Abstract
Superantigens, i.e., staphylococcal enterotoxins and toxic shock syndrome toxin-1, interact with T cells in a different manner in comparison to conventional antigens. In fact, they activate a larger contingent of T lymphocytes, binding outside the peptide-binding groove of the major histocompatibility complex class II. Involvement of many T cells by superantigens leads to a massive release of pro-inflammatory cytokines, such as interleukin (IL)-1, IL-2, IL-6, tumor necrosis factor-alpha and interferon-gamma. Such a storm of mediators has been shown to account for tissue damage, multiorgan failure and shock. Besides conventional drugs and biotherapeutics, experiments with natural and biological products have been undertaken to attenuate the toxic effects exerted by superantigens. In this review, emphasis will be placed on polyphenols, probiotics, beta-glucans and antimicrobial peptides. In fact, these substances share a common functional denominator, since they skew the immune response toward an anti-inflammatory profile, thus mitigating the cytokine wave evoked by superantigens. However, clinical applications of these products are still scarce, and more trials are needed to validate their usefulness in humans.
1. Introduction
Daily, a myriad of noxious antigens enters the host through both respiratory and intestinal tracts. However, the mucosal immune arsenal plays a protective role against pathogenic microorganisms and their excreted products [1,2,3]. Bacteria and their toxins, which can cause certain diseases (some already known in ancient medicine such as that caused by Corynebacterium diphtheriae), are the most frequent invaders of humans and animals, but the host can trigger a sequence of defensive immunological events. A first defense line is represented by the innate immune cells, while for more specific responses a second category of cells intervene, i.e., T and B lymphocytes, which govern the so-called adaptive immunity. In this framework, pathogen-associated molecular patterns (PAMPs) expressed on the surface of both Gram-positive and Gram-negative bacteria bind specific receptors on the surface of monocytes–macrophages and neutrophils, thus eliciting a pro-host response [4,5]. Quite importantly, innate immune cells do not retain immunological memory, which is a prerogative of the adaptive immunity, composed of various subsets of T and B cells. Nevertheless, as recently reported [6], phagocytes, as major components of the innate immunity, are endowed with so-called trained immunity, which allows a faster response towards a reencounter with the same pathogen. On the other hand, T cells respond to antigens presented in the context of the MHC class II molecules, with T cytotoxic (CD8+) cells, as killers of infected cells and T helper (h) (CD4+) cells, as supporters of memory CD8+ cell responses [7,8,9]. Furthermore, Th cells can kill pathogens activating macrophage-mediated phagocytosis and B cell-induced production of specific antibodies [10]. T cells are endowed with alpha-beta T cell receptors (TCRs) to detect foreign antigens presented by antigen-presenting cells (APCs), e.g., macrophages and dendritic cells (DCs), through the major histocompatibility complex (MHC), class I or class II [11]. Then, following antigen presentation, naïve alpha-beta T cells become activated effector T cells with the participation of cytokines and costimulatory signals [12,13]. In the case of microorganisms, T cell response leads to the elimination of a given pathogen with the formation of memory cells, which harbor in the blood, lymphoid organs and mucosal sites and are maintained by interleukin (IL)-7 and IL-15 [14,15,16]. Therefore, re-exposure to the same pathogen leads to a prompter and more effective response. Conversely, the so-called superantigens, i.e., staphylococcal enterotoxins (SEs) and toxic shock syndrome toxin-1 (TSST-1), interact with T cells in a different manner. The superantigen family includes several 22–29 kDa proteins, highly resistant to proteases and heat denaturation. Analysis of the three-dimensional structure of superantigens reveals a common molecular architecture, consisting of a C-terminal domain with a ubiquitin-like motif and an N-terminal domain with a characteristic OB fold that binds oligosaccharides and oligonucleotides (oligosaccharide/oligonucleotide-binding fold), separated by a long α-helix extending down the center of the molecule. They are unconventional antigens, which trigger a response by binding outside the complementary determining regions of their target immune receptor macromolecules (antibodies or T cell receptors [17]. In fact, they activate a large contingent (5–30%) of T cells in comparison to conventional antigens, which stimulate <0.01% of T cells [18]. Furthermore, superantigens bind outside the peptide-binding groove of MHC class II without internalization and processing and are not MHC class II-restricted. The binding of the superantigen/MHC II complex to specific V-beta regions of TCR represents the first signal for T cell activation that is followed by binding of costimulatory molecules, as a second signal, with an early cytokine storm and massive polyclonal T cell proliferation [19]. In fact, superantigens stimulate human peripheral blood mononuclear cells (PBMCs) with early release of a plethora of cytokines, such as IL-1, Il-2, Il-6, tumor necrosis factor (TNF)-alpha and interferon (IFN)-gamma [20]. In turn, IL-1 and TNF-alpha activate other cells, as well as tissue factor and matrix-metalloproteinases, thus contributing to damage of the immune and cardiovascular system, culminating in multiorgan dysfunction and shock [21,22].
Over the recent decades, besides the use of conventional drugs and monoclonal antibodies to limit the superantigen-mediated injury, experimental studies with natural and biological products have been carried out. However, clinical attempts to evaluate their putative antimicrobial and anti-inflammatory role against superantigen effects are still scarce, but to date some promising experimental results have been published. In this framework, polyphenols are compounds largely present in the vegetal kingdom that are currently used in the treatment of several chronic inflammatory diseases [23]. Probiotics and, mostly, lactobacilli, when administered, provide the host with beneficial effects in terms of anti-inflammatory and antimicrobial activities [24]. Beta-glucans present in mushrooms and bacteria inhibit the activation of NF-kB with diminished release of pro-inflammatory cytokines [25].
Among biological products, antimicrobial peptides (AMPs) are produced by epithelial cells and innate immune cells, and they are very effective against a broad range of bacteria, even including antibiotic resistant bugs [26,27].
In the light of the above notions, the present review is aimed at describing the mechanisms of interactions of superantigens with host cells and related inflammatory damage. Then, the use of polyphenols, beta-glucans, probiotics and AMPs in different experimental settings to block the toxic activity of superantigens will be described.
2. T Cell Receptor and T Cell Activation
According to the TCRs expressed on their membrane, T cells can be divided into alpha-beta or gamma-delta T cells that play different functions [28,29,30]. Alpha-beta T cells recognize small peptide antigens on the cell surface of antigen-presenting cells (APCs) complexed to MHC class I or class II molecules. Usually, CD4+ T cells recognize MHC class II molecules, while CD8+ T cells recognize MHC class I molecules, even if the latter are also able to recognize MHC class II molecules [28,31]. On the other hand, gamma-delta T cells recognize antigens in an MHC-unrestricted manner, engaging CD1 molecules that are encoded outside the MHC locus and able to present microbial lipids to T cells [32].
With special reference to alpha-beta T cells, they can respond to an enormous array of microbial antigens in view of a diversity of TCR sequences that are based on alpha- and beta chain rearrangements [33]. Then, the so-called clonotypic diversity relies on somatic recombination of V, D and J gene segments and junctional adaptations, which create three complementary determining region (CDR) loops [34]. CDR1 and CDR2 loops bind the TCR to its target MHC, whereas hypervariable loops engage the exposed regions of the MHC bound peptide. The above mechanisms of clonal expansion can enhance the numbers of antigen-specific effector T cells [35].
Briefly, in response to microbial antigens, naïve alpha-beta T cells differentiate into Th1, Th2, Th17, T regulatory (TREG) cellular subsets, with Th1 lymphocytes governing the cellular immunity and Th2 cells directing the humoral immunity [36]. CD4+ Th1 cells use T-bet, STAT1 and STAT4 for transcriptional regulation, with release of IL-2, IFN-gamma, TNF-alpha and IL-12, thus leading to a protective response against intracellular pathogens. IL-2 and IFN-gamma help CD8+ cells and phagocytes in bacterial killing [37]. On the other hand, CD4 + Th2 cells utilize GATA3, STAT5 and STAT6 for transcriptional regulation and produce IL-4, IL-5 and IL-13 for antibody production against extracellular pathogens, even including parasites. Memory CD8+ T cells generate fast recall responses to bacterial antigens while maintaining long-term immunity [38]. Memory cells have been divided into central memory T, effector memory T and tissue resident memory T (TRM) cells [39]. CD8+ TRM cells mostly protect against pathogens that invade mucosal tissues via local proliferation, with release of IFN-gamma, TNF-alpha and cytotoxic molecules, such as granzyme B [40]. Gamma-delta T cells express TCRs composed of rearranged TCR-gamma and TCR-delta chains distinct from alpha-beta TCRs. They are encoded by a lower number of V, D and J segments with a limited clonotypic diversity [41]. Functionally, gamma-delta T cells rapidly respond to microbial antigens, with the production of IFN-gamma, TNF-alpha and IL-17 [42]. It has been reported that gamma-delta T cells from long-lived memory cells, following bacterial challenge, can permanently reside in the affected tissue.
3. Modalities of Superantigen Binding to TCR and Induction of the Inflammatory Pathway
Superantigens bind outside the peptide-binding groove on MHC molecules through two distinct binding sites [43,44,45]. In this respect, the invariant alpha chain of MHC II is used by the majority of superantigens, while, among others, SEA and SED bind the polymorphic beta chain [46,47,48]. The binding of the superantigen/MHC II complex to TCR represents the first signal for T cell activation to occur, while the second signal is delivered by the binding of costimulatory molecules CD80 and CD86 on APCs with CD28 expressed on T cells [49,50,51,52,53]. Furthermore, the intervention of CD2, intercellular adhesion molecule-1 (ICAM-1) and endothelial adhesion molecule activate endothelial cells and T cells via SEB, while CD11a/ICAM-1 and CD28/CD80 stimulation activate T cells via SEA [51,54]. All the above-described events culminate in the activation of protein tyrosine kinases (PTKs) and of calcineurin phosphatase with translocation of the nuclear factor of activated T cells to the nucleus that, in turn, enhances the expression of IL-2 and other T cell-derived cytokines [54]. Among the transcriptional factors activated, NF-kB binds the promoter region of several pro-inflammatory cytokines, T cell growth and differentiation factors, thus leading to the release of a variety of mediators outside cells, as an expression of the third signal [55]. In this scenario, the role played by NOD-like-receptors (NLRs), and particularly NLRP3, as triggers of pro-inflammatory cytokines needs to be clarified. Of note, the NLR inflammasome is an essential component of the innate immune system, leading to caspase-1 activation, with secretion of IL-1 beta and IL-18 in response to microbes and or their toxins [55]. Recent in vitro study has demonstrated that recombinant TSST-1 could activate NLRP3 inflammasome in murine primary macrophages [56]. Release of IL-1 beta and TNF-alpha by stimulated macrophages occurred in the presence of Toll-like receptor (TLR)-4, as a first signal.
Cytokines represent the main players of superantigen-induced damage. Among them, IL-1 and TNF-alpha contribute to extending the inflammatory status, increasing vascular permeability and, ultimately, provoking multiorgan failure and shock [57]. Furthermore, the chemokines, IL-8, monocyte chemoattractant protein-1, macrophage inflammatory protein (MIP)-1 alpha and MIP-1 beta, are induced via SEA, SEB and TSST-1 [58,59]. Systemic and intranasal exposure to SEB has been shown to induce infiltration of neutrophils and mononuclear cells into tissue with increased vascular permeability and acute lung injury (ALI) [60,61]. Furthermore, superantigens can also drive polyclonal IgE production, the so-called “T-cell dependent super allergen” with degranulation of mast cells and basophils [62,63]. For instance, SE sensitization was associated with allergic poly-sensitization against food and inhaled allergens in adolescents [64].
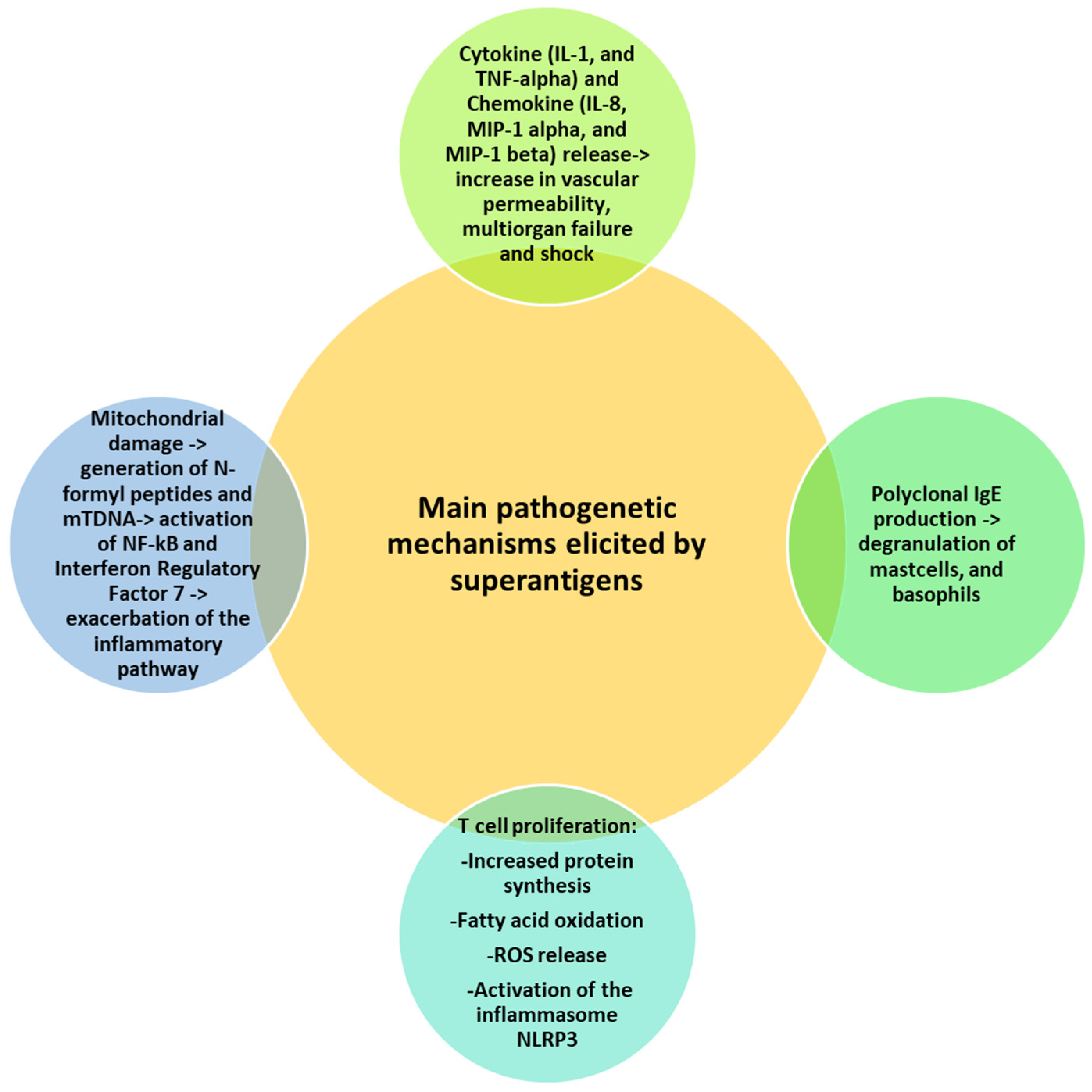
Massive T cell proliferation induced by superantigens is followed by metabolic activities, such as increased protein synthesis, fatty acid oxidation and reactive oxygen species (ROS) generation with endoplasmic reticular (ER) stress and mitochondrial damage [65]. ER stress activates the inflammasome NLRP3, with further release of IL-1 beta in a caspase 8-dependent manner [66]. Superantigen-induced mitochondrial damage promotes the release of cytochrome c, ATP, N-formyl peptides (NFPs) and mtDNA [67]. NFPs are chemoattractants for neutrophils, while mtDNA binds endosomal TLR9 and activates NF-kB and interferon regulatory factor 7, thus exacerbating the inflammatory pathway [68]. Figure 1 summarizes the main mechanisms of superantigen-mediated damage.
Figure 1.
Superantigen-mediated damage. Various mechanisms cooperate in the superantigen-induced harmful effects, including release of cytokines and chemokines, T cell proliferation and mitochondrial damage. All mechanisms share a common denominator represented by the induction of the inflammatory pathway.
4. Superantigen-Mediated Disease
The most frequent disease caused by superantigens is toxicosis, especially that associated with staphylococcal food poisoning with nausea and vomiting [69,70]. It seems that superantigens bind a yet unidentified receptor in the gut, thus evoking release of 5-hydroxy tryptamine that, in turn, leads to the vomiting reflex through depolarization of the vagal afferent nerves.
Penetration of superantigens into the bloodstream causes TSS, characterized by high fever, inflammation, vascular leakage, hypotension, erythematous rash and multiorgan failure [57]. Notably, TSS is a rare disease, since humans possess high titers of neutralizing antibodies [71].
Kawasaki syndrome (KS) is an acute multisystem vasculitis in children with coronary artery lesions [72,73]. Even if the etiology of KS is still unknown, many similarities have been observed between KS and TSS [74]. The common pathogenetic denominator seems to rely on the cytokine storm they elicit with hypotension, vascular capillary leak syndrome and vasculitis. Quite interestingly, COVID-19 infection in children seems to share clinical symptoms with KS and TSS and occurs 4–5 weeks post-COVID-19 [75].
Furthermore, it is noteworthy that some superantigens, such as staphylococcal or mycoplasmal toxins, can induce nonspecific activation of large subsets of T cells directly binding to MHC molecules expressed on them, without processing. Because the peptides expressed on the cell together with the MHC proteins derive not only from antigens but also from cellular metabolism, being “self-molecules” of the organism itself can trigger an autoimmune response which is expressed with different clinical pictures (e.g., systemic lupus erythematosus, rheumatoid arthritis and inflammatory bowel disease) [73].
5. Natural and Biological Products as an Alternative Treatment of Superantigen-Mediated Damage
Treatment of superantigen-induced toxic shock is still limited. Experimental attempts have been undertaken with immunosuppressants, such as dexamethasone, cyclosporine A and rapamycin with the aim of interrupting T cell proliferation [53,76,77,78,79]. Furthermore, intravenous immunoglobulin and, recently, humanized monoclonal antibodies (MoAbs) to neutralize SEs and TSST-1 have been used [80,81,82,83]. However, MoAbs exert a limited inhibition of the binding of superantigens to their receptors in the early phase of toxin exposure and, therefore, a downstream blockade seems to be the only means of intervening, in order to inhibit the cytokine storm. Of note, most of the studies are still experimental, and mice represent the best model to investigate the immunological involvement during superantigen-mediated shock [84]. In parallel, the potential ability of natural and biological products to attenuate the superantigen-induced damage in in vitro and in in vivo experimental models is under investigation, as described below.
5.1. Polyphenols
Polyphenols are largely present in the vegetal kingdom, especially in fruits, vegetables, cereals, extra virgin olive oil and red wine. There is a large body of evidence that polyphenols exert antioxidant and anti-inflammatory activities, and, for this reason, they are currently used for the treatment of various chronic disease [85,86,87]. Among major mechanisms of action, polyphenols can inhibit the activation of the NF-kB pathway with dampening of proinflammatory cytokine release while activating T regulatory (TREG) cells with production of the anti-inflammatory cytokine IL-10 [88,89]. A few experimental data are available about the effects of polyphenols on superantigens.
Resveratrol (RES) is a stilbene that has been investigated during superantigen-mediated acute respiratory syndrome (ARDS) [90]. SEB administration induced release of proinflammatory cytokines and ARDS in mice, increasing Proteobacteria phylum and Cutibacterium acnes (formerly Propionibacterium acnes) species in the lung. RES treatment mitigated the inflammatory profile and reduced mortality, also increasing beneficial bacteria in the colon and lungs, such as Limosilactobacillus reuteri (previously named Lactobacillus reuteri). In another study, severity of SEB-mediated ALI in mice was mitigated by RES treatment through regulation of miR-193a and induction of the anti-inflammatory cytokine, transforming growth factor (TGF)-beta [91]. Altogether, these data demonstrate the ability of RES to reduce the inflammatory process during ARDS and ALI. Involvement of TGF-beta via RES represents a potential mechanism of immunosuppression, even including its ability to induce TREG cell activation [92]. In another study, apple juice and apple polyphenols inhibited the biological activity of SEA, without any cytotoxic effects on spleen cells [93]. SEA was irreversibly bound to apple juice constituents, thus suggesting the possibility to use polyphenols in vivo. Furthermore, it has been reported that epigallocatechin gallate (EGCG) contained in green tea could block peripheral blood mononuclear cell activation via SEB, preventing the dysfunction of the intestinal epithelial barrier [94].
5.2. Beta-Glucans
B-1-3-D-glucans (beta-glucans) represent main components of the microbial cell wall or can be secreted by fungi, such as Saccharomyces cerevisiae or Candida albicans [95]. Beta-glucans bind a specific receptor, dectin-1, on dendritic cells, monocytes–macrophages and neutrophils, whose binding accounts for activation of the NF-kB pathway with production of pro-inflammatory cytokines [96,97,98]. Conversely, in a murine polymicrobial sepsis model, beta-glucan treatment diminished morbidity and mortality, inhibiting NF-kB, while triggering phosphoinositide-3-kinase. With special reference to superantigens, PBMCs were exposed to glucan phosphate (GP) and then stimulated with TSST-1 for 48 h [99]. Determination of cytokine production demonstrated that GP could lead to an anti-inflammatory shift with production of IL-1 receptor antagonist. Such a protective function exerted by GP in vitro was supported by in vivo data of beta-glucan-mediated cardio protection [100]. In another study, stimulation of murine lymphocytes isolated from soluble beta-glucan-treated mice with SEB or TSST-1 increased production of IFN-gamma while abrogating production of IL-2 and TNF-alpha [101]. These results indicate that beta-glucans may represent immunomodulators for controlling release of pro-inflammatory cytokines from superantigen-activated lymphocytes and monocytes with mitigation of septic shock.
5.3. Lactobacilli
Lactobacilli are commensal bacteria belonging to the class of probiotics. They are dietary supplements that provide the host with beneficial effects, acting as immunomodulators [102,103]. In this respect, Lactobacilli, as well as their cell-free supernatants, exert different functions, such as induction of TREG cells with production of IL-10 and a shift towards an anti-inflammatory profile [104,105]. A few studies have been focused on the ability of lactobacilli to neutralize the toxic effects of SE. Colonization of S. aureus in early life was associated with a massive release of cytokines, while co-colonization with lactobacilli in vitro decreased immune response [106]. Also, soluble factors derived from lactobacilli were able to reduce the S. aureus-induced activation of T cells and release of pro-inflammatory cytokines [107]. In another study, PBMCs were cultured with S. aureus cell-free supernatants (CFSs) and SEA in the presence of L. rhamnosus CFS and L. reuteri [108]. Then, activation of T cells and natural killer cells was evaluated. Results showed that these cells were activated by S. aureus-CFS, while SEA and CFS derived from lactobacilli in vitro decreased immune functions through direct cellular contact. In fact, immune suppression occurred in the absence of antigen presentation or APC-derived IL-10. Furthermore, during S. aureus bloodstream infection in mice, an exopolysaccharide (EPS) from Bacillus subtilis reduced mortality [109]. In this test system, EPS abrogated the IL-12-derived production of IFN-gamma by NK cells in a TLR-4-dependent manner.
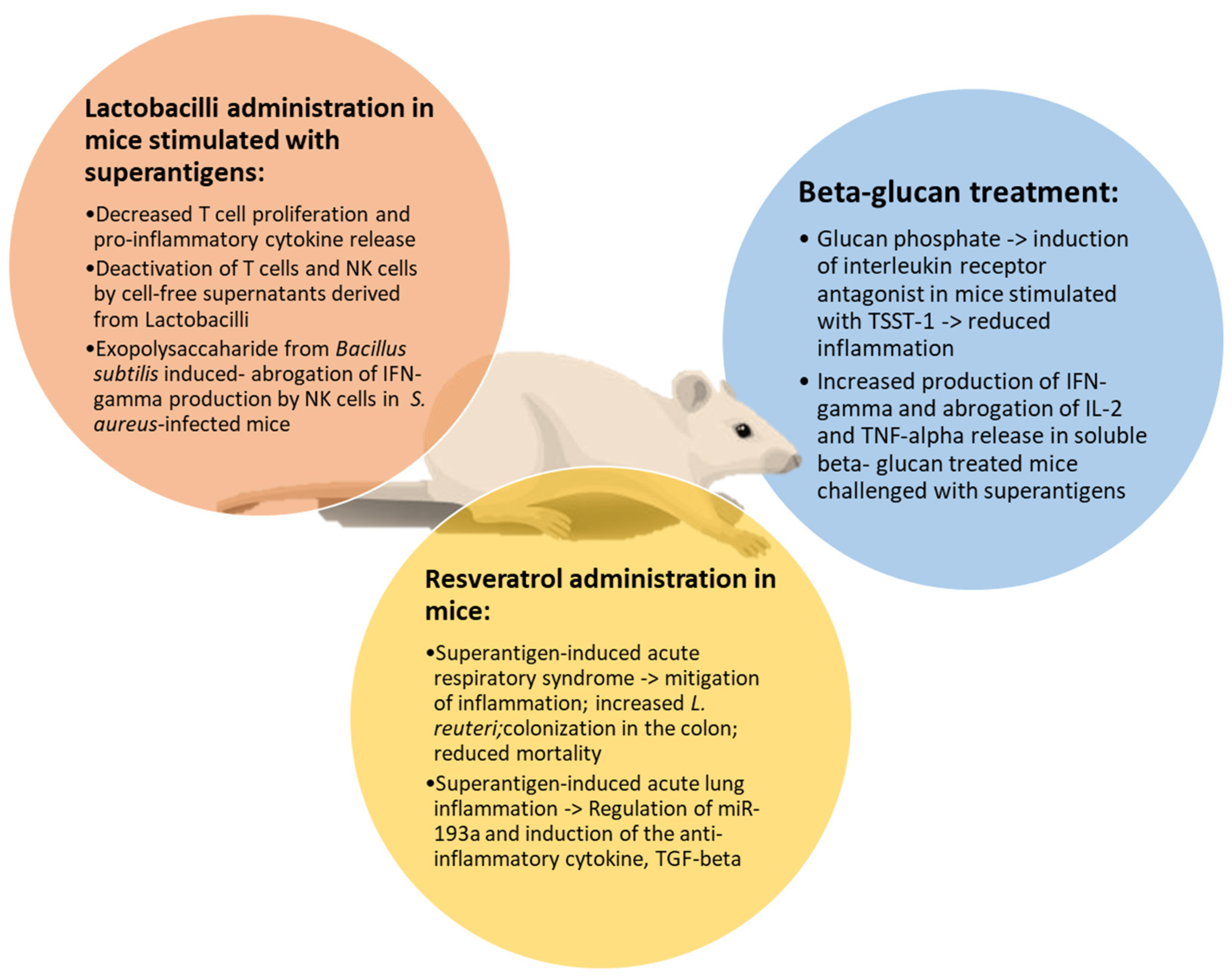
In Figure 2, the effects of natural products against superantigen-mediated damage are illustrated.
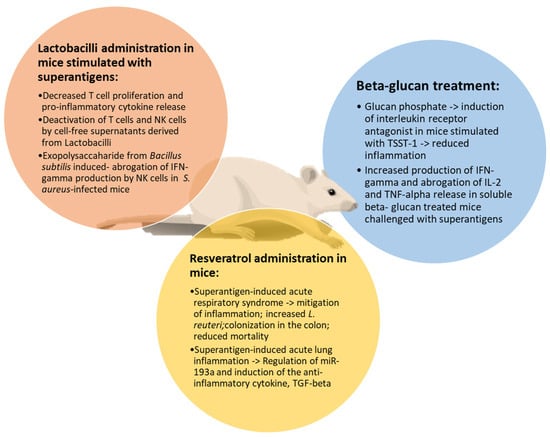
Figure 2.
Main effects of natural products on superantigen-induced damage. Resveratrol, beta-glucans and lactobacilli share common activities in attenuating inflammation, reducing T cell proliferation and cytokine release in mice stimulated by superantigens.
5.4. Antimicrobial Peptides
AMPs are small peptides produced by innate immune cells that exert broad antibacterial activity against both Gram-positive and Gram-negative bacteria [26,27]. The emergence of bacteria resistant to antibiotics represents a serious health care problem, and AMPs are currently used as a potential alternative treatment to antibiotics [110]. Very few studies focused on the ability of AMPs to neutralize superantigens.
Evidence has been provided that naturally occurring hemoglobin alpha chain peptides can inhibit TSST-1 from S. aureus, likely interfering with plasma membrane signal transduction in view of their positive charges [111]. Moreover, this AMP was nontoxic to human vaginal cells, without inhibiting the normal microbiotal member L. crispatus that produces anti-inflammatory mediators. In another report, expression of IL-26 has been demonstrated in skin wounds infected with S. aureus [112]. In this context, SE was responsible for IL-26 expression in T cell lines and primary skin T cells. Il-26 was able to inhibit growth of S. aureus and biofilm formation.
In conclusion, IL-26 behaves as an AMP, hampering T cell responses to SE. Lactoferrin (LF) is an AMP that is largely present in colostrum, milk and other exocrine secretions [113,114]. LF has bacteriostatic and bactericidal activities in view of its ability to chelate iron and prevent LPS binding to TLR-4 [115]. Bovine (b)LF could mitigate SEB- induced proliferation, IL-2 release and CD25 expression by transgenic murine T cells [116]. Furthermore, bLF hampered cytokine secretion from Jurkat T cell lines and PBMCs from healthy donors in response to SEB. In the light of these findings, bLF may represent a potential therapeutic tool to protect the host during the toxic shock syndrome. Conversely, it has been reported that human transferrin and LF could modulate expression of streptococcal pyrogenic exotoxins (Spes) [117]. Down-regulation of SpeB may favor bacterial growth, preserving the integrity of M proteins and superantigens [118,119].
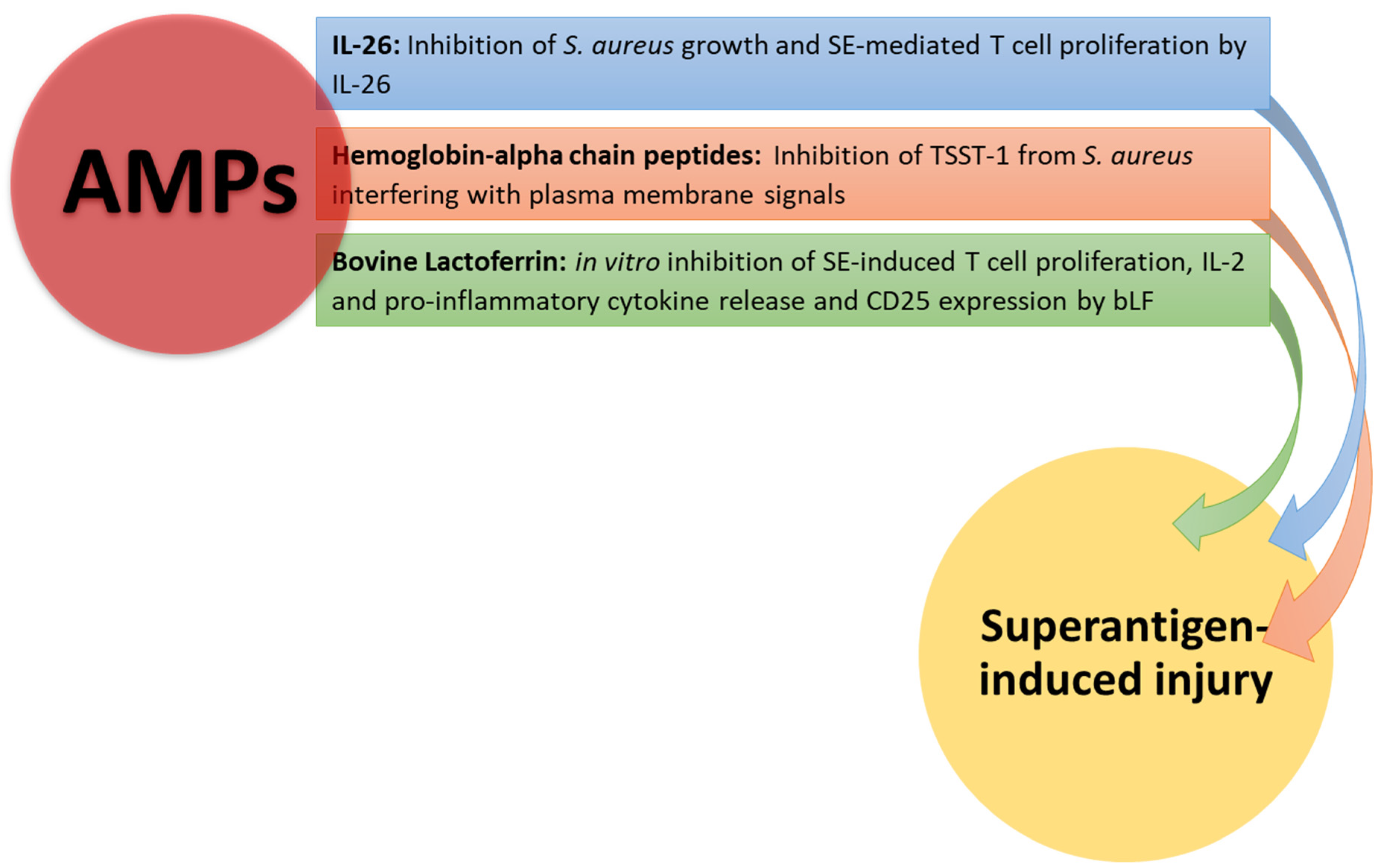
In Figure 3, the effects of AMPs on superantigen-induced injury are shown.
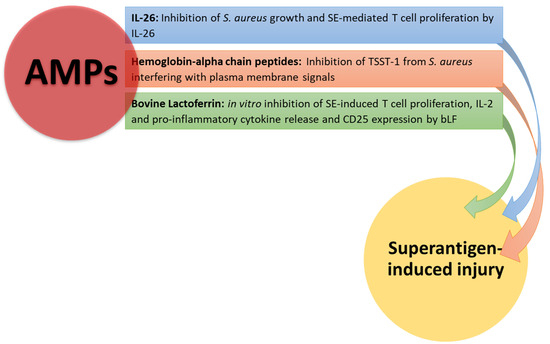
Figure 3.
AMP neutralizing effects on superantigen-mediated damage. Hemoglobin-alpha chain peptides and IL-26 inhibit S. aureus growth, interfering with plasma membrane signals. Il-26 and bLF inhibit T cell proliferation and cytokine production by T cells stimulated with SEB.
6. Conclusions
Superantigens are very dangerous bacterial products, since they bind the TCR on T cells outside the peptide-binding groove, thus involving a far larger number of lymphocytes. Binding is followed by the liberation of the cascade of pro-inflammatory cytokines, which may lead to a condition of multiorgan failure and death. Attempts to block the above cited binding and neutralize the liberation of pro-inflammatory cytokines systemically with the use of immunosuppressants and biotherapeutics, such as MoAbs, are under investigation. However, clinical trials are still scarce, and drugs like MoAbs can induce side effects. On these grounds, the attention of scientists has recently been focused on the use of natural and biological products as potential anti-inflammatory and antimicrobial agents. In fact, polyphenols, beta-glucans, probiotics and AMPs share common activities in terms of inhibition of the NF-kB pathway and release of anti-inflammatory cytokines, mainly IL-10, also arresting the growth of pathogenic bacteria. Moreover, they exert immunomodulating activities, mitigating the noxious effects of superantigens. The advantages of these products are the absence of side effects and limited costs in comparison to MoAbs. Despite that, there are very few clinical trials, and, therefore, exploitation of the properties of natural and biological products against superantigen-mediated damage is needed. Natural products may represent a prospective therapeutic tool to prevent or mitigate the deleterious effects exerted by superantigens.
Author Contributions
Conceptualization: E.J. and L.S.; Investigation: P.L., R.L., S.T. and R.P.; Writing—original: E.J. and I.A.C.; Resources: S.T.; Project administration: E.J., S.T. and R.P.; Validation: L.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All available data have been reported in the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| ALI | Acute lung injury |
| AMPs | Antimicrobial peptides |
| APCs | Antigen-presenting cells |
| b-LF | Bovine lactoferrin |
| CDR | Complementary determining regions |
| CFs | Cell-free supernatants |
| DCs | Dendritic cells |
| EPs | Exopolysaccharides |
| ER | Endoplasmic reticular |
| ICAM-1 | Intercellular adhesion molecule-1 |
| IFN | Interferon |
| IL | Interleukin |
| LF | Lactoferrin |
| MHC | Major histocompatibility complex |
| MIP-1 | Macrophage inflammatory protein-1 |
| NFPs | N-formyl peptides |
| NLRs | Nod-like receptors |
| PAMPS | Pathogen-associated molecular patterns |
| PTKs | Protein tyrosine kinases |
| RES | Resveratrol |
| ROS | Reactive oxygen species |
| SEs | Staphylococcal enterotoxins |
| SPEs | Streptococcal pyrogenic exotoxins |
| Th | T helper |
| TGF-beta | Transforming growth factor-beta |
| TLRs | Toll-like receptors |
| TNF-alpha | Tumor necrosis factor-alpha |
| TREG | T regulatory |
| TRM | T resident memory |
| TSS | Toxic shock syndrome |
| TSST-1 | Toxic shock syndrome toxin-1 |
References
- Le, J.; Kulatheepan, Y.; Jeyaseelan, S. Role of toll-like receptors and nod-like receptors in acute lung infection. Front. Immunol. 2023, 14, 1249098. [Google Scholar] [CrossRef] [PubMed]
- Gül, E.; Fattinger, S.A.; Sellin, M.E.; Hardt, W.D. Epithelial inflammasomes, gasdermins, and mucosal inflammation—Lessons from Salmonella and Shigella infected mice. Semin. Immunol. 2023, 70, 101812. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.S.; Oh, S.F. Unconventional immune cells in the gut mucosal barrier: Regulation by symbiotic microbiota. Exp. Mol. Med. 2023, 55, 1905–1912. [Google Scholar] [CrossRef] [PubMed]
- Santacroce, L.; Imbimbo, C.; Ballini, A.; Crocetto, F.; Scacco, S.; Cantore, S.; Di Zazzo, E.; Colella, M.; Jirillo, E. Testicular Immunity and Its Connection with the Microbiota. Physiological and Clinical Implications in the Light of Personalized Medicine. J. Pers. Med. 2022, 12, 1335. [Google Scholar] [CrossRef] [PubMed]
- Gray, J.I.; Farber, D.L. Tissue-Resident Immune Cells in Humans. Annu. Rev. Immunol. 2022, 40, 195–220. [Google Scholar] [CrossRef] [PubMed]
- Al, B.; Suen, T.K.; Placek, K.; Netea, M.G. Innate (learned) memory. J. Allergy Clin. Immunol. 2023, 152, 551–566. [Google Scholar] [CrossRef]
- Nofi, C.P.; Wang, P.; Aziz, M. Chromatin-Associated Molecular Patterns (CAMPs) in sepsis. Cell Death Dis. 2022, 13, 700. [Google Scholar] [CrossRef]
- Kaplan, M.J. Casting a Wide NET. J. Immunol. 2022, 209, 843–844. [Google Scholar] [CrossRef]
- Netea, M.G.; Joosten, L.A.; Latz, E.; Mills, K.H.; Natoli, G.; Stunnenberg, H.G.; O’Neill, L.A.; Xavier, R.J. Trained immunity: A program of innate immune memory in health and disease. Science 2016, 352, aaf1098. [Google Scholar] [CrossRef]
- Janssen, E.M.; Lemmens, E.E.; Wolfe, T.; Christen, U.; von Herrath, M.G.; Schoenberger, S.P. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature 2003, 421, 852–856. [Google Scholar] [CrossRef]
- Shedlock, D.J.; Shen, H. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science 2003, 300, 337–339. [Google Scholar] [CrossRef] [PubMed]
- Jiao, A.; Liu, H.; Ding, R.; Zheng, H.; Zhang, C.; Feng, Z.; Lei, L.; Wang, X.; Su, Y.; Yang, X.; et al. Med1 Controls Effector CD8+ T Cell Differentiation and Survival through C/EBPβ-Mediated Transcriptional Control of T-bet. J. Immunol. 2022, 209, 855–863. [Google Scholar] [CrossRef] [PubMed]
- Crotty, S. A brief history of T cell help to B cells. Nat. Rev. Immunol. 2015, 15, 185–189. [Google Scholar] [CrossRef]
- La Gruta, N.L.; Gras, S.; Daley, S.R.; Thomas, P.G.; Rossjohn, J. Understanding the drivers of MHC restriction of T cell receptors. Nat. Rev. Immunol. 2018, 18, 467–478. [Google Scholar] [CrossRef] [PubMed]
- Kaech, S.M.; Cui, W. Transcriptional control of effector and memory CD8+ T cell differentiation. Nat. Rev. Immunol. 2012, 12, 749–761. [Google Scholar] [CrossRef]
- Laidlaw, B.J.; Craft, J.E.; Kaech, S.M. The multifaceted role of CD4(+) T cells in CD8(+) T cell memory. Nat. Rev. Immunol. 2016, 16, 102–111. [Google Scholar] [CrossRef]
- Surh, C.D.; Sprent, J. Homeostasis of naive and memory T cells. Immunity. 2008, 29, 848–862. [Google Scholar] [CrossRef]
- Noli Truant, S.; Redolfi, D.M.; Sarratea, M.B.; Malchiodi, E.L.; Fernández, M.M. Superantigens, a Paradox of the Immune Response. Toxins 2022, 14, 800. [Google Scholar] [CrossRef]
- Jensen, I.J.; Farber, D.L. Gutsy memory T cells stand their ground against pathogens. Sci. Immunol. 2022, 7, eade7168. [Google Scholar] [CrossRef]
- Li, H.; Llera, A.; Malchiodi, E.L.; Mariuzza, R.A. The structural basis of T cell activation by superantigens. Annu. Rev. Immunol. 1999, 17, 435–466. [Google Scholar] [CrossRef]
- Kolla, H.B.; Tirumalasetty, C.; Sreerama, K.; Ayyagari, V.S. An immunoinformatics approach for the design of a multi-epitope vaccine targeting super antigen TSST-1 of Staphylococcus aureus. J. Genet. Eng. Biotechnol. 2021, 19, 69. [Google Scholar] [CrossRef] [PubMed]
- Balzanelli, M.G.; Distratis, P.; Aityan, S.K.; Amatulli, F.; Catucci, O.; Cefalo, A.; De Michele, A.; Dipalma, G.; Inchingolo, F.; Lazzaro, R.; et al. An Alternative “Trojan Horse” Hypothesis for COVID-19: Immune Deficiency of IL-10 and SARS-CoV-2 Biology. Endocr. Metab. Immune Disord. Drug Targets 2022, 22, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Jupin, C.; Anderson, S.; Damais, C.; Alouf, J.E.; Parant, M. Toxic shock syndrome toxin 1 as an inducer of human tumor necrosis factors and gamma interferon. J. Exp. Med. 1988, 167, 752–761. [Google Scholar] [CrossRef] [PubMed]
- Tessier, P.A.; Naccache, P.H.; Diener, K.R.; Gladue, R.P.; Neote, K.S.; Clark-Lewis, I.; McColl, S.R. Induction of acute inflammation in vivo by staphylococcal superantigens. II. Critical role for chemokines, ICAM-1, and TNF-alpha. J. Immunol. 1998, 161, 1204–1211. [Google Scholar] [CrossRef] [PubMed]
- Krakauer, T. Costimulatory receptors for the superantigen staphylococcal enterotoxin B on human vascular endothelial cells and T cells. J. Leukoc. Biol. 1994, 56, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Magrone, T.; Magrone, M.; Russo, M.A.; Jirillo, E. Recent Advances on the Anti-Inflammatory and Antioxidant Properties of Red Grape Polyphenols: In Vitro and In Vivo Studies. Antioxidants 2019, 9, 35. [Google Scholar] [CrossRef] [PubMed]
- Kazemifard, N.; Dehkohneh, A.; Baradaran Ghavami, S. Probiotics and probiotic-based vaccines: A novel approach for improving vaccine efficacy. Front. Med. 2022, 9, 940454. [Google Scholar] [CrossRef]
- Han, X.; Luo, R.; Ye, N.; Hu, Y.; Fu, C.; Gao, R.; Fu, S.; Gao, F. Research progress on natural β-glucan in intestinal diseases. Int. J. Biol. Macromol. 2022, 219, 1244–1260. [Google Scholar] [CrossRef]
- Reddy, K.V.; Yedery, R.D.; Aranha, C. Antimicrobial peptides: Premises and promises. Int. J. Antimicrob. Agents 2004, 24, 536–547. [Google Scholar] [CrossRef]
- Magrone, T.; Russo, M.A.; Jirillo, E. Antimicrobial peptides in human disease: Therapeutic approaches. Second of two parts. Curr. Pharm. Des. 2018, 24, 1148–1156. [Google Scholar] [CrossRef]
- Galperin, M.; Farenc, C.; Mukhopadhyay, M.; Jayasinghe, D.; Decroos, A.; Benati, D.; Tan, L.L.; Ciacchi, L.; Reid, H.H.; Rossjohn, J.; et al. CD4+ T cell-mediated HLA class II cross-restriction in HIV controllers. Sci. Immunol. 2018, 3, eaat0687. [Google Scholar] [CrossRef] [PubMed]
- Willcox, B.E.; Willcox, C.R. γδ TCR ligands: The quest to solve a 500-million-year-old mystery [published correction appears. Nat. Immunol. 2019, 20, 516. [Google Scholar] [CrossRef] [PubMed]
- Burn, T.N.; Miot, C.; Gordon, S.M.; Culberson, E.J.; Diamond, T.; Kreiger, P.A.; Hayer, K.E.; Bhattacharyya, A.; Jones, J.M.; Bassing, C.H.; et al. The RAG1 Ubiquitin Ligase Domain Stimulates Recombination of TCRβ and TCRα Genes and Influences Development of αβ T Cell Lineages. J. Immunol. 2022, 209, 938–949. [Google Scholar] [CrossRef] [PubMed]
- Ranasinghe, S.; Lamothe, P.A.; Soghoian, D.Z.; Kazer, S.W.; Cole, M.B.; Shalek, A.K.; Yosef, N.; Jones, R.B.; Donaghey, F.; Nwonu, C.; et al. Antiviral CD8+ T Cells Restricted by Human Leukocyte Antigen Class II Exist during Natural HIV Infection and Exhibit Clonal Expansion. Immunity 2016, 45, 917–930. [Google Scholar] [CrossRef] [PubMed]
- Calabi, F.; Jarvis, J.M.; Martin, L.; Milstein, C. Two classes of CD1 genes. Eur. J. Immunol. 1989, 19, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Arstila, T.P.; Casrouge, A.; Baron, V.; Even, J.; Kanellopoulos, J.; Kourilsky, P. A direct estimate of the human alphabeta T cell receptor diversity. Science 1999, 286, 958–961. [Google Scholar] [CrossRef]
- Tian, L.; Zhou, W.; Wu, X.; Hu, Z.; Qiu, L.; Zhang, H.; Chen, X.; Zhang, S.; Lu, Z. CTLs: Killers of intracellular bacteria. Front. Cell Infect. Microbiol. 2022, 12, 967679. [Google Scholar] [CrossRef]
- Jenkins, M.K.; Moon, J.J. The role of naive T cell precursor frequency and recruitment in dictating immune response magnitude. J. Immunol. 2012, 188, 4135–4140. [Google Scholar] [CrossRef]
- Thakur, A.; Mikkelsen, H.; Jungersen, G. Intracellular Pathogens: Host Immunity and Microbial Persistence Strategies. J. Immunol. Res. 2019, 2019, 1356540. [Google Scholar] [CrossRef]
- Cruz-Adalia, A.; Ramirez-Santiago, G.; Calabia-Linares, C.; Torres-Torresano, M.; Feo, L.; Galán-Díez, M.; Fernandez-Ruiz, E.; Pereiro, E.; Guttmann, P.; Chiappi, M.; et al. T cells kill bacteria captured by transinfection from dendritic cells and confer protection in mice. Cell Host Microbe 2014, 15, 611–622. [Google Scholar] [CrossRef]
- Liu, H.; Wang, X.; Ding, R.; Jiao, A.; Zheng, H.; Zhang, C.; Feng, Z.; Su, Y.; Yang, X.; Lei, L.; et al. The Transcription Factor Zfp335 Promotes Differentiation and Persistence of Memory CD8+ T Cells by Regulating TCF-1. J. Immunol. 2022, 209, 886–895. [Google Scholar] [CrossRef] [PubMed]
- Li, G.Q.; Xia, J.; Zeng, W.; Luo, W.; Liu, L.; Zeng, X.; Cao, D. The intestinal γδ T cells: Functions in the gut and in the distant organs. Front. Immunol. 2023, 14, 1206299. [Google Scholar] [CrossRef] [PubMed]
- Park, S.L.; Zaid, A.; Hor, J.L.; Christo, S.N.; Prier, J.E.; Davies, B.; Alexandre, Y.O.; Gregory, J.L.; Russell, T.A.; Gebhardt, T.; et al. Local proliferation maintains a stable pool of tissue-resident memory T cells after antiviral recall responses. Nat. Immunol. 2018, 19, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Adams, E.J.; Gu, S.; Luoma, A.M. Human gamma delta T cells: Evolution and ligand recognition. Cell Immunol. 2015, 296, 31–40. [Google Scholar] [CrossRef]
- Khairallah, C.; Chu, T.H.; Sheridan, B.S. Tissue Adaptations of Memory and Tissue-Resident Gamma Delta T Cells. Front. Immunol. 2018, 9, 2636. [Google Scholar] [CrossRef]
- Abrahmsén, L.; Dohlsten, M.; Segrén, S.; Björk, P.; Jonsson, E.; Kalland, T. Characterization of two distinct MHC class II binding sites in the superantigen staphylococcal enterotoxin A. EMBO J. 1995, 14, 2978–2986. [Google Scholar] [CrossRef]
- Hudson, K.R.; Tiedemann, R.E.; Urban, R.G.; Lowe, S.C.; Strominger, J.L.; Fraser, J.D. Staphylococcal enterotoxin A has two cooperative binding sites on major histocompatibility complex class II. J. Exp. Med. 1995, 182, 711–720. [Google Scholar] [CrossRef]
- Ulrich, R.G.; Bavari, S.; Olson, M.A. Staphylococcal enterotoxins A and B share a common structural motif for binding class II major histocompatibility complex molecules. Nat. Struct. Biol. 1995, 2, 554–560. [Google Scholar] [CrossRef]
- Hopkins, P.A.; Fraser, J.D.; Pridmore, A.C.; Russell, H.H.; Read, R.C.; Sriskandan, S. Superantigen recognition by HLA class II on monocytes up-regulates toll-like receptor 4 and enhances proinflammatory responses to endotoxin. Blood 2005, 105, 3655–3662. [Google Scholar] [CrossRef]
- Grundström, S.; Cederbom, L.; Sundstedt, A.; Scheipers, P.; Ivars, F. Superantigen-induced regulatory T cells display different suppressive functions in the presence or absence of natural CD4+CD25+ regulatory T cells in vivo. J. Immunol. 2003, 170, 5008–5017. [Google Scholar] [CrossRef]
- Li, S.J.; Hu, D.L.; Maina, E.K.; Shinagawa, K.; Omoe, K.; Nakane, A. Superantigenic activity of toxic shock syndrome toxin-1 is resistant to heating and digestive enzymes. J. Appl. Microbiol. 2011, 110, 729–736. [Google Scholar] [CrossRef] [PubMed]
- Smith-Garvin, J.E.; Koretzky, G.A.; Jordan, M.S. T cell activation. Annu. Rev. Immunol. 2009, 27, 591–619. [Google Scholar] [CrossRef] [PubMed]
- Linsley, P.S.; Ledbetter, J.A. The role of the CD28 receptor during T cell responses to antigen. Annu. Rev. Immunol. 1993, 11, 191–212. [Google Scholar] [CrossRef]
- Isakov, N.; Altman, A. PKC-theta-mediated signal delivery from the TCR/CD28 surface receptors. Front. Immunol. 2012, 3, 273. [Google Scholar] [CrossRef]
- Kelley, N.; Jeltema, D.; Duan, Y.; He, Y. The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int. J. Mol. Sci. 2019, 20, 3328. [Google Scholar] [CrossRef] [PubMed]
- Krakauer, T.; Stiles, B.G. The staphylococcal enterotoxin (SE) family: SEB and siblings. Virulence 2013, 4, 759–773. [Google Scholar] [CrossRef] [PubMed]
- Isakov, N.; Altman, A. Regulation of immune system cell functions by protein kinase C. Front. Immunol. 2013, 4, 384. [Google Scholar] [CrossRef] [PubMed]
- Santacroce, L.; Colella, M.; Charitos, I.A.; Di Domenico, M.; Palmirotta, R.; Jirillo, E. Microbial and Host Metabolites at the Backstage of Fever: Current Knowledge about the Co-Ordinate Action of Receptors and Molecules Underlying Pathophysiology and Clinical Implications. Metabolites 2023, 13, 461. [Google Scholar] [CrossRef]
- Peng, L.; Jiang, J.; Chen, T.; Xu, D.; Hou, F.; Huang, Q.; Peng, Y.; Ye, C.; Hu, D.L.; Fang, R. Toxic Shock Syndrome Toxin 1 Induces Immune Response via the Activation of NLRP3 Inflammasome. Toxins 2021, 13, 68. [Google Scholar] [CrossRef]
- Krakauer, T. Staphylococcal Superantigens: Pyrogenic Toxins Induce Toxic Shock. Toxins 2019, 11, 178. [Google Scholar] [CrossRef]
- Huzella, L.M.; Buckley, M.J.; Alves, D.A.; Stiles, B.G.; Krakauer, T. Central roles for IL-2 and MCP-1 following intranasal exposure to SEB: A new mouse model. Res. Vet. Sci. 2009, 86, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.X.; Gilmore, K.J.; Szabo, P.A.; Zeppa, J.J.; Baroja, M.L.; Haeryfar, S.M.; McCormick, J.K. Superantigens subvert the neutrophil response to promote abscess formation and enhance Staphylococcus aureus survival in vivo. Infect. Immun. 2014, 82, 3588–3598. [Google Scholar] [CrossRef] [PubMed]
- Neumann, B.; Engelhardt, B.; Wagner, H.; Holzmann, B. Induction of acute inflammatory lung injury by staphylococcal enterotoxin B. J. Immunol. 1997, 158, 1862–1871. [Google Scholar] [CrossRef] [PubMed]
- Santacroce, L.; Muzio, E.L.; Bottalico, L.; Spirito, F.; Charitos, I.A.; Passarelli, P.C.; Jirillo, E. Subversion of the Oral Microbiota and Induction of Immune-Mediated Systemic Inflammation with Special Reference to Periodontitis. Current Knowledge and Perspectives. Endocr. Metab. Immune Disord. Drug Targets 2023, 23, 470–484. [Google Scholar] [CrossRef] [PubMed]
- Bouvet, J.P.; Marone, G. Protein Fv: An endogenous immunoglobulin superantigen and superallergen. Chem. Immunol. Allergy 2007, 93, 58–76. [Google Scholar] [CrossRef]
- Marone, G.; Rossi, F.W.; Detoraki, A.; Granata, F.; Marone, G.; Genovese, A.; Spadaro, G. Role of superallergens in allergic disorders. Chem. Immunol. Allergy 2007, 93, 195–213. [Google Scholar] [CrossRef]
- Sørensen, M.; Klingenberg, C.; Wickman, M.; Sollid, J.U.; Furberg, A.S.; Bachert, C.; Bousquet, J. Staphylococcus aureus enterotoxin sensitization is associated with allergic poly-sensitization and allergic multimorbidity in adolescents. Allergy 2017, 72, 1548–1555. [Google Scholar] [CrossRef]
- Solinas, G.; Karin, M. JNK1 and IKKbeta: Molecular links between obesity and metabolic dysfunction. FASEB J. 2010, 24, 2596–2611. [Google Scholar] [CrossRef]
- Shenderov, K.; Riteau, N.; Yip, R.; Mayer-Barber, K.D.; Oland, S.; Hieny, S.; Fitzgerald, P.; Oberst, A.; Dillon, C.P.; Green, D.R.; et al. Cutting edge: Endoplasmic reticulum stress licenses macrophages to produce mature IL-1β in response to TLR4 stimulation through a caspase-8- and TRIF-dependent pathway. J. Immunol. 2014, 192, 2029–2033. [Google Scholar] [CrossRef]
- Santacroce, L.; Palmirotta, R.; Bottalico, L.; Charitos, I.A.; Colella, M.; Topi, S.; Jirillo, E. Crosstalk between the Resident Microbiota and the Immune Cells Regulates Female Genital Tract Health. Life 2023, 13, 1531. [Google Scholar] [CrossRef]
- Nakahira, K.; Hisata, S.; Choi, A.M. The Roles of Mitochondrial Damage-Associated Molecular Patterns in Diseases. Antioxid. Redox Signal. 2015, 23, 1329–1350. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.L.; Nakane, A. Mechanisms of staphylococcal enterotoxin-induced emesis. Eur. J. Pharmacol. 2014, 722, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Di Serio, F.; Lovero, R.; D’Agostino, D.; Nisi, L.; Miragliotta, G.; Contino, R.; Man, A.; Ciccone, M.M.; Santacroce, L. Evaluation of procalcitonin, Vitamin D and C-reactive protein levels in septic patients with positive emocoltures. Our preliminary experience. Acta Med. Mediterr. 2016, 32, 1911–1914. [Google Scholar] [CrossRef]
- Grumann, D.; Ruotsalainen, E.; Kolata, J.; Kuusela, P.; Järvinen, A.; Kontinen, V.P.; Bröker, B.M.; Holtfreter, S. Characterization of infecting strains and superantigen-neutralizing antibodies in Staphylococcus aureus bacteremia. Clin. Vaccine Immunol. 2011, 18, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Burns, J.C. New perspectives on Kawasaki disease. Arch. Dis. Child. 2019, 104, 616–617. [Google Scholar] [CrossRef] [PubMed]
- Topi, S.; Bottalico, L.; Charitos, I.A.; Colella, M.; Di Domenico, M.; Palmirotta, R.; Santacroce, L. Biomolecular Mechanisms of Autoimmune Diseases and Their Relationship with the Resident Microbiota: Friend or Foe? Pathophysiology 2022, 29, 507–536. [Google Scholar] [CrossRef]
- Leung, D.Y.M.; Schlievert, P.M. Kawasaki syndrome: Role of superantigens revisited. FEBS J. 2021, 288, 1771–1777. [Google Scholar] [CrossRef]
- Carretta, D.M.; Silva, A.M.; D’Agostino, D.; Topi, S.; Lovero, R.; Charitos, I.A.; Wegierska, A.E.; Montagnani, M.; Santacroce, L. Cardiac Involvement in COVID-19 Patients: A Contemporary Review. Infect. Dis. Rep. 2021, 13, 494–517. [Google Scholar] [CrossRef]
- Halloran, P.F. Immunosuppressive drugs for kidney transplantation [published correction appears. N. Engl. J. Med. 2005, 352, 1056. [Google Scholar] [CrossRef]
- Komisar, J.L.; Weng, C.F.; Oyejide, A.; Hunt, R.E.; Briscoe, C.; Tseng, J. Cellular and cytokine responses in the circulation and tissue reactions in the lung of rhesus monkeys (Macaca mulatta) pretreated with cyclosporin A and challenged with staphylococcal enterotoxin B. Toxicol. Pathol. 2001, 29, 369–378. [Google Scholar] [CrossRef]
- Tilahun, A.Y.; Karau, M.J.; Clark, C.R.; Patel, R.; Rajagopalan, G. The impact of tacrolimus on the immunopathogenesis of staphylococcal enterotoxin-induced systemic inflammatory response syndrome and pneumonia. Microbes Infect. 2012, 14, 528–536. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, M.; Stabilini, A.; Roncarolo, M.G. Rapamycin selectively expands CD4+CD25+FoxP3+ regulatory T cells. Blood 2005, 105, 4743–4748. [Google Scholar] [CrossRef] [PubMed]
- Darenberg, J.; Söderquist, B.; Normark, B.H.; Norrby-Teglund, A. Differences in potency of intravenous polyspecific immunoglobulin G against streptococcal and staphylococcal superantigens: Implications for therapy of toxic shock syndrome. Clin. Infect. Dis. 2004, 38, 836–842. [Google Scholar] [CrossRef] [PubMed]
- Tilahun, M.E.; Rajagopalan, G.; Shah-Mahoney, N.; Lawlor, R.G.; Tilahun, A.Y.; Xie, C.; Natarajan, K.; Margulies, D.H.; Ratner, D.I.; Osborne, B.A.; et al. Potent neutralization of staphylococcal enterotoxin B by synergistic action of chimeric antibodies. Infect. Immun. 2010, 78, 2801–2811. [Google Scholar] [CrossRef] [PubMed]
- Larkin, J.; Hatswell, A.J.; Nathan, P.; Lebmeier, M.; Lee, D. The Predicted Impact of Ipilimumab Usage on Survival in Previously Treated Advanced or Metastatic Melanoma in the UK. PLoS ONE 2015, 10, e0145524. [Google Scholar] [CrossRef] [PubMed]
- Varshney, A.K.; Wang, X.; Cook, E.; Dutta, K.; Scharff, M.D.; Goger, M.J.; Fries, B.C. Generation, characterization, and epitope mapping of neutralizing and protective monoclonal antibodies against staphylococcal enterotoxin B-induced lethal shock. J. Biol. Chem. 2014, 289, 13706. [Google Scholar] [CrossRef]
- Whitfield, S.J.C.; Taylor, C.; Risdall, J.E.; Griffiths, G.D.; Jones, J.T.A.; Williamson, E.D.; Rijpkema, S.; Saraiva, L.; Vessillier, S.; Green, A.C.; et al. Interference of the T Cell and Antigen-Presenting Cell Costimulatory Pathway Using CTLA4-Ig (Abatacept) Prevents Staphylococcal Enterotoxin B Pathology. J. Immunol. 2017, 198, 3989–3998. [Google Scholar] [CrossRef]
- Magrone, T.; Magrone, M.; Russo, M.A.; Jirillo, E. Taking Advantage of Plant Defense Mechanisms to Promote Human Health. The Plant Immune System. First of Two Parts. Endocr. Metab. Immune Disord. Drug Targets 2021, 21, 1183–1195. [Google Scholar] [CrossRef]
- Magrone, T.; Russo, M.A.; Jirillo, E. Antimicrobial Peptides: Phylogenic Sources and Biological Activities. First of Two Parts. Curr. Pharm. Des. 2018, 24, 1043–1053. [Google Scholar] [CrossRef]
- Nagy-Bota, M.C.; Man, A.; Santacroce, L.; Brinzaniuc, K.; Pap, Z.; Pacurar, M.; Pribac, M.; Ciurea, C.N.; Pintea-Simon, I.A.; Kovacs, M. Essential Oils as Alternatives for Root-Canal Treatment and Infection Control against Enterococcus faecalis—A Preliminary Study. Appl. Sci. 2021, 11, 1422. [Google Scholar] [CrossRef]
- Marzulli, G.; Magrone, T.; Kawaguchi, K.; Kumazawa, Y.; Jirillo, E. Fermented grape marc (FGM): Immunomodulating properties and its potential exploitation in the treatment of neurodegenerative diseases. Curr. Pharm. Des. 2012, 18, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Fujiki, T.; Shinozaki, R.; Udono, M.; Katakura, Y. Identification and Functional Evaluation of Polyphenols That Induce Regulatory T Cells. Nutrients 2022, 14, 2862. [Google Scholar] [CrossRef] [PubMed]
- Rasooly, R.; Do, P.M.; Friedman, M. Inhibition of biological activity of staphylococcal enterotoxin A (SEA) by apple juice and apple polyphenols. J. Agric. Food Chem. 2010, 58, 5421–5426. [Google Scholar] [CrossRef] [PubMed]
- Watson, J.L.; Vicario, M.; Wang, A.; Moreto, M.; McKay, D.M. Immune cell activation and subsequent epithelial dysfunction by Staphylococcus enterotoxin B is attenuated by the green tea polyphenol (-)-epigallocatechin gallate. Cell Immunol. 2005, 237, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Alghetaa, H.; Mohammed, A.; Zhou, J.; Singh, N.; Nagarkatti, M.; Nagarkatti, P. Resveratrol-mediated attenuation of superantigen-driven acute respiratory distress syndrome is mediated by microbiota in the lungs and gut. Pharmacol. Res. 2021, 167, 105548. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Zhao, L.; Sun, Y.; Wang, X.; Shi, X. HMGB1 and Toll-like receptors: Potential therapeutic targets in autoimmune diseases. Mol. Med. 2023, 29, 117. [Google Scholar] [CrossRef]
- Lyu, Y.; Wang, T.; Huang, S.; Zhang, Z. Mitochondrial Damage-Associated Molecular Patterns and Metabolism in the Regulation of Innate Immunity. J. Innate Immun. 2023, 15, 665–679. [Google Scholar] [CrossRef]
- Tirunavalli, S.K.; Pramatha, S.; Eedara, A.C.; Walvekar, K.P.; Immanuel, C.; Potdar, P.; Nayak, P.G.; Chamallamudi, M.R.; Sistla, R.; Chilaka, S.; et al. Protective effect of β-glucan on Poly(I:C)-induced acute lung injury/inflammation: Therapeutic implications of viral infections in the respiratory system. Life Sci. 2023, 330, 122027. [Google Scholar] [CrossRef]
- Gordon, S. Pattern recognition receptors: Doubling up for the innate immune response. Cell 2002, 111, 927–930. [Google Scholar] [CrossRef]
- Battle, J.; Ha, T.; Li, C.; Della Beffa, V.; Rice, P.; Kalbfleisch, J.; Browder, W.; Williams, D. Ligand binding to the (1 --> 3)-beta-D-glucan receptor stimulates NFkappaB activation, but not apoptosis in U937 cells. Biochem. Biophys. Res. Commun. 1998, 249, 499–504. [Google Scholar] [CrossRef]
- Altstaedt, J.; Kirchner, H.; Rink, L. Cytokine production of neutrophils is limited to interleukin-8. Immunology 1996, 89, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.L.; Ha, T.; Li, C.; Kalbfleisch, J.H.; Laffan, J.J.; Ferguson, D.A. Inhibiting early activation of tissue nuclear factor-kappa B and nuclear factor interleukin 6 with (1-->3)-beta-D-glucan increases long-term survival in polymicrobial sepsis. Surgery 1999, 126, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.L.; Li, C.; Ha, T.; Ozment-Skelton, T.; Kalbfleisch, J.H.; Preiszner, J.; Brooks, L.; Breuel, K.; Schweitzer, J.B. Modulation of the phosphoinositide 3-kinase pathway alters innate resistance to polymicrobial sepsis. J. Immunol. 2004, 172, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Luhm, J.; Langenkamp, U.; Hensel, J.; Frohn, C.; Brand, J.M.; Hennig, H.; Rink, L.; Koritke, P.; Wittkopf, N.; Williams, D.L.; et al. Beta-(1-->3)-D-glucan modulates DNA binding of nuclear factors kappaB, AT and IL-6 leading to an anti-inflammatory shift of the IL-1beta/IL-1 receptor antagonist ratio. BMC Immunol. 2006, 7, 5. [Google Scholar] [CrossRef] [PubMed]
- Latif, A.; Shehzad, A.; Niazi, S.; Zahid, A.; Ashraf, W.; Iqbal, M.W.; Rehman, A.; Riaz, T.; Aadil, R.M.; Khan, I.M.; et al. Probiotics: Mechanism of action, health benefits and their application in food industries. Front. Microbiol. 2023, 14, 1216674. [Google Scholar] [CrossRef]
- Soltys, J.; Quinn, M.T. Modulation of endotoxin- and enterotoxin-induced cytokine release by in vivo treatment with beta-(1,6)-branched beta-(1,3)-glucan. Infect. Immun. 1999, 67, 244–252. [Google Scholar] [CrossRef]
- Yang, X.; Chi, H.; Wu, M.; Wang, Z.; Lang, Q.; Han, Q.; Wang, X.; Liu, X.; Li, Y.; Wang, X.; et al. Discovery and characterization of SARS-CoV-2 reactive and neutralizing antibodies from humanized CAMouseHG mice through rapid hybridoma screening and high-throughput single-cell V(D)J sequencing. Front. Immunol. 2022, 13, 992787. [Google Scholar] [CrossRef]
- Arrigoni, R.; Ballini, A.; Topi, S.; Bottalico, L.; Jirillo, E.; Santacroce, L. Antibiotic Resistance to Mycobacterium tuberculosis and Potential Use of Natural and Biological Products as Alternative Anti-Mycobacterial Agents. Antibiotics 2022, 11, 1431. [Google Scholar] [CrossRef]
- de Moreno de Leblanc, A.; Del Carmen, S.; Zurita-Turk, M.; Santos Rocha, C.; Van De Guchte, M.; Azevedo, V.; Miyoshi, A.; LeBlanc, J.G. Importance of IL-10 modulation by probiotic microorganisms in gastrointestinal inflammatory diseases. ISRN Gastroenterol. 2011, 2011, 892971. [Google Scholar] [CrossRef]
- Ashraf, R.; Vasiljevic, T.; Smith, S.C.; Donkor, O.N. Effect of cell-surface components and metabolites of lactic acid bacteria and probiotic organisms on cytokine production and induction of CD25 expression in human peripheral mononuclear cells. J. Dairy Sci. 2014, 97, 2542–2558. [Google Scholar] [CrossRef]
- Colella, M.; Charitos, I.A.; Ballini, A.; Cafiero, C.; Topi, S.; Palmirotta, R.; Santacroce, L. Microbiota revolution: How gut microbes regulate our lives. World J. Gastroenterol. 2023, 29, 4368–4383. [Google Scholar] [CrossRef] [PubMed]
- Haileselassie, Y.; Johansson, M.A.; Zimmer, C.L.; Björkander, S.; Petursdottir, D.H.; Dicksved, J.; Petersson, M.; Persson, J.O.; Fernandez, C.; Roos, S.; et al. Lactobacilli regulate Staphylococcus aureus 161:2-induced pro-inflammatory T-cell responses in vitro. PLoS ONE 2013, 8, e77893. [Google Scholar] [CrossRef] [PubMed]
- Johansson, M.A.; Björkander, S.; Mata Forsberg, M.; Qazi, K.R.; Salvany Celades, M.; Bittmann, J.; Eberl, M.; Sverremark-Ekström, E. Probiotic Lactobacilli Modulate Staphylococcus aureus-Induced Activation of Conventional and Unconventional T cells and NK Cells. Front. Immunol. 2016, 7, 273. [Google Scholar] [CrossRef] [PubMed]
- Ballini, A.; Charitos, I.A.; Cantore, S.; Topi, S.; Bottalico, L.; Santacroce, L. About Functional Foods: The Probiotics and Prebiotics State of Art. Antibiotics 2023, 12, 635. [Google Scholar] [CrossRef]
- Santacroce, L.; Cagiano, R.; Del Prete, R.; Bottalico, L.; Sabatini, R.; Carlaio, R.G.; Prejbeanu, R.; Vermesan, H.; Dragulescu, S.I.; Vermesan, D.; et al. Helicobacter pylori infection and gastric MALTomas: An up-to-date and therapy highlight. Clin. Ter. 2008, 159, 457–462. [Google Scholar] [PubMed]
- Merriman, J.A.; Nemeth, K.A.; Schlievert, P.M. Novel antimicrobial peptides that inhibit gram positive bacterial exotoxin synthesis. PLoS ONE 2014, 9, e95661. [Google Scholar] [CrossRef] [PubMed]
- Woetmann, A.; Alhede, M.; Dabelsteen, S.; Bjarnsholt, T.; Rybtke, M.; Nastasi, C.; Krejsgaard, T.; Andersen, M.H.; Bonefeld, C.M.; Geisler, C.; et al. Interleukin-26 (IL-26) is a novel anti-microbial peptide produced by T cells in response to staphylococcal enterotoxin. Oncotarget 2018, 9, 19481–19489. [Google Scholar] [CrossRef]
- Yami, H.A.; Tahmoorespur, M.; Javadmanesh, A.; Tazarghi, A.; Sekhavati, M.H. The immunomodulatory effects of lactoferrin and its derived peptides on NF-κB signaling pathway: A systematic review and meta-analysis. Immun. Inflamm. Dis. 2023, 11, e972. [Google Scholar] [CrossRef]
- Ianiro, G.; Rosa, L.; Bonaccorsi di Patti, M.C.; Valenti, P.; Musci, G.; Cutone, A. Lactoferrin: From the structure to the functional orchestration of iron homeostasis. Biometals 2023, 36, 391–416. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).