Sperm Adhesion Molecule 1 (SPAM1) Distribution in Selected Human Sperm by Hyaluronic Acid Test
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Semen Samples Analysis
2.3. Sperm Capacitation by Swim Up
2.4. Hyaluronic Acid Sperm Selection
2.5. Induction and Evaluation of Acrosomal Reaction
2.6. Fixation
2.7. Immunolocation of Tyrosine Phosphorylation
2.8. Immunolocation of SPAM1
2.9. Statistical Analysis
3. Results
3.1. Sperm Parameters
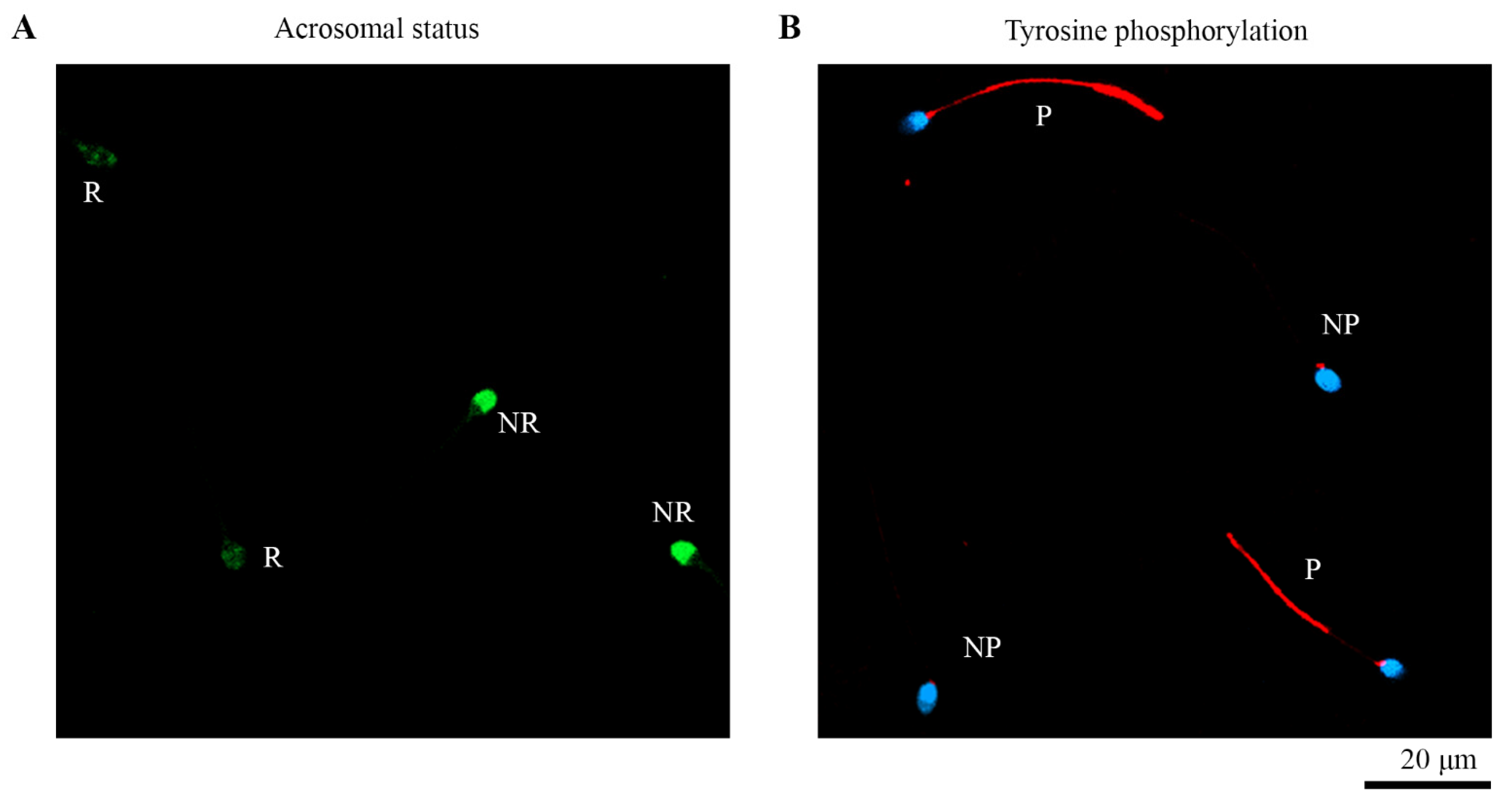
3.2. Assessment of Acrosomal Status
3.3. Assessment of Tyrosine Phosphorylation
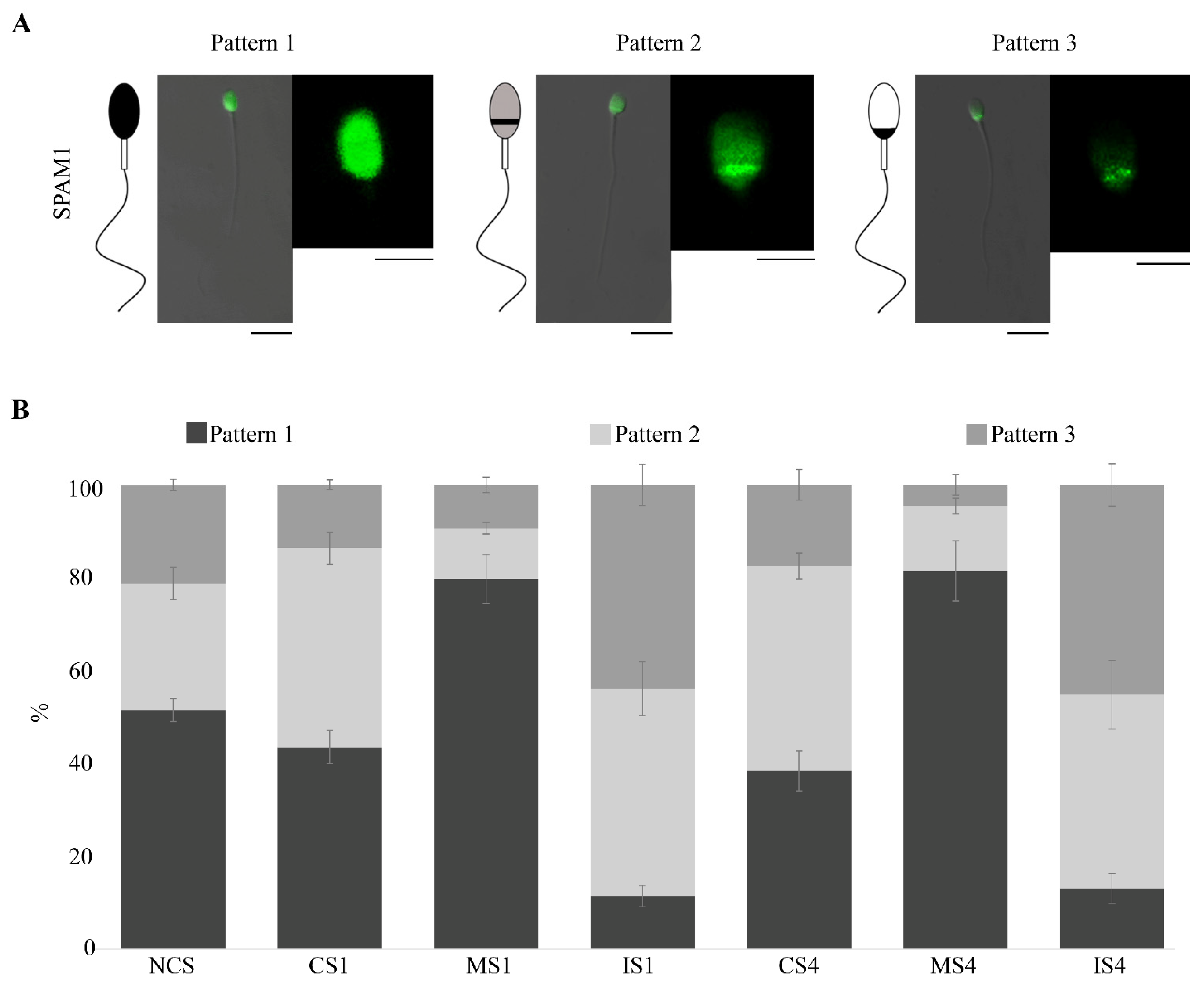
3.4. SPAM1 Immunolocation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chang, M.C. Fertilizing capacity of spermatozoa deposited into the fallopian tubes. Nature 1951, 168, 697–698. [Google Scholar] [CrossRef] [PubMed]
- Yanagimachi, R. Fertility of mammalian spermatozoa: Its development and relativity. Zygote 1994, 2, 371–372. [Google Scholar] [CrossRef]
- Wassarman, P.M. Gamete interactions during mammalian fertilization. Theriogenology 1994, 41, 31–44. [Google Scholar] [CrossRef]
- Wassarman, P.M. Mammalian Fertilization: Molecular Aspects of Gamete Adhesion, Exocytosis, and Fusion. Cell 1999, 96, 175–183. [Google Scholar] [CrossRef] [Green Version]
- Redgrove, K.A.; Nixon, B.; Baker, M.A.; Hetherington, L.; Baker, G.; Liu, D.-Y.; Aitken, R.J. The molecular chaperone HSPA2 plays a key role in regulating the expression of sperm surface receptors that mediate sperm-egg recognition. PLoS ONE 2012, 7, e50851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Redgrove, K.A.; Anderson, A.L.; McLaughlin, E.A.; O’Bryan, M.K.; Aitken, R.J.; Nixon, B. Investigation of the mechanisms by which the molecular chaperone HSPA2 regulates the expression of sperm surface receptors involved in human sperm-oocyte recognition. Mol. Hum. Reprod. 2013, 19, 120–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cherr, G.N.; Yudin, A.I.; Overstreet, J.W. The dual functions of GPI-anchored PH-20: Hyaluronidase and intracellular signaling. Matrix Biol. 2001, 20, 515–525. [Google Scholar] [CrossRef]
- Evans, E.A.; Zhang, H.; Martin-DeLeon, P.A. SPAM1 (PH-20) protein and mRNA expression in the epididymides of humans and macaques: Utilizing laser microdissection/RT-PCR. Reprod. Biol. Endocrinol. 2003, 1, 54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.; Griffiths, G.; Galileo, D.S.; Martin-DeLeon, P.A. Epididymal SPAM1 is a marker for sperm maturation in the mouse. Biol. Reprod. 2006, 74, 923–930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin-DeLeon, P.A. Germ-cell hyaluronidases: Their roles in sperm function. Int. J. Androl. 2011, 34, e306–e318. [Google Scholar] [CrossRef] [PubMed]
- Martin-DeLeon, P.A. Epididymal SPAM1 and its impact on sperm function. Mol. Cell. Endocrinol. 2006, 250, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Hinton, B.; Robaire, B. The Epididymis. In Knobil and Neill’s Physiology of Reproduction; Academic Press: Cambridge, MA, USA, 2015; Volume 1, pp. 691–771. ISBN 9780123971753. [Google Scholar]
- Myles, D.G.; Primakoff, P. Why did the sperm cross the cumulus? To get to the oocyte. Functions of the sperm surface proteins PH-20 and fertilin in arriving at, and fusing with, the egg. Biol. Reprod. 1997, 56, 320–327. [Google Scholar] [CrossRef] [Green Version]
- Sabeur, K.; Cherr, G.N.; Yudin, A.I.; Primakoff, P.; Li, M.W.; Overstreet, J.W. The PH-20 protein in human spermatozoa. J. Androl. 1997, 18, 151–158. [Google Scholar] [PubMed]
- Lin, Y.; Mahan, K.; Lathrop, W.F.; Myles, D.G.; Primakoff, P. A hyaluronidase activity of the sperm plasma membrane protein PH-20 enables sperm to penetrate the cumulus cell layer surrounding the egg. J. Cell Biol. 1994, 125, 1157–1163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhuo, L.; Yoneda, M.; Zhao, M.; Yingsung, W.; Yoshida, N.; Kitagawa, Y.; Kawamura, K.; Suzuki, T.; Kimata, K. Defect in SHAP-hyaluronan complex causes severe female infertility. A study by inactivation of the bikunin gene in mice. J. Biol. Chem. 2001, 276, 7693–7696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhuo, L.; Kimata, K. Cumulus Oophorus Extracellular Matrix: Its Construction and Regulation. Cell Struct. Funct. 2001, 26, 189–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huszar, G.; Ozenci, C.C.; Cayli, S.; Zavaczki, Z.; Hansch, E.; Vigue, L. Hyaluronic acid binding by human sperm indicates cellular maturity, viability, and unreacted acrosomal status. Fertil. Steril. 2003, 79, 1616–1624. [Google Scholar] [CrossRef]
- Rashki Ghaleno, L.; Rezazadeh Valojerdi, M.; Chehrazi, M.; Sahraneshin Samani, F.; Salman Yazdi, R. Hyaluronic Acid Binding Assay Is Highly Sensitive to Select Human Spermatozoa with Good Progressive Motility, Morphology, and Nuclear Maturity. Gynecol. Obstet. Investig. 2016, 81, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Long, Y.; Liu, M.; Chen, X.-L.; Wu, W.-Y.; Cheng, J.-E. Quality characteristics of human spermatozoa with hyaluronic acid receptors. Zhonghua Nan Ke Xue 2014, 20, 37–43. [Google Scholar]
- Worrilow, K.C.; Eid, S.; Woodhouse, D.; Perloe, M.; Smith, S.; Witmyer, J.; Ivani, K.; Khoury, C.; Ball, G.D.; Elliot, T.; et al. Use of hyaluronan in the selection of sperm for intracytoplasmic sperm injection (ICSI): Significant improvement in clinical outcomes--multicenter, double-blinded and randomized controlled trial. Hum. Reprod. 2013, 28, 306–314. [Google Scholar] [CrossRef]
- Parmegiani, L.; Cognigni, G.E.; Ciampaglia, W.; Pocognoli, P.; Marchi, F.; Filicori, M. Efficiency of hyaluronic acid (HA) sperm selection. J. Assist. Reprod. Genet. 2010, 27, 13–16. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Feng, G.; Shu, J.; Zhou, H.; Zhang, B.; Chen, H.; Lin, R.; Gan, X.; Wu, Z.; Wei, T. Cumulus oophorus complexes favor physiologic selection of spermatozoa for intracytoplasmic sperm injection. Fertil. Steril. 2018, 109, 823–831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, D.; Pavitt, S.; Sharma, V.; Forbes, G.; Hooper, R.; Bhattacharya, S.; Kirkman-Brown, J.; Coomarasamy, A.; Lewis, S.; Cutting, R.; et al. Physiological, hyaluronan-selected intracytoplasmic sperm injection for infertility treatment (HABSelect): A parallel, two-group, randomised trial. Lancet 2019, 393, 416–422. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. World Health Organization Laboratory Manual for the Examination and Processing of Human Semen; WHO Press: Geneve, Switzerland, 2010. [Google Scholar]
- Baibakov, B.; Boggs, N.A.; Yauger, B.; Baibakov, G.; Dean, J. Human sperm bind to the N-terminal domain of ZP2 in humanized zonae pellucidae in transgenic mice. J. Cell Biol. 2012, 197, 897–905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sáez-Espinosa, P.; Huerta-Retamal, N.; Robles-Gómez, L.; Avilés, M.; Aizpurua, J.; Velasco, I.; Romero, A.; Gómez-Torres, M.J. Influence of in vitro capacitation time on structural and functional human sperm parameters. Asian J. Androl. 2020, 22, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Sáez-Espinosa, P.; López-Huedo, A.; Robles-Gómez, L.; Huerta-Retamal, N.; Aizpurua, J.; Gómez-Torres, M.J. Characterization of Human Spermatic Subpopulations by ConA-Binding Sites and Tyrosine Phosphorylation during in vitro Capacitation and Acrosome Reaction. Cells Tissues Organs 2021, 210, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Torres, M.J.; Huerta-Retamal, N.; Sáez-Espinosa, P.; Robles-Gómez, L.; Avilés, M.; Aizpurua, J. Molecular Chaperone HSPA2 Distribution During Hyaluronic Acid Selection in Human Sperm. Reprod. Sci. 2022, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Sáez-Espinosa, P.; Ferrández-Rives, M.; Huerta-Retamal, N.; Robles-Gómez, L.; Aizpurua, J.; Romero, A.; Gómez-Torres, M.J. Proper cytoskeleton α-tubulin distribution is concomitant to tyrosine phosphorylation during in vitro capacitation and acrosomal reaction in human spermatozoa. Cytoskeleton 2020, 77, 333–341. [Google Scholar] [CrossRef]
- Huerta-Retamal, N.; Sáez-Espinosa, P.; Robles-Gómez, L.; Avilés, M.; Romero, A.; Aizpurua, J.; Gómez-Torres, M.J. Human sperm chaperone HSPA2 distribution during in vitro capacitation. J. Reprod. Immunol. 2020, 143, 103246. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.S.; Leong, J.Y.; Ramasamy, R. Prediction of male infertility by the World Health Organization laboratory manual for assessment of semen analysis: A systematic review. Arab J. Urol. 2018, 16, 96–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oseguera-López, I.; Ruiz-Díaz, S.; Ramos-Ibeas, P.; Pérez-Cerezales, S. Novel Techniques of Sperm Selection for Improving IVF and ICSI Outcomes. Front. Cell Dev. Biol. 2019, 7, 298. [Google Scholar] [CrossRef] [PubMed]
- Florman, H.; Fissore, R. Fertilization in Mammals. In Knobil and Neill’s Physiology of Reproduction: Two-Volume Set; Academic Press: Cambridge, MA, USA, 2015; Volume 1, pp. 149–196. ISBN 9780123971753. [Google Scholar]
- Mahi, C.A.; Yanagimachi, R. Capacitation acrosome reaction, and egg penetration by canine spermatozoa in a simple defined medium. Gamete Res. 1978, 1, 101–109. [Google Scholar] [CrossRef]
- Sosa, C.M.; Pavarotti, M.A.; Zanetti, M.N.; Zoppino, F.C.M.; De Blas, G.A.; Mayorga, L.S. Kinetics of human sperm acrosomal exocytosis. Mol. Hum. Reprod. 2014, 21, 244–254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cardona Maya, W.D.; Olivera Angel, M.; Cadavid, A.P. Evaluation of the calcium ionophore induced acrosome reaction: A more realistic approach to the fecundant capacity of the spermatozoid. Arch. Esp. Urol. 2006, 59, 501–510. [Google Scholar] [CrossRef] [Green Version]
- Sakkas, D.; Leppens-Luisier, G.; Lucas, H.; Chardonnens, D.; Campana, A.; Franken, D.R.; Urner, F. Localization of tyrosine phosphorylated proteins in human sperm and relation to capacitation and zona pellucida binding. Biol. Reprod. 2003, 68, 1463–1469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Battistone, M.A.; Alvau, A.; Salicioni, A.M.; Visconti, P.E.; Da Ros, V.G.; Cuasnicu, P.S. Evidence for the involvement of proline-rich tyrosine kinase 2 in tyrosine phosphorylation downstream of protein kinase A activation during human sperm capacitation. Mol. Hum. Reprod. 2014, 20, 1054–1066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbonetti, A.; Vassallo, M.R.; Cinque, B.; Antonangelo, C.; Sciarretta, F.; Santucci, R.; D’Angeli, A.; Francavilla, S.; Francavilla, F. Dynamics of the global tyrosine phosphorylation during capacitation and acquisition of the ability to fuse with oocytes in human spermatozoa. Biol. Reprod. 2008, 79, 649–656. [Google Scholar] [CrossRef] [Green Version]
- Si, Y.; Okuno, M. Role of tyrosine phosphorylation of flagellar proteins in hamster sperm hyperactivation. Biol. Reprod. 1999, 61, 240–246. [Google Scholar] [CrossRef]
- Urner, F.; Leppens-Luisier, G.; Sakkas, D. Protein tyrosine phosphorylation in sperm during gamete interaction in the mouse: The influence of glucose. Biol. Reprod. 2001, 64, 1350–1357. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.Y.; Clarke, G.N.; Baker, H.W. Tyrosine phosphorylation on capacitated human sperm tail detected by immunofluorescence correlates strongly with sperm-zona pellucida (ZP) binding but not with the ZP-induced acrosome reaction. Hum. Reprod. 2006, 21, 1002–1008. [Google Scholar] [CrossRef] [Green Version]
- Hereng, T.H.; Elgstoen, K.B.; Cederkvist, F.H.; Eide, L.; Jahnsen, T.; Skalhegg, B.S.; Rosendal, K.R. Exogenous pyruvate accelerates glycolysis and promotes capacitation in human spermatozoa. Hum. Reprod. 2011, 26, 3249–3263. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Leon, E.; Osycka-Salut, C.; Signorelli, J.; Pozo, P.; Perez, B.; Kong, M.; Morales, P.; Perez-Martinez, S.; Diaz, E.S. Fibronectin stimulates human sperm capacitation through the cyclic AMP/protein kinase A pathway. Hum. Reprod. 2015, 30, 2138–2151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nassar, A.; Mahony, M.; Morshedi, M.; Lin, M.H.; Srisombut, C.; Oehninger, S. Modulation of sperm tail protein tyrosine phosphorylation by pentoxifylline and its correlation with hyperactivated motility. Fertil. Steril. 1999, 71, 919–923. [Google Scholar] [CrossRef]
- Lamb, D.J. Semen analysis in 21st century medicine: The need for sperm function testing. Asian J. Androl. 2010, 12, 64–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Swerdloff, R.S. Limitations of semen analysis as a test of male fertility and anticipated needs from newer tests. Fertil. Steril. 2014, 102, 1502–1507. [Google Scholar] [CrossRef] [Green Version]
- López-Botella, A.; Velasco, I.; Acién, M.; Sáez-Espinosa, P.; Todolí-Torró, J.-L.; Sánchez-Romero, R.; Gómez-Torres, M.J. Impact of Heavy Metals on Human Male Fertility—An Overview. Antioxidants 2021, 10, 1473. [Google Scholar] [CrossRef]
- Lettieri, G.; D’Agostino, G.; Mele, E.; Cardito, C.; Esposito, R.; Cimmino, A.; Giarra, A.; Trifuoggi, M.; Raimondo, S.; Notari, T.; et al. Discovery of the Involvement in DNA Oxidative Damage of Human Sperm Nuclear Basic Proteins of Healthy Young Men Living in Polluted Areas. Int. J. Mol. Sci. 2020, 21, 4198. [Google Scholar] [CrossRef]
- Lettieri, G.; Marra, F.; Moriello, C.; Prisco, M.; Notari, T.; Trifuoggi, M.; Giarra, A.; Bosco, L.; Montano, L.; Piscopo, M. Molecular Alterations in Spermatozoa of a Family Case Living in the Land of Fires—A First Look at Possible Transgenerational Effects of Pollutants. Int. J. Mol. Sci. 2020, 21, 6710. [Google Scholar] [CrossRef]
- Barratt, C.L.R.; Björndahl, L.; Menkveld, R.; Mortimer, D. ESHRE special interest group for andrology basic semen analysis course: A continued focus on accuracy, quality, efficiency and clinical relevance†. Hum. Reprod. 2011, 26, 3207–3212. [Google Scholar] [CrossRef] [Green Version]
- Oehninger, S.; Franken, D.R.; Ombelet, W. Sperm functional tests. Fertil. Steril. 2014, 102, 1528–1533. [Google Scholar] [CrossRef]
- Khatun, A.; Rahman, M.S.; Pang, M. Clinical assessment of the male fertility. Obstet. Gynecol. Sci. 2018, 61, 179. [Google Scholar] [CrossRef] [PubMed]
- Kimura, M.; Kim, E.; Kang, W.; Yamashita, M.; Saigo, M.; Yamazaki, T.; Nakanishi, T.; Kashiwabara, S.; Baba, T. Functional Roles of Mouse Sperm Hyaluronidases, HYAL5 and SPAM1, in Fertilization1. Biol. Reprod. 2009, 81, 939–947. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hunnicutt, G.R.; Primakoff, P.; Myles, D.G. Sperm surface protein PH-20 is bifunctional: One activity is a hyaluronidase and a second, distinct activity is required in secondary sperm-zona binding. Biol. Reprod. 1996, 55, 80–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, S.-J.; Chiu, P.C.-N.; Lee, K.-F.; Tse, J.Y.-M.; Ho, P.-C.; Yeung, W.S.-B. Cumulus cells and their extracellular matrix affect the quality of the spermatozoa penetrating the cumulus mass. Fertil. Steril. 2009, 92, 971–978. [Google Scholar] [CrossRef] [Green Version]
- Hong, S.; Tse, J.Y.M.; Ho, P.; Yeung, W.S. Cumulus cells reduce the spermatozoa–zona binding inhibitory activity of human follicular fluid. Fertil. Steril. 2003, 79, 802–807. [Google Scholar] [CrossRef]
- Franken, D.R.; Bastiaan, H.S. Can a cumulus cell complex be used to select spermatozoa for assisted reproduction? Andrologia 2009, 41, 369–376. [Google Scholar] [CrossRef]
- Chiu, P.C.N.; Chung, M.-K.; Koistinen, R.; Koistinen, H.; Seppala, M.; Ho, P.-C.; Ng, E.H.Y.; Lee, K.-F.; Yeung, W.S.B. Cumulus Oophorus-associated Glycodelin-C Displaces Sperm-bound Glycodelin-A and -F and Stimulates Spermatozoa-Zona Pellucida Binding *. J. Biol. Chem. 2007, 282, 5378–5388. [Google Scholar] [CrossRef] [Green Version]
- Huszar, G.; Ozkavukcu, S.; Jakab, A.; Celik-Ozenci, C.; Sati, G.L.; Cayli, S. Hyaluronic acid binding ability of human sperm reflects cellular maturity and fertilizing potential: Selection of sperm for intracytoplasmic sperm injection. Curr. Opin. Obstet. Gynecol. 2006, 18, 260–267. [Google Scholar] [CrossRef]
- Parmegiani, L.; Cognigni, G.E.; Bernardi, S.; Troilo, E.; Ciampaglia, W.; Filicori, M. “Physiologic ICSI”: Hyaluronic acid (HA) favors selection of spermatozoa without DNA fragmentation and with normal nucleus, resulting in improvement of embryo quality. Fertil. Steril. 2010, 93, 598–604. [Google Scholar] [CrossRef]
- Saylan, A.; Duman, S. Efficacy of hyaluronic acid in the selection of human spermatozoa with intact DNA by the swim-up method. Cell J. 2016, 18, 83–88. [Google Scholar]
- De Jonge, C. Biological basis for human capacitation-revisited. Hum. Reprod. Update 2017, 23, 289–299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buffone, M.G.; Doncel, G.F.; Marín Briggiler, C.I.; Vazquez-Levin, M.H.; Calamera, J.C. Human sperm subpopulations: Relationship between functional quality and protein tyrosine phosphorylation. Hum. Reprod. 2004, 19, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Puga Molina, L.C.; Luque, G.M.; Balestrini, P.A.; Marín-Briggiler, C.I.; Romarowski, A.; Buffone, M.G. Molecular Basis of Human Sperm Capacitation. Front. Cell Dev. Biol. 2018, 6, 72. [Google Scholar] [CrossRef] [PubMed]
| Parameter | NCS Mean ± SD | CS1 Mean ± SD | CS4 Mean ± SD |
|---|---|---|---|
| Volume (mL) | 3.16 ± 0.97 | - | - |
| pH | 7.28 ± 0.96 | - | - |
| Normal morphology (%) | 13.35 ± 3.99 | - | - |
| Concentration (106/mL) | 62.93 ± 22.29 | 19.00 ± 18.42 * | 11.05 ± 7.96 * |
| Total motility (%) | 73.95 ± 10.26 | 97.54 ± 1.26 * | 96.48 ± 1.78 * |
| Viability (%) | 80.87 ± 8.09 | 98.75 ± 3.15 * | 96.22 ± 2.67 * |
| Spontaneous acrosome reaction (%) | 19.24 ± 5.54 | 22.15 ± 7.18 | 25.74 ± 6.47 |
| Induced acrosome reaction (%) | 37.95 ± 10.88 | 57.61 ± 12.36 * | 62.76 ± 15.76 * |
| Tyrosine phosphorylation (%) | 9.36 ± 4.52 | 28.41 ± 8.27 | 37.63 ± 10.32 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez-Torres, M.J.; Sáez-Espinosa, P.; Manzano-Santiago, P.; Robles-Gómez, L.; Huerta-Retamal, N.; Aizpurua, J. Sperm Adhesion Molecule 1 (SPAM1) Distribution in Selected Human Sperm by Hyaluronic Acid Test. Biomedicines 2022, 10, 2553. https://doi.org/10.3390/biomedicines10102553
Gómez-Torres MJ, Sáez-Espinosa P, Manzano-Santiago P, Robles-Gómez L, Huerta-Retamal N, Aizpurua J. Sperm Adhesion Molecule 1 (SPAM1) Distribution in Selected Human Sperm by Hyaluronic Acid Test. Biomedicines. 2022; 10(10):2553. https://doi.org/10.3390/biomedicines10102553
Chicago/Turabian StyleGómez-Torres, María José, Paula Sáez-Espinosa, Paula Manzano-Santiago, Laura Robles-Gómez, Natalia Huerta-Retamal, and Jon Aizpurua. 2022. "Sperm Adhesion Molecule 1 (SPAM1) Distribution in Selected Human Sperm by Hyaluronic Acid Test" Biomedicines 10, no. 10: 2553. https://doi.org/10.3390/biomedicines10102553







