, 43, 100910. [Google Scholar] [CrossRef] [PubMed]
- Habib, G.; Khan, M.S.Z.; Gul, H.; Hayat, A.; Rehman, M.U. A persistent high ambient temperature waned the community spread of Severe Acute Respiratory Syndrome Coronavirus-2 in Pakistan. In New Microbe and New Infect; Elsevier: Amsterdam, The Netherlands, 2022; p. 100961. [Google Scholar]
- Maslennikov, R.; Ivashkin, V.; Ufimtseva, A.; Poluektova, E.; Ulyanin, A. Clostridioides difficile co-infection in patients with COVID-19. Future Microbiol. 2022, 17, 653–663. [Google Scholar] [CrossRef]
- Russell, C.D.; Fairfield, C.J.; Drake, T.M.; Turtle, L.; Seaton, R.A.; Wootton, D.G.; Sigfrid, L.; Harrison, E.M.; Docherty, A.B.; de Silva, T.I.; et al. Co-infections, secondary infections, and antimicrobial use in patients hospitalised with COVID-19 during the first pandemic wave from the ISARIC WHO CCP-UK study: A multicentre, prospective cohort study. Lancet Microbe 2021, 2, e354–e365. [Google Scholar] [CrossRef]
- Langford, B.J.; So, M.; Raybardhan, S.; Leung, V.; Westwood, D.; MacFadden, D.R.; Soucy, J.P.; Daneman, N. Bacterial co-infection and secondary infection in patients with COVID-19: A living rapid review and meta-analysis. Clin. Microbiol. Infect. 2020, 26, 1622–1629. [Google Scholar] [CrossRef]
- Lansbury, L.; Lim, B.; Baskaran, V.; Lim, W.S. Co-infections in people with COVID-19: A systematic review and meta-analysis. J. Infect. 2020, 81, 266–275. [Google Scholar] [CrossRef]
- Wikler, M.; Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Disk Susceptibility Tests: Approved Standard, 10th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2009; Volume 29, pp. 1–53. [Google Scholar]
- Rakiro, J.; Shah, J.; Waweru-Siika, W.; Wanyoike, I.; Riunga, F. Microbial coinfections and superinfections in critical COVID-19: A Kenyan retrospective cohort analysis. IJID Reg. 2021, 1, 41–46. [Google Scholar] [CrossRef]
- Cataño-Correa, J.C.; Cardona-Arias, J.A.; Porras Mancilla, J.P.; García, M.T. Bacterial superinfection in adults with COVID-19 hospitalized in two clinics in Medellín-Colombia, 2020. PLoS ONE 2021, 16, e0254671. [Google Scholar] [CrossRef] [PubMed]
- Asokan, G.V.; Vanitha, A. WHO global priority pathogens list on antibiotic resistance: An urgent need for action to integrate One Health data. Perspect. Public Health 2018, 138, 87–88. [Google Scholar]
- Musuuza, J.S.; Watson, L.; Parmasad, V.; Putman-Buehler, N.; Christensen, L.; Safdar, N. Prevalence and outcomes of co-infection and superinfection with SARS-CoV-2 and other pathogens: A systematic review and meta-analysis. PLoS ONE 2021, 16, e0251170. [Google Scholar] [CrossRef] [PubMed]
- Sharifipour, E.; Shams, S.; Esmkhani, M.; Khodadadi, J.; Fotouhi-Ardakani, R.; Koohpaei, A.; Doosti, Z.; Ej Golzari, S. Evaluation of bacterial co-infections of the respiratory tract in COVID-19 patients admitted to ICU. BMC Infect. Dis. 2020, 20, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ding, Q.; Lu, P.; Fan, Y.; Xia, Y.; Liu, M. The clinical characteristics of pneumonia patients coinfected with 2019 novel coronavirus and influenza virus in Wuhan, China. J. Med. Virol. 2020, 92, 1549–1555. [Google Scholar] [CrossRef]
- Sharif, A.F.; Mattout, S.K.; Mitwally, N.A. Coronavirus disease-19 spread in the Eastern Mediterranean Region, updates and prediction of disease progression in Kingdom of Saudi Arabia, Iran, and Pakistan. Int. J. Health Sci. 2020, 14, 32. [Google Scholar]

| Antibiotic Resistance | Number of Isolates | mecA Gene Detected | Pneumonia Cases | MIC of mecA-Positive Strains (Interquartile Range µg/mL) |
|---|---|---|---|---|
| Resistance to methicillin | 224 | 159 | 192 | 32–512 |
| Resistance to tazobactam | 110 | 36 | 66 | 16–128 |
| Resistance to ciprofloxacin | 53 | 4 | 12 | 4–16 |
| Resistance to gentamicin | 23 | 3 | 7 | 2–16 |
| Resistance to azithromycin | 11 | 6 | 6 | 4–32 |
| Resistance to vancomycin | 3 | 2 | 3 | 1–4 |
| Resistance to methicillin and tazobactam | 68 | 68 | 64 | ND |
| Resistance to methicillin and azithromycin | 17 | 6 | 10 | ND |
| Resistance to tazobactam and azithromycin | 26 | ND | 7 | ND |
| MRSA patients | Age > 65 | Age ≥ 50 | Age < 50 | Total |
| 7-day hospitalization | 21 | 30 | 13 | 64 |
| 14-day hospitalization | 33 | 51 | 22 | 106 |
| 25-day hospitalization | 14 | 21 | 9 | 44 |
| Total MRSA positive | 68 | 102 | 44 | 214 |
| Total number of patients tested for MRSA | 1240 | 1390 | 862 | 3492 |
| COVID-MRSA Prevalence (%) | 5.48 | 7.33 | 5.10 | 5.52 |
| Mortality rate (%) | 27.9 | 25.49 | 20.45 | 25.23 |
| Variables (Reference Range) | COVID-19 with Pneumonia | COVID-19 | p-Value |
|---|---|---|---|
| Body temperature | 39–41 °C | 38–39.5 °C | |
| Cough | More | Less | |
| Chest pain | High | Low | |
| Breathing problem | High | Low | |
| Sputum | More | Less | |
| Sweating | High | Less | |
| WBC (4–10 × 109/L) | 8–15.6 × 109 | 1.5–8.6 × 109 | <0.05 |
| Lymphocytes (1.1–3.2 × 109/L) | 0.2–1.0 × 109 | 0.75–2.1 × 109 | <0.05 |
| Neutrophils (45–75%) | 55–110 | 35–80 | <0.05 |
| Procalcitonin (<0.15 ng/mL) | 0.5–22.0 | 0.2–6.5 | <0.001 |
| Albumin (30–55 mg/L) | 10–24 | 24–40 | <0.05 |
| EST (1–20 mm/h) | 40–100 | 30–50 | <0.001 |
| AST (15–40 U/L) | 45–105 | 35–75 | <0.05 |
| ALT (9–50 U/L) | 12–65 | 15–85 | >0.05 |
| LDH (120–250 U/L) | 640–1780 | 300–1050 | <0.001 |
| CK (50–310 U/L) | 65–460 | 50–550 | <0.05 |
| CRP (0–4 mg/L) | 20–130 | 20–90 | <0.05 |
| D-dimer (<200 ng/mL) | 600–1800 | 650–1200 | <0.05 |
| RBC (4–6 × 106 cell/µL) | 3.0–6.5 × 106 | 3.0–7.0 × 106 | >0.05 |
| Platelets (150–450 × 103/µL) | 110–400 × 103 | 130–450 × 103 | >0.05 |
| Monocytes (2–10%) | 2–9 | 1–11 | >0.05 |
| Eosinophils (0–0.6%) | 0.1–0.7 | 0.1–0.7 | >0.05 |
| Ferritin (30–400 ng/mL) | 50–600 | 60–800 | >0.05 |
| Troponin-I (<0.6 ng/mL) | 3.5–8.0 | 5.5–10.5 | >0.05 |
| Blood urea (18–45 mg/dL) | 15–50 | 15–50 | >0.05 |
| Creatinine (0.5–1.2 mg/dL) | 0.5–2.0 | 0.5–2.0 | >0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Habib, G.; Mahmood, K.; Gul, H.; Tariq, M.; Ain, Q.U.; Hayat, A.; Rehman, M.U. Pathophysiology of Methicillin-Resistant Staphylococcus aureus Superinfection in COVID-19 Patients. Pathophysiology 2022, 29, 405-413. https://doi.org/10.3390/pathophysiology29030032
Habib G, Mahmood K, Gul H, Tariq M, Ain QU, Hayat A, Rehman MU. Pathophysiology of Methicillin-Resistant Staphylococcus aureus Superinfection in COVID-19 Patients. Pathophysiology. 2022; 29(3):405-413. https://doi.org/10.3390/pathophysiology29030032
Chicago/Turabian StyleHabib, Gul, Khalid Mahmood, Haji Gul, Muhammad Tariq, Qurat Ul Ain, Azam Hayat, and Mujaddad Ur Rehman. 2022. "Pathophysiology of Methicillin-Resistant Staphylococcus aureus Superinfection in COVID-19 Patients" Pathophysiology 29, no. 3: 405-413. https://doi.org/10.3390/pathophysiology29030032






