Breast Cancer Treatment Decreases Serum Levels of TGF-β1, VEGFR2, and TIMP-2 Compared to Healthy Volunteers: Significance for Therapeutic Outcomes?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
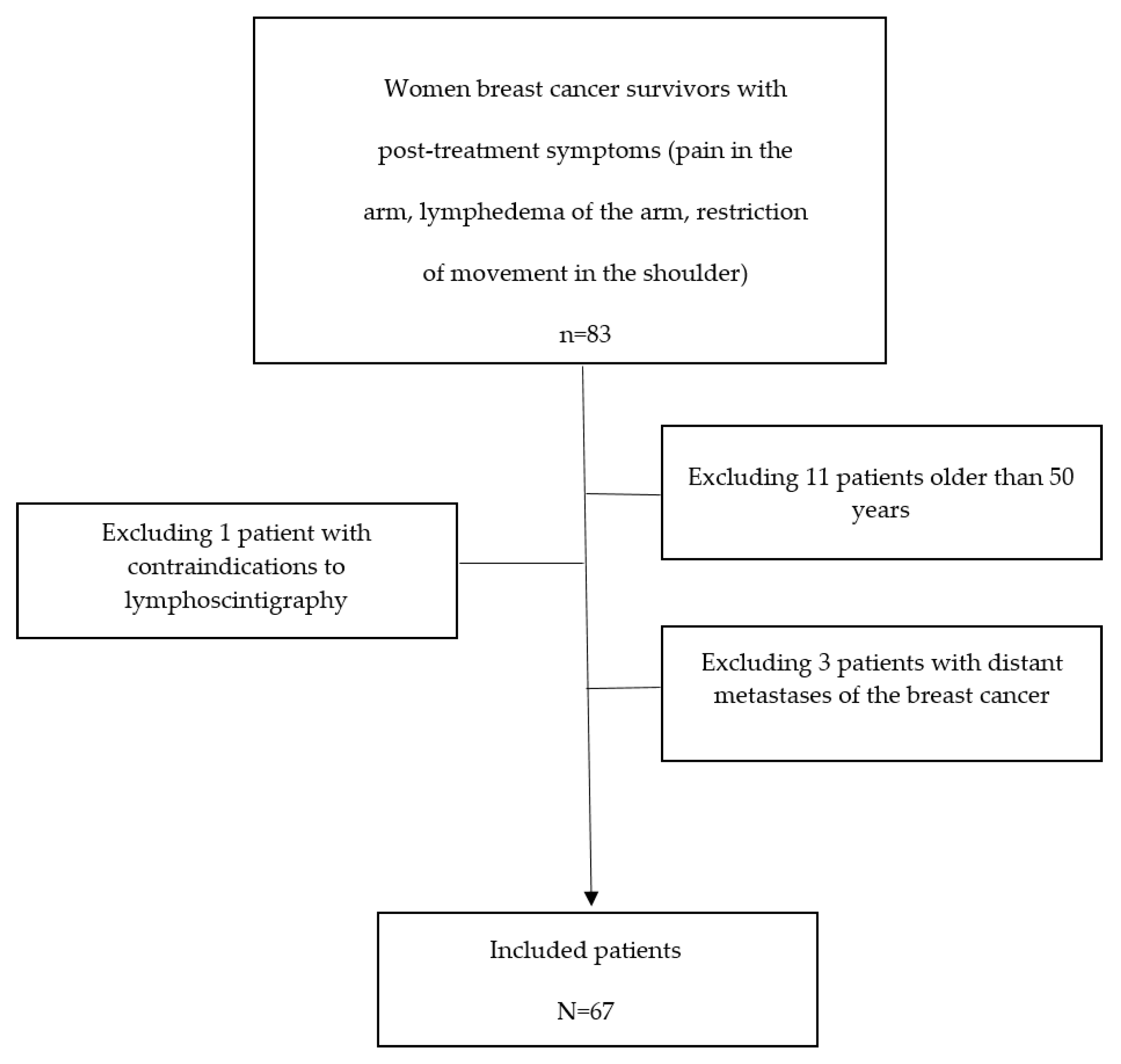
2.1.1. Inclusion Criteria
2.1.2. Exclusion Criteria
2.2. Clinical Assessment
2.3. Assessment of the Serum Levels of TGF-β1, VEGF-R2, and TIMP-2 Molecules
2.4. Upper Limb Lymphoscintigraphy
2.5. Statistical Analysis
3. Results
3.1. Clinical Evaluation of Patients
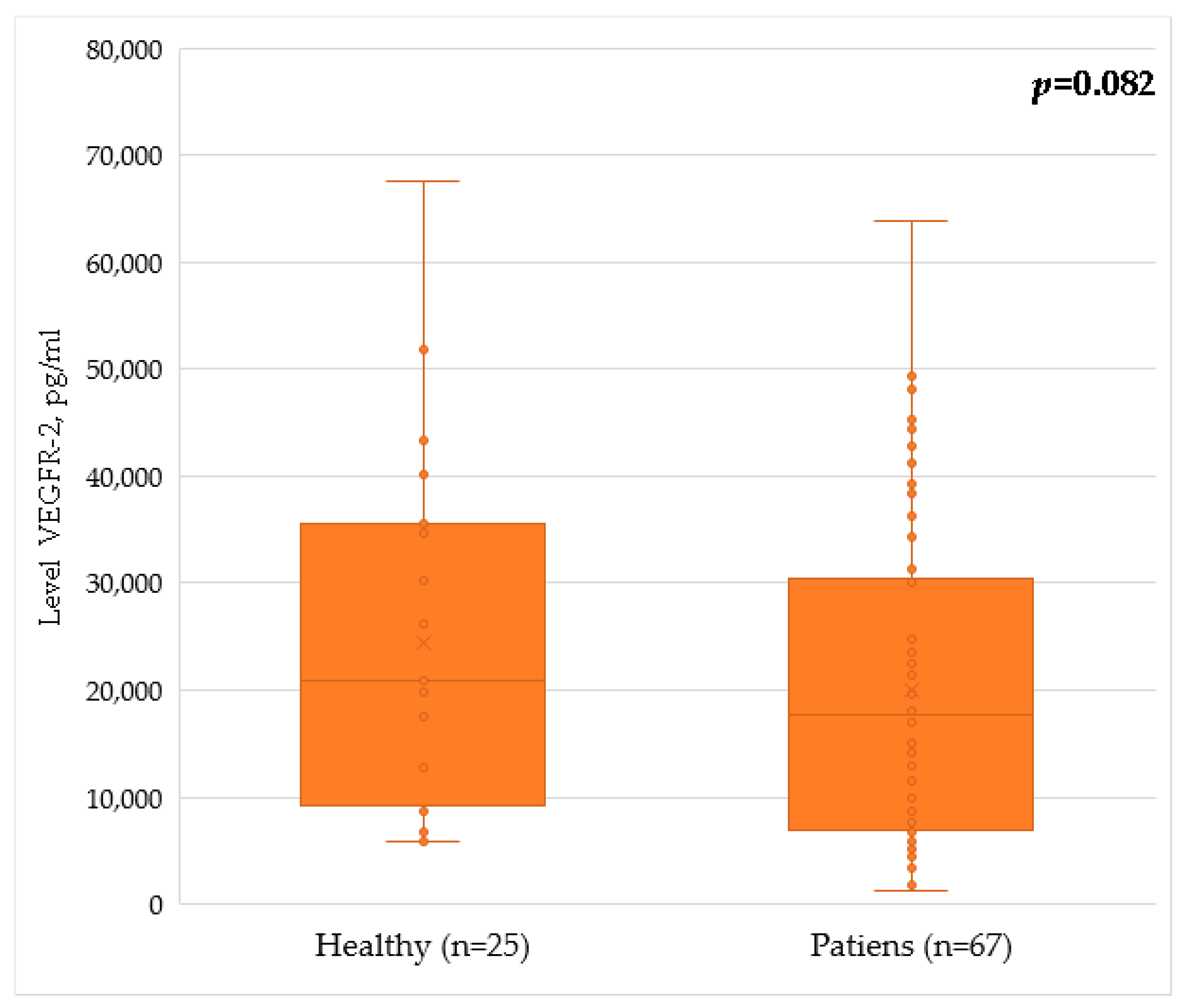
3.2. TGF-β1, VEGFR-2, and TIMP-2 Serum Levels
3.3. Correlation Analysis of the Level of Fibrosis Molecules
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rojas, K.; Stuckey, A. Breast Cancer Epidemiology and Risk Factors. Clin. Obstet. Gynecol. 2016, 59, 651–672. [Google Scholar] [CrossRef] [PubMed]
- Lopes, J.V.; Bergerot, C.D.; Barbosa, L.R.; Calux, N.M.C.T.; Elias, S.; Ashing, K.T.; Domenico, E.B.L. Impact of breast cancer and quality of life of women survivors. Rev. Bras. Enferm. 2018, 71, 2916–2921. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Qu, H.; Wu, Q.; Song, Y. Lymphedema in survivors of breast cancer. Oncol. Lett. 2020, 19, 2085–2096. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Tan, Q.; Qin, Q.; Wei, C. Prevalence of postmastectomy pain syndrome and associated risk factors: A large single-institution cohort study. Medicine 2020, 99, e19834. [Google Scholar] [CrossRef] [PubMed]
- Staff, N.P.; Grisold, A.; Grisold, W.; Windebank, A.J. Chemotherapy-induced peripheral neuropathy: A current review. Ann. Neurol. 2017, 81, 772–781. [Google Scholar] [CrossRef]
- Dinas, K.; Kalder, M.; Zepiridis, L.; Mavromatidis, G.; Pratilas, G. Axillary web syndrome: Incidence, pathogenesis, and management. Curr. Probl. Cancer 2019, 43, 100470. [Google Scholar] [CrossRef]
- Roxane, M.; Erik, B.; Young, F.; Selwyn, R.; Iacono, D.; Rittase, W.B.; Regina, M. Day Chapter Two—Effects of radiation on endothelial barrier and vascular integrity. In Tissue Barriers in Disease, Injury and Regeneration; Elsevier: Amsterdam, The Netherlands, 2021; pp. 43–94. [Google Scholar] [CrossRef]
- Young, E.F.; Smilenov, L.B. Impedance-based surveillance of transient permeability changes in coronary endothelial monolayers after exposure to ionizing radiation. Radiat. Res. 2011, 176, 415–424. [Google Scholar] [CrossRef]
- Guéguen, Y.; Bontemps, A.; Ebrahimian, T.G. Adaptive responses to low doses of radiation or chemicals: Their cellular and molecular mechanisms. Cell Mol. Life Sci. 2019, 76, 1255–1273. [Google Scholar] [CrossRef]
- Baselet, B.; Sonveaux, P.; Baatout, S.; Aerts, A. Pathological effects of ionizing radiation: Endothelial activation and dysfunction. Cell Mol. Life Sci. 2019, 76, 699–728. [Google Scholar] [CrossRef] [Green Version]
- Cheki, M.; Shirazi, A.; Mahmoudzadeh, A.; Bazzaz, J.T.; Hosseinimehr, S.J. The radioprotective effect of metformin against cytotoxicity and genotoxicity induced by ionizing radiation in cultured human blood lymphocytes. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2016, 809, 24–32. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, B.; Zhang, H.; Wang, J. Erythrocyte stiffness during morphological remodeling induced by carbon ion radiation. PLoS ONE 2014, 9, e112624. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Hu, M.; Chen, Z.; Ling, Z. The roles and mechanisms of hypoxia in liver fibrosis. J. Transl. Med. 2021, 19, 186. [Google Scholar] [CrossRef] [PubMed]
- Kataru, R.P.; Wiser, I.; Baik, J.E.; Park, H.J.; Rehal, S.; Shin, J.Y.; Mehrara, B.J. Fibrosis and secondary lymphedema: Chicken or egg? Transl. Res. 2019, 209, 68–76. [Google Scholar] [CrossRef]
- Mortimer, P.S.; Rockson, S.G. New developments in clinical aspects of lymphatic disease. J. Clin. Investig. 2014, 124, 915–921. [Google Scholar] [CrossRef] [PubMed]
- Rutkowski, J.M.; Moya, M.; Johannes, J.; Goldman, J.; Swartz, M.A. Secondary lymphedema in the mouse tail: Lymphatic hyperplasia, VEGF-C upregulation, and the protective role of MMP-9. Microvasc. Res. 2006, 72, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Mihara, M.; Hara, H.; Hayashi, Y.; Narushima, M.; Yamamoto, T.; Todokoro, T.; Iida, T.; Sawamoto, N.; Araki, J.; Kikuchi, K.; et al. Pathological steps of cancer-related lymphedema: Histological changes in the collecting lymphatic vessels after lymphadenectomy. PLoS ONE 2012, 7, e41126. [Google Scholar] [CrossRef]
- Ly, C.L.; Kataru, R.P.; Mehrara, B.J. Inflammatory Manifestations of Lymphedema. Int. J. Mol. Sci. 2017, 18, 171. [Google Scholar] [CrossRef] [PubMed]
- Shin, K.; Kataru, R.P.; Park, H.J.; Kwon, B.I.; Kim, T.W.; Hong, Y.K.; Lee, S.H. TH2 cells and their cytokines regulate formation and function of lymphatic vessels. Nat. Commun. 2015, 6, 6196. [Google Scholar] [CrossRef]
- Shaitelman, S.F.; Cromwell, K.D.; Rasmussen, J.C.; Stout, N.L.; Armer, J.M.; Lasinski, B.B.; Cormier, J.N. Recent progress in the treatment and prevention of cancer-related lymphedema. CA Cancer J. Clin. 2015, 65, 55–81. [Google Scholar] [CrossRef] [Green Version]
- Henderson, N.C.; Rieder, F.; Wynn, T.A. Fibrosis: From mechanisms to medicines. Nature 2020, 587, 555–566. [Google Scholar] [CrossRef]
- Chen, Z.; Wu, Z.; Ning, W. Advances in Molecular Mechanisms and Treatment of Radiation-Induced Pulmonary Fibrosis. Transl. Oncol. 2019, 12, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Guarino, M.; Tosoni, A.; Nebuloni, M. Direct contribution of epithelium to organ fibrosis: Epithelial-mesenchymal transition. Hum. Pathol. 2009, 40, 1365–1376. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.H.; Chen, D.Q.; Wang, Y.N.; Feng, Y.L.; Cao, G.; Vaziri, N.D.; Zhao, Y.Y. New insights into TGF-β/Smad signaling in tissue fibrosis. Chem. Biol. Interact. 2018, 292, 76–83. [Google Scholar] [CrossRef]
- Caja, L.; Dituri, F.; Mancarella, S.; Caballero-Diaz, D.; Moustakas, A.; Giannelli, G.; Fabregat, I. TGF-β and the Tissue Microenvironment: Relevance in Fibrosis and Cancer. Int. J. Mol. Sci. 2018, 19, 1294. [Google Scholar] [CrossRef] [PubMed]
- Horiguchi, M.; Ota, M.; Rifkin, D.B. Matrix control of transforming growth factor-β function. J. Biochem. 2012, 152, 321–329. [Google Scholar] [CrossRef]
- Zilberberg, L.; Todorovic, V.; Dabovic, B.; Horiguchi, M.; Couroussé, T.; Sakai, L.Y.; Rifkin, D.B. Specificity of latent TGF-β binding protein (LTBP) incorporation into matrix: Role of fibrillins and fibronectin. J. Cell Physiol. 2012, 227, 3828–3836. [Google Scholar] [CrossRef]
- Richter, K.; Kietzmann, T. Reactive oxygen species and fibrosis: Further evidence of a significant liaison. Cell Tissue Res. 2016, 365, 591–605. [Google Scholar] [CrossRef]
- Imaizumi, N.; Monnier, Y.; Hegi, M.; Mirimanoff, R.O.; Rüegg, C. Radiotherapy suppresses angiogenesis in mice through TGF-betaRI/ALK5-dependent inhibition of endothelial cell sprouting. PLoS ONE 2010, 5, e11084. [Google Scholar] [CrossRef]
- Chai, Y.; Calaf, G.M.; Zhou, H.; Ghandhi, S.A.; Elliston, C.D.; Wen, G.; Nohmi, T.; Amundson, S.A.; Hei, T.K. Radiation induced COX-2 expression and mutagenesis at non-targeted lung tissues of gpt delta transgenic mice. Br. J. Cancer. 2013, 108, 91–98. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Zhai, Y.; Xi, B.; Ma, W.; Zhang, J.; Ma, X.; Miao, Y.; Zhao, Y.; Ning, W.; Zhou, H.; et al. Pinocembrin Ameliorates Skin Fibrosis via Inhibiting TGF-β1 Signaling Pathway. Biomolecules 2021, 11, 1240. [Google Scholar] [CrossRef]
- Zhang, Z.; Gao, X.; He, Y.; Kang, Y.; Jin, F.; Li, Y.; Li, T.; Wei, Z.; Li, S.; Cai, W.; et al. MicroRNA-411-3p inhibits bleomycin-induced skin fibrosis by regulating transforming growth factor-β/Smad ubiquitin regulatory factor-2 signalling. J. Cell Mol. Med. 2021, 25, 11290–11299. [Google Scholar] [CrossRef] [PubMed]
- Puthawala, K.; Hadjiangelis, N.; Jacoby, S.C.; Bayongan, E.; Zhao, Z.; Yang, Z.; Devitt, M.L.; Horan, G.S.; Weinreb, P.H.; Lukashev, M.E.; et al. Inhibition of integrin alpha(v)beta6, an activator of latent transforming growth factor-beta, prevents radiation-induced lung fibrosis. Am. J. Respir. Crit. Care Med. 2008, 177, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Duan, L.; Yuan, S.; Zhuang, X.; Qiao, T.; He, J. Ferroptosis inhibitor alleviates Radiation-induced lung fibrosis (RILF) via down-regulation of TGF-β1. J. Inflamm. 2019, 16, 11. [Google Scholar] [CrossRef] [PubMed]
- Di, S.; Ziyou, Y.; Liu, N.F. Pathological Changes of Lymphedematous Skin: Increased Mast Cells, Related Proteases, and Activated Transforming Growth Factor-β1. Lymphat. Res. Biol. 2016, 14, 162–171. [Google Scholar] [CrossRef]
- Avraham, T.; Yan, A.; Zampell, J.C.; Daluvoy, S.V.; Haimovitz-Friedman, A.; Cordeiro, A.P.; Mehrara, B.J. Radiation therapy causes loss of dermal lymphatic vessels and interferes with lymphatic function by TGF-beta1-mediated tissue fibrosis. Am. J. Physiol. Cell Physiol. 2010, 299, C589–C605. [Google Scholar] [CrossRef]
- Guo, S.; Colbert, L.S.; Fuller, M.; Zhang, Y.; Gonzalez-Perez, R.R. Vascular endothelial growth factor receptor-2 in breast cancer. Biochim Biophys Acta. 2010, 1806, 108–121. [Google Scholar] [CrossRef]
- Holmes, K.; Roberts, O.L.; Thomas, A.M.; Cross, M.J. Vascular endothelial growth factor receptor-2: Structure, function, intracellular signalling and therapeutic inhibition. Cell Signal 2007, 19, 2003–2012. [Google Scholar] [CrossRef]
- Park, S.A.; Jeong, M.S.; Ha, K.T.; Jang, S.B. Structure and function of vascular endothelial growth factor and its receptor system. BMB Rep. 2018, 51, 73–78. [Google Scholar] [CrossRef]
- Apte, R.S.; Chen, D.S.; Ferrara, N. VEGF in Signaling and Disease: Beyond Discovery and Development. Cell 2019, 176, 1248–1264. [Google Scholar] [CrossRef] [Green Version]
- Fu, M.R.; Aouizerat, B.E.; Yu, G.; Conley, Y.; Axelrod, D.; Guth, A.A.; Gagner, J.P.; Qiu, J.M.; Zagzag, D. Model-Based Patterns of Lymphedema Symptomatology: Phenotypic and Biomarker Characterization. Curr. Breast Cancer Rep. 2021, 13, 1–18. [Google Scholar] [CrossRef]
- Visser, J.; van Geel, M.; Cornelissen, A.J.M.; van der Hulst, R.R.W.J.; Qiu, S.S. Breast Cancer-Related Lymphedema and Genetic Predisposition: A Systematic Review of the Literature. Lymphat. Res. Biol. 2019, 17, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Arpino, V.; Brock, M.; Gill, S.E. The role of TIMPs in regulation of extracellular matrix proteolysis. Matrix Biol. 2015, 44–46, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Brew, K.; Nagase, H. The tissue inhibitors of metalloproteinases (TIMPs): An ancient family with structural and functional diversity. Biochim. Biophys. Acta 2010, 1803, 55–71. [Google Scholar] [CrossRef] [PubMed]
- Seo, D.W.; Li, H.; Guedez, L.; Wingfield, P.T.; Diaz, T.; Salloum, R.; Wei, B.Y.; Stetler-Stevenson, W.G. TIMP-2 mediated inhibition of angiogenesis: An MMP-independent mechanism. Cell 2003, 114, 171–180. [Google Scholar] [CrossRef]
- Ratajczak-Wielgomas, K.; Gosk, J.; Rabczyński, J.; Augoff, K.; Podhorska-Okołów, M.; Gamian, A.; Rutowski, R. Expression of MMP-2, TIMP-2, TGF-β1, and decorin in Dupuytren’s contracture. Connect. Tissue Res. 2012, 53, 469–477. [Google Scholar] [CrossRef]
- Aoki, M.; Miyake, K.; Ogawa, R.; Dohi, T.; Akaishi, S.; Hyakusoku, H.; Shimada, T. siRNA knockdown of tissue inhibitor of metalloproteinase-1 in keloid fibroblasts leads to degradation of collagen type I. J. Investig. Dermatol. 2014, 134, 818–826. [Google Scholar] [CrossRef]
- Lluri, G.; Langlois, G.D.; McClellan, B.; Soloway, P.D.; Jaworski, D.M. Tissue inhibitor of metalloproteinase-2 (TIMP-2) regulates neuromuscular junction development via a beta1 integrin-mediated mechanism. J. Neurobiol. 2006, 66, 1365–1377. [Google Scholar] [CrossRef]
- Peuckmann, V.; Ekholm, O.; Rasmussen, N.K.; Groenvold, M.; Christiansen, P.; Møller, S.; Eriksen, J.; Sjøgren, P. Chronic pain and other sequelae in long-term breast cancer survivors: Nationwide survey in Denmark. Eur. J. Pain. 2009, 13, 478–485. [Google Scholar] [CrossRef]
- International Society of Lymphology. The diagnosis and treatment of peripheral lymphedema: 2013 Consensus Document of the International Society of Lymphology. Lymphology 2013, 46, 1–11. [Google Scholar]
- Pappalardo, M.; Cheng, M.H. Lymphoscintigraphy for the diagnosis of extremity lymphedema: Current controversies regarding protocol, interpretation, and clinical application. J. Surg. Oncol. 2020, 121, 37–47. [Google Scholar] [CrossRef] [Green Version]
- Lodyga, M.; Hinz, B. TGF-β1—A truly transforming growth factor in fibrosis and immunity. Semin Cell Dev. Biol. 2020, 101, 123–139. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, Y.; Eren, F. Serum biomarkers of fibrosis and extracellular matrix remodeling in patients with nonalcoholic fatty liver disease: Association with liver histology. Eur. J. Gastroenterol. Hepatol. 2019, 31, 43–46. [Google Scholar] [CrossRef] [PubMed]
- Rossato, F.A.; Su, Y.; Mackey, A.; Ng, Y.S.E. Fibrotic Changes and Endothelial-to-Mesenchymal Transition Promoted by VEGFR2 Antagonism Alter the Therapeutic Effects of VEGFA Pathway Blockage in a Mouse Model of Choroidal Neovascularization. Cells 2020, 9, 2057. [Google Scholar] [CrossRef]
- Lyngholm, C.D.; Christiansen, P.M.; Damsgaard, T.E.; Overgaard, J. Long-term follow-up of late morbidity, cosmetic outcome and body image after breast conserving therapy. A study from the Danish Breast Cancer Cooperative Group (DBCG). Acta Oncol. 2013, 52, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Melincovici, C.S.; Boşca, A.B.; Şuşman, S.; Mărginean, M.; Mihu, C.; Istrate, M.; Moldovan, I.M.; Roman, A.L.; Mihu, C.M. Vascular endothelial growth factor (VEGF)—key factor in normal and pathological angiogenesis. Rom. J. Morphol. Embryol. 2018, 59, 455–467. [Google Scholar]
- Haubner, F.; Ohmann, E.; Pohl, F.; Prantl, L.; Strutz, J.; Gassner, H.G. Effects of radiation on the expression of adhesion molecules and cytokines in a static model of human dermal microvascular endothelial cells. Clin. Hemorheol. Microcirc. 2013, 54, 371–379. [Google Scholar] [CrossRef]
- Liang, X.; Zheng, S.; Cui, J.; Yu, D.; Yang, G.; Zhou, L.; Wang, B.; Cai, L.; Li, W. Alterations of MicroRNA Expression in the Liver, Heart, and Testis of Mice Upon Exposure to Repeated Low-Dose Radiation. Dose Response 2018, 16, 1559325818799561. [Google Scholar] [CrossRef]
- Wang, B.; Wang, H.; Zhang, M.; Ji, R.; Wei, J.; Xin, Y.; Jiang, X. Radiation-induced myocardial fibrosis: Mechanisms underlying its pathogenesis and therapeutic strategies. J. Cell Mol. Med. 2020, 24, 7717–7729. [Google Scholar] [CrossRef]
- Grainger, D.J. Transforming growth factor beta and atherosclerosis: So far, so good for the protective cytokine hypothesis. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 399–404. [Google Scholar] [CrossRef]
- Battegay, E.J.; Raines, E.W.; Seifert, R.A.; Bowen-Pope, D.F.; Ross, R. TGF-beta induces bimodal proliferation of connective tissue cells via complex control of an autocrine PDGF loop. Cell 1990, 63, 515–524. [Google Scholar] [CrossRef]
- Shull, M.M.; Ormsby, I.; Kier, A.B.; Pawlowski, S.; Diebold, R.J.; Yin, M.; Allen, R.; Sidman, C.; Proetzel, G.; Calvin, D.; et al. Targeted disruption of the mouse transforming growth factor-β 1 gene results in multifocal inflammatory disease. Nature 1992, 359, 693–699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gamble, J.R.; Khew-Goodall, Y.; Vadas, M.A. Transforming growth factor-beta inhibits E-selectin expression on human endothelial cells. J. Immunol. 1993, 150, 4494–4503. [Google Scholar] [PubMed]
- Pospelova, M.; Krasnikova, V.; Fionik, O.; Alekseeva, T.; Samochernykh, K.; Ivanova, N.; Trofimov, N.; Vavilova, T.; Vasilieva, E.; Topuzova, M.; et al. Adhesion Molecules ICAM-1 and PECAM-1 as Potential Biomarkers of Central Nervous System Damage in Women Breast Cancer Survivors. Pathophysiology 2022, 29, 52–65. [Google Scholar] [CrossRef] [PubMed]
- Hoefer, J.; Dal-Pont, C.; Jochberger, S.; Fantin, R.; Schennach, H. The ‘rejuvenating factor’ TIMP-2 is detectable in human blood components for transfusion. Vox Sang 2021, 116, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Hurria, A.; Somlo, G.; Ahles, T. Renaming “chemobrain”. Cancer Investig. 2007, 25, 373–377. [Google Scholar] [CrossRef]
- Ahles, T.A.; Saykin, A.J. Candidate mechanisms for chemotherapy-induced cognitive changes. Nat Rev. Cancer. 2007, 7, 192–201. [Google Scholar] [CrossRef]
- Tzavlaki, K.; Moustakas, A. TGF-β Signaling. Biomolecules 2020, 10, 487. [Google Scholar] [CrossRef]
- Roldán, V.; Marín, F.; Gimeno, J.R.; Ruiz-Espejo, F.; González, J.; Feliu, E.; García-Honrubia, A.; Saura, D.; de la Morena, G.; Valdés, M.; et al. Matrix metalloproteinases and tissue remodeling in hypertrophic cardiomyopathy. Am. Heart J. 2008, 156, 85–91. [Google Scholar] [CrossRef]
- Nastri, C.O.; Martins Wde, P.; Reis, F.J.; Ferriani, R.A. Câncer de mama e disfunção endotelial [Breast cancer and endothelial dysfunction]. Rev. Assoc. Med. Bras. 2008, 54, 467–470. [Google Scholar] [CrossRef]
- Stouten-Kemperman, M.M.; de Ruiter, M.B.; Koppelmans, V.; Boogerd, W.; Reneman, L.; Schagen, S.B. Neurotoxicity in breast cancer survivors ≥10 years post-treatment is dependent on treatment type. Brain Imaging Behav. 2015, 9, 275–284. [Google Scholar] [CrossRef]
- Hartmann, M.C.; Dwyer, R.M.; Costello, M.; Potter, S.M.; Curran, C.; Hennessy, E.; Newell, J.; Griffin, D.G.; Kerin, M.J. Relationship between CCL5 and transforming growth factor-β1 (TGFβ1) in breast cancer. Eur. J. Cancer. 2011, 47, 1669–1675. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Qian, K.; Sun, Y.; Xiao, L.; Shi, X. Application of TGF-β1, TIMP-1 and TIMP-2 small interfering RNAs can alleviate CCl4-induced hepatic fibrosis in rats by rebalancing Th1/Th2 cytokines. Exp. Ther. Med. 2021, 22, 963. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Tsang, P.S.; Diaz, T.M.; Wei, B.Y.; Stetler-Stevenson, W.G. TIMP-2 modulates VEGFR-2 phosphorylation and enhances phosphodiesterase activity in endothelial cells. Lab. Investig. 2010, 90, 374–382. [Google Scholar] [CrossRef] [PubMed]
- Westergren-Thorsson, G.; Bagher, M.; Andersson-Sjöland, A.; Thiman, L.; Löfdahl, C.G.; Hallgren, O.; Bjermer, L.; Larsson-Callerfelt, A.K. VEGF synthesis is induced by prostacyClin. and TGF-β in distal lung fibroblasts from COPD patients and control subjects: Implications for pulmonary vascular remodelling. Respirology 2018, 23, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Kim, L.B.; Russkih, G.S.; Putyatina, A.N.; Tsypysheva, O.B. Age-related dynamics of the contents of matrix metalloproteinases (MMP-1, -2, -3, -9) and tissue inhibitors of matrix metalloproteinases (TIMP-1, -2, -4) in blood plasma of residents of the European part of the Arctic zone of the Russian Federation. Adv. Gerontol. 2018, 31, 223–230. [Google Scholar] [CrossRef]


| Group Characteristics of Patients | Patients after Breast Cancer Treatment n = 67 | Healthy n = 25 |
|---|---|---|
| Age (years) | 47.0 [44; 49] | 42.0 [38; 47] |
| Years since treatment | 3.0 [2; 5] | - |
| Number of patients TNM stage | ||
| I (T1N0M0) | 8 (12%) | - |
| II A (T2N1M0) | 46 (68%) | - |
| II B (T3N1M0) | 3 (5%) | - |
| III A (T3N2M0) | 2 (3%) | - |
| III B (T4N2M0) | 8 (12%) | - |
| Types of breast cancer | ||
| Ductal carcinoma in situ (DCIS) | 7 (11%) | - |
| Invasive ductal carcinoma (IDC) | 49 (73%) | - |
| Invasive lobular carcinoma (ILC) | 11 (16%) | - |
| Breast cancer hormone receptor status | ||
| Hormone receptor-positive (HR+) | 55 (72%) | - |
| Hormone receptor-negative (HR−) | 12 (18%) | - |
| Major pathological grades of breast cancer | ||
| Grade 1 | 10 (15%) | - |
| Grade 2 | 35 (52%) | - |
| Grade 3 | 22 (33%) | - |
| Treatment of breast cancer | ||
| Complex treatment (surgical treatment, radiotherapy, chemotherapy) | 37 (55%) | - |
| Combination of surgical treatment and chemotherapy | 18 (27%) | - |
| Combination of surgical treatment and radiotherapy | 7 (10%) | - |
| Only surgical treatment | 5 (7%) | - |
| Type of surgical treatment | ||
| Modified unilateral mastectomy Madden | 53 (79%) | - |
| Sector mastectomy | 14 (21%) | - |
| Hormonal therapy (tamoxifen vs. GH-LH analogues) | ||
| Do not take the medicine | 12 (18%) | |
| Take the medicine | 50 (75%) | |
| Completed the course | 5 (7%) | |
| Clinical Characteristics | Number of Patients (N, %) |
|---|---|
| Restriction of movement in the shoulder | 37 (55%) |
| Lymphedema of the arm | 27 (40%) |
| Change Type | Number of Patients (N, %) |
|---|---|
| Dermal backflow | 36 (53%) |
| Compensatory changes | 31 (47%) |
| Dermal backflow without clinical lymphedema | 10 (15%) |
| Clinical lymphedema without dermal backflow | 8 (12%) |
| Fibrosis Molecules | Patients (n = 67) | Healthy (n = 25) | Mann–Whitney U-Test | Significance (p) |
|---|---|---|---|---|
| TGF-β1 | 6356 [551; 11,706] | 17,374 [8802; 17,152] | 666 | <0.001 * |
| VEGFR2 | 17,750 [6865; 30,200] | 20,850 [10,137; 35,402] | 1345 | 0.082 |
| TIMP-2 | 85 [74; 95] | 100 [92; 113] | 637 | <0.001 * |
| Sign of Separation | Characteristic of the Sign | Number of Patients (and Age) | TGF-β1 | Kruskal–Wallis Test | p | VEGFR2 | Kruskal–Wallis Test | p | TIMP-2 | Kruskal–Wallis Test | p |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Presence of lymphedema (LE) | yes | 27 (42.0 [40; 46]) | 6087 [1065; 1008] | 32.231 | <0.001 * | 16,328 [8224; 21,223] | 3.127 | 0.209 | 82 [75; 92] | 30.749 | <0.001 * |
| no | 40 (48.0 [46; 50]) | 6498 [292; 11,229] | 19,050 [6086; 31,110] | 88 [72; 99] | |||||||
| Limitation of movement in the shoulder joint (LSh) | yes | 37 (47.5 [43.5; 49]) | 8277 [3502; 9223] | 22.589 | <0.001 * | 9223 [7559; 12,122] | 2.328 | 0.312 | 91 [77; 101] | 22.589 | <0.001 * |
| no | 30 (44.6 [41; 46.5]) | 6932 [1823; 8993] | 16,468 [8014; 25,183] | 81 [74; 97] | |||||||
| Hormone receptor status of breast cancer (HRS) | HR+ | 55 (42.0 [39; 47.4]) | 6356 [512; 10,133] | 21.62 | <0.001 * | 18,250 [6749; 28,800] | 4.344 | 0.227 | 86 [75; 96] | 24.397 | <0.001 * |
| HR− | 12 (47.4 [44; 49]) | 6230 [1950; 1284] | 13,355 [9022; 29,200] | 81 [71; 101] | |||||||
| Major pathological grades of breast cancer (G) | G1 | 10 (48.0 [43; 49]) | 6041 [627; 8749] | 34.061 | <0.001 * | 22,750 [12,248; 36,509] | 4.274 | 0.233 | 73 [69; 82] | 43.015 | <0.001 * |
| G2 | 35 (47.0 [44; 48]) | 6340 [870; 9628] | 17,450 [6321; 24,950] | 91 [82; 101] | |||||||
| G3 | 22 (46.0 [42; 49]) | 4811 [864; 12,505] | 15,132 [10,096; 20,100] | 81 [72; 87] | |||||||
| Treatment history | Only surgical treatment (OS) | 5 (45.0 [43; 48.7]) | 7941 [7437; 14,676] | 37.287 | <0.001 * | 6086 [4373; 23,050] | 2.261 | 0.262 | 93 [85; 102] | 36.643 | <0.001 * |
| Surgical treatment and radiotherapy (S + R) | 7 (46.5 [44; 48]) | 8868 [7604; 11,705] | 3574 [3508; 13,612] | 91 [84; 101] | |||||||
| Surgical treatment and chemotherapy (S + Ch) | 18 (46.0 [43.8; 49]) | 5725 [959; 8307] | 14,391 [6787; 30,200] | 81 [68; 94] | |||||||
| Complex treatment (CT) | 37 (47.0 [44; 49)] | 18,250 [9022; 31,995] | 18,250 [9022; 31,995] | 87 [73; 96] | |||||||
| Operation type | Modified unilateral mastectomy Madden (M) | 53 (45.3 [42.5; 47]) | 6940 [870; 12,568] | 36.297 | <0.001 * | 18,050 [7255; 27,550] | 4.221 | 0.239 | 87 [75; 98] | 31.876 | <0.001 * |
| Sector mastectomy (SM) | 14 (47,8 [44.8; 49)] | 7437 [5460; 11,251] | 21,450 [9207; 27,150] | 82 [72; 95] | |||||||
| Lymphoscintigraphy changes | Dermal backflow (DB) | 36 (48.0 [45; 49]) | 4876 [704; 9787] | 22.526 | <0.001 * | 19,650 [8392; 31,529] | 4.029 | 0.258 | 82 [73; 94] | 22.757 | <0.001 * |
| Compensatory changes (CCh) | 31 (46.3 [43.3; 47]) | 6624 [704; 12,381] | 15,058 [5930; 23,350] | 83 [74; 101] | |||||||
| Changes in lymphoscintigraphy with clinic | Dermal backflow without clinical lymphedema (DB without LY) | 10 (44.0 [42; 46]) | 2412 [152; 6349] | 21.152 | <0.001 * | 28,880 [7657; 42,449] | 3.567 | 0.312 | 89 [75; 95] | 23.784 | <0.001 * |
| Clinical lymphedema without dermal backflow (LY without DB) | 8 (47.0 [44; 49)] | 6024 [777; 9707] | 15,132 [9121; 19,145] | 85 [78; 91] |
| Sign of Separation | (I) Criterion | (J) Criterion | Mean Difference (I-J) | Std. Error | p | 95% Confidence Interval (CI) | |
|---|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | ||||||
| LY | Healthy | Yes | 7076 | 1528 | <0.001 * | 3405 | 10,075 |
| Healthy | No | 6979 | 1530 | <0.001 * | 3309 | 10,650 | |
| Yes | No | −96 | 1487 | 1 | −3669 | 3576 | |
| LSh | Healthy | Yes | 7705 | 1668 | <0.001 * | 3670 | 9293 |
| Healthy | No | 6668 | 1445 | <0.001 * | 3205 | 9223 | |
| Yes | No | −288 | 4102 | 0.78 | −4799 | 2727 | |
| HRS | Healthy | HR+ | 7315 | 1379 | <0.001 * | 4077 | 10,553 |
| Healthy | HR− | 5455 | 2600 | 0.13 | −1392 | 12,303 | |
| HR+ | HR− | −1860 | 2472 | 0.74 | −8540 | 4819 | |
| G | Healthy | G1 | 8013 | 1923 | 0.005 * | 2380 | 9223 |
| Healthy | G2 | 7368 | 1235 | <0.001 * | 4128 | 9223 | |
| Healthy | G3 | 6836 | 1647 | 0.001 * | 2394 | 9223 | |
| G1 | G2 | −644, | 1976 | 0.98 | −6363 | 5073 | |
| G1 | G3 | −1177 | 2257 | 0.95 | −7465 | 5111 | |
| G2 | G3 | −532 | 1709 | 0.98 | −5123 | 4058 | |
| Treatment history | Healthy | OS | 2083 | 3160 | 0.96 | −10,928 | 15,095 |
| Healthy | S + R | 1648 | 2576 | 0.96 | −13,882 | 17,128 | |
| Healthy | S + Ch | 5597 | 1567 | 0.01 * | 1053 | 10,141 | |
| Healthy | CT | 3828 | 1474 | 0.04 * | −517 | 7973 | |
| OS | S + R | −435 | 3883 | 1 | −15,068 | 14,196 | |
| OS | S + Ch | 3513 | 3300 | 0.82 | −9210 | 16,237 | |
| OS | CT | 1745 | 3227 | 0.98 | −11,034 | 1 | |
| S + R | S + Ch | 3949.23 | 2747.39 | 0.65 | −9768.73 | 17,667.19 | |
| S + R | CT | 2180.70 | 2695.26 | 0.91 | −11,926.71 | 16,288.11 | |
| S + Ch | CT | −1768.53 | 1756.00 | 0.85 | −6802.28 | 3265.23 | |
| Operation type | Healthy | SM | 6464.78 | 1293.53 | <0.001 * | 3069.14 | 9860.43 |
| Healthy | M | 5664.95 | 1825.40 | 0.03 * | 417.75 | 10,912.15 | |
| M | Healthy | −799.83 | 1920.63 | 0.98 | −6218.37 | 4618.70 | |
| Lymphoscintigraphy changes | Healthy | DB | 7485.27 | 1548.83 | <0.001 * | 3397.93 | 11,572.61 |
| Healthy | CCh | 6453.91 | 1587.79 | <0.001 * | 2253.70 | 10,654.11 | |
| DB | Cch | 1031.36 | 1566.33 | 0.91 | −3117.97 | 5180.69 | |
| Changes in lymphoscintigraphy with clinic | Healthy | DB without LY | 9164.26 | 2095.13 | <0.001 * | 3198.09 | 15,130.44 |
| Healthy | LY without DB | 7742.97 | 2136.88 | 0.02 * | 1455.36 | 14,030.58 | |
| DB without LY | LY without DB | −1421.30 | 2547.40 | 0.94 | −8728.40 | 5885.80 | |
| Sign of Separation | (I) Criterion | (J) Criterion | Mean Difference (I-J) | Std. Error | p | 95% Confidence Interval (CI) | |
|---|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | ||||||
| LY | Healthy | Yes | 17.52 | 4.64 | <0.001 * | 6.38 | 28.67 |
| Healthy | No | 17.01 | 4.13 | <0.001 * | 7.11 | 26.92 | |
| Yes | No | −0.51 | 4.48 | 0.99 | −11.30 | 10.28 | |
| LSh | Healthy | Yes | 16.58 | 5.61 | 0.02 * | 2.89 | 30.27 |
| Healthy | No | 17.61 | 3.81 | <0.001 * | 8.46 | 26.75 | |
| Yes | No | 1.03 | 5.25 | 0.98 | −11.90 | 13.96 | |
| HRS | Healthy | HR+ | 16.70 | 3.87 | <0.001 * | 7.44 | 25.96 |
| Healthy | HR− | 20.24 | 6.48 | 0.02 * | 3.35 | 37.13 | |
| HR+ | HR− | 3.54 | 6.20 | 0.84 | −12.94 | 20.03 | |
| G | Healthy | G1 | 31.24 | 3.98 | <0.001 * | 20.22 | 42.27 |
| Healthy | G2 | 13.67 | 3.86 | 0.004 * | 3.53 | 23.80 | |
| Healthy | G3 | 23.29 | 4.29 | <0.001 * | 11.84 | 34.74 | |
| G1 | G2 | −17.58 | 4.28 | 0.002 * | −29.29 | −5.86 | |
| G1 | G3 | −7.95 | 4.67 | 0.341 | −20.72 | 4.81 | |
| G2 | G3 | 9.62 | 4.56 | 0.165 | −2.53 | 21.78 | |
| Treatment history | Healthy | OS | 10.97 | 5.80 | 0.41 | −11.10 | 33.04 |
| Healthy | S + R | 9.40 | 10.13 | 0.87 | −59.65 | 78.45 | |
| Healthy | S + Ch | 20.57 | 4.60 | <0.001 * | 7.12 | 34.02 | |
| Healthy | CT | 12.07 | 4.43 | 0.04 * | −0.43 | 24.56 | |
| OS | S + R | −1.57 | 11.18 | 1.00 | −58.42 | 55.29 | |
| OS | S + Ch | 9.60 | 6.60 | 0.61 | −12.63 | 31.83 | |
| OS | CT | 1.10 | 6.48 | 1.00 | −20.86 | 23.06 | |
| S + R | S + Ch | 11.17 | 10.61 | 0.82 | −50.13 | 72.46 | |
| S + R | CT | 2.67 | 10.54 | 1.00 | −59.57 | 64.90 | |
| S + Ch | CT | −8.50 | 5.44 | 0.53 | −24.06 | 7.06 | |
| Operation type | Healthy | SM | 17.03 | 3.99 | <0.001 * | 6.55 | 27.51 |
| Healthy | M | 22.73 | 5.00 | <0.001* | 8.56 | 36.90 | |
| M | Healthy | 5.70 | 5.34 | 0.71 | −9.14 | 20.54 | |
| Lymphoscintigraphy changes | Healthy | DB | 18.03 | 4.65 | <0.001 * | 5.75 | 30.31 |
| Healthy | CCh | 17.32 | 4.36 | <0.001 * | 5.77 | 28.87 | |
| DB | Cch | 0.71 | 4.72 | 1.00 | −11.78 | 13.20 | |
| Changes in lymphoscintigraphy with clinic | Healthy | DB without LY | 17.14 | 5.97 | 0.04 * | 0.03 | 34.25 |
| Healthy | LY without DB | 17.50 | 5.45 | 0.03 * | 1.67 | 33.33 | |
| DB without LY | LY without DB | 0.36 | 6.85 | 1.00 | −19.24 | 19.97 | |
| TGF-β1 | VEGFR2 | TIMP-2 | Age (Years) | Years since Treatment | |
|---|---|---|---|---|---|
| TGF-β1 | - | ρ = −0.264, p = 0.034 * | ρ = 0.328, p = 0.008 * | ρ = 0.093, p = 0.463 | ρ = 0.09, p = 0.474 |
| VEGFR2 | ρ = −0.264, p = 0.034 * | - | ρ = −0.369, p = 0.002 * | ρ = −0.055, p = 0.662 | ρ = 0.105, p = 0.403 |
| TIMP-2 | ρ = 0.328, p = 0.008 * | ρ = −0.369, p = 0.002 * | - | ρ = −0.13, p = 0.919 | ρ = −0.317, p = 0.010 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krasnikova, V.; Pospelova, M.; Fionik, O.; Alekseeva, T.; Samochernykh, K.; Ivanova, N.; Trofimov, N.; Vavilova, T.; Vasilieva, E.; Makhanova, A.; et al. Breast Cancer Treatment Decreases Serum Levels of TGF-β1, VEGFR2, and TIMP-2 Compared to Healthy Volunteers: Significance for Therapeutic Outcomes? Pathophysiology 2022, 29, 537-554. https://doi.org/10.3390/pathophysiology29030042
Krasnikova V, Pospelova M, Fionik O, Alekseeva T, Samochernykh K, Ivanova N, Trofimov N, Vavilova T, Vasilieva E, Makhanova A, et al. Breast Cancer Treatment Decreases Serum Levels of TGF-β1, VEGFR2, and TIMP-2 Compared to Healthy Volunteers: Significance for Therapeutic Outcomes? Pathophysiology. 2022; 29(3):537-554. https://doi.org/10.3390/pathophysiology29030042
Chicago/Turabian StyleKrasnikova, Varvara, Maria Pospelova, Olga Fionik, Tatyana Alekseeva, Konstantin Samochernykh, Nataliya Ivanova, Nikita Trofimov, Tatyana Vavilova, Elena Vasilieva, Albina Makhanova, and et al. 2022. "Breast Cancer Treatment Decreases Serum Levels of TGF-β1, VEGFR2, and TIMP-2 Compared to Healthy Volunteers: Significance for Therapeutic Outcomes?" Pathophysiology 29, no. 3: 537-554. https://doi.org/10.3390/pathophysiology29030042
APA StyleKrasnikova, V., Pospelova, M., Fionik, O., Alekseeva, T., Samochernykh, K., Ivanova, N., Trofimov, N., Vavilova, T., Vasilieva, E., Makhanova, A., Tonyan, S., Nikolaeva, A., Kayumova, E., & Shevtsov, M. (2022). Breast Cancer Treatment Decreases Serum Levels of TGF-β1, VEGFR2, and TIMP-2 Compared to Healthy Volunteers: Significance for Therapeutic Outcomes? Pathophysiology, 29(3), 537-554. https://doi.org/10.3390/pathophysiology29030042










