Depth of SCUBA Diving Affects Cardiac Autonomic Nervous System
Abstract
1. Introduction
2. Methods
2.1. Participants
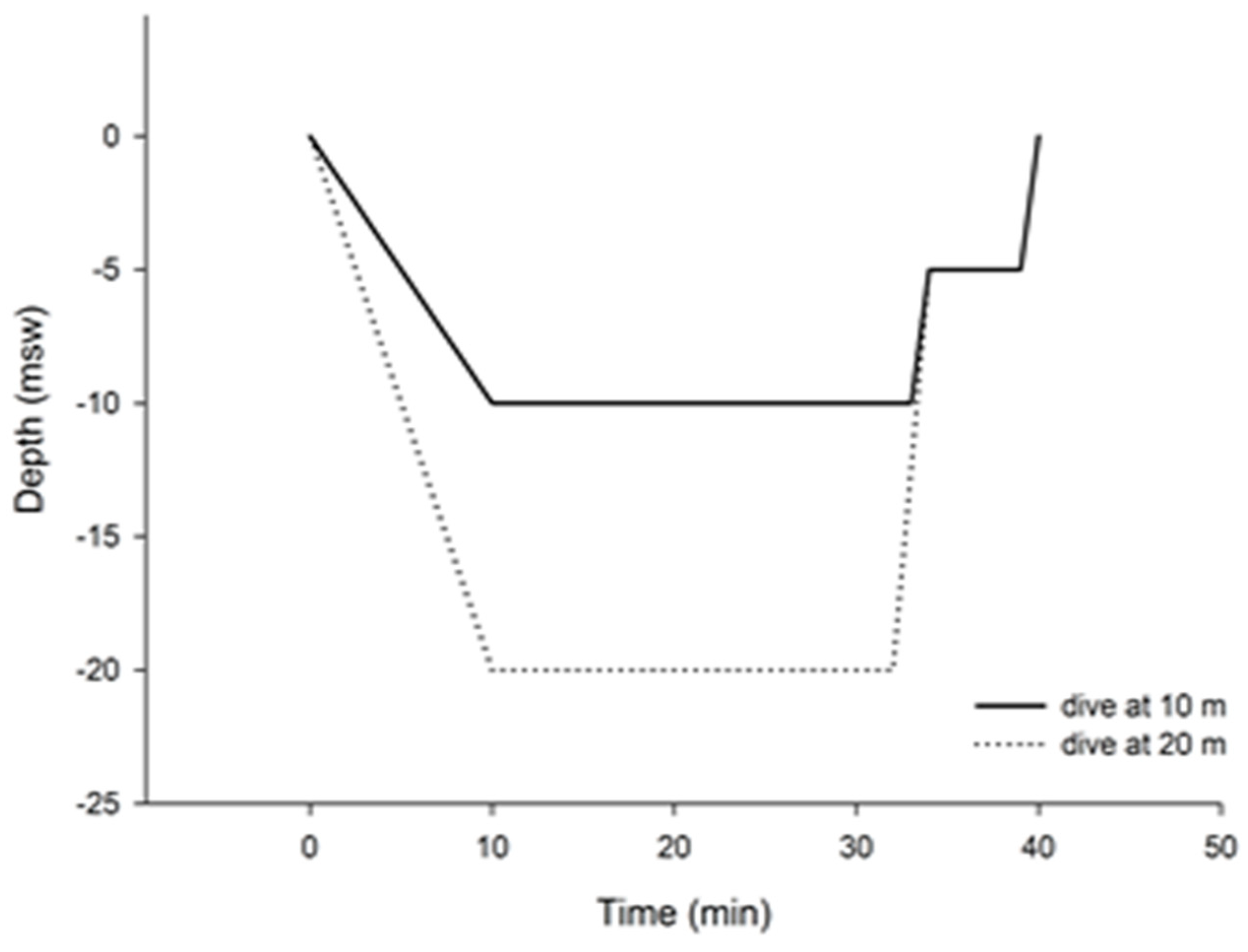
2.2. Study Design
2.3. HRV Assessment and Analysis
2.4. Statistics
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Noh, Y.; Posada-Quintero, H.F.; White, J.; Florian, J.P.; Brink, P.R.; Chon, K.H. Effect of Shallow and Deep SCUBA Dives on Heart Rate Variability. Front. Physiol. 2018, 9, 110. [Google Scholar] [CrossRef] [PubMed]
- Lafère, P.; Lambrechts, K.; Germonpré, P. Heart Rate Variability During a Standard Dive: A Role for Inspired Oxygen Pressure? Front. Physiol. 2021, 12, 1060. [Google Scholar] [CrossRef] [PubMed]
- Schipke, J.D.; Pelzer, M. Effect of immersion, submersion, and scuba diving on heart rate variability. Br. J. Sports Med. 2001, 35, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Vuoti, A.O.; Tulppo, M.P.; Ukkola, O.H.; Junttila, M.J.; Huikuri, H.V.; Kiviniemi, A.M.; Perkiömäki, J.S. Prognostic value of heart rate variability in patients with coronary artery disease in the current treatment era. PLoS ONE 2021, 16, e0254107. [Google Scholar] [CrossRef] [PubMed]
- Task Force of the European Society of Cardiology; The North American Society of Pacing and Electrophysiology. Heart rate variability: Standards of measurement, physiological interpretation, and clinical use. Eur. Heart J. 1996, 17, 354–381. [Google Scholar] [CrossRef]
- Pendergast, D.R.; Lundgren, C.E. The physiology and pathophysiology of the hyperbaric and diving environments. J. Appl. Physiol. 2009, 106, 274–275. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gunes, A.E.; Cimsit, M. The prevalence of electrocardiogram abnormalities in professional divers. Diving Hyperb. Med. 2017, 47, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Hexdall, E.J.; Cooper, J.S. Patent Foramen Ovale in Diving; StatPearls: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK431111/ (accessed on 1 January 2020).
- Tso, J.V.T.; Powers, J.M.; Kim, J.H. Cardiovascular considerations for skuba divers. Heart 2022, 108, 1084–1089. [Google Scholar] [CrossRef] [PubMed]
- Bosco, G.; De Marzi, E.; Michieli, P.; Omar, H.R.; Camporesi, E.M.; Padulo, J.; Paoli, A.; Mangar, D.; Schiavon, M. 12-lead Holter monitoring in diving and water sports: A preliminary investigation. Diving Hyperb. Med. 2014, 44, 202–207. [Google Scholar] [PubMed]
- Shaffer, F.; Ginsberg, J.P. An Overview of Heart Rate Variability Metrics and Norms. Front. Public Health 2017, 5, 258. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, E.; García-Manso, J.M.; Martín-González, J.M.; Sarmiento, S.; Calderón, F.J.; Da Silva-Grigoletto, M.E. Effect of Hyperbaric Pressure During Scuba Diving on Autonomic Modulation of the Cardiac Response:Application of the Continuous Wavelet Transform to the Analysis of Heart Rate Variability. Mil. Med. 2010, 175, 61. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Klugar, M.; Stejskal, P.; Krejčíř, V.; Bartáková, O.; Drbošalová, V.; Kozáková, J.; Štěpaník, P. Changes in autonomic nervous system activity in connection with scuba diving. Acta Univ. Palacki. Olomuc. Gymnica 2009, 39, 7–12. [Google Scholar]
- Winklewski, P.J.; Kot, J.; Frydrychowski, A.F.; Nuckowska, M.K.; Tkachenko, Y. Effects of diving and oxygen on autonomic nervous system and cerebral blood flow. Diving Hyperb. Med. 2013, 43, 148–156. [Google Scholar] [PubMed]
- Christoforidi, V.; Koutlianos, N.; Deligiannis, P.; Kouidi, E.; Deligiannis, A. Heart rate variability in free diving athletes. Clin. Physiol. Funct. Imaging 2012, 32, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Sacha, J. Interaction between heart rate and heart rate variability. Ann. Noninvasive Electrocardiol. 2014, 19, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Lundell, R.V.; Ojanen, T. A systematic review of HRV during diving in very cold water. Int. J. Circumpolar. Health 2023, 82, 2203369. [Google Scholar] [CrossRef] [PubMed]
- Schaller, C.; Fümm, A.; Bachmann, S.; Oechslin, L.; Nakahara, Y.; Melliger, R.; Biaggi, P.; Wyss, C.A. Heart rate profiles and heart rate variability during scuba diving. Swiss Med. Wkly. 2021, 151, w30039. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.-C.; Wu, Y.-L.; Tsai, P.-S. Heart Rate Variability and Risk of All-Cause Death and Cardiovascular Events in Patients With Cardiovascular Disease: A Meta-Analysis of Cohort Studies. Biol. Res. Nurs. 2020, 22, 45–56. [Google Scholar] [CrossRef] [PubMed]
| Baseline | Bottom | Recovery | Time | Depth | Interaction | |
|---|---|---|---|---|---|---|
| HR (bpm) | ||||||
| 10 m depth | 101 ± 18 | 103 ± 17 | 92 ± 13 $ | - | - | - |
| 20 m depth | 100 ± 14 | 104 ± 21 | 90 ± 16 $ | 0.001 | 0.054 | 0.077 |
| SDNN (ms) | ||||||
| 10 m depth | 57.6 ± 18.6 | 35.2 ± 20.7 $ | 62.1 ± 23.1 $ | - | - | - |
| 20 m depth | 57.6 ± 19.1 | 37.6 ± 16.2 $ | 71.9 ± 33.8 $ | 0.001 | 0.510 | 0.402 |
| RMSSD (ms) | ||||||
| 10 m depth | 19.4 ± 7.7 | 21.4 ± 12.4 | 24.2 ± 12.2 | |||
| 20 m depth | 24.8 ± 13.9 | 26.1 ± 17.1 | 29.3 ± 14.1 | 0.226 | 0.352 | 0.991 |
| pNN50 (%) | ||||||
| 10 m depth | 3.2 ± 3.1 | 4.6 ± 6.0 | 5.5 ± 5.7 | - | - | - |
| 20 m depth | 7.9 ± 13.2 | 9.8 ± 16.9 | 9.5 ± 10.7 | 0.448 | 0.311 | 0.794 |
| ULF | ||||||
| 10 m depth | 662.6 ± 703.1 | 252.8 ± 491.2 $ | 417.0 ± 396.7 $ | - | - | - |
| 20 m depth | 619.9 ± 1149.5 | 76.9 ± 46.6 $ | 1169.4 ± 2086.1 $ | 0.043 | 0.431 | 0.208 |
| VLF | ||||||
| 10 m depth | 1734.9 ± 1366.4 | 518.8 ± 519.7 $ | 1465.3 ± 999.4 $ | - | - | - |
| 20 m depth | 1313.9 ± 885.7 | 454.6 ± 337.3 $ | 1960.0 ± 2076.4 $ | 0.003 | 0.991 | 0.258 |
| LF | ||||||
| 10 m depth | 695.5 ± 419.0 | 655.2 ± 891.7 | 1191.4 ± 1142.7 $ | - | - | - |
| 20 m depth | 1098.8 ± 768.4 | 684.6 ± 665.5 $ | 1427.7 ± 1376.6 $ | 0.014 | 0.482 | 0.762 |
| HF | ||||||
| 10 m depth | 96.95 ± 64.6 | 300.5 ± 483.2 | 189.2 ± 211.3 | |||
| 20 m depth | 177.2 ± 135.4 | 352.3 ± 411.7 | 294.8 ± 394.4 | 0.117 | 0.482 | 0.922 |
| TP | ||||||
| 10 m depth | 3190 ± 2046 | 1727 ± 1989 $ | 3263 ± 1958 $ | |||
| 20 m depth | 3210 ± 2255 | 1568 ± 1363 $ | 4851 ± 4233 $ | 0.004 | 0.492 | 0.231 |
| Log LF/HF | ||||||
| 10 m depth | 0.88 ± 0.20 | 0.44 ± 0.22 $ | 0.80 ± 0.23 | |||
| 20 m depth | 0.80 ± 0.32 | 0.32 ± 0.42 $ | 0.75 ± 0.23 | 0.001 | 0.368 | 0.845 |
| ULF, % | ||||||
| 10 m depth | 18.3 ± 12.2 | 11.5 ± 9.3 | 15.03 ± 14.8 | |||
| 20 m depth | 13.7 ± 12.4 | 9.97 ± 9.5 | 15.8 ± 14.6 | 0.172 | 0.373 | 0.284 |
| VLF, % | ||||||
| 10 m depth | 51.4 ± 10.6 | 38.9 ± 12.0 $ | 46.0 ± 11.2 $ | |||
| 20 m depth | 43.2 ± 13.3 * | 33.5 ± 14.9 $ | 38.9 ± 12.2 $ | 0.026 | 0.032 | 0.937 |
| LF, % | ||||||
| 10 m depth | 26.7 ± 15.9 | 35.6 ± 11.5 | 33.7 ± 18.7 | |||
| 20 m depth | 37.1 ± 18.6 | 36.9 ± 18.5 | 37.8 ± 19.4 | 0.495 | 0.237 | 0.663 |
| HF, % | ||||||
| 10 m depth | 3.5 ± 1.7 | 13.9 ± 7.5 $ | 5.2 ± 2.99 $ | |||
| 20 m depth | 5.8 ± 3.4 | 20.6 ± 18.9 $ | 7.4 ± 5.3 $ | 0.003 | 0.145 | 0.401 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vulić, M.; Milovanovic, B.; Obad, A.; Glavaš, D.; Glavicic, I.; Zubac, D.; Valic, M.; Valic, Z. Depth of SCUBA Diving Affects Cardiac Autonomic Nervous System. Pathophysiology 2024, 31, 183-189. https://doi.org/10.3390/pathophysiology31020014
Vulić M, Milovanovic B, Obad A, Glavaš D, Glavicic I, Zubac D, Valic M, Valic Z. Depth of SCUBA Diving Affects Cardiac Autonomic Nervous System. Pathophysiology. 2024; 31(2):183-189. https://doi.org/10.3390/pathophysiology31020014
Chicago/Turabian StyleVulić, Marina, Branislav Milovanovic, Ante Obad, Duška Glavaš, Igor Glavicic, Damir Zubac, Maja Valic, and Zoran Valic. 2024. "Depth of SCUBA Diving Affects Cardiac Autonomic Nervous System" Pathophysiology 31, no. 2: 183-189. https://doi.org/10.3390/pathophysiology31020014
APA StyleVulić, M., Milovanovic, B., Obad, A., Glavaš, D., Glavicic, I., Zubac, D., Valic, M., & Valic, Z. (2024). Depth of SCUBA Diving Affects Cardiac Autonomic Nervous System. Pathophysiology, 31(2), 183-189. https://doi.org/10.3390/pathophysiology31020014





