NO Addition during Gas Oxygenation Reduces Liver and Kidney Injury during Prolonged Cardiopulmonary Bypass
Abstract
:1. Introduction
2. Methods
2.1. Anesthesia and Cardiopulmonary Bypass
2.2. Surgical Procedure
2.3. Adding NO to the Sweep Gas of the Oxygenator
2.4. Assessment of Liver and Renal Function
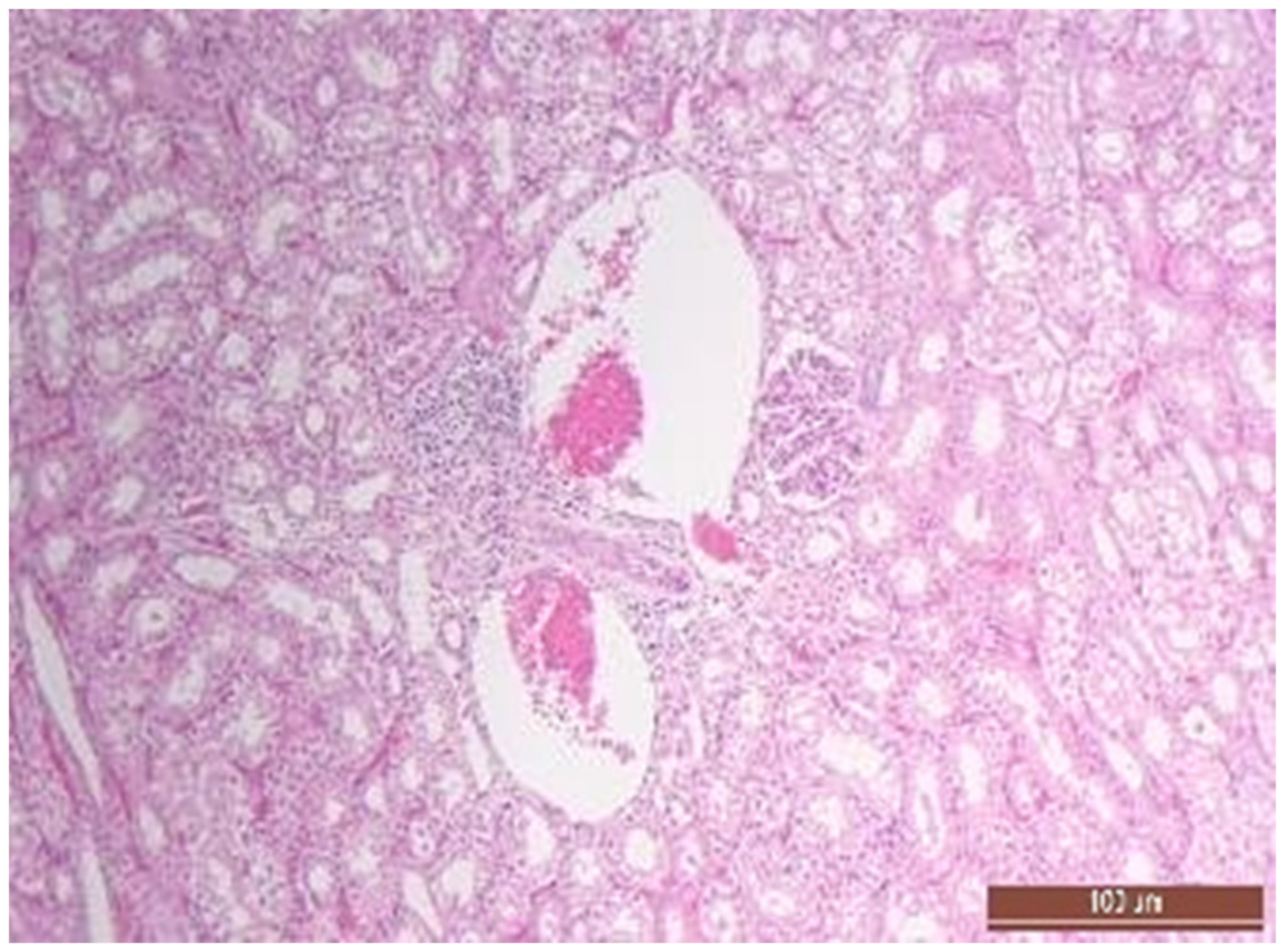
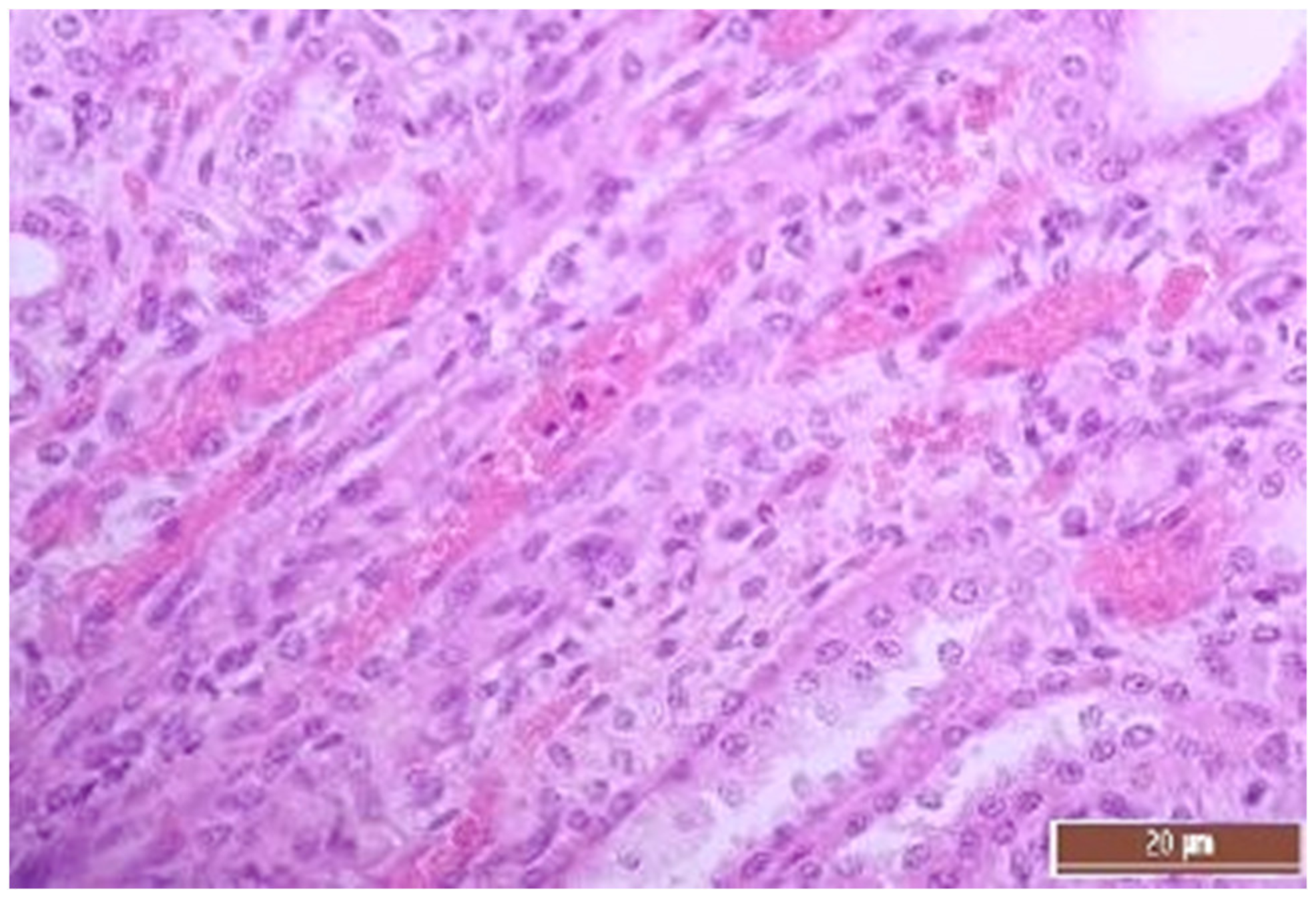
2.5. Pathomorphology
2.6. Statistical Analysis
3. Results
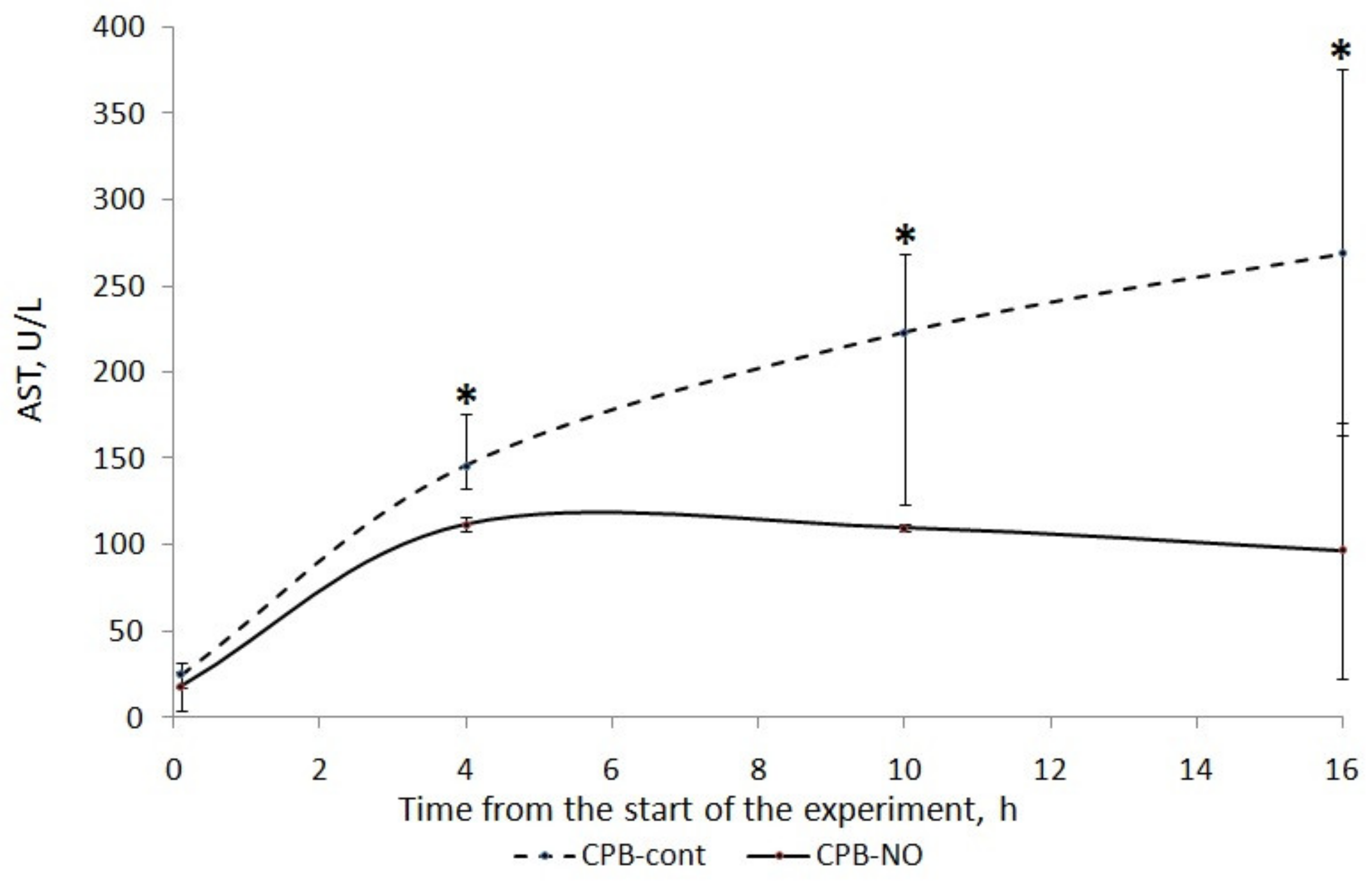
3.1. Assessment of Liver Function
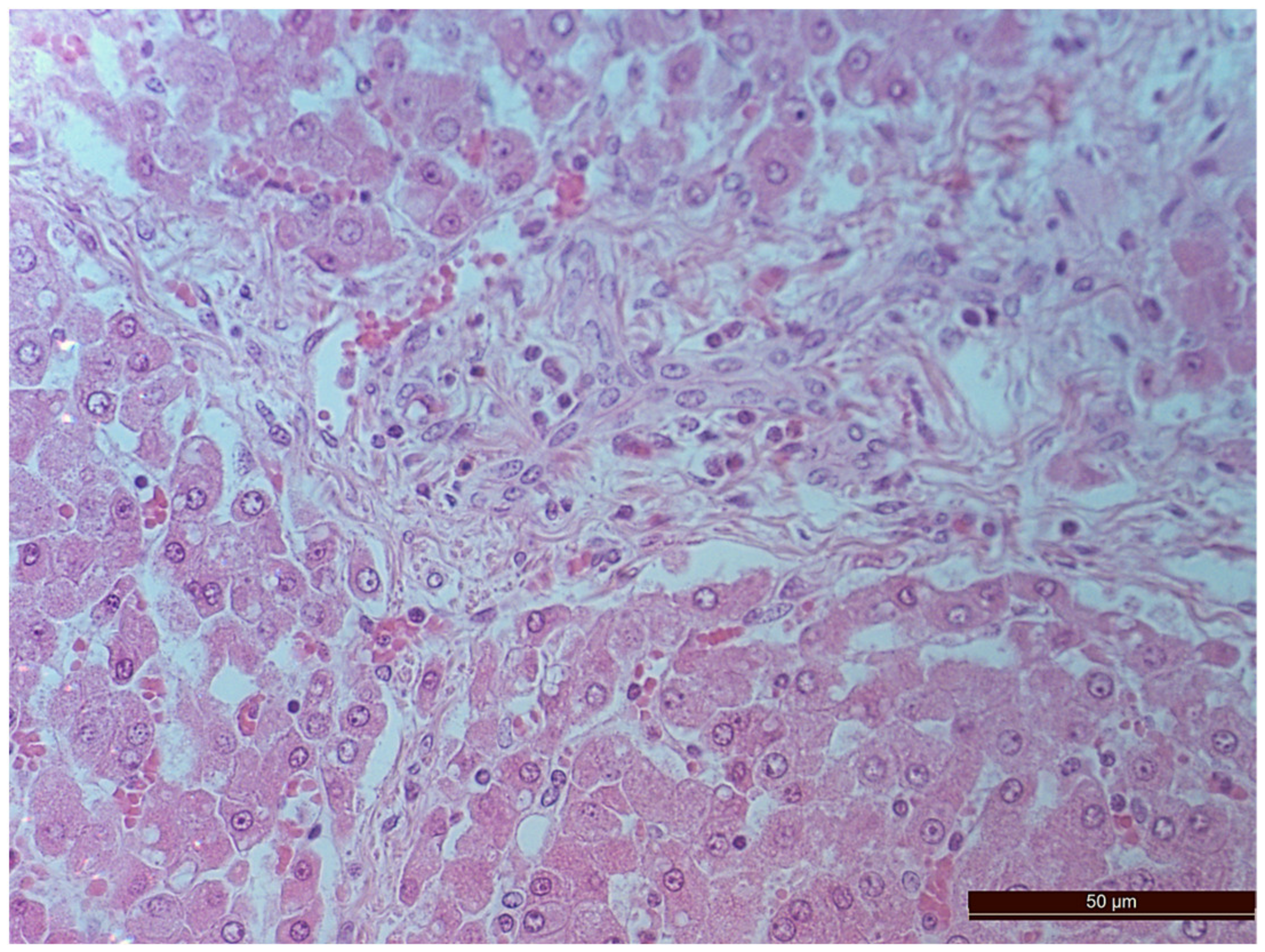
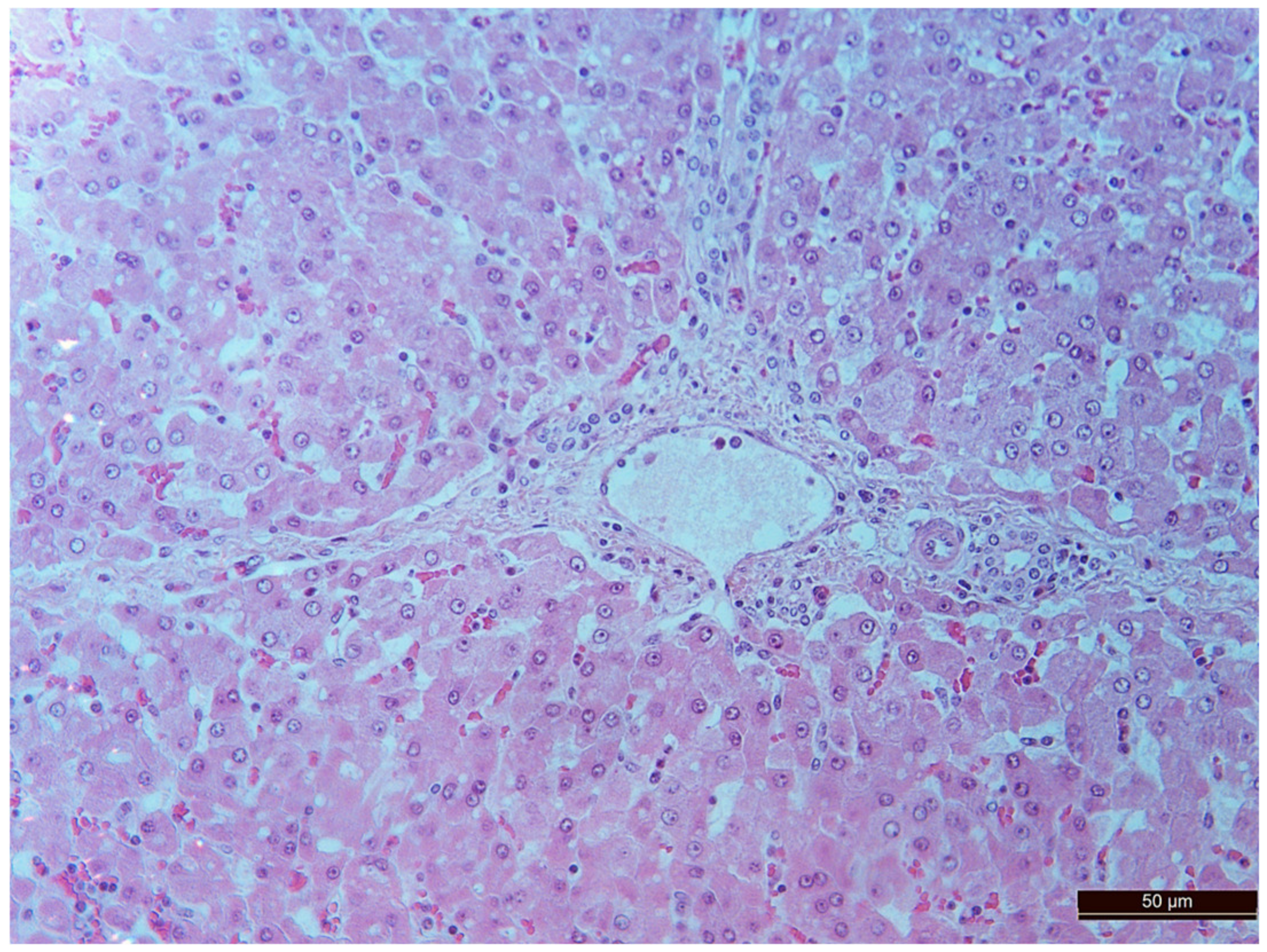
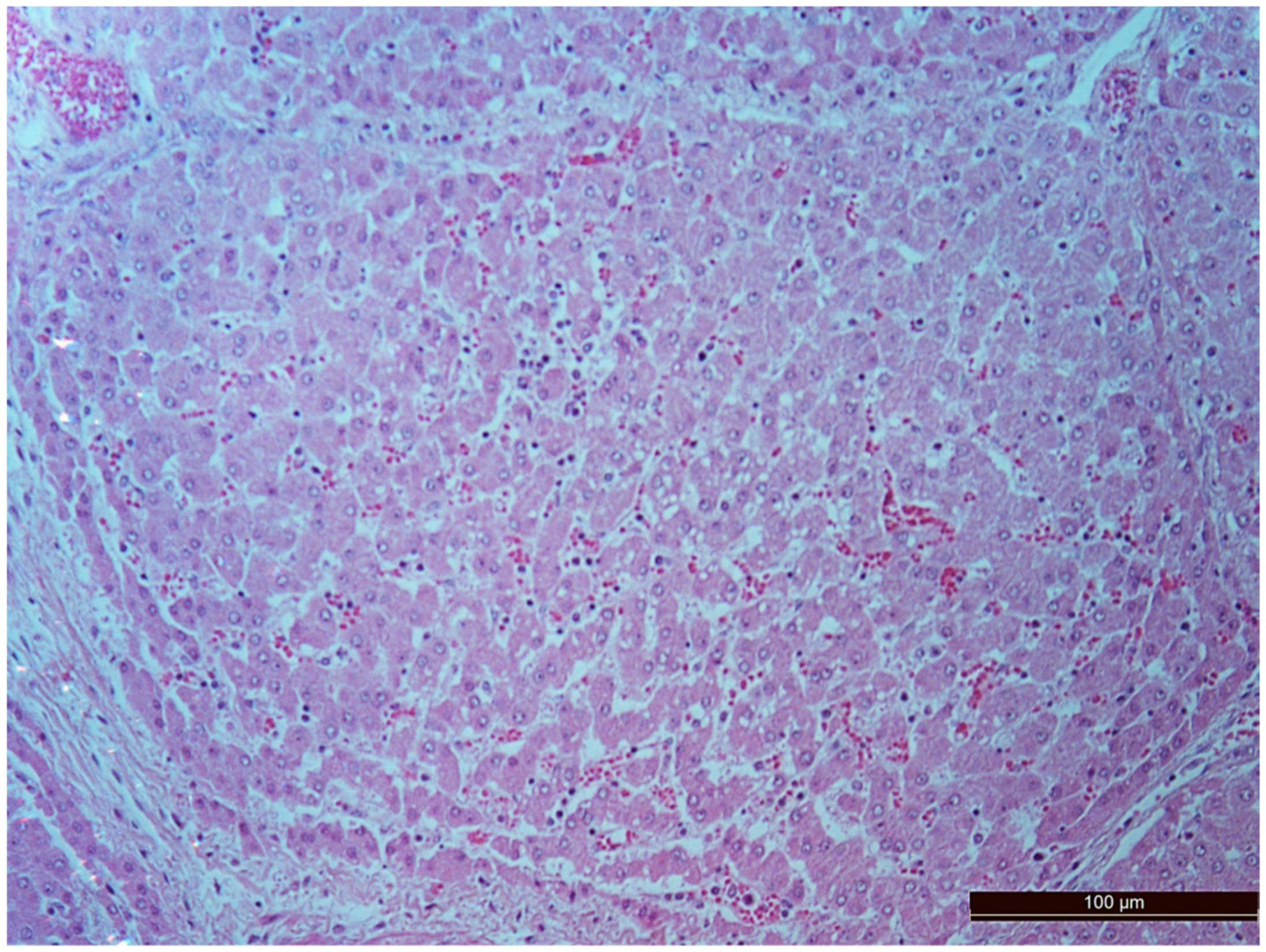
3.2. Pathology of the Liver
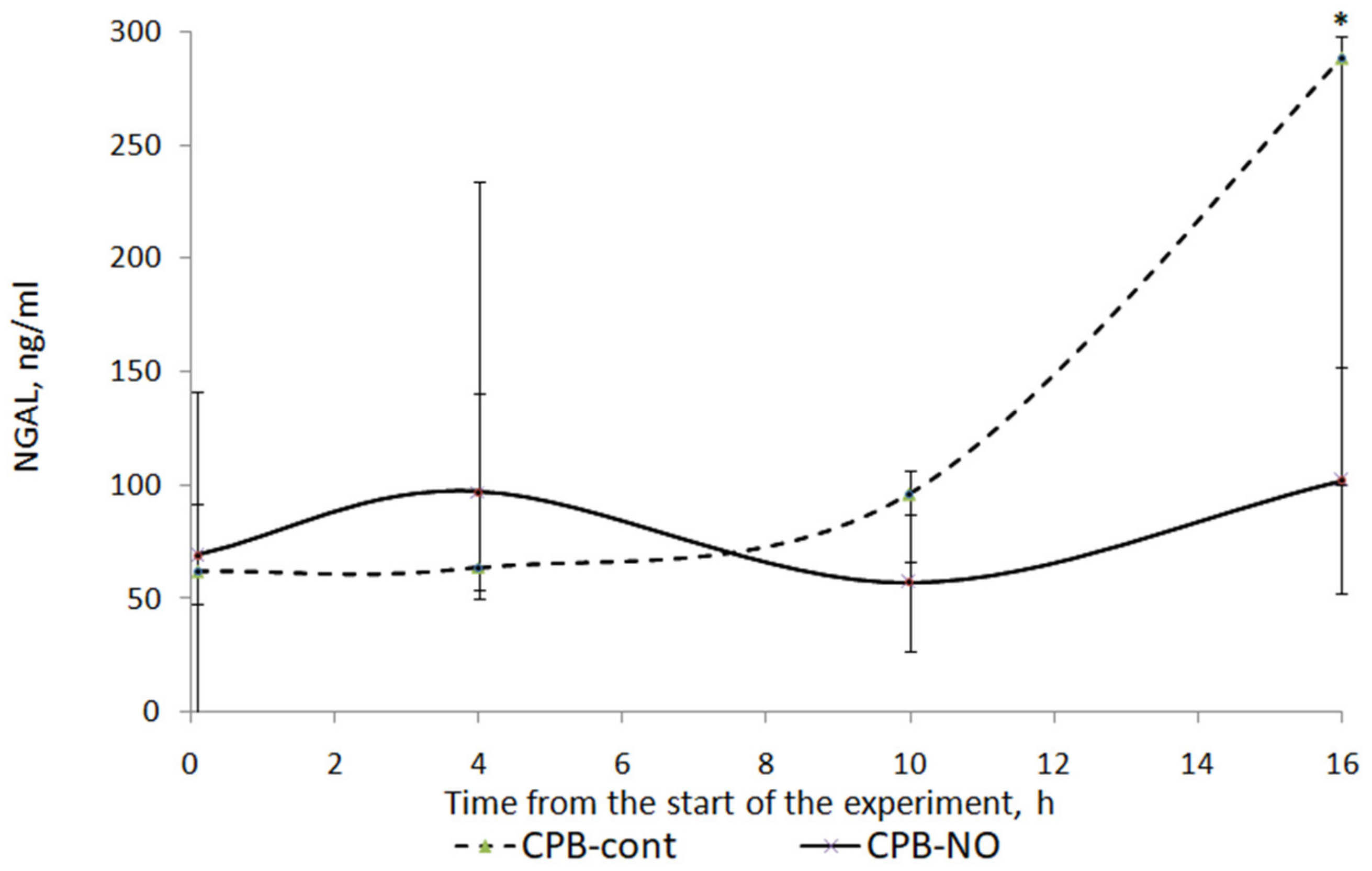
3.3. Renal Function
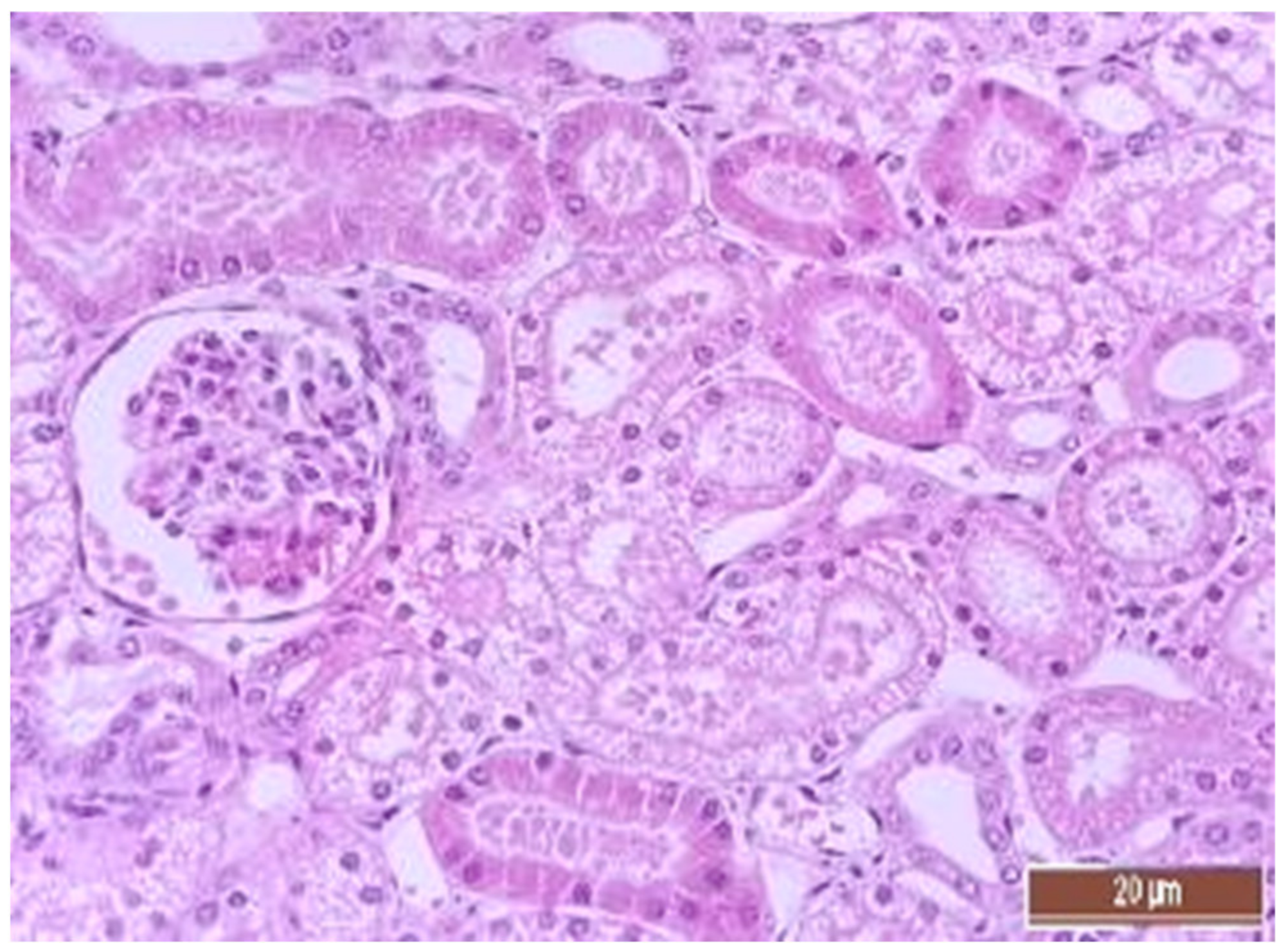
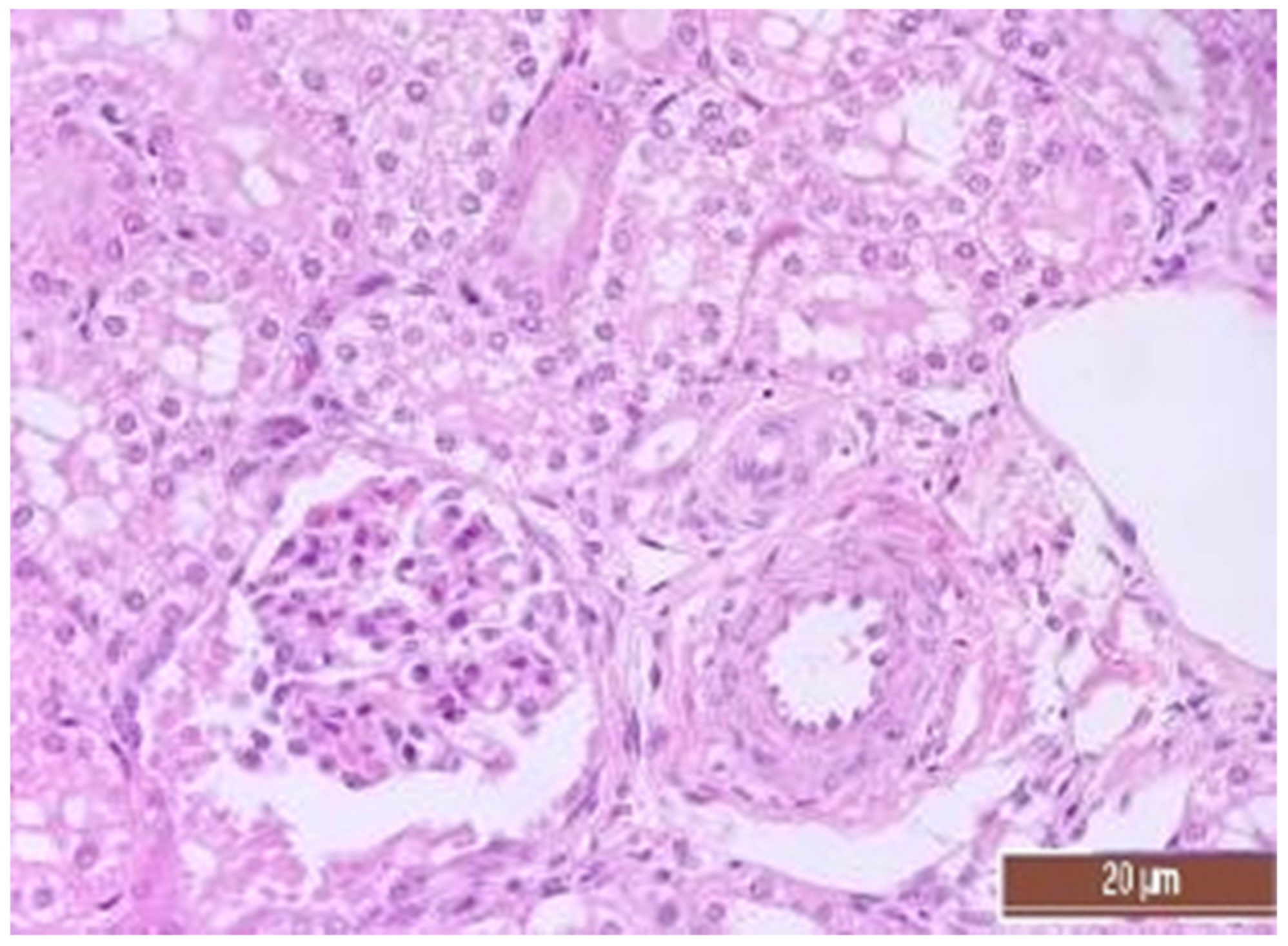
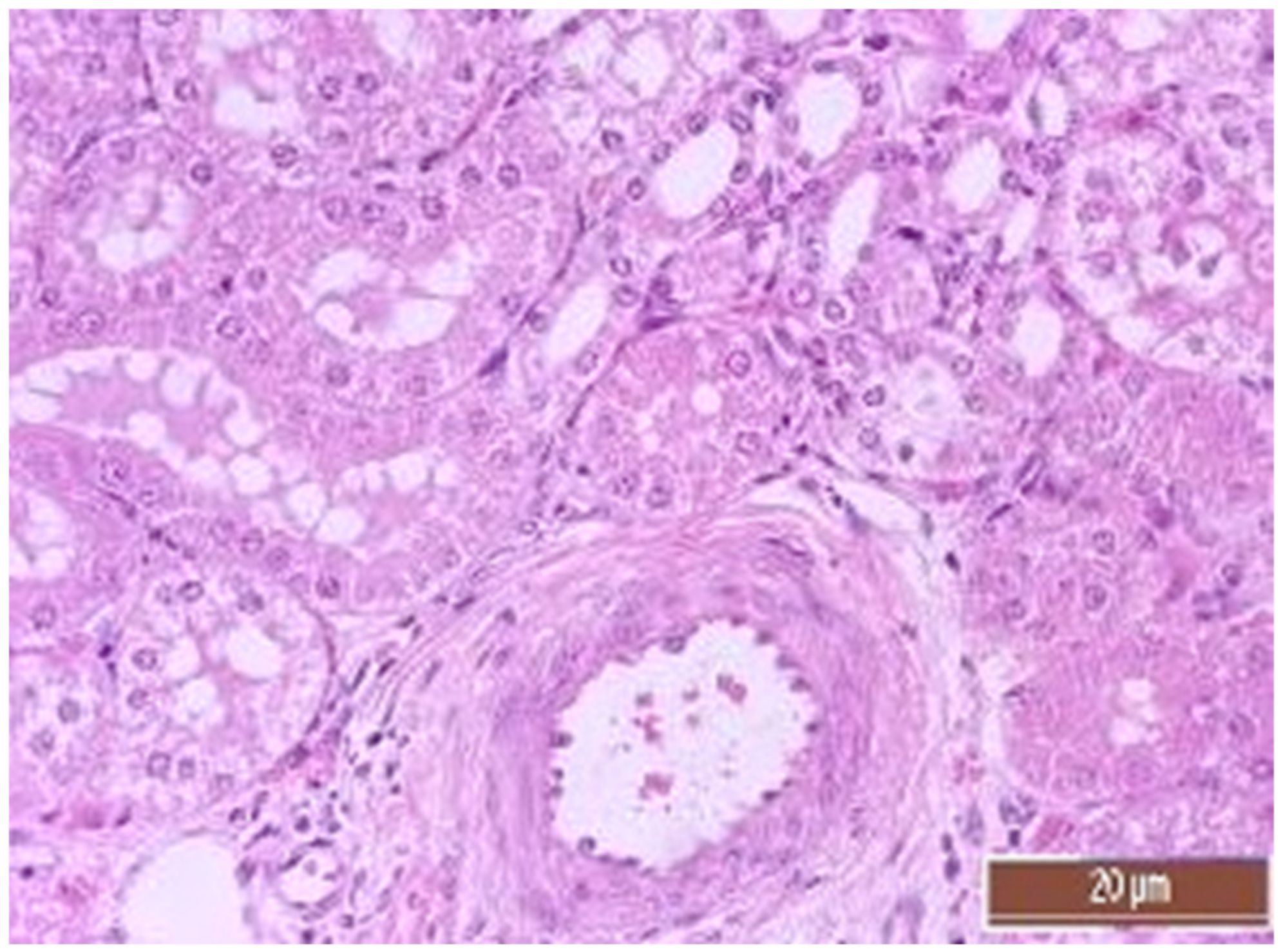
3.4. Pathology of Kidneys
4. Discussion
5. Study Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chaney, M.A. Corticosteroids and Cardiopulmonary Bypass: A review of clinical investigations. Chest 2002, 121, 921–931. [Google Scholar] [CrossRef] [PubMed]
- Paparella, D.; Yau, T.; Young, E. Cardiopulmonary bypass induced inflammation: Pathophysiology and treatment. An update. Eur. J. Cardio-Thorac. Surg. 2002, 21, 232–244. [Google Scholar] [CrossRef] [PubMed]
- Shang, L.; Ao, Y.; Lv, L.; Zhang, Y.; Hou, J.; Yao, J.; Wu, Z. Preoperative Liver Function Test Abnormalities Were Associated with Short-Term and Long-Term Prognosis in Cardiac Surgery Patients without Liver Disease. Front. Cardiovasc. Med. 2021, 8, 772430. [Google Scholar] [CrossRef]
- Lopez-Delgado, J.C.; Putzu, A.; Landoni, G. The importance of liver function assessment before cardiac surgery: A narrative review. Front. Surg. 2022, 9, 1053019. [Google Scholar] [CrossRef]
- Di Tomasso, N.; Monaco, F.; Landoni, G. Hepatic and renal effects of cardiopulmonary bypass. Best Pr. Res. Clin. Anaesthesiol. 2015, 29, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Ryan, T.A.; Rady, M.Y.; Bashour, C.A.; Leventhal, M.; Lytle, B.; Starr, N.J. Predictors of Outcome in Cardiac Surgical Patients with Prolonged Intensive Care Stay. Chest 1997, 112, 1035–1042. [Google Scholar] [CrossRef]
- Hessel, E.A. Abdominal Organ Injury After Cardiac Surgery. Semin. Cardiothorac. Vasc. Anesthesia 2004, 8, 243–263. [Google Scholar] [CrossRef]
- Luckraz, H.; Gravenor, M.B.; George, R.; Taylor, S.; Williams, A.; Ashraf, S.; Argano, V.; Youhana, A. Long and short-term outcomes in patients requiring continuous renal replacement therapy post cardiopulmonary bypass. Eur. J. Cardio-Thorac. Surg. 2005, 27, 906–909. [Google Scholar] [CrossRef]
- Lewicki, M.; Ng, I.; Schneider, A.G. HMG CoA reductase inhibitors (statins) for preventing acute kidney injury after surgical procedures requiring cardiac bypass. Cochrane Database Syst. Rev. 2015, 11, CD010480. [Google Scholar] [CrossRef]
- Pickering, J.W.; James, M.T.; Palmer, S.C. Acute Kidney Injury and Prognosis After Cardiopulmonary Bypass: A Meta-analysis of Cohort Studies. Am. J. Kidney Dis. 2015, 65, 283–293. [Google Scholar] [CrossRef]
- Kertai, M.D.; Zhou, S.; Karhausen, J.A.; Cooter, M.; Jooste, E.; Li, Y.-J.; White, W.D.; Aronson, S.; Podgoreanu, M.V.; Gaca, J.; et al. Platelet Counts, Acute Kidney Injury, and Mortality after Coronary Artery Bypass Grafting Surgery. Anesthesiology 2016, 124, 339–352. [Google Scholar] [CrossRef] [PubMed]
- Rosner, M.H.; Okusa, M.D. Acute Kidney Injury Associated with Cardiac Surgery. Clin. J. Am. Soc. Nephrol. 2006, 1, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Junior, F.U.V.; Antunes, N.; Vieira, R.W.; Álvares, L.M.P.; Costa, E.T. Hemolysis in extracorporeal circulation: Relationship between time and procedures. Rev. Bras. de Cir. Cardiovasc. 2012, 27, 535–541. [Google Scholar] [CrossRef]
- Wang, X.; Tanus-Santos, J.E.; Reiter, C.D.; Dejam, A.; Shiva, S.; Smith, R.D.; Hogg, N.; Gladwin, M.T. Biological activity of nitric oxide in the plasmatic compartment. Proc. Natl. Acad. Sci. USA 2004, 101, 11477–11482. [Google Scholar] [CrossRef] [PubMed]
- Signori, D.; Magliocca, A.; Hayashida, K.; Graw, J.A.; Malhotra, R.; Bellani, G.; Berra, L.; Rezoagli, E. Inhaled nitric oxide: Role in the pathophysiology of cardio-cerebrovascular and respiratory diseases. Intensiv. Care Med. Exp. 2022, 10, 28. [Google Scholar] [CrossRef]
- Redaelli, S.; Magliocca, A.; Malhotra, R.; Ristagno, G.; Citerio, G.; Bellani, G.; Berra, L.; Rezoagli, E. Nitric oxide: Clinical applications in critically ill patients. Nitric Oxide 2022, 121, 20–33. [Google Scholar] [CrossRef]
- Lei, C.; Berra, L.; Rezoagli, E.; Yu, B.; Dong, H.; Yu, S.; Hou, L.; Chen, M.; Chen, W.; Wang, H.; et al. Nitric Oxide Decreases Acute Kidney Injury and Stage 3 Chronic Kidney Disease after Cardiac Surgery. Am. J. Respir. Crit. Care Med. 2018, 198, 1279–1287. [Google Scholar] [CrossRef]
- Kamenshchikov, N.O.; Anfinogenova, Y.J.; Kozlov, B.N.; Svirko, Y.S.; Pekarskiy, S.E.; Evtushenko, V.V.; Lugovsky, V.A.; Shipulin, V.M.; Lomivorotov, V.V.; Podoksenov, Y.K. Nitric oxide delivery during cardiopulmonary bypass reduces acute kidney injury: A randomized trial. J. Thorac. Cardiovasc. Surg. 2022, 163, 1393–1403.e9. [Google Scholar] [CrossRef]
- James, C.; Millar, J.; Horton, S.; Brizard, C.; Molesworth, C.; Butt, W. Nitric oxide administration during paediatric cardiopulmonary bypass: A randomised controlled trial. Intensiv. Care Med. 2016, 42, 1744–1752. [Google Scholar] [CrossRef]
- Gasthuys, E.; Devreese, M.; Millecam, J.; Sys, S.; Vanderperren, K.; Delanghe, J.; Vande Walle, J.; Heyndrickx, M.; Croubels, S. Postnatal Maturation of the Glomerular Filtration Rate in Conventional Growing Piglets As Potential Juvenile Animal Model for Preclinical Pharmaceutical Research. Front. Pharmacol. 2017, 29, 431. [Google Scholar] [CrossRef]
- Schlapbach, L.J.; Gibbons, K.S.; Horton, S.B.; Johnson, K.; Long, D.A.; Buckley, D.H.F.; Erickson, S.; Festa, M.; D’udekem, Y.; Alphonso, N.; et al. Effect of Nitric Oxide via Cardiopulmonary Bypass on Ventilator-Free Days in Young Children Undergoing Congenital Heart Disease Surgery: The NITRIC Randomized Clinical Trial. JAMA 2022, 328, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Wang, Z.; Zhang, J.; Jing, H. Hepatic injury in a rat cardiopulmonary bypass model. Interact. Cardiovasc. Thorac. Surg. 2008, 7, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Cai, D.-S.; Jin, B.-B.; Pei, L.; Jin, Z. Protective effects of penehyclidine hydrochloride on liver injury in a rat cardiopulmonary bypass model. Eur. J. Anaesthesiol. 2010, 27, 824–828. [Google Scholar] [CrossRef]
- Cooper, W.A.; Duarte, I.G.; Thourani, V.H.; Nakamura, M.; Wang, N.-P.; Brown, W.; Gott, J.P.; Vinten-Johansen, J.; Guyton, R.A. Hypothermic circulatory arrest causes multisystem vascular endothelial dysfunction and apoptosis. Ann. Thorac. Surg. 2000, 69, 696–702. [Google Scholar] [CrossRef] [PubMed]
- Viaro, F.; Baldo, C.F.; Capellini, V.K.; Celotto, A.C.; Bassetto, S.; Rodrigues, A.J.; Evora, P.R.B. Plasma Nitrate/Nitrite (NOx) Is Not a Useful Biomarker to Predict Inherent Cardiopulmonary Bypass Inflammatory Response. J. Card. Surg. 2008, 23, 336–338. [Google Scholar] [CrossRef] [PubMed]
- Sobieski, M.A.; Graham, J.D.; Pappas, P.S.; Tatooles, A.J.; Slaughter, M.S. Reducing the Effects of the Systemic Inflammatory Response to Cardiopulmonary Bypass: Can Single Dose Steroids Blunt Systemic Inflammatory Response Syndrome? ASAIO J. 2008, 54, 203–206. [Google Scholar] [CrossRef]
- Zhang, Y.-Q.; Ding, N.; Zeng, Y.-F.; Xiang, Y.-Y.; Yang, M.-W.; Hong, F.-F.; Yang, S.-L. New progress in roles of nitric oxide during hepatic ischemia reperfusion injury. World J. Gastroenterol. 2017, 23, 2505–2510. [Google Scholar] [CrossRef]
- Dezfulian, C.; Raat, N.; Shiva, S.; Gladwin, M.T. Role of the anion nitrite in ischemia-reperfusion cytoprotection and therapeutics. Cardiovasc. Res. 2007, 75, 327–338. [Google Scholar] [CrossRef]
- Hataishi, R.; Rodrigues, A.C.; Neilan, T.G.; Morgan, J.G.; Buys, E.; Shiva, S.; Tambouret, R.; Jassal, D.S.; Raher, M.J.; Furutani, E.; et al. Inhaled nitric oxide decreases infarction size and improves left ventricular function in a murine model of myocardial ischemia-reperfusion injury. Am. J. Physiol. Circ. Physiol. 2006, 291, H379–H384. [Google Scholar] [CrossRef]
- Zhang, Y.-P.; Liu, X.-R.; Yang, M.-W.; Yang, S.-L.; Hong, F.-F. New progress in understanding roles of nitric oxide during hepatic ischemia-reperfusion injury. World J. Hepatol. 2022, 14, 504–515. [Google Scholar] [CrossRef]
- Lefer, A.M. Attenuation of myocardial ischemia-reperfusion injury with nitric oxide replacement therapy. Ann. Thorac. Surg. 1995, 60, 847–851. [Google Scholar] [CrossRef] [PubMed]
- Abe, Y.; Hines, I.N.; Zibari, G.; Pavlick, K.; Gray, L.; Kitagawa, Y.; Grisham, M.B. Mouse model of liver ischemia and reperfusion injury: Method for studying reactive oxygen and nitrogen metabolites in vivo. Free Radic. Biol. Med. 2009, 46, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Rezoagli, E.; Ichinose, F.; Strelow, S.; Roy, N.; Shelton, K.; Matsumine, R.; Chen, L.; Bittner, E.A.; Bloch, D.B.; Zapol, W.M.; et al. Pulmonary and Systemic Vascular Resistances after Cardiopulmonary Bypass: Role of Hemolysis. J. Cardiothorac. Vasc. Anesthesia 2017, 31, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Windsant, I.C.V.; Snoeijs, M.G.; Hanssen, S.J.; Altintas, S.; Heijmans, J.H.; Koeppel, T.A.; Schurink, G.W.H.; Buurman, W.A.; Jacobs, M.J. Hemolysis is associated with acute kidney injury during major aortic surgery. Kidney Int. 2010, 77, 913–920. [Google Scholar] [CrossRef]
- Windsant, I.C.V.; de Wit, N.C.J.; Sertorio, J.T.C.; van Bijnen, A.A.; Ganushchak, Y.M.; Heijmans, J.H.; Tanus-Santos, J.E.; Jacobs, M.J.; Maessen, J.G.; Buurman, W.A. Hemolysis during cardiac surgery is associated with increased intravascular nitric oxide consumption and perioperative kidney and intestinal tissue damage. Front. Physiol. 2014, 5, 340. [Google Scholar] [CrossRef]
- Hu, J.; Rezoagli, E.; Zadek, F.; Bittner, E.A.; Lei, C.; Berra, L. Free Hemoglobin Ratio as a Novel Biomarker of Acute Kidney Injury after On-Pump Cardiac Surgery: Secondary Analysis of a Randomized Controlled Trial. Obstet. Anesth. Dig. 2021, 132, 1548–1558. [Google Scholar] [CrossRef]
- Minneci, P.C.; Deans, K.J.; Zhi, H.; Yuen, P.S.; Star, R.A.; Banks, S.M.; Schechter, A.N.; Natanson, C.; Gladwin, M.T.; Solomon, S.B. Hemolysis-associated endothelial dysfunction mediated by accelerated NO inactivation by decompartmentalized oxyhemoglobin. J. Clin. Investig. 2005, 115, 3409–3417. [Google Scholar] [CrossRef]






| Parameters | Point | CPB-contr, n = 5 | CPB-NO, n = 5 | Mann–Whitney U-Test |
|---|---|---|---|---|
| MAP, mm Hg | Baseline | 75 (67.5; 85) | 78 (75; 81) | p = 0.832 |
| 2 h CPB | 68 (65; 70) | 69 (66; 70) | p = 0.833 | |
| Weaning CPB | 72 (70; 75) | 78 (76.5; 79) | p = 0.036 | |
| 6 h after CPB | 71 (70; 75) | 78 (70; 83) | p = 0.599 | |
| 12 h after CPB | 70.5 (70; 79) | 75 (72.5; 77) | p = 0.834 | |
| CVP, mm Hg | Baseline | 12 (9.5; 14) | 12.2 (8.1; 13) | p = 1.000 |
| 2 h CPB | 12 (8; 12) | 10 (8; 14) | p = 0.833 | |
| Weaning CPB | 16 (12; 17) | 13.6 (12; 15) | p = 0.675 | |
| 6 h after CPB | 13.5 (11; 14.9) | 12.2 (12.2; 13.6) | p = 0.596 | |
| 12 h after CPB | 13.8 (13.6; 14.5) | 14.6 (13.8; 17) | p = 0.195 | |
| HR, min−1 | Baseline | 105 (85; 105) | 104 (85; 105) | p = 0.592 |
| 2 h CPB | 90 (85; 95) | 92 (85; 95) | p = 1.000 | |
| Weaning CPB | 103 (100; 105) | 96 (93; 104) | p = 0.530 | |
| 6 h after CPB | 92 (75; 95) | 98 (95; 106) | p = 0.143 | |
| 12 h after CPB | 97 (95; 108) | 94 (90; 107) | p = 0.346 | |
| FiO2 | Baseline | 0.5 (0.45; 0.5) | 0.45 (0.4; 0.5) | p = 0.496 |
| 2 h CPB | 0.4 (0.4; 0.5) | 0.4 (0.45; 0.4) | p = 0.513 | |
| Weaning CPB | 0.5 (0.4; 0.5) | 0.5 (0.5; 0.5) | p = 0.513 | |
| 6 h after CPB | 0.45 (0.45; 0.45) | 0.4 (0.4; 0.4) | p = 0.174 | |
| 12 h after CPB | 0.45 (0.45; 0.5) | 0.45 (0.45; 0.45) | p = 0.343 | |
| PaO2, mm Hg | Baseline | 190 (150; 190) | 190 (150; 195) | p = 0.827 |
| 2 h CPB | 185 (180; 190) | 180 (150; 190) | p = 0.527 | |
| Weaning CPB | 190 (172; 190) | 184 (150; 184) | p = 0.916 | |
| 6 h after CPB | 197 (189; 200) | 189 (180; 190) | p = 0.31 | |
| 12 h after CPB | 166 (111; 190) | 150 (150; 187) | p = 1.000 | |
| PaCO2, mm Hg | Baseline | 39 (37; 45.7) | 39.1 (36.6; 42.5) | p = 0.675 |
| 2 h CPB | 34 (33; 34) | 35 (33; 35) | p = 0.455 | |
| Weaning CPB | 38.3 (38; 42.3) | 40.7 (33.3; 42.9) | p = 0.841 | |
| 6 h after CPB | 38 (34.9; 41) | 38.9 (38; 39.5) | p = 0.53 | |
| 12 h after CPB | 38.3 (35.2; 42) | 38 (37; 38.4) | p = 0.841 | |
| T, °C | Baseline | 37 (36.6; 37.3) | 36.8 (36.8; 37.4) | p = 0.906 |
| 2 h CPB | 36 (35.9; 36.2) | 36 (35.8; 36.2) | p = 1.000 | |
| Weaning CPB | 38.5 (38.4; 38.8) | 38.6 (38.6; 38.8) | p = 0.674 | |
| 6 h after CPB | 39.4 (39.2; 39.9) | 39.6 (39.6; 40.1) | p = 0.461 | |
| 12 h after CPB | 39.5 (39.5; 39.6) | 39.4 (39.3; 39.7) | p = 0.381 | |
| Lactate, mmol/L | Baseline | 1.98 (1.27; 2.35) | 2.28 (1.81; 3.89) | p = 0.548 |
| 2 h CPB | 1.55 (1.26; 2.99) | 2 (1.75; 4.05) | p = 0.31 | |
| Weaning CPB | 2.96 (1.83; 3.42) | 2.66 (2.06; 3.24) | p = 1.000 | |
| 6 h after CPB | 0.96 (0.72; 1.02) | 0.72 (0.39; 1.0) | p = 0.566 | |
| 12 h after CPB | 1.65 (0.82; 2.4) | 0.71 (0.66; 0.86) | p = 0.064 | |
| Drainage blood loss, mL | 280 (160; 400) | 200 (150; 300) | p = 0.69 | |
| Point | CPB-contr, n = 5 | CPB-NO, n = 5 | Mann–Whitney U-Test |
|---|---|---|---|
| Weaning CPB | 3 (0; 6) | 5 (1.5; 6) | p = 1.000 |
| 6 h after CPB | 4 (0; 7) | 3.5 (0.5; 6.5) | p = 1.000 |
| 12 h after CPB | 5 (1.5; 6.5) | 3.5 (0.5; 7.5) | p = 1.000 |
| Parameters | Point | CPB-contr, n = 5 | CPB-NO, n = 5 | Mann–Whitney U-Test |
|---|---|---|---|---|
| Hemoglobin, g/L | Baseline | 86 (86; 90) | 89 (86; 101) | p = 0.597 |
| 2 h CPB | 64 (57; 65) | 61 (61; 69) | p = 0.753 | |
| Weaning CPB | 59 (55; 62) | 59 (54; 64) | p = 0.916 | |
| 12 h after CPB | 66 (63; 76) | 66 (54; 78) | p = 0.525 | |
| Leukocytes, ×109/L | Baseline | 15.4 (15.3; 16.5) | 19.5 (19.2; 20.8) | p = 0.31 |
| 2 h CPB | 10.2 (10.1; 10.6) | 11.7 (10.1; 12.8) | p = 0.675 | |
| Weaning CPB | 12.5 (12.3; 15) | 14.9 (10.4; 18.1) | p = 1.000 | |
| 12 h after CPB | 14.2 (12.7; 17.5) | 14.9 (11.8; 17.7) | p = 0.841 | |
| Platelets, ×1012/L | Baseline | 487 (472; 569) | 487 (332; 572) | p = 0.834 |
| 2 h CPB | 325 (311; 343) | 322 (245; 350) | p = 0.691 | |
| Weaning CPB | 295 (202; 312) | 225 (194; 276) | p = 0.421 | |
| 12 h after CPB | 280 (182; 295) | 167 (132; 272) | p = 0.31 |
| Point | CPB-contr, n = 5 | CPB-NO, n = 5 | Mann–Whitney U-Test |
|---|---|---|---|
| Baseline (1) | 1.95 (1.9; 2) | 2.05 (1.95; 2.05) | p = 0.4 |
| Weaning CPB (2) | 2.2 (1.9; 2.7) | 3.2 (3.05; 3.65) | p = 0.032 |
| 6 h after CPB (3) | 1.6 (1.6; 1.8) | 2.8 (2.5; 3) | p = 0.016 |
| 12 h after CPB (4) | 1.6 (1.2; 1.9) | 2 (1.9; 2.1) | p = 0.143 |
| Wilcoxon test | p1–2 = 0.715 p1–3 = 0.5 p1–4 = 0.138 | p1–2 = 0.043 p1–3 = 0.042 p1–4 = 0.687 |
| Point | CPB-contr, n = 5 | CPB-NO, n = 5 | Mann–Whitney U-Test |
|---|---|---|---|
| Baseline (1) | 43 (34; 44) | 36 (36; 37) | p = 0.691 |
| Weaning CPB (2) | 43 (31; 45) | 33 (29; 38) | p = 0.222 |
| 6 h after CPB (3) | 66 (41; 70) | 38 (37; 38) | p = 0.222 |
| 12 h after CPB (4) | 82 (53; 99) | 50 (49; 50) | p = 0.151 |
| AUC ALT (U/L/16 h) | 775 (464; 855) | 451 (440; 489) | p = 0.175 |
| Wilcoxon test | p1–2 = 0.682 p1–3 = 0.08 p1–4 = 0.043 | p1–2 = 0.225 p1–3 = 1.000 p1–4 = 0.08 | |
| Max. ALT (U/L) | 82 (53; 99) | 50 (49; 50) | p = 0.151 |
| Point | CPB-contr, n = 5 | CPB-NO, n = 5 | Mann–Whitney U-Test |
|---|---|---|---|
| Baseline (1) | 25 (17; 26) | 18 (16; 32) | p = 0.91 |
| Weaning CPB (2) | 146 (129; 174) | 112 (99; 116) | p = 0.421 |
| 6 h after CPB (3) | 223 (124; 269) | 110 (99; 112) | p = 0.175 |
| 12 h after CPB (4) | 269 (164; 376) | 97 (94; 171) | p = 0.175 |
| Wilcoxon test | p1–2 = 0.043 p1–3 = 0.043 p1–4 = 0.043 | p1–2 = 0.043 p1–3 = 0.068 p1–4 = 0.08 | |
| AUC AST (U/L/16 h) | 2325 (1255; 2669) | 1094 (1013; 1248) | p = 0.117 |
| Max. AST (U/L) | 269 (174; 376) | 112 (111; 283) | p = 0.076 |
| Point | CPB-contr, n = 5 | CPB-NO, n = 5 | Mann–Whitney U-Test |
|---|---|---|---|
| Baseline | 1.75 (1.59; 2.60) | 1.84 (1.51; 8.42) | p = 0.752 |
| Weaning CPB | 2.60 (2.55; 5.6) | 3.65 (3.54; 5.53) | p = 0.751 |
| 6 h after CPB | 2.34 (2.17; 2.87) | 2.42 (2.10; 2.52) | p = 0.753 |
| 12 h after CPB | 2.93 (2.39; 3.23) | 2.14 (1.70; 2.58) | p = 0.352 |
| Localization | Groups | Number of Binuclear Hepatocytes per 100 Cells | Kruskal–Wallis Test |
|---|---|---|---|
| The center of the lobule | CPB-contr, n = 25 | 4 (4; 4) | p = 0.170 |
| CPB-NO, n = 25 | 6 (3; 6) | ||
| Control-noCPB, n = 5 | 4 (4; 5) | ||
| The periphery of the lobule | CPB-contr, n = 25 | 2 (1; 3) | p = 0.426 |
| CPB-NO, n = 25 | 4 (3; 6) | ||
| Control-noCPB, n = 5 | 3 (3; 5) |
| Point | CPB-contr, n = 5 | CPB-NO, n = 5 | Mann–Whitney U-Test |
|---|---|---|---|
| Baseline (1) | 131 (129; 133) | 136 (129; 145) | p = 0.42 |
| Weaning CPB (2) | 157 (154; 167) | 133 (130; 157) | p = 0.291 |
| 6 h after CPB (3) | 191 (169; 215) | 146 (141; 148) | p = 0.076 |
| 12 h after CPB (4) | 273 (241; 306) | 183 (168; 196) | p = 0.008 |
| Wilcoxon test | p1–2 = 0.08 p1–3 = 0.043 p1–4 = 0.043 | p1–2 = 0.5 p1–3 = 0.144 p1–4 = 0.225 |
| Point | CPB-contr, n = 5 | CPB-NO, n = 5 | Mann–Whitney U-Test |
|---|---|---|---|
| Baseline (1) | 81.7 (79.2; 86.5) | 83.6 (81.5; 83.9) | p = 1.00 |
| Weaning CPB (2) | 73.3 (68.5; 76.6) | 83.5 (80.2; 84.5) | p = 0.548 |
| 6 h after CPB (3) | 67.9 (62.3; 69.2) | 78.9 (77.8; 82.3) | p = 0.016 |
| 12 h after CPB (4) | 50.3 (48.7; 54.9) (0.042) | 67.7 (65.5; 68.0) | p = 0.032 |
| Wilcoxon test | p1–2 = 0.008 p1–3 = 0.043 p1–4 = 0.042 | p1–2 = 0.5 p1–3 = 0.144 p1–4 = 0.043 | |
| Urine output in operation procedure mL/kg/h | 2 (1.75; 2.55) | 4.5 (4; 4.5) | p = 0.015 |
| Urine output in postoperative period mL/kg/h | 1.67 (1.35; 2.19) | 2.09 (1.67; 2.29) | p = 0.53 |
| Point | CPB-contr, n = 5 | CPB-NO, n = 5 | Mann–Whitney U-Test |
|---|---|---|---|
| Baseline | 0 (0; 0) | 0 (0; 0.2) | p = 0.134 |
| Weaning CPB | 0.2 (0.1; 0.2) | 0 (0; 0) | p = 0.106 |
| 6 h after CPB | 0 (0; 0) | 0 (0; 0) | p = 1.000 |
| 12 h after CPB | 0 (0; 0) | 0 (0; 0) | p = 0.317 |
| Point | CPB-contr, n = 5 | CPB-NO, n = 5 | Mann–Whitney U-Test |
|---|---|---|---|
| Baseline (1) | 62.1 (48.3; 92.8) | 69.4 (43.3; 140.6) | p = 0.77 |
| Weaning CPB (2) | 63.6 (48.2; 234.4) | 96.7 (84.3; 140.1) | p = 0.6 |
| 6 h after CPB (3) | 95.9 (64; 101.7) | 57 (41.8; 87.8) | p = 0.22 |
| 12 h after CPB (4) | 287.9 (100.1; 296.3) | 102 (53.2; 150.9) | p = 0.14 |
| Wilcoxon test | p1–2 = 0.686 p1–3 = 0.345 p1–4 = 0.043 | p1–2 = 0.715 p1–3 = 0.144 p1–4 = 1.000 |
| Value | Group | Value, Point | Mann–Whitney U-Test |
|---|---|---|---|
| Dystrophy of the epithelium of the tubules | CPB-contr, n = 25 | 2 (1; 3) | p = 0.49 |
| CPB-NO, n = 25 | 1 (1; 2) | ||
| Lymphoplasmocytic infiltration | CPB-contr, n = 25 | 0 (0; 1) | p = 0.37 |
| CPB-NO, n = 25 | 1 (1; 1) | ||
| Hyperemia | CPB-contr, n = 25 | 2 (2; 3) | p = 0.55 |
| CPB-NO, n = 25 | 3 (2; 3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radovskiy, A.M.; Bautin, A.E.; Marichev, A.O.; Osovskikh, V.V.; Semenova, N.Y.; Artyukhina, Z.E.; Murashova, L.A.; Zinserling, V.A. NO Addition during Gas Oxygenation Reduces Liver and Kidney Injury during Prolonged Cardiopulmonary Bypass. Pathophysiology 2023, 30, 484-504. https://doi.org/10.3390/pathophysiology30040037
Radovskiy AM, Bautin AE, Marichev AO, Osovskikh VV, Semenova NY, Artyukhina ZE, Murashova LA, Zinserling VA. NO Addition during Gas Oxygenation Reduces Liver and Kidney Injury during Prolonged Cardiopulmonary Bypass. Pathophysiology. 2023; 30(4):484-504. https://doi.org/10.3390/pathophysiology30040037
Chicago/Turabian StyleRadovskiy, Aleksey Maksimovich, Andrey Evgenevich Bautin, Alexander Olegovich Marichev, Victor Vasilyevich Osovskikh, Natalia Yuryevna Semenova, Zoya Evgenyevna Artyukhina, Lada Aleksandrovna Murashova, and Vsevolod Alexandrovich Zinserling. 2023. "NO Addition during Gas Oxygenation Reduces Liver and Kidney Injury during Prolonged Cardiopulmonary Bypass" Pathophysiology 30, no. 4: 484-504. https://doi.org/10.3390/pathophysiology30040037
APA StyleRadovskiy, A. M., Bautin, A. E., Marichev, A. O., Osovskikh, V. V., Semenova, N. Y., Artyukhina, Z. E., Murashova, L. A., & Zinserling, V. A. (2023). NO Addition during Gas Oxygenation Reduces Liver and Kidney Injury during Prolonged Cardiopulmonary Bypass. Pathophysiology, 30(4), 484-504. https://doi.org/10.3390/pathophysiology30040037






