Prerequisite for COVID-19 Prediction: A Review on Factors Affecting the Infection Rate
Abstract
:1. Introduction
2. Face Mask Design and Material
3. Meteorological Factors
4. Distance
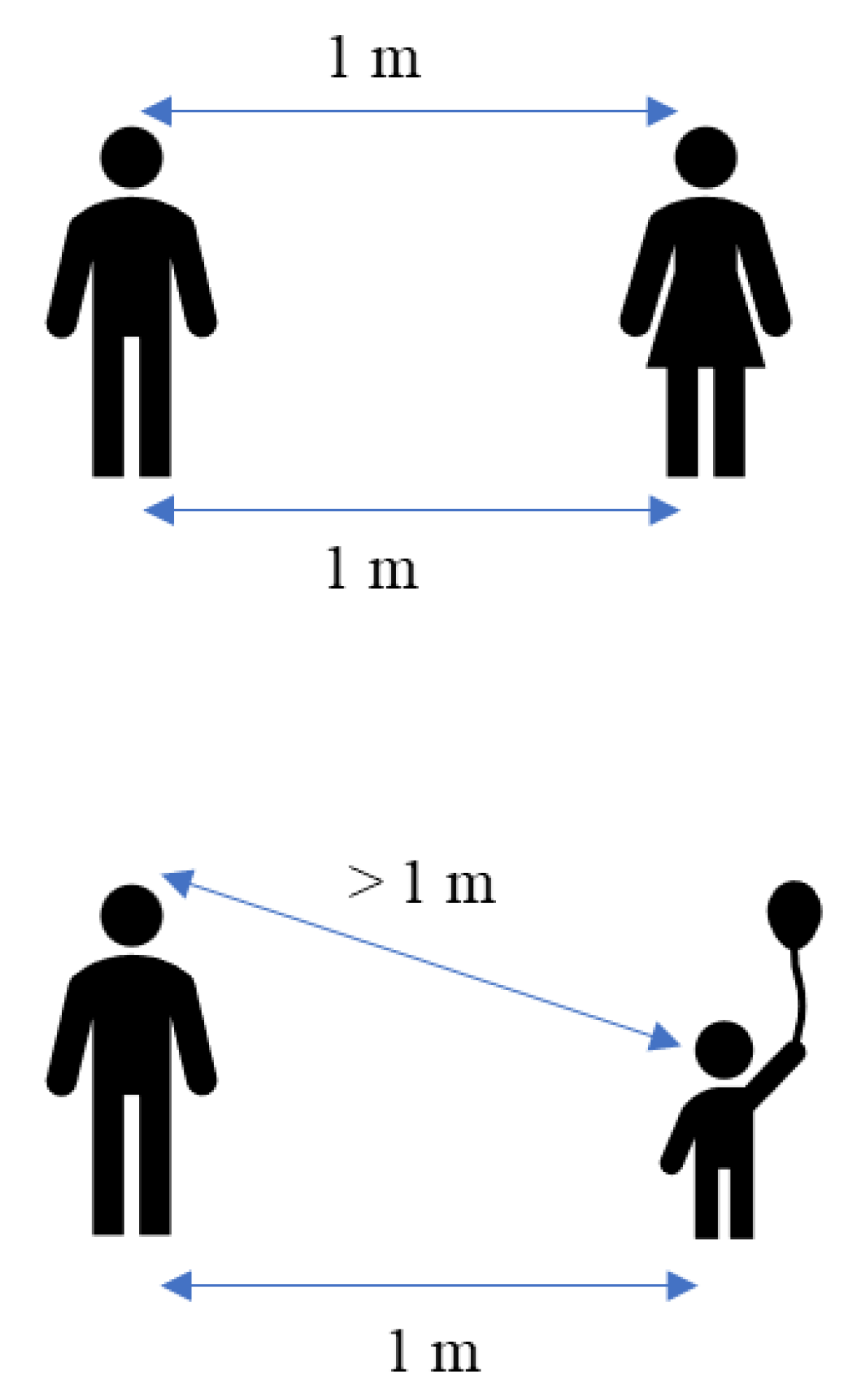
4.1. Physical Distance
4.2. Mobility Reduction
5. Ventilation
6. Vaccines
6.1. Vaccine Equity
6.2. Vaccine Hesitancy
6.3. Vaccine Efficacy
7. Socioeconomic Factors
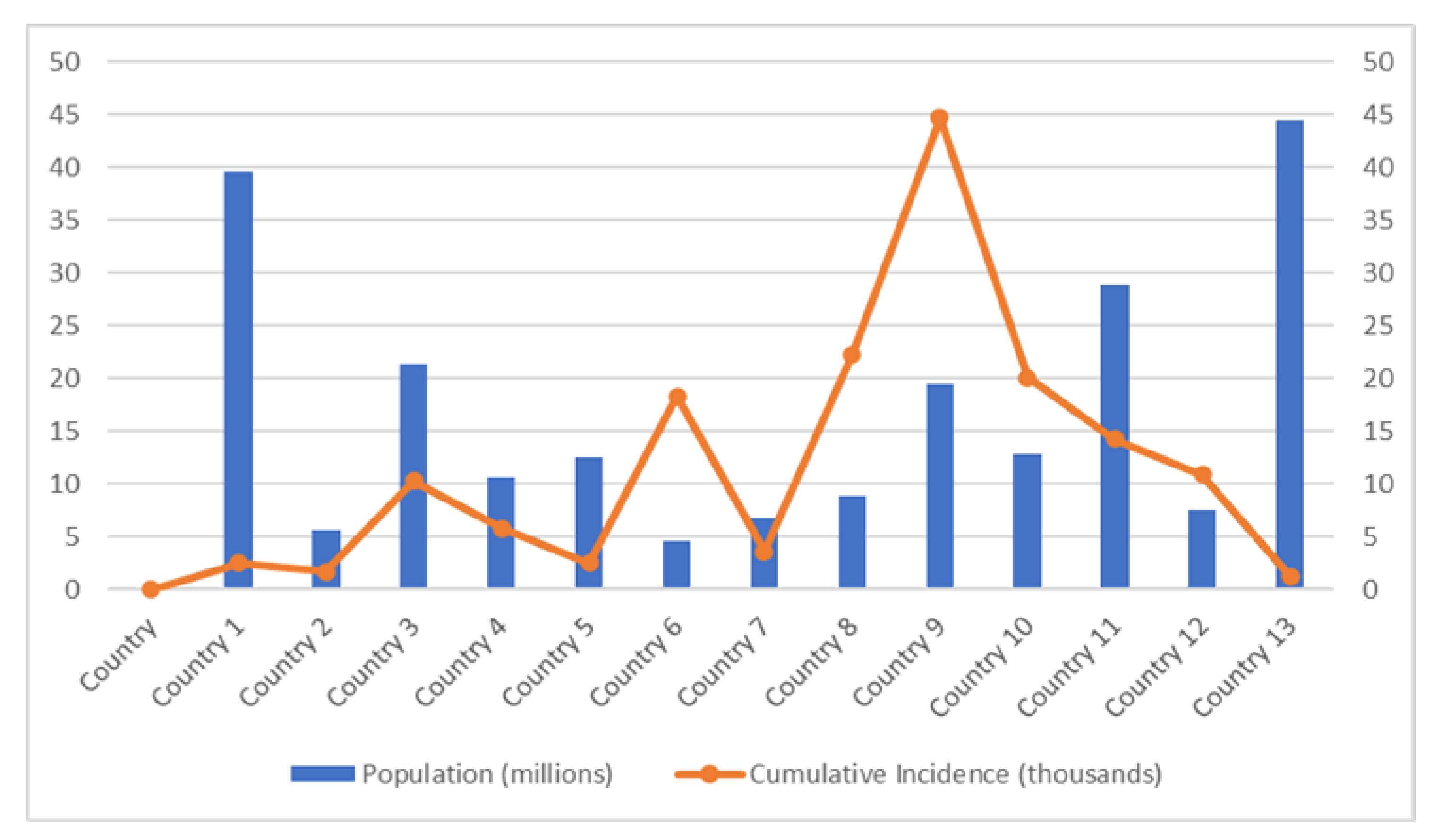
7.1. Population Density
7.2. Housing Condition
7.3. Economic Status
7.4. Education Level
8. Host Factors
9. SARS-CoV-2 Variants
10. COVID-19 Testing Availability
11. Challenges and Recommendations
12. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cronkleton, E. The Biggest Causes of Death in 2020. Available online: https://www.medicalnewstoday.com/articles/death-statistics-by-cause-2020 (accessed on 27 July 2022).
- WHO. WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 27 July 2022).
- Wu, F.; Zhao, S.; Yu, B.; Chen, Y.M.; Wang, W.; Song, Z.G.; Hu, Y.; Tao, Z.W.; Tian, J.H.; Pei, Y.Y.; et al. A New Coronavirus Associated with Human Respiratory Disease in China. Nature 2020, 579, 265–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, P.; Yang, X.L.; Wang, X.G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.R.; Zhu, Y.; Li, B.; Huang, C.L.; et al. A Pneumonia Outbreak Associated with a New Coronavirus of Probable Bat Origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef] [Green Version]
- Wang, N.; Li, S.Y.; Yang, X.L.; Huang, H.M.; Zhang, Y.J.; Guo, H.; Luo, C.M.; Miller, M.; Zhu, G.; Chmura, A.A.; et al. Serological Evidence of Bat SARS-Related Coronavirus Infection in Humans, China. Virol. Sin. 2018, 33, 104–107. [Google Scholar] [CrossRef] [Green Version]
- Zhou, H.; Ji, J.; Chen, X.; Bi, Y.; Li, J.; Wang, Q.; Hu, T.; Song, H.; Zhao, R.; Chen, Y.; et al. Identification of Novel Bat Coronaviruses Sheds Light on the Evolutionary Origins of SARS-CoV-2 and Related Viruses. Cell 2021, 184, 4380–4391.e14. [Google Scholar] [CrossRef]
- Mallapaty, S. The Search for Animals Harbouring Coronavirus—and Why It Matters. Nature 2021, 591, 26–28. [Google Scholar] [CrossRef]
- Nga, N.T.T.; Latinne, A.; Thuy, H.B.; Van Long, N.; Ngoc, P.T.B.; Anh, N.T.L.; Van Thai, N.; Phuong, T.Q.; Van Thai, H.; Hai, L.K.; et al. Evidence of SARS-CoV-2 Related Coronaviruses Circulating in Sunda Pangolins (Manis Javanica) Confiscated from the Illegal Wildlife Trade in Viet Nam. Front. Public Health 2022, 10, 826116. [Google Scholar] [CrossRef]
- Wang, W.; Tian, J.-H.; Chen, X.; Hu, R.-X.; Lin, X.-D.; Pei, Y.-Y.; Lv, J.-X.; Zheng, J.-J.; Dai, F.-H.; Song, Z.-G.; et al. Coronaviruses in Wild Animals Sampled in and around Wuhan at the Beginning of COVID-19 Emergence. Virus Evol. 2022, 8, veac046. [Google Scholar] [CrossRef]
- Murphy, S.L.; Xu, J.; Kochanek, K.D.; Arias, E. Mortality in the United States, 2017. NCHS Data Brief 2018, 328, 1–8. [Google Scholar]
- Shi, Y.; Wang, G.; Cai, X.P.; Deng, J.W.; Zheng, L.; Zhu, H.H.; Zheng, M.; Yang, B.; Chen, Z. An Overview of COVID-19. J. Zhejiang Univ. Sci. B 2020, 21, 343–360. [Google Scholar] [CrossRef] [PubMed]
- Wilk-Sledziewska, K.; Sielatycki, P.J.; Uscinska, N.; Bujno, E.; Rosolowski, M.; Kakareko, K.; Sledziewski, R.; Rydzewska-Rosolowska, A.; Hryszko, T.; Zbroch, E. The Impact of Cardiovascular Risk Factors on the Course of COVID-19. J. Clin. Med. 2022, 11, 2250. [Google Scholar] [CrossRef]
- WHO Team-Emergency Response. Weekly Epidemiological Update on COVID-19-7 September 2022, Edition 108. World Health Organization. Available online: https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---7-september-2022 (accessed on 7 September 2022).
- World Health Organization. Coronavirus Disease 2019 (COVID-19): Situation Report, 46. Available online: https://apps.who.int/iris/handle/10665/331443 (accessed on 23 September 2022).
- World Health Organization. Expanding Our Understanding of Post COVID-19 Condition: Report of a WHO Webinar-9 February 2021. Available online: https://www.who.int/publications/i/item/9789240025035 (accessed on 23 September 2022).
- Raveendran, A.V.; Jayadevan, R.; Sashidharan, S. Long COVID: An Overview. Diabetes Metab. Syndr. Clin. Res. Rev. 2021, 15, 869–875. [Google Scholar] [CrossRef] [PubMed]
- Crook, H.; Raza, S.; Nowell, J.; Young, M.; Edison, P. Long COVID—Mechanisms, Risk Factors, and Management. BMJ 2021, 374, n1648. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.; Ali, F.M.; Nixon, S.J.; Ingram, J.R.; Salek, S.M.; Finlay, A.Y. Measuring the Impact of COVID-19 on the Quality of Life of the Survivors, Partners and Family Members: A Cross-Sectional International Online Survey. BMJ Open 2021, 11, e047680. [Google Scholar] [CrossRef]
- O’ Mahony, L.; Buwalda, T.; Blair, M.; Forde, B.; Lunjani, N.; Ambikan, A.; Neogi, U.; Barrett, P.; Geary, E.; O’Connor, N.; et al. Impact of Long COVID on Health and Quality of Life. HRB Open Res. 2022, 5, 31. [Google Scholar] [CrossRef]
- Tabatabaeizadeh, S.A. Airborne Transmission of COVID-19 and the Role of Face Mask to Prevent It: A Systematic Review and Meta-Analysis. Eur. J. Med. Res. 2021, 26, 1. [Google Scholar] [CrossRef]
- Shirvanimoghaddam, K.; Czech, B.; Yadav, R.; Gokce, C.; Fusco, L.; Delogu, L.G.; Yilmazer, A.; Brodie, G.; Al-Othman, A.; Al-Tamimi, A.K.; et al. Facemask Global Challenges: The Case of Effective Synthesis, Utilization, and Environmental Sustainability. Sustainability 2022, 14, 737. [Google Scholar] [CrossRef]
- Howard, J.; Huang, A.; Li, Z.; Tufekci, Z.; Zdimal, V.; van der Westhuizen, H.M.; von Delft, A.; Price, A.; Fridman, L.; Tang, L.H.; et al. An Evidence Review of Face Masks against COVID-19. Proc. Natl. Acad. Sci. USA 2021, 118, e2014564118. [Google Scholar] [CrossRef] [PubMed]
- Chan, L.Y.H.; Yuan, B.; Convertino, M. COVID-19 Non-Pharmaceutical Intervention Portfolio Effectiveness and Risk Communication Predominance. Sci. Rep. 2021, 11, 10605. [Google Scholar] [CrossRef]
- Beca-Martínez, M.T.; Romay-Barja, M.; Falcón-Romero, M.; Rodríguez-Blázquez, C.; Benito-Llanes, A.; Forjaz, M.J. Compliance with the Main Preventive Measures of COVID-19 in Spain: The Role of Knowledge, Attitudes, Practices, and Risk Perception. Transbound. Emerg. Dis. 2021, 69, e871–e882. [Google Scholar] [CrossRef] [PubMed]
- Aquino, E.M.L.; Silveira, I.H.; Pescarini, J.M.; Aquino, R.; de Souza-Filho, J.A.; Rocha, A.D.S.; Ferreira, A.; Victor, A.; Teixeira, C.; Machado, D.B.; et al. Social Distancing Measures to Control the COVID-19 Pandemic: Potential Impacts and Challenges in Brazil. Cienc. Saude Coletiva 2020, 25, 2423–2446. [Google Scholar] [CrossRef]
- Khandker, S.S.; Godman, B.; Jawad, M.I.; Meghla, B.A.; Tisha, T.A.; Khondoker, M.U.; Haq, M.A.; Charan, J.; Talukder, A.A.; Azmuda, N.; et al. A Systematic Review on COVID-19 Vaccine Strategies, Their Effectiveness, and Issues. Vaccines 2021, 9, 1387. [Google Scholar] [CrossRef] [PubMed]
- Cowling, B.J.; Ali, S.T.; Ng, T.W.Y.; Tsang, T.K.; Li, J.C.M.; Fong, M.W.; Liao, Q.; Kwan, M.Y.; Lee, S.L.; Chiu, S.S.; et al. Impact Assessment of Non-Pharmaceutical Interventions against Coronavirus Disease 2019 and Influenza in Hong Kong: An Observational Study. Lancet Public Health 2020, 5, e279–e288. [Google Scholar] [CrossRef]
- Wang, Y.; Tian, H.; Zhang, L.; Zhang, M.; Guo, D.; Wu, W.; Zhang, X.; Kan, G.L.; Jia, L.; Huo, D.; et al. Reduction of Secondary Transmission of SARS-CoV-2 in Households by Face Mask Use, Disinfection and Social Distancing: A Cohort Study in Beijing, China. BMJ Glob. Health 2020, 5, e002794. [Google Scholar] [CrossRef]
- Maged, A.; Ahmed, A.; Haridy, S.; Baker, A.W.; Xie, M. SEIR Model to Address the Impact of Face Masks amid COVID-19 Pandemic. Risk Anal. 2022, 1–15. [Google Scholar] [CrossRef]
- Hui, D.S.; Chow, B.K.; Chu, L.; Ng, S.S.; Lee, N.; Gin, T.; Chan, M.T.V. Exhaled Air Dispersion during Coughing with and without Wearing a Surgical or N95 Mask. PLoS ONE 2012, 7, e50845. [Google Scholar] [CrossRef]
- Chiera, S.; Cristoforetti, A.; Benedetti, L.; Nollo, G.; Borro, L.; Mazzei, L.; Tessarolo, F. A Simple Method to Quantify Outward Leakage of Medical Face Masks and Barrier Face Coverings: Implication for the Overall Filtration Efficiency. Int. J. Environ. Res. Public Health 2022, 19, 3548. [Google Scholar] [CrossRef]
- Soriano, J.B.; Anzueto, A.; Bosnic Anticevich, S.; Kaplan, A.; Miravitlles, M.; Usmani, O.; Papadopoulos, N.G.; Puggioni, F.; Canonica, G.W.; Roche, N. Face Masks, Respiratory Patients and COVID-19. Eur. Respir. J. 2020, 56, 2003325. [Google Scholar] [CrossRef] [PubMed]
- UK Health Security Agency. People with Symptoms of a Respiratory Infection Including COVID-19. Available online: https://www.gov.uk/guidance/people-with-symptoms-of-a-respiratory-infection-including-covid-19 (accessed on 23 September 2022).
- Brenner, M.J.; Roy-Faderman, I.; Roy, S.; Osazuwa-Peters, N.; Jackler, R.K.; Holt, G.R. When Should Patients Receive Mask Exemptions During the COVID-19 Pandemic? Ethics in Practice: Point-Counterpoint. Otolaryngol. Neck Surg. 2022, 166, 652–656. [Google Scholar] [CrossRef]
- Esposito, S.; Principi, N. To Mask or Not to Mask Children to Overcome COVID-19. Eur. J. Pediatr. 2020, 179, 1267–1270. [Google Scholar] [CrossRef]
- Raz, M.; Dorfman, D. Bans on COVID-19 Mask Requirements vs Disability Accommodations. JAMA Health Forum 2021, 2, e211912. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Order: Wearing of Face Masks While on Conveyances and at Transportation Hubs. Available online: https://www.cdc.gov/quarantine/masks/mask-travel-guidance.html (accessed on 2 September 2022).
- GBD 2019 Diseases and Injuries Collaborators. Global Burden of 369 Diseases and Injuries in 204 Countries and Territories, 1990–2019: A Systematic Analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef]
- Wang, Q.; Xu, R.; Volkow, N.D. Increased Risk of COVID-19 Infection and Mortality in People with Mental Disorders: Analysis from Electronic Health Records in the United States. World Psychiatry 2021, 20, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Damette, O.; Mathonnat, C.; Goutte, S. Meteorological Factors against COVID-19 and the Role of Human Mobility. PLoS ONE 2021, 16, e0252405. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Liu, S.; Liu, Y.; Liu, B.; Gong, X. The Impact of Meteorological Factors on COVID-19 of California and Its Lag Effect. Meteorol. Appl. 2022, 29, e2045. [Google Scholar] [CrossRef]
- Lashley, F.R. Factors Contributing to the Occurrence of Emerging Infectious Diseases. Biol. Res. Nurs. 2003, 4, 258–267. [Google Scholar] [CrossRef]
- Wu, X.; Lu, Y.; Zhou, S.; Chen, L.; Xu, B. Impact of Climate Change on Human Infectious Diseases: Empirical Evidence and Human Adaptation. Environ. Int. 2016, 86, 14–23. [Google Scholar] [CrossRef] [Green Version]
- Chen, B.; Liang, H.; Yuan, X.; Hu, Y.; Xu, M.; Zhao, Y.; Zhang, B.; Tian, F.; Zhu, X. Roles of Meteorological Conditions in COVID-19 Transmission on a Worldwide Scale. medRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Sarkodie, S.A.; Owusu, P.A. Impact of Meteorological Factors on COVID-19 Pandemic: Evidence from Top 20 Countries with Confirmed Cases. Environ. Res. 2020, 191, 110101. [Google Scholar] [CrossRef]
- Yuan, J.; Wu, Y.; Jing, W.; Liu, J.; Du, M.; Wang, Y.; Liu, M. Association between Meteorological Factors and Daily New Cases of COVID-19 in 188 Countries: A Time Series Analysis. Sci. Total Environ. 2021, 780, 146538. [Google Scholar] [CrossRef]
- Kassem, A.Z.E. Does Temperature Affect COVID-19 Transmission? Front. Public Health 2020, 8, 554964. [Google Scholar] [CrossRef]
- Wan, X.; Cheng, C.; Zhang, Z. Early Transmission of COVID-19 Has an Optimal Temperature but Late Transmission Decreases in Warm Climate; Preprint; Public and Global Health. medRxiv 2020. [Google Scholar] [CrossRef]
- V’kovski, P.; Kratzel, A.; Steiner, S.; Stalder, H.; Thiel, V. Coronavirus Biology and Replication: Implications for SARS-CoV-2. Nat. Rev. Microbiol. 2021, 19, 155–170. [Google Scholar] [CrossRef]
- Bezabeh, Y.M.; Mequanint, A.; Alamneh, E.; Bezabih, A.; Sabiiti, W.; Roujeinikova, A.; Bezabhe, W.M. Correlation of the Global Spread of Coronavirus Disease-19 with Atmospheric Air Temperature; Preprint; Infectious Diseases (except HIV/AIDS). medRxiv 2020. [Google Scholar] [CrossRef]
- Rath, S.L.; Kumar, K. Investigation of the Effect of Temperature on the Structure of SARS-CoV-2 Spike Protein by Molecular Dynamics Simulations. Front. Mol. Biosci. 2020, 7, 583523. [Google Scholar] [CrossRef]
- Das, P.; Manna, S.; Basak, P. Analyzing the Effect of Environmental Factors (Temperature and Humidity) on the Outspread of COVID-19 around the Globe. Ecol. Environ. Conserv. 2021, 27, S386–S394. [Google Scholar]
- Audi, A.; AlIbrahim, M.; Kaddoura, M.; Hijazi, G.; Yassine, H.M.; Zaraket, H. Seasonality of Respiratory Viral Infections: Will COVID-19 Follow Suit? Front. Public Health 2020, 8, 567184. [Google Scholar] [CrossRef] [PubMed]
- Ujiie, M.; Tsuzuki, S.; Ohmagari, N. Effect of Temperature on the Infectivity of COVID-19. Int. J. Infect. Dis. 2020, 95, 301–303. [Google Scholar] [CrossRef] [PubMed]
- Landier, J.; Paireau, J.; Rebaudet, S.; Legendre, E.; Lehot, L.; Fontanet, A.; Cauchemez, S.; Gaudart, J. Cold and Dry Winter Conditions Are Associated with Greater SARS-CoV-2 Transmission at Regional Level in Western Countries during the First Epidemic Wave. Sci. Rep. 2021, 11, 12756. [Google Scholar] [CrossRef] [PubMed]
- Abdollahi, A.; Rahbaralam, M. Effect of Temperature on the Transmission of COVID-19: A Machine Learning Case Study in Spain; Preprint; Infectious Diseases (except HIV/AIDS). medRxiv 2020. [Google Scholar] [CrossRef]
- Prata, D.N.; Rodrigues, W.; Bermejo, P.H. Temperature Significantly Changes COVID-19 Transmission in (Sub)Tropical Cities of Brazil. Sci. Total Environ. 2020, 729, 138862. [Google Scholar] [CrossRef]
- Wang, Y.; Sibaii, F.; Lee, K.; Gill, M.J.; Hatch, J.L. Meta-analytic findings on lower reading skills in children with cochlear implants; Preprint; Otolaryngology. medRxiv 2021. [Google Scholar] [CrossRef]
- Biryukov, J.; Boydston, J.A.; Dunning, R.A.; Yeager, J.J.; Wood, S.; Reese, A.L.; Ferris, A.; Miller, D.; Weaver, W.; Zeitouni, N.E.; et al. Increasing Temperature and Relative Humidity Accelerates Inactivation of SARS-CoV-2 on Surfaces. mSphere 2020, 5, e00441-20. [Google Scholar] [CrossRef]
- Biryukov, J.; Boydston, J.A.; Dunning, R.A.; Yeager, J.J.; Wood, S.; Ferris, A.; Miller, D.; Weaver, W.; Zeitouni, N.E.; Freeburger, D.; et al. SARS-CoV-2 Is Rapidly Inactivated at High Temperature. Environ. Chem. Lett. 2021, 19, 1773–1777. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Chin, Y.; Yu, S.; Huang, J.; Zhang, C.J.P.; Zhu, K.; Azarakhsh, N.; Sheng, J.; He, Y.; Jayavanth, P.; et al. The Influence of Average Temperature and Relative Humidity on New Cases of COVID-19. JMIR Public Health Surveill. 2021, 7, e20495. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, J.; Yao, J.; Zhang, X.; Li, L.; Xu, X.; He, X.; Wang, B.; Fu, S.; Niu, T.; et al. Impact of Meteorological Factors on the COVID-19 Transmission: A Multi-City Study in China. Sci. Total Environ. 2020, 726, 138513. [Google Scholar] [CrossRef] [PubMed]
- Lowen, A.C.; Mubareka, S.; Steel, J.; Palese, P. Influenza Virus Transmission Is Dependent on Relative Humidity and Temperature. PLoS Pathog. 2008, 3, e151. [Google Scholar] [CrossRef]
- Sun, Z.; Thilakavathy, K.; Kumar, S.S.; He, G.; Liu, S.V. Potential Factors Influencing Repeated SARS Outbreaks in China. Int. J. Environ. Res. Public Health 2020, 17, 1633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kudo, E.; Song, E.; Yockey, L.J.; Rakib, T.; Wong, P.W.; Homer, R.J.; Iwasaki, A. Low Ambient Humidity Impairs Barrier Function and Innate Resistance against Influenza Infection. Proc. Natl. Acad. Sci. USA 2019, 166, 10905–10910. [Google Scholar] [CrossRef] [Green Version]
- Jaakkola, K.; Saukkoriipi, A.; Jokelainen, J.; Juvonen, R.; Kauppila, J.; Vainio, O.; Ziegler, T.; Rönkkö, E.; Jaakkola, J.J.K.; Ikäheimo, T.M. Decline in Temperature and Humidity Increases the Occurrence of Influenza in Cold Climate. Environ. Health A Glob. Access Sci. Source 2014, 13, 22. [Google Scholar] [CrossRef] [Green Version]
- Rendana, M. Impact of the Wind Conditions on COVID-19 Pandemic: A New Insight for Direction of the Spread of the Virus. Urban Clim. 2020, 34, 100680. [Google Scholar] [CrossRef]
- Bashir, M.F.; Ma, B.; Komal, B.; Bashir, M.A.; Tan, D.; Bashir, M. Correlation between Climate Indicators and COVID-19 Pandemic in New York, USA. Sci. Total Environ. 2020, 728, 138835. [Google Scholar] [CrossRef] [PubMed]
- Clouston, S.A.P.; Morozova, O.; Meliker, J.R. A Wind Speed Threshold for Increased Outdoor Transmission of Coronavirus: An Ecological Study. BMC Infect. Dis. 2021, 21, 1194. [Google Scholar] [CrossRef]
- Coccia, M. How Do Low Wind Speeds and High Levels of Air Pollution Support the Spread of COVID-19? Atmos. Pollut. Res. 2021, 12, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Nakada, L.Y.K.; Urban, R.C. COVID-19 Pandemic: Environmental and Social Factors Influencing the Spread of SARS-CoV-2 in São Paulo, Brazil. Environ. Sci. Pollut. Res. 2021, 28, 40322–40328. [Google Scholar] [CrossRef]
- Whittemore, P.B. COVID-19 fatalities, latitude, sunlight, and vitamin D. Am. J. Infect. Control 2020, 48, 1042–1044. [Google Scholar] [CrossRef] [PubMed]
- Mercola, J.; Grant, W.B.; Wagner, C.L. Evidence Regarding Vitamin D and Risk of COVID-19 and Its Severity. Nutrients 2020, 12, 3361. [Google Scholar] [CrossRef]
- Hassan, M.S.; Bhuiyan, M.A.H.; Tareq, F.; Bodrud-Doza, M.; Tanu, S.M.; Rabbani, K.A. Relationship between COVID-19 Infection Rates and Air Pollution, Geo-Meteorological, and Social Parameters. Environ. Monit. Assess. 2021, 193, 29. [Google Scholar] [CrossRef] [PubMed]
- Gupta, Y.; Raghuwanshi, G.; Ahmadini, A.A.H.; Sharma, U.; Mishra, A.K.; Mashwani, W.K.; Goktas, P.; Alshqaq, S.S.; Balogun, O.S. Impact of Weather Predictions on COVID-19 Infection Rate by Using Deep Learning Models. Complexity 2021, 2021, 5520663. [Google Scholar] [CrossRef]
- Sarmadi, M.; Ahmadi-Soleimani, S.M.; Fararouei, M.; Dianatinasab, M. COVID-19, Body Mass Index and Cholesterol: An Ecological Study Using Global Data. BMC Public Health 2021, 21, 1712. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Zhai, Z. The Efficacy of Social Distance and Ventilation Effectiveness in Preventing COVID-19 Transmission. Sustain. Cities Soc. 2020, 62, 102390. [Google Scholar] [CrossRef]
- WHO. Novel Coronavirus Advice for Public. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/advice-for-public (accessed on 27 July 2022).
- CDC. Coronavirus Social Distancing. Available online: https://www.cdc.gov/coronavirus/2019-ncov/community/tribal/social-distancing.html (accessed on 7 September 2022).
- Kelland, K. One Meter or Two? How Social Distancing Affects COVID-19 Risk. Available online: https://www.reuters.com/article/us-health-coronavirus-distance-explainer-idUSKBN23U22W (accessed on 2 September 2022).
- Setti, L.; Passarini, F.; De Gennaro, G.; Barbieri, P.; Perrone, M.G.; Borelli, M.; Palmisani, J.; Di Gilio, A.; Piscitelli, P.; Miani, A. Airborne Transmission Route of COVID-19: Why 2 Meters/6 Feet of Inter-Personal Distance Could Not Be Enough. Int. J. Environ. Res. Public Health 2020, 17, 2932. [Google Scholar] [CrossRef] [Green Version]
- WHO. Infection Prevention and Control of Epidemic- and Pandemic-Prone Acute Respiratory Infections in Health Care; WHO Guidelines; WHO: Geneva, Switzerland, 2014. Available online: https://www.who.int/publications/i/item/infection-prevention-and-control-of-epidemic-and-pandemic-prone-acute-respiratory-infections-in-health-care (accessed on 23 September 2022).
- Bourouiba, L. Turbulent Gas Clouds and Respiratory Pathogen Emissions: Potential Implications for Reducing Transmission of COVID-19. JAMA—J. Am. Med. Assoc. 2020, 323, 1837–1838. [Google Scholar] [CrossRef] [PubMed]
- Lane, J.; Means, A.R.; Bardosh, K.; Shapoval, A.; Vio, F.; Anderson, C.; Cushnie, A.; Forster, N.; Ledikwe, J.; O’Malley, G.; et al. Comparing COVID-19 Physical Distancing Policies: Results from a Physical Distancing Intensity Coding Framework for Botswana, India, Jamaica, Mozambique, Namibia, Ukraine, and the United States. Glob. Health 2021, 17, 124. [Google Scholar] [CrossRef]
- El-shabasy, R.M.; Nayel, M.A.; Taher, M.M.; Abdelmonem, R. Three waves changes, new variant strains, and vaccination effect against COVID-19 pandemic. Int. J. Biol. Micromol. 2022, 204, 161–168. [Google Scholar] [CrossRef]
- Ivorra, B.; Ferrández, M.R.; Vela-pérez, M.; Ramos, A.M. Mathematical modeling of the spread of the coronavirus disease 2019 (COVID-19) taking into account the undetected infections. The case of China. Commun. Nonlinear Sci. Numer. Simul. 2020, 88, 105303. [Google Scholar] [CrossRef]
- Rhodes, T.; Lancaster, K. Mathematical Models as Public Troubles in COVID-19 Infection Control: Following the Numbers. Health Sociol. Rev. 2020, 29, 177–194. [Google Scholar] [CrossRef] [PubMed]
- Boseley, S. New Data, New Policy: Why UK’s Coronavirus Strategy Changed. Guardian. 16 March 2020. Available online: https://www.theguardian.com/world/2020/mar/16/new-data-new-policy-why-uks-coronavirus-strategy-has-changed (accessed on 22 September 2022).
- Sample, I. Coronavirus Exposes the Problems and Pitfalls of Modelling. Guardian. 25 March 2020. Available online: https://www.theguardian.com/science/2020/mar/25/coronavirus-exposes-the-problems-and-pitfalls-of-modelling (accessed on 22 September 2022).
- Panovska-Griffiths, J. Can Mathematical Modelling Solve the Current COVID-19 Crisis? BMC Public Health 2020, 20, 551. [Google Scholar] [CrossRef] [PubMed]
- Eker, S. Validity and Usefulness of COVID-19 Models. Humanit. Soc. Sci. Commun. 2020, 7, 54. [Google Scholar] [CrossRef]
- McGrail, D.J.; Dai, J.; McAndrews, K.M.; Kalluri, R. Enacting National Social Distancing Policies Corresponds with Dramatic Reduction in COVID19 Infection Rates. PLoS ONE 2020, 15, e0236619. [Google Scholar] [CrossRef]
- Prem, K.; Liu, Y.; Russell, T.W.; Kucharski, A.J.; Eggo, R.M.; Davies, N.; Flasche, S.; Clifford, S.; Pearson, C.A.B.; Munday, J.D.; et al. The Effect of Control Strategies to Reduce Social Mixing on Outcomes of the COVID-19 Epidemic in Wuhan, China: A Modelling Study. Lancet Public Health 2020, 5, e261–e270. [Google Scholar] [CrossRef] [Green Version]
- Dahl, E. Coronavirus (COVID-19) Outbreak on the Cruise Ship Diamond Princess. Int. Marit. Health 2020, 71, 5–8. [Google Scholar] [CrossRef] [Green Version]
- Mohindra, R.; Ghai, A.; Brar, R.; Khandelwal, N.; Biswal, M.; Suri, V.; Goyal, K.; Singh, M.P.; Bhalla, A.; Rana, K.; et al. Superspreaders: A Lurking Danger in the Community. J. Prim. Care Community Health 2021, 12, 215013272098743. [Google Scholar] [CrossRef]
- Domènech-Montoliu, S.; Pac-Sa, M.R.; Vidal-Utrillas, P.; Latorre-Poveda, M.; Del Rio-González, A.; Ferrando-Rubert, S.; Ferrer-Abad, G.; Sánchez-Urbano, M.; Aparisi-Esteve, L.; Badenes-Marques, G.; et al. Mass Gathering Events and COVID-19 Transmission in Borriana (Spain): A Retrospective Cohort Study. PLoS ONE 2021, 16, e0256747. [Google Scholar] [CrossRef] [PubMed]
- Whaley, C.M.; Cantor, J.; Pera, M.; Jena, A.B. Assessing the Association between Social Gatherings and COVID-19 Risk Using Birthdays. JAMA Intern. Med. 2021, 181, 1090–1099. [Google Scholar] [CrossRef]
- Brown, K.A.; Jones, A.; Daneman, N.; Chan, A.K.; Schwartz, K.L.; Garber, G.E.; Costa, A.P.; Stall, N.M. Association between Nursing Home Crowding and COVID-19 Infection and Mortality in Ontario, Canada. JAMA Intern. Med. 2021, 181, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Hongoh, V.; Maybury, D.; Levesque, J.; Fazil, A.; Otten, A.; Turgeon, P.; Waddell, L.; Ogden, N.H. Decision Analysis Support for Evaluating Transmission Risk of COVID-19 in Places Where People Gather. Can. Commun. Dis. Rep. 2021, 47, 446–460. [Google Scholar] [CrossRef]
- Karia, R.; Gupta, I.; Khandait, H.; Yadav, A.; Yadav, A. COVID-19 and Its Modes of Transmission. SN Compr. Clin. Med. 2020, 2, 1798–1801. [Google Scholar] [CrossRef]
- Onakpoya, I.J.; Heneghan, C.J.; Spencer, E.A.; Brassey, J.; Plüddemann, A.; Evans, D.H.; Conly, J.M.; Jefferson, T. SARS-CoV-2 and the role of fomite transmission: A systematic review. F1000Research 2021, 10, 233. [Google Scholar] [CrossRef]
- Casanova, L.M.; Jeon, S.; Rutala, W.A.; Weber, D.J.; Sobsey, M.D. Effects of air temperature and relative humidity on coronavirus survival on surfaces. Appl. Environ. Microbiol. 2010, 76, 2712–2717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kratzel, A.; Steiner, S.; Todt, D.; V’kovski, P.; Brueggemann, Y.; Steinmann, J.; Steinmann, E.; Thiel, V.; Pfaender, S. Temperature-Dependent Surface Stability of SARS-CoV-2. J. Infect. 2020, 81, 452–482. [Google Scholar] [CrossRef]
- Riddell, S.; Goldie, S.; Hill, A.; Eagles, D.; Drew, T.W. The Effect of Temperature on Persistence of SARS-CoV-2 on Common Surfaces. Virol. J. 2020, 17, 145. [Google Scholar] [CrossRef]
- Chatterjee, S.; Murallidharan, J.S.; Agrawal, A.; Bhardwaj, R. Why Coronavirus Survives Longer on Impermeable than Porous Surfaces. Phys. Fluids 2021, 33, 021701. [Google Scholar] [CrossRef]
- Chin, A.; Chu, J.; Perera, M.; Hui, K.; Yen, H.L.; Chan, M.C.W.; Peiris, M.; Poon, L.L.M. Stability of SARS-CoV-2 in Different Environmental Conditions. Lancet Microbe 2020, 1, e10. [Google Scholar] [CrossRef]
- van Doremalen, N.; Bushmaker, T.; Morris, D.H.; Holbrook, M.G.; Gamble, A.; Williamson, B.N.; Tamin, A.; Harcourt, J.L.; Thornburg, N.J.; Gerber, S.I.; et al. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N. Engl. J. Med. 2020, 382, 1564–1567. [Google Scholar] [CrossRef]
- Pitol, A.K.; Julian, T.R. Community Transmission of SARS-CoV-2 by Fomites: Risks and Risk Reduction Strategies. Environ. Sci. Technol. Lett. 2020, 8, 263–269. [Google Scholar] [CrossRef]
- Zimmermann, P.; Curtis, N. Why Is COVID-19 Less Severe in Children? A Review of the Proposed Mechanisms Underlying the Age-Related Difference in Severity of SARS-CoV-2 Infections. Arch. Dis. Child. 2020, 106, 429–439. [Google Scholar] [CrossRef]
- Lelieveld, J.; Helleis, F.; Borrmann, S.; Cheng, Y.; Drewnick, F.; Haug, G.; Klimach, T.; Sciare, J.; Su, H.; Pöschl, U. Model Calculations of Aerosol Transmission and Infection Risk of COVID-19 in Indoor Environments. Int. J. Environ. Res. Public Health 2020, 17, 8114. [Google Scholar] [CrossRef]
- Park, S.; Choi, Y.; Song, D.; Kim, E.K. Natural Ventilation Strategy and Related Issues to Prevent Coronavirus Disease 2019 (COVID-19) Airborne Transmission in a School Building. Sci. Total Environ. 2021, 789, 147764. [Google Scholar] [CrossRef] [PubMed]
- AMA. COVID 19 Endemic. Available online: https://www.ama-assn.org/delivering-care/public-health/how-we-will-know-when-COVID-19-has-become-endemic (accessed on 8 September 2022).
- Szabo, L. Better Ventilation Can Prevent COVID Spread. But Are Companies Paying Attention? KHN. Available online: https://khn.org/news/article/ventilation-covid-prevention-indoor-air-quality-businesses-invest/ (accessed on 8 September 2022).
- Lipinski, T.; Ahmad, D.; Serey, N.; Jouhara, H. Review of Ventilation Strategies to Reduce the Risk of Disease Transmission in High Occupancy Buildings. Int. J. Thermofluids 2020, 7–8, 100045. [Google Scholar] [CrossRef]
- Higdon, M.M.; Wahl, B.; Jones, C.B.; Rosen, J.G.; Truelove, S.A.; Baidya, A.; Nande, A.A.; ShamaeiZadeh, P.A.; Walter, K.K.; Feikin, D.R.; et al. A Systematic Review of Coronavirus Disease 2019 Vaccine Efficacy and Effectiveness Against Severe Acute Respiratory Syndrome Coronavirus 2 Infection and Disease. Open Forum. Infect. Dis. 2022, 9, ofac138. [Google Scholar] [CrossRef] [PubMed]
- Rapaka, R.R.; Hammershaimb, E.A.; Neuzil, K.M. Are Some COVID-19 Vaccines Better Than Others? Interpreting and Comparing Estimates of Efficacy in Vaccine Trials. Clin. Infect. Dis. 2022, 74, 352–358. [Google Scholar] [CrossRef]
- World Health Organization. Evaluation of COVID-19 vaccine effectiveness: Interim Guidance. 17 March 2021; License: CC BY-NC-SA 3. Available online: https://apps.who.int/iris/handle/10665/340301 (accessed on 17 March 2022).
- WHO. Vaccine Equity. Available online: https://www.who.int/campaigns/vaccine-equity (accessed on 30 July 2022).
- WHO. Situation by Region, Country, Territory & Area. Available online: https://covid19.who.int/table (accessed on 28 July 2022).
- Puri, N.; Coomes, E.A.; Haghbayan, H.; Gunaratne, K. Social Media and Vaccine Hesitancy: New Updates for the Era of COVID-19 and Globalized Infectious Diseases. Hum. Vaccines Immunother. 2020, 16, 2586–2593. [Google Scholar] [CrossRef] [PubMed]
- Solís Arce, J.S.; Warren, S.S.; Meriggi, N.F.; Scacco, A.; McMurry, N.; Voors, M.; Syunyaev, G.; Malik, A.A.; Aboutajdine, S.; Adeojo, O.; et al. COVID-19 Vaccine Acceptance and Hesitancy in Low- and Middle-Income Countries. Nat. Med. 2021, 27, 1385–1394. [Google Scholar] [CrossRef]
- Sallam, M. COVID-19 Vaccine Hesitancy Worldwide: A Concise Systematic Review of Vaccine Acceptance Rates. Vaccines 2021, 9, 160. [Google Scholar] [CrossRef] [PubMed]
- Momplaisir, F.M.; Kuter, B.J.; Ghadimi, F.; Browne, S.; Nkwihoreze, H.; Feemster, K.A.; Frank, I.; Faig, W.; Shen, A.K.; Offit, P.A.; et al. Racial/Ethnic Differences in COVID-19 Vaccine Hesitancy among Health Care Workers in 2 Large Academic Hospitals. JAMA Netw. Open 2021, 4, e2121931. [Google Scholar] [CrossRef] [PubMed]
- Bellon, M. Gender Differences in COVID-19 Vaccine Hesitancy. The Clayman Institute for Gender Research, Standford University. Available online: https://gender.stanford.edu/news-publications/gender-news/gender-differences-COVID-19-vaccine-hesitancy (accessed on 21 September 2022).
- Leigh, J.P.; Moss, S.J.; White, T.M.; Picchio, C.A.; Rabin, K.H.; Ratzan, S.C.; Wyka, K.; El-Mohandes, A.; Lazarus, J.V. Factors Affecting COVID-19 Vaccine Hesitancy among Healthcare Providers in 23 Countries. Vaccine 2022, 40, 4081–4089. [Google Scholar] [CrossRef]
- Bianchi, F.P.; Stefanizzi, P.; Di Gioia, M.C.; Brescia, N.; Lattanzio, S.; Tafuri, S. COVID-19 Vaccination Hesitancy in Pregnant and Breastfeeding Women and Strategies to Increase Vaccination Compliance: A Systematic Review and Meta-Analysis. Expert Rev. Vaccines 2022, 21, 1443–1454. [Google Scholar] [CrossRef]
- Saitoh, A.; Takaku, M.; Saitoh, A. High Rates of Vaccine Hesitancy among Pregnant Women during the Coronavirus Disease 2019 (COVID-19) Pandemic in Japan. Hum. Vaccines Immunother. 2022, 18, 2064686. [Google Scholar] [CrossRef] [PubMed]
- Gerussi, V.; Peghin, M.; Palese, A.; Bressan, V.; Visintini, E.; Bontempo, G.; Graziano, E.; De Martino, M.; Isola, M.; Tascini, C. Vaccine Hesitancy among Italian Patients Recovered from COVID-19 Infection towards Influenza and SARS-CoV Vaccination. Vaccines 2021, 9, 172. [Google Scholar] [CrossRef] [PubMed]
- Millner, V.S.; Eichold, B.H.; Franks, R.D.; Johnson, G.D. Influenza Vaccination Acceptance and Refusal Rates Among Health Care Personnel. South. Med. J. 2010, 103, 993–998. [Google Scholar] [CrossRef] [PubMed]
- Schmid, P.; Rauber, D.; Betsch, C.; Lidolt, G.; Denker, M.-L. Barriers of Influenza Vaccination Intention and Behavior—A Systematic Review of Influenza Vaccine Hesitancy, 2005–2016. PLoS ONE 2017, 12, e0170550. [Google Scholar] [CrossRef] [Green Version]
- Haque, A.; Pant, A.B. Mitigating COVID-19 in the Face of Emerging Virus Variants, Breakthrough Infections and Vaccine Hesitancy. J. Autoimmun. 2022, 127, 102792. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, J. Populist Politics and Vaccine Hesitancy in Western Europe: An Analysis of National-Level Data. Eur. J. Public Health 2019, 29, 512–516. [Google Scholar] [CrossRef]
- Troiano, G.; Nardi, A. Vaccine Hesitancy in the Era of COVID-19. Public Health 2021, 194, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Harder, T.; Koch, J.; Vygen-Bonnet, S.; Külper-Schiek, W.; Pilic, A.; Reda, S.; Scholz, S.; Wichmann, O. Efficacy and Effectiveness of COVID-19 Vaccines against SARS-CoV-2 Infection: Interim Results of a Living Systematic Review, 1 January to 14 May 2021. Euro Surveill 2021, 26, 2100563. [Google Scholar] [CrossRef]
- Pouwels, K.B.; Pritchard, E.; Matthews, P.C.; Stoesser, N.; Eyre, D.W.; Vihta, K.D.; House, T.; Hay, J.; Bell, J.I.; Newton, J.N.; et al. Effect of Delta Variant on Viral Burden and Vaccine Effectiveness against New SARS-CoV-2 Infections in the UK. Nat. Med. 2021, 27, 2127–2135. [Google Scholar] [CrossRef]
- Chambers, C. Comparing Vaccines: Efficacy, Safety and Side Effects. Healthy Debate. Available online: https://healthydebate.ca/2021/03/topic/comparing-vaccines/ (accessed on 22 September 2022).
- Mallapaty, S. Can COVID Vaccines Stop Transmission? Scientists Race to Find Answers. Nature. 19 February 2021. Available online: https://www.nature.com/articles/d41586-021-00450-z (accessed on 8 September 2022).
- Levine-Tiefenbrun, M.; Yelin, I.; Katz, R.; Herzel, E.; Golan, Z.; Schreiber, L.; Wolf, T.; Nadler, V.; Ben-Tov, A.; Kuint, J.; et al. Initial Report of Decreased SARS-CoV-2 Viral Load after Inoculation with the BNT162b2 Vaccine. Nat. Med. 2021, 27, 790–792. [Google Scholar] [CrossRef] [PubMed]
- Ioannou, P.; Karakonstantis, S.; Astrinaki, E.; Saplamidou, S.; Vitsaxaki, E.; Hamilos, G.; Sourvinos, G.; Kofteridis, D.P. Transmission of SARS-CoV-2 Variant, B.1.1.7 among Vaccinated Health Care Workers. Infect. Dis. 2021, 53, 876–879. [Google Scholar] [CrossRef]
- Hsu, L.; Hurraß, J.; Kossow, A.; Klobucnik, J.; Nießen, J.; Wiesmüller, G.A.; Grüne, B.; Joisten, C. Breakthrough Infections with the SARS-CoV-2 Delta Variant: Vaccinations Halved Transmission Risk. Public Health 2022, 204, 40–42. [Google Scholar] [CrossRef] [PubMed]
- Feikin, D.R.; Higdon, M.M.; Abu-Raddad, L.J.; Andrews, N.; Araos, R.; Goldberg, Y.; Groome, M.J.; Huppert, A.; O’Brien, K.L.; Smith, P.G.; et al. Duration of Effectiveness of Vaccines against SARS-CoV-2 Infection and COVID-19 Disease: Results of a Systematic Review and Meta-Regression. Lancet 2022, 399, 924–944. [Google Scholar] [CrossRef]
- Wald, A. Booster Vaccination to Reduce SARS-CoV-2 Transmission and Infection. JAMA—J. Am. Med. Assoc. 2022, 327, 327–328. [Google Scholar] [CrossRef] [PubMed]
- Abu-Raddad, L.J.; Chemaitelly, H.; Ayoub, H.H.; AlMukdad, S.; Yassine, H.M.; Al-Khatib, H.A.; Smatti, M.K.; Tang, P.; Hasan, M.R.; Coyle, P.; et al. Effect of MRNA Vaccine Boosters against SARS-CoV-2 Omicron Infection in Qatar. N. Engl. J. Med. 2022, 386, 1804–1816. [Google Scholar] [CrossRef]
- Tenforde, M.W.; Self, W.H.; Naioti, E.A.; Ginde, A.A.; Douin, D.J.; Olson, S.M.; Talbot, H.K.; Casey, J.D.; Mohr, N.M.; Zepeski, A.; et al. Sustained Effectiveness of Pfizer-BioNTech and Moderna Vaccines Against COVID-19 Associated Hospitalizations Among Adults—United States, March–July 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 1156–1162. [Google Scholar] [CrossRef]
- Roy, S.; Ghosh, P. Factors Affecting COVID-19 Infected and Death Rates Inform Lockdown-Related Policymaking. PLoS ONE 2020, 15, e0241165. [Google Scholar] [CrossRef] [PubMed]
- Yazdani, S.; Heydari, M.; Foroughi, Z.; Jabali, H. Factors Affecting COVID-19 Transmission and Modelling of Close Contact Tracing Strategies. Iran J. Public Health 2021, 50, 2121–2131. [Google Scholar] [CrossRef] [PubMed]
- Velasco, J.M.; Tseng, W.C.; Chang, C.L. Factors Affecting the Cases and Deaths of COVID-19 Victims. Int. J. Environ. Res. Public Health 2021, 18, 674. [Google Scholar] [CrossRef] [PubMed]
- Aabed, K.; Lashin, M.M.A. An Analytical Study of the Factors That Influence COVID-19 Spread. Saudi J. Biol. Sci. 2021, 28, 1177–1195. [Google Scholar] [CrossRef]
- Ganasegeran, K.; Jamil, M.F.A.; Ch’ng, A.S.H.; Looi, I.; Peariasamy, K.M. Influence of Population Density for COVID-19 Spread in Malaysia: An Ecological Study. Int. J. Environ. Res. Public Health 2021, 18, 9866. [Google Scholar] [CrossRef]
- Aldridge, R.W.; Pineo, H.; Fragaszy, E.; Eyre, M.T.; Kovar, J.; Nguyen, V.; Beale, S.; Byrne, T.; Aryee, A.; Smith, C.; et al. Household Overcrowding and Risk of SARS-CoV-2: Analysis of the Virus Watch Prospective Community Cohort Study in England and Wales. Wellcome Open Res. 2021, 6, 347. [Google Scholar] [CrossRef]
- Ahmad, K.; Erqou, S.; Shah, N.; Nazir, U.; Morrison, A.R.; Choudhary, G.; Wu, W.C. Association of Poor Housing Conditions with COVID-19 Incidence and Mortality across US Counties. PLoS ONE 2020, 15, e0241327. [Google Scholar] [CrossRef]
- Varshney, K.; Glodjo, T.; Adalbert, J. Overcrowded Housing Increases Risk for COVID-19 Mortality: An Ecological Study. BMC Res. Notes 2022, 15, 126. [Google Scholar] [CrossRef] [PubMed]
- Daras, K.; Alexiou, A.; Rose, T.C.; Buchan, I.; Taylor-Robinson, D.; Barr, B. How Does Vulnerability to COVID-19 Vary between Communities in England? Developing a Small Area Vulnerability Index (SAVI). J. Epidemiol. Community Health 2021, 75, 729–734. [Google Scholar] [CrossRef] [PubMed]
- Arceo-Gomez, E.O.; Campos-Vazquez, R.M.; Esquivel, G.; Alcaraz, E.; Martinez, L.A.; Lopez, N.G. The Income Gradient in COVID-19 Mortality and Hospitalisation: An Observational Study with Social Security Administrative Records in Mexico. Lancet Reg. Health-Am. 2022, 6, 100115. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Zhao, G. Wealth, Health, and beyond: Is COVID-19 Less Likely to Spread in Rich Neighborhoods? PLoS ONE 2022, 17, 8–10. [Google Scholar] [CrossRef] [PubMed]
- Oronce, C.I.A.; Scannell, C.A.; Kawachi, I.; Tsugawa, Y. Association Between State-Level Income Inequality and COVID-19 Cases and Mortality in the USA. J. Gen. Intern. Med. 2020, 35, 2791–2793. [Google Scholar] [CrossRef]
- Liao, T.F.; De Maio, F. Association of Social and Economic Inequality with Coronavirus Disease 2019 Incidence and Mortality across US Counties. JAMA Netw. Open 2021, 4, e2034578. [Google Scholar] [CrossRef]
- Tan, A.X.; Hinman, J.A.; Abdel Magid, H.S.; Nelson, L.M.; Odden, M.C. Association between Income Inequality and County-Level COVID-19 Cases and Deaths in the US. JAMA Netw. Open 2021, 4, e218799. [Google Scholar] [CrossRef]
- WHO. Literacy Rate among Adults Aged ≥ 15 Years (%). Available online: https://www.who.int/data/gho/indicator-metadata-registry/imr-details/77 (accessed on 23 September 2022).
- Archila, P.A.; Danies, G.; Molina, J.; Truscott de Mejía, A.-M.; Restrepo, S. Towards COVID-19 Literacy. Sci. Educ. 2021, 30, 785–808. [Google Scholar] [CrossRef]
- CDC. Framework for Implementation of COVID-19 Community Mitigation Measures for Lower-Resource Countries. Available online: https://www.cdc.gov/coronavirus/2019-ncov/global-COVID-19/community-mitigation-measures.html (accessed on 22 September 2022).
- Rattay, P.; Michalski, N.; Domanska, O.M.; Kaltwasser, A.; de Bock, F.; Wieler, L.H.; Jordan, S. Differences in Risk Perception, Knowledge and Protective Behaviour Regarding COVID-19 by Education Level among Women and Men in Germany. Results from the COVID-19 Snapshot Monitoring (COSMO) Study. PLoS ONE 2021, 16, e0251694. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Asaba, K. Educational Attainment Decreases the Risk of COVID-19 Severity in the European Population: A Two-Sample Mendelian Randomization Study. Front. Public Health 2021, 9, 673451. [Google Scholar] [CrossRef]
- Marzo, R.R.; Sami, W.; Alam, M.Z.; Acharya, S.; Jermsittiparsert, K.; Songwathana, K.; Pham, N.T.; Respati, T.; Faller, E.M.; Baldonado, A.M.; et al. Hesitancy in COVID-19 Vaccine Uptake and Its Associated Factors among the General Adult Population: A Cross-Sectional Study in Six Southeast Asian Countries. Trop. Med. Health 2022, 50, 4. [Google Scholar] [CrossRef]
- Eitze, S.; Vaccination60+ Study Group; Heinemeier, D.; Schmid-Küpke, N.K.; Betsch, C. Decreasing Vaccine Hesitancy with Extended Health Knowledge: Evidence from a Longitudinal Randomized Controlled Trial. Health Psychol. 2021, 40, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Bigdelou, B.; Sepand, M.R.; Najafikhoshnoo, S.; Negrete, J.A.T.; Sharaf, M.; Ho, J.Q.; Sullivan, I.; Chauhan, P.; Etter, M.; Shekarian, T.; et al. COVID-19 and Preexisting Comorbidities: Risks, Synergies, and Clinical Outcomes. Front. Immunol. 2022, 13, 890517. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.R.; Kabir, M.R.; Reza, S. Comorbidities Might Be a Risk Factor for the Incidence of COVID-19: Evidence from a Web-Based Survey. Prev. Med. Rep. 2021, 21, 101319. [Google Scholar] [CrossRef] [PubMed]
- Conti, P.; Younes, A. Coronavirus COV-19/SARS-CoV-2 Affects Women Less than Men: Clinical Response to Viral Infection. J. Biol. Regul. Homeost. Agents 2020, 34, 339–343. [Google Scholar] [CrossRef]
- Sama, I.E.; Voors, A.A. Men More Vulnerable to COVID-19: Explained by ACE2 on the X Chromosome? Eur. Heart J. 2020, 41, 3096. [Google Scholar] [CrossRef]
- Gotluru, C.; Roach, A.; Cherry, S.H.; Runowicz, C.D. Sex, Hormones, Immune Functions, and Susceptibility to Coronavirus Disease 2019 (COVID-19)–Related Morbidity. Obstet. Gynecol. 2021, 137, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Gudbjartsson, D.F.; Helgason, A.; Jonsson, H.; Magnusson, O.T.; Melsted, P.; Norddahl, G.L.; Saemundsdottir, J.; Sigurdsson, A.; Sulem, P.; Agustsdottir, A.B.; et al. Spread of SARS-CoV-2 in the Icelandic Population. N. Engl. J. Med. 2020, 382, 2302–2315. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.-M.; Bai, P.; He, W.; Wu, F.; Liu, X.-F.; Han, D.-M.; Liu, S.; Yang, J.-K. Gender Differences in Patients With COVID-19: Focus on Severity and Mortality. Front. Public Health 2020, 8, 152. [Google Scholar] [CrossRef]
- Viveiros, A.; Gheblawi, M.; Aujla, P.K.; Sosnowski, D.K.; Seubert, J.M.; Kassiri, Z.; Oudit, G.Y. Sex- and Age-Specific Regulation of ACE2: Insights into Severe COVID-19 Susceptibility. J. Mol. Cell Cardiol. 2022, 164, 13–16. [Google Scholar] [CrossRef]
- Fagyas, M.; Fejes, Z.; Sütő, R.; Nagy, Z.; Székely, B.; Pócsi, M.; Ivády, G.; Bíró, E.; Bekő, G.; Nagy, A.; et al. Circulating ACE2 Activity Predicts Mortality and Disease Severity in Hospitalized COVID-19 Patients. Int. J. Infect. Dis. 2022, 115, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Li, M.-Y.; Li, L.; Zhang, Y.; Wang, X.-S. Expression of the SARS-CoV-2 Cell Receptor Gene ACE2 in a Wide Variety of Human Tissues. Infect. Dis. Poverty 2020, 9, 45. [Google Scholar] [CrossRef] [PubMed]
- Esteve, A.; Permanyer, I.; Boertien, D.; Vaupel, J.W. National Age and Coresidence Patterns Shape COVID-19 Vulnerability. Proc. Natl. Acad. Sci. USA 2020, 117, 16118–16120. [Google Scholar] [CrossRef]
- Bukowska, A.; Spiller, L.; Wolke, C.; Lendeckel, U.; Weinert, S.; Hoffmann, J.; Bornfleth, P.; Kutschka, I.; Gardemann, A.; Isermann, B.; et al. Protective Regulation of the ACE2/ACE Gene Expression by Estrogen in Human Atrial Tissue from Elderly Men. Exp. Biol. Med. 2017, 242, 1412–1423. [Google Scholar] [CrossRef] [Green Version]
- Pijls, B.G.; Jolani, S.; Atherley, A.; Derckx, R.T.; Dijkstra, J.I.R.; Franssen, G.H.L.; Hendriks, S.; Richters, A.; Venemans-Jellema, A.; Zalpuri, S.; et al. Demographic Risk Factors for COVID-19 Infection, Severity, ICU Admission and Death: A Meta-Analysis of 59 Studies. BMJ Open 2021, 11, e044640. [Google Scholar] [CrossRef] [PubMed]
- Pang, X. Evaluation of Control Measures Implemented in the Severe Acute Respiratory Syndrome Outbreak in Beijing, 2003. JAMA 2003, 290, 3215. [Google Scholar] [CrossRef] [Green Version]
- Guan, W.; Ni, Z.; Hu, Y.; Liang, W.; Ou, C.; He, J.; Liu, L.; Shan, H.; Lei, C.; Hui, D.S.C.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef]
- Antos, A.; Kwong, M.L.; Balmorez, T.; Villanueva, A.; Murakami, S. Unusually High Risks of COVID-19 Mortality with Age-Related Comorbidities: An Adjusted Meta-Analysis Method to Improve the Risk Assessment of Mortality Using the Comorbid Mortality Data. Infect. Dis. Rep. 2021, 13, 700–711. [Google Scholar] [CrossRef]
- Furuhashi, M.; Moniwa, N.; Mita, T.; Fuseya, T.; Ishimura, S.; Ohno, K.; Shibata, S.; Tanaka, M.; Watanabe, Y.; Akasaka, H.; et al. Urinary Angiotensin-Converting Enzyme 2 in Hypertensive Patients May Be Increased by Olmesartan, an Angiotensin II Receptor Blocker. Am. J. Hypertens. 2015, 28, 15–21. [Google Scholar] [CrossRef] [Green Version]
- Cure, E.; Cumhur Cure, M. Angiotensin-Converting Enzyme Inhibitors and Angiotensin Receptor Blockers May Be Harmful in Patients with Diabetes during COVID-19 Pandemic. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 349–350. [Google Scholar] [CrossRef]
- Liu, D.; Zeng, X.; Ding, Z.; Lv, F.; Mehta, J.L.; Wang, X. Adverse Cardiovascular Effects of Anti-COVID-19 Drugs. Front. Pharmacol. 2021, 12, 699949. [Google Scholar] [CrossRef]
- Boutin, S.; Hildebrand, D.; Boulant, S.; Kreuter, M.; Rüter, J.; Pallerla, S.R.; Velavan, T.P.; Nurjadi, D. Host Factors Facilitating SARS-CoV-2 Virus Infection and Replication in the Lungs. Cell Mol. Life Sci. 2021, 78, 5953–5976. [Google Scholar] [CrossRef]
- Contoli, M.; Message, S.D.; Laza-Stanca, V.; Edwards, M.R.; Wark, P.A.B.; Bartlett, N.W.; Kebadze, T.; Mallia, P.; Stanciu, L.A.; Parker, H.L.; et al. Role of Deficient Type III Interferon-λ Production in Asthma Exacerbations. Nat. Med. 2006, 12, 1023–1026. [Google Scholar] [CrossRef] [PubMed]
- Cai, G.; Bossé, Y.; Xiao, F.; Kheradmand, F.; Amos, C.I. Tobacco Smoking Increases the Lung Gene Expression of ACE2, the Receptor of SARS-CoV-2. Am. J. Respir. Crit. Care Med. 2020, 201, 1557–1559. [Google Scholar] [CrossRef] [PubMed]
- Leung, J.M.; Yang, C.X.; Tam, A.; Shaipanich, T.; Hackett, T.-L.; Singhera, G.K.; Dorscheid, D.R.; Sin, D.D. ACE-2 Expression in the Small Airway Epithelia of Smokers and COPD Patients: Implications for COVID-19. Eur. Respir. J. 2020, 55, 2000688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.M.; Koh, H.Y.; Moon, S.Y.; Yoo, I.K.; Ha, E.K.; You, S.; Kim, S.Y.; Yon, D.K.; Lee, S.W. Allergic Disorders and Susceptibility to and Severity of COVID-19: A Nationwide Cohort Study. J. Allergy Clin. Immunol. 2020, 146, 790–798. [Google Scholar] [CrossRef]
- Atkins, J.L.; Masoli, J.A.H.; Delgado, J.; Pilling, L.C.; Kuo, C.-L.; Kuchel, G.A.; Melzer, D. Preexisting Comorbidities Predicting COVID-19 and Mortality in the UK Biobank Community Cohort. J. Gerontol. Ser. A 2020, 75, 2224–2230. [Google Scholar] [CrossRef]
- Popkin, B.M.; Du, S.; Green, W.D.; Beck, M.A.; Algaith, T.; Herbst, C.H.; Alsukait, R.F.; Alluhidan, M.; Alazemi, N.; Shekar, M. Individuals with Obesity and COVID-19: A Global Perspective on the Epidemiology and Biological Relationships. Obes. Rev. 2020, 21, e13128. [Google Scholar] [CrossRef]
- Ritter, A.; Kreis, N.-N.; Louwen, F.; Yuan, J. Obesity and COVID-19: Molecular Mechanisms Linking Both Pandemics. Int. J. Mol. Sci. 2020, 21, 5793. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Chi, J.; Lv, W.; Wang, Y. Obesity and Diabetes as High-risk Factors for Severe Coronavirus Disease 2019 (COVID-19). Diabetes. Metab. Res. Rev. 2021, 37, e3377. [Google Scholar] [CrossRef]
- Mancuso, P. The Role of Adipokines in Chronic Inflammation. Immunotargets Ther. 2016, 5, 47–56. [Google Scholar] [CrossRef] [Green Version]
- Jia, X.; Yin, C.; Lu, S.; Chen, Y.; Liu, Q.; Bai, J.; Lu, Y. Two Things about COVID-19 Might Need Attention. Preprints 2020, 2020020315. [Google Scholar] [CrossRef]
- Ryan, P.M.; Caplice, N.M. Is Adipose Tissue a Reservoir for Viral Spread, Immune Activation, and Cytokine Amplification in Coronavirus Disease 2019? Obesity 2020, 28, 1191–1194. [Google Scholar] [CrossRef] [Green Version]
- Tao, K.; Tzou, P.L.; Nouhin, J.; Gupta, R.K.; de Oliveira, T.; Kosakovsky Pond, S.L.; Fera, D.; Shafer, R.W. The Biological and Clinical Significance of Emerging SARS-CoV-2 Variants. Nat. Rev. Genet. 2021, 22, 757–773. [Google Scholar] [CrossRef] [PubMed]
- Candido, D.S.; Claro, I.M.; De Jesus, J.G.; Souza, W.M.; Moreira, F.R.R.; Dellicour, S.; Mellan, T.A.; Du Plessis, L.; Pereira, R.H.M.; Sales, F.C.S.; et al. Evolution and Epidemic Spread of SARS-CoV-2 in Brazil. Science 2020, 369, 1255–1260. [Google Scholar] [CrossRef]
- Faria, N.R.; Mellan, T.A.; Whittaker, C.; Claro, I.M.; Candido, D.D.S.; Mishra, S.; Crispim, M.A.E.; Sales, F.C.S.; Hawryluk, I.; McCrone, J.T.; et al. Genomics and Epidemiology of the P.1 SARS-CoV-2 Lineage in Manaus, Brazil. Science 2021, 372, 815–821. [Google Scholar] [CrossRef] [PubMed]
- Tegally, H.; Wilkinson, E.; Giovanetti, M.; Iranzadeh, A.; Fonseca, V.; Giandhari, J.; Doolabh, D.; Pillay, S.; San, E.J.; Msomi, N.; et al. Detection of a SARS-CoV-2 Variant of Concern in South Africa. Nature 2021, 592, 438–443. [Google Scholar] [CrossRef]
- Salehi-Vaziri, M.; Fazlalipour, M.; Seyed Khorrami, S.M.; Azadmanesh, K.; Pouriayevali, M.H.; Jalali, T.; Shoja, Z.; Maleki, A. The Ins and Outs of SARS-CoV-2 Variants of Concern (VOCs). Arch. Virol. 2022, 167, 327–344. [Google Scholar] [CrossRef]
- World Health Organization. WHO Tracking SARS-CoV-2 Variants. Available online: https://www.who.int/en/activities/tracking-SARS-CoV-2-variants/ (accessed on 21 September 2022).
- Andeweg, S.P.; Vennema, H.; Veldhuijzen, I.; Smorenburg, N.; Schmitz, D.; Zwagemaker, F.; van Gageldonk-Lafeber, A.B.; Hahné, S.J.M.; Reusken, C.; Knol, M.J.; et al. Elevated Risk of Infection with SARS-CoV-2 Beta, Gamma, and Delta Variant Compared to Alpha Variant in Vaccinated Individuals. Sci. Transl. Med. 2022, eabn4338. [Google Scholar] [CrossRef]
- Walker, A.S.; Vihta, K.-D.; Gethings, O.; Pritchard, E.; Jones, J.; House, T.; Bell, I.; Bell, J.I.; Newton, J.N.; Farrar, J.; et al. COVID-19 Infection Survey Team. Tracking the Emergence of SARS-CoV-2 Alpha Variant in the United Kingdom. N. Engl. J. Med. 2021, 385, 2582–2585. [Google Scholar] [CrossRef]
- Davies, N.G.; Abbott, S.; Barnard, R.C.; Jarvis, C.I.; Kucharski, A.J.; Munday, J.D.; Pearson, C.A.B.; Russell, T.W.; Tully, D.C.; Washburne, A.D.; et al. Estimated Transmissibility and Impact of SARS-CoV-2 Lineage, B.1.1.7 in England. Science 2021, 372, eabg3055. [Google Scholar] [CrossRef] [PubMed]
- Gravagnuolo, A.M.; Faqih, L.; Cronshaw, C.; Wynn, J.; Burglin, L.; Klapper, P.; Wigglesworth, M. Epidemiological Investigation of New SARS-CoV-2 Variant of Concern 202012/01 in England; Preprint; Epidemiology. medRxiv 2021. [Google Scholar] [CrossRef]
- Parra-Lucares, A.; Segura, P.; Rojas, V.; Pumarino, C.; Saint-Pierre, G.; Toro, L. Emergence of SARS-CoV-2 Variants in the World: How Could This Happen? Life 2022, 12, 194. [Google Scholar] [CrossRef]
- Volz, E.; Mishra, S.; Chand, M.; Barrett, J.C.; Johnson, R.; Geidelberg, L.; Hinsley, W.R.; Laydon, D.J.; Dabrera, G.; O’Toole, Á.; et al. Assessing Transmissibility of SARS-CoV-2 Lineage, B.1.1.7 in England. Nature 2021, 593, 266–269. [Google Scholar] [CrossRef]
- Garcia Borrega, J.; Naendrup, J.-H.; Heindel, K.; Hamacher, L.; Heger, E.; Di Cristanziano, V.; Deppe, A.-C.; Dusse, F.; Wetsch, W.A.; Eichenauer, D.A.; et al. Clinical Course and Outcome of Patients with SARS-CoV-2 Alpha Variant Infection Compared to Patients with SARS-CoV-2 Wild-Type Infection Admitted to the ICU. Microorganisms 2021, 9, 1944. [Google Scholar] [CrossRef] [PubMed]
- Davies, N.G.; Jarvis, C.I.; Edmunds, W.J.; Jewell, N.P.; Diaz-Ordaz, K.; Keogh, R.H. Increased Mortality in Community-Tested Cases of SARS-CoV-2 Lineage, B.1.1.7. Nature 2021, 593, 270–274. [Google Scholar] [CrossRef]
- Yadav, P.D.; Sarkale, P.; Razdan, A.; Gupta, N.; Nyayanit, D.A.; Sahay, R.R.; Potdar, V.; Patil, D.Y.; Baradkar, S.; Kumar, A.; et al. Isolation and Characterization of SARS-CoV-2 Beta Variant from UAE Travelers. J. Infect. Public Health 2022, 15, 182–186. [Google Scholar] [CrossRef]
- Wang, P.; Nair, M.S.; Liu, L.; Iketani, S.; Luo, Y.; Guo, Y.; Wang, M.; Yu, J.; Zhang, B.; Kwong, P.D.; et al. Antibody Resistance of SARS-CoV-2 Variants, B.1.351 and B.1.1.7. Nature 2021, 593, 130–135. [Google Scholar] [CrossRef]
- Zhao, S.; Peng, Z.; Wang, M.H. A Tentative Assessment of the Changes in Transmissibility and Fatality Risk Associated with Beta SARS-CoV-2 Variants in South Africa: An Ecological Study. Pathog. Glob. Health 2022, 116, 137–139. [Google Scholar] [CrossRef]
- Pearson, C.A.B.; Russel, T.W.; Davies, N.; Kucharski, A.J.; CMMID COVID-19 Working Group; Edmunds, W.J.; Eggo, R.M. Estimates of severity and transmissibility of novel SARS-CoV-2 variant 501Y.V2 in South Africa. CMMID Repos. 2021. preprint. Available online: https://cmmid.github.io/topics/covid19/sa-novel-variant.html (accessed on 8 September 2022).
- Pyke, A.T.; Nair, N.; van den Hurk, A.F.; Burtonclay, P.; Nguyen, S.; Barcelon, J.; Kistler, C.; Schlebusch, S.; McMahon, J.; Moore, F. Replication Kinetics of B.1.351 and B.1.1.7 SARS-CoV-2 Variants of Concern Including Assessment of a B.1.1.7 Mutant Carrying a Defective ORF7a Gene. Viruses 2021, 13, 1087. [Google Scholar] [CrossRef]
- Dejnirattisai, W.; Zhou, D.; Ginn, H.M.; Duyvesteyn, H.M.; Supasa, P.; Case, J.B.; Zhao, Y.; Walter, T.S.; Mentzer, A.J.; Liu, C.; et al. The Antigenic Anatomy of SARS-CoV-2 Receptor Binding Domain. Cell 2021, 184, 2183–2200. [Google Scholar] [CrossRef] [PubMed]
- Supasa, P.; Zhou, D.; Dejnirattisai, W.; Liu, C.; Mentzer, A.J.; Ginn, H.M.; Zhao, Y.; Duyvesteyn, H.M.E.; Nutalai, R.; Tuekprakhon, A.; et al. Reduced Neutralization of SARS-CoV-2 B.1.1.7 Variant by Convalescent and Vaccine Sera. Cell 2021, 184, 2201–2211.e7. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Liu, Y.; Tang, X.; He, D. The Disease Severity and Clinical Outcomes of the SARS-CoV-2 Variants of Concern. Front. Public Health 2021, 9, 775224. [Google Scholar] [CrossRef] [PubMed]
- Naveca, F.G.; Nascimento, V.; Souza, V.; Corado, A.; Corado, A.D.L.; Nascimento, F.; Silva, G.; Mejía, M.C.; Brandão, M.J.; Costa, Á; et al. Spread of Gamma (P.1) Sub-Lineages Carrying Spike Mutations Close to the Furin Cleavage Site and Deletions in the N-Terminal Domain Drives Ongoing Transmission of SARS-CoV-2 in Amazonas, Brazil. Microbiol. Spectr. 2022, 10, e0236621. [Google Scholar] [CrossRef]
- Khan, A.; Zia, T.; Suleman, M.; Khan, T.; Ali, S.S.; Abbasi, A.A.; Mohammad, A.; Wei, D. Higher Infectivity of the SARS-CoV-2 New Variants Is Associated with K417N/T, E484K, and N501Y Mutants: An Insight from Structural Data. J. Cell Physiol. 2021, 236, 7045–7057. [Google Scholar] [CrossRef] [PubMed]
- Freitas, A.R.R.; Lemos, D.R.Q.; Beckedorff, O.A.; de Góes Cavalcanti, L.P.; Siqueira, A.M.; de Mello, R.C.S.; Barros, E.N.C. The Increase in the Risk of Severity and Fatality Rate of COVID-19 in Southern Brazil after the Emergence of the Variant of Concern (VOC) SARS-CoV-2 P.1 Was Greater among Young Adults without Pre-Existing Risk Conditions; Preprint; Infectious Diseases (except HIV/AIDS). medRxiv 2021. [Google Scholar] [CrossRef]
- Padilha, D.A.; Benetti Filho, V.; Moreira, R.S.; Soratto, T.A.T.; Maia, G.A.; Christoff, A.P.; Barazzetti, F.H.; Schörner, M.A.; Ferrari, F.L.; Martins, C.L.; et al. Emergence of Two Distinct SARS-CoV-2 Gamma Variants and the Rapid Spread of P.1-like-II SARS-CoV-2 during the Second Wave of COVID-19 in Santa Catarina, Southern Brazil. Viruses 2022, 14, 695. [Google Scholar] [CrossRef] [PubMed]
- Fiolet, T.; Kherabi, Y.; MacDonald, C.-J.; Ghosn, J.; Peiffer-Smadja, N. Comparing COVID-19 Vaccines for Their Characteristics, Efficacy and Effectiveness against SARS-CoV-2 and Variants of Concern: A Narrative Review. Clin. Microbiol. Infect. 2022, 28, 202–221. [Google Scholar] [CrossRef]
- Shiehzadegan, S.; Alaghemand, N.; Fox, M.; Venketaraman, V. Analysis of the Delta Variant, B.1.617.2 COVID-19. Clin. Pract. 2021, 11, 778–784. [Google Scholar] [CrossRef]
- Ashley Hagen, M.S. How Dangerous Is the Delta Variant (B.1.617.2)? American Society for Microbiology. Available online: https://asm.org/Articles/2021/July/How-Dangerous-is-the-Delta-Variant-B-1-617-2# (accessed on 21 September 2022).
- Badan Litbangkes Kementerian Kesehatan RI. Peta Sebaran Sekuens dan Varian COVID-19 di Indonesia. Available online: https://www.litbang.kemkes.go.id/peta-sebaran-sekuens-dan-varian-COVID-19/ (accessed on 21 September 2022).
- Lang, K. Delta Variant has 235% Higher Risk of ICU Admission than Original Virus. Medical News Today. Available online: https://www.medicalnewstoday.com/articles/COVID-19-which-vaccines-are-effective-against-the-delta-variant#Very-high-levels-of-protection-against-hospitalization (accessed on 21 September 2022).
- Lee, B.U. Why Does the SARS-CoV-2 Delta VOC Spread So Rapidly? Universal Conditions for the Rapid Spread of Respiratory Viruses, Minimum Viral Loads for Viral Aerosol Generation, Effects of Vaccination on Viral Aerosol Generation, and Viral Aerosol Clouds. Int. J. Environ. Res. Public Health 2021, 18, 9804. [Google Scholar] [CrossRef]
- Thakur, N.; Gallo, G.; Newman, J.; Peacock, T.P.; Biasetti, L.; Hall, C.N.; Wright, E.; Barclay, W.; Bailey, D. SARS-CoV-2 Variants of Concern Alpha, Beta, Gamma and Delta Have Extended ACE2 Receptor Host Ranges. J. Gen. Virol. 2022, 103, 1735. [Google Scholar] [CrossRef]
- Planas, D.; Veyer, D.; Baidaliuk, A.; Staropoli, I.; Guivel-Benhassine, F.; Rajah, M.M.; Planchais, C.; Porrot, F.; Robillard, N.; Puech, J.; et al. Reduced Sensitivity of SARS-CoV-2 Variant Delta to Antibody Neutralization. Nature 2021, 596, 276–280. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, J.; Johnson, B.A.; Xia, H.; Ku, Z.; Schindewolf, C.; Widen, S.G.; An, Z.; Weaver, S.C.; Menachery, V.D.; et al. Delta Spike P681R Mutation Enhances SARS-CoV-2 Fitness over Alpha Variant. Cell Rep. 2022, 39, 110829. [Google Scholar] [CrossRef]
- Hu, Z.; Huang, X.; Zhang, J.; Fu, S.; Ding, D.; Tao, Z. Differences in Clinical Characteristics Between Delta Variant and Wild-Type SARS-CoV-2 Infected Patients. Front. Med. 2022, 8, 792135. [Google Scholar] [CrossRef] [PubMed]
- Twohig, K.A.; Nyberg, T.; Zaidi, A.; Thelwall, S.; Sinnathamby, M.A.; Aliabadi, S.; Seaman, S.R.; Harris, R.J.; Hope, R.; Lopez-Bernal, J.; et al. Hospital Admission and Emergency Care Attendance Risk for SARS-CoV-2 Delta (B.1.617.2) Compared with Alpha (B.1.1.7) Variants of Concern: A Cohort Study. Lancet Infect. Dis. 2022, 22, 35–42. [Google Scholar] [CrossRef]
- Sheikh, A.; McMenamin, J.; Taylor, B.; Robertson, C. SARS-CoV-2 Delta VOC in Scotland: Demographics, Risk of Hospital Admission, and Vaccine Effectiveness. Lancet 2021, 397, 2461–2462. [Google Scholar] [CrossRef]
- Torjesen, I. COVID-19: Delta Variant Is Now UK’s Most Dominant Strain and Spreading through Schools. BMJ 2021, 373, n1445. [Google Scholar] [CrossRef]
- Rahman, F.I.; Ether, S.A.; Islam, M.R. The “Delta Plus” COVID-19 Variant Has Evolved to Become the next Potential Variant of Concern: Mutation History and Measures of Prevention. J. Basic Clin. Physiol. Pharmacol. 2022, 33, 109–112. [Google Scholar] [CrossRef]
- Ferré, V.M.; Peiffer-Smadja, N.; Visseaux, B.; Descamps, D.; Ghosn, J.; Charpentier, C. Omicron SARS-CoV-2 Variant: What We Know and What We Don’t. Anaesth. Crit. Care Pain Med. 2022, 41, 100998. [Google Scholar] [CrossRef] [PubMed]
- Meo, S.A.; Meo, A.S.; Al-Jassir, F.F.; Klonoff, D.C. Omicron SARS-CoV-2 New Variant: Global Prevalence and Biological and Clinical Characteristics. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 8012–8018. [Google Scholar] [CrossRef] [PubMed]
- WHO. COVID-19 Weekly Epidemiological Update Edition 110. Available online: https://www.who.int/publications/m/item/weekly-epidemiological-update-on-COVID-19---21-september-2022 (accessed on 23 September 2022).
- Wei, C.; Shan, K.-J.; Wang, W.; Zhang, S.; Huan, Q.; Qian, W. Evidence for a Mouse Origin of the SARS-CoV-2 Omicron Variant. J. Genet. Genom. 2021, 48, 1111–1121. [Google Scholar] [CrossRef]
- Thakur, V.; Ratho, R.K. OMICRON (B.1.1.529): A New SARS-CoV-2 Variant of Concern Mounting Worldwide Fear. J. Med. Virol. 2022, 94, 1821–1824. [Google Scholar] [CrossRef]
- Bhattacharyya, R.P.; Hanage, W.P. Challenges in Inferring Intrinsic Severity of the SARS-CoV-2 Omicron Variant. N. Engl. J. Med. 2022, 386, e14. [Google Scholar] [CrossRef]
- Sofonea, M.T.; Roquebert, B.; Foulongne, V.; Morquin, D.; Verdurme, L.; Trombert-Paolantoni, S.; Roussel, M.; Bonetti, J.-C.; Zerah, J.; Haim-Boukobza, S.; et al. Analyzing and Modeling the Spread of SARS-CoV-2 Omicron Lineages BA.1 and BA.2, France, September 2021-February 2022. Emerg. Infect. Dis. 2022, 28, 1355–1365. [Google Scholar] [CrossRef]
- Ito, K.; Piantham, C.; Nishiura, H. Relative Instantaneous Reproduction Number of Omicron SARS-CoV-2 Variant with Respect to the Delta Variant in Denmark. J. Med. Virol. 2022, 94, 2265–2268. [Google Scholar] [CrossRef] [PubMed]
- Dejnirattisai, W.; Huo, J.; Zhou, D.; Zahradník, J.; Supasa, P.; Liu, C.; Duyvesteyn, H.M.; Ginn, H.M.; Mentzer, A.J.; Tuekprakhon, A.; et al. SARS-CoV-2 Omicron-B.1.1.529 Leads to Widespread Escape from Neutralizing Antibody Responses. Cell 2022, 185, 467–484.e15. [Google Scholar] [CrossRef]
- Andrews, N.; Stowe, J.; Kirsebom, F.; Toffa, S.; Rickeard, T.; Gallagher, E.; Gower, C.; Kall, M.; Groves, N.; O’Connell, A.-M.; et al. COVID-19 Vaccine Effectiveness against the Omicron (B.1.1.529) Variant. N. Engl. J. Med. 2022, 386, 1532–1546. [Google Scholar] [CrossRef]
- Minka, S.; Minka, F. A Tabulated Summary of the Evidence on Humoral and Cellular Responses to the SARS-CoV-2 Omicron VOC, as Well as Vaccine Efficacy against This Variant. Immunol. Lett. 2022, 243, 38–43. [Google Scholar] [CrossRef]
- Po, A.L.W. Omicron Variant as Nature’s Solution to the COVID-19 Pandemic. J. Clin. Pharm. Ther. 2022, 47, 3–5. [Google Scholar] [CrossRef]
- Arons, M.M.; Hatfield, K.M.; Reddy, S.C.; Kimball, A.; James, A.; Jacobs, J.R.; Taylor, J.; Spicer, K.; Bardossy, A.C.; Oakley, L.P.; et al. Presymptomatic SARS-CoV-2 Infections and Transmission in a Skilled Nursing Facility. N. Engl. J. Med. 2020, 382, 2081–2090. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO Director-General’s opening remarks at the media briefing on COVID-19—16 March 2020. Available online: https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-COVID-19---16-march-2020 (accessed on 23 September 2022).
- Mercer, T.R.; Salit, M. Testing at Scale during the COVID-19 Pandemic. Nat. Rev. Genet. 2021, 22, 415–426. [Google Scholar] [CrossRef]
- Cauchemez, S.; Kiem, C.T.; Paireau, J.; Rolland, P.; Fontanet, A. Lockdown Impact on COVID-19 Epidemics in Regions across Metropolitan France. Lancet 2020, 396, 1068–1069. [Google Scholar] [CrossRef]
- Zhou, Y.; Jiang, H.; Wang, Q.; Yang, M.; Chen, Y.; Jiang, Q. Use of Contact Tracing, Isolation, and Mass Testing to Control Transmission of COVID-19 in China. BMJ 2021, 375, n2330. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Wong, G.W.K.; Ni, W.; Hu, X.; Xing, Q. Rapid Response to an Outbreak in Qingdao, China. N. Engl. J. Med. 2020, 383, e129. [Google Scholar] [CrossRef]
- Cousins, S. New Zealand Eliminates COVID-19. Lancet 2020, 395, 1474. [Google Scholar] [CrossRef]
- Guan, D.; Wang, D.; Hallegatte, S.; Davis, S.J.; Huo, J.; Li, S.; Bai, Y.; Lei, T.; Xue, Q.; Coffman, D.; et al. Global Supply-Chain Effects of COVID-19 Control Measures. Nat. Hum. Behav. 2020, 4, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Songok, E. A Locally Sustainable Approach to COVID-19 Testing in Africa. Lancet Microbe 2020, 1, e197. [Google Scholar] [CrossRef]
- Hotez, P.; Batista, C.; Ergonul, O.; Figueroa, J.P.; Gilbert, S.; Gursel, M.; Hassanain, M.; Kang, G.; Kim, J.H.; Lall, B.; et al. Correcting COVID-19 Vaccine Misinformation. EClinicalMedicine 2021, 33, 100780. [Google Scholar] [CrossRef]
- Batista, C.; Hotez, P.; Amor, Y.B.; Kim, J.H.; Kaslow, D.; Lall, B.; Ergonul, O.; Figueroa, J.P.; Gursel, M.; Hassanain, M.; et al. The Silent and Dangerous Inequity around Access to COVID-19 Testing: A Call to Action. eClinicalMedicine 2022, 43, 101230. [Google Scholar] [CrossRef] [PubMed]
- FIND Diagnosis for All. SARS-CoV TEST TRACKER. Available online: https://www.finddx.org/COVID-19/test-tracker/ (accessed on 16 September 2022).
- Munharo, S.; Nayupe, S.; Mbulaje, P.; Patel, P.; Banda, C.; Gacutno, K.J.A.; Lin, X.; Shawa, I.T.; Lucero-Prisno III, D.E. Challenges of COVID-19 Testing in Low-Middle Income Countries (LMICs): The Case of Malawi. J. Lab. Precis. Med. 2020, 5, 32. [Google Scholar] [CrossRef]
- Torres, I.; Sippy, R.; Sacoto, F. Assessing Critical Gaps in COVID-19 Testing Capacity: The Case of Delayed Results in Ecuador. BMC Public Health 2021, 21, 637. [Google Scholar] [CrossRef]
- Government of Canada. Priority Strategies to Optimize Testing and Screening for COVID-19 in Canada: Report. Available online: https://www.canada.ca/en/health-canada/services/drugs-health-products/covid19-industry/medical-devices/testing-screening-advisory-panel/reports-summaries/priority-strategies.html (accessed on 23 September 2022).
- Connor, A.; Hariharan, N.; Carson, S.; Sanders, K.C.; Vosburg, K.B.; Sabot, O. Access To COVID-19 Testing In Low- and Middle-Income Countries Is Still Critical To Achieving Health Equity. Health Aff. Forefr. 2021. [Google Scholar] [CrossRef]
- Clipman, S.J.; Wesolowski, A.; Mehta, S.H.; Agarwal, S.; Cobey, S.E.; Cummings, D.A.T.; Gibson, D.G.; Labrique, A.B.; Kirk, G.D.; Solomon, S.S. SARS-CoV-2 Testing in Florida, Illinois, and Maryland: Access and Barriers. medRxiv 2020. [Google Scholar] [CrossRef]
- Cordes, J.; Castro, M.C. Spatial Analysis of COVID-19 Clusters and Contextual Factors in New York City. Spat. Spatiotemporal. Epidemiol. 2020, 34, 100355. [Google Scholar] [CrossRef]
- Maxmen, A. Why More Coronavirus Testing Won’t Automatically Help the Hardest Hit. Nature. 12 June 2020. Available online: https://www.nature.com/articles/d41586-020-01781-z (accessed on 23 September 2022).
- Rader, B.; Astley, C.M.; Sy, K.T.L.; Sewalk, K.; Hswen, Y.; Brownstein, J.S.; Kraemer, M.U.G. Geographic Access to United States SARS-CoV-2 Testing Sites Highlights Healthcare Disparities and May Bias Transmission Estimates. J. Travel Med. 2020, 27, taaa076. [Google Scholar] [CrossRef]
- Peeling, R.W.; Olliaro, P. Rolling out COVID-19 Antigen Rapid Diagnostic Tests: The Time Is Now. Lancet Infect. Dis. 2021, 21, 1052–1053. [Google Scholar] [CrossRef]
- Kenarkoohi, A.; Noorimotlagh, Z.; Falahi, S.; Amarloei, A.; Mirzaee, S.A.; Pakzad, I.; Bastani, E. Hospital Indoor Air Quality Monitoring for the Detection of SARS-CoV-2 (COVID-19) Virus. Sci. Total Environ. 2020, 748, 141324. [Google Scholar] [CrossRef]
- Lednicky, J.A.; Lauzardo, M.; Fan, Z.H.; Jutla, A.; Tilly, T.B.; Gangwar, M.; Usmani, M.; Shankar, S.N.; Mohamed, K.; Eiguren-Fernandez, A.; et al. Viable SARS-CoV-2 in the Air of a Hospital Room with COVID-19 Patients. Int. J. Infect. Dis. 2020, 100, 476–482. [Google Scholar] [CrossRef]
- Ong, S.W.X.; Tan, Y.K.; Coleman, K.K.; Tan, B.H.; Leo, Y.-S.; Wang, D.L.; Ng, C.G.; Ng, O.-T.; Wong, M.S.Y.; Marimuthu, K. Lack of Viable Severe Acute Respiratory Coronavirus Virus 2 (SARS-CoV-2) among PCR-Positive Air Samples from Hospital Rooms and Community Isolation Facilities. Infect. Control Hosp. Epidemiol. 2021, 42, 1327–1332. [Google Scholar] [CrossRef]
- Breshears, L.E.; Nguyen, B.T.; Mata Robles, S.; Wu, L.; Yoon, J.-Y. Biosensor Detection of Airborne Respiratory Viruses Such as SARS-CoV-2. SLAS Technol. 2022, 27, 4–17. [Google Scholar] [CrossRef]
- Makhsous, S.; Segovia, J.M.; He, J.; Chan, D.; Lee, L.; Novosselov, I.V.; Mamishev, A.V. Methodology for Addressing Infectious Aerosol Persistence in Real-Time Using Sensor Network. Sensors 2021, 21, 3928. [Google Scholar] [CrossRef] [PubMed]
- Obermeyer, F.; Jankowiak, M.; Barkas, N.; Schaffner, S.F.; Pyle, J.D.; Yurkovetskiy, L.; Bosso, M.; Park, D.J.; Babadi, M.; MacInnis, B.L.; et al. Analysis of 6.4 Million SARS-CoV-2 Genomes Identifies Mutations Associated with Fitness. Science 2022, 376, 1327–1332. [Google Scholar] [CrossRef]




| Type of Facemask | Material | Protection Level | BFE (%) |
|---|---|---|---|
| Surgical | Non-woven fabrics with a multi-layered structure consisting of a leak-proof layer, a high-density filter layer, and a direct contact skin layer | Prevent cross-contamination between respiratory particles of a wearer and body fluid of patients | >99 |
| Respirator (N95) | Multiple layers of non-woven fabric, often composed of polypropylene, and pre-filtration layer | Filter contaminants, bacteria | >99 |
| Cloth | Cotton/cloth | Mitigate aerosol dispersal, reduced transmission through droplets | 96 |
| Mask ID | Mask Type (Manufacturer Claim) | Filter Material | No. of Layers | DP | BFE (%) | Fibre Structure | Filtering Area (cm2) | Total Mask Size (cm2) | Fitting System | Nose Piece |
|---|---|---|---|---|---|---|---|---|---|---|
| CM-1 | Community, reusable | 100% cotton | 2 | 315 |  | Woven fabric | 329 | 393 | Ear loops | Metal wire |
| CM-2 | Community, reusable | 92% cotton, 8% PU | 2 | 56 |  | Knitted fabric | 225 | 259 | Ear loops | None |
| CM-3 | Community, reusable | PP | 3 (SSS) | 10 |  | Non-woven | 308 | 347 | Ear loops | None |
| CM-4 | Community, single-use | PP | 1 (S) | 7 |  | Non-woven | 356 | 396 | Ear loops | None |
| SM-1 | Surgical Type I, single-use | PP | 3 (SMS) | 28 |  | Non-woven | 188 | 271 | Ear loops | Metal wire |
| SM-2 | Surgical Type I, single-use | PP | 3 (SSS) | 77 |  | Non-woven | 207 | 286 | Ear loops | Metal wire |
| SM-3 | Surgical Type I, single-use | PP | 3 (SMS) | 35 |  | Non-woven | 200 | 277 | Ear loops | Metal wire |
| SM-4 | Surgical Type IIR, single-use | PP | 3 (SMS) | 30 |  | Non-woven | 200 | 272 | Ear loops | Metal wire |
| FP-1 | FFP2 respirator | PP | 3 (SMS) | 58 |  | Non-woven | 192 | 252 | Ear loops | Metal wire |
| FP-2 | FFP2 respirator | PP | 3 (SMS) | 53 |  | Non-woven | 173 | 255 | Head loop | Metal wire + foam |
| Type of Exemptions | Factors of Exemptions |
|---|---|
| Exempted |
|
| May be exempted |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, S.G.H.; Hadi, M.H.H.; Arsad, S.R.; Ker, P.J.; Ramanathan, S.; Afandi, N.A.M.; Afzal, M.M.; Yaw, M.W.; Krishnan, P.S.; Chen, C.P.; et al. Prerequisite for COVID-19 Prediction: A Review on Factors Affecting the Infection Rate. Int. J. Environ. Res. Public Health 2022, 19, 12997. https://doi.org/10.3390/ijerph192012997
Tang SGH, Hadi MHH, Arsad SR, Ker PJ, Ramanathan S, Afandi NAM, Afzal MM, Yaw MW, Krishnan PS, Chen CP, et al. Prerequisite for COVID-19 Prediction: A Review on Factors Affecting the Infection Rate. International Journal of Environmental Research and Public Health. 2022; 19(20):12997. https://doi.org/10.3390/ijerph192012997
Chicago/Turabian StyleTang, Shirley Gee Hoon, Muhamad Haziq Hasnul Hadi, Siti Rosilah Arsad, Pin Jern Ker, Santhi Ramanathan, Nayli Aliah Mohd Afandi, Madihah Mohd Afzal, Mei Wyin Yaw, Prajindra Sankar Krishnan, Chai Phing Chen, and et al. 2022. "Prerequisite for COVID-19 Prediction: A Review on Factors Affecting the Infection Rate" International Journal of Environmental Research and Public Health 19, no. 20: 12997. https://doi.org/10.3390/ijerph192012997
APA StyleTang, S. G. H., Hadi, M. H. H., Arsad, S. R., Ker, P. J., Ramanathan, S., Afandi, N. A. M., Afzal, M. M., Yaw, M. W., Krishnan, P. S., Chen, C. P., & Tiong, S. K. (2022). Prerequisite for COVID-19 Prediction: A Review on Factors Affecting the Infection Rate. International Journal of Environmental Research and Public Health, 19(20), 12997. https://doi.org/10.3390/ijerph192012997








