Abstract
Energy plays a crucial role in the sustainable development of modern nations. Today, hydrogen is considered the most promising alternative fuel as it can be generated from clean and green sources. Moreover, it is an efficient energy carrier because hydrogen burning only generates water as a byproduct. Currently, it is generated from natural gas. However, it can be produced using other methods, i.e., physicochemical, thermal, and biological. The biological method is considered more environmentally friendly and pollution free. This paper aims to provide an updated review of biohydrogen production via photofermentation, dark fermentation, and microbial electrolysis cells using different waste materials as feedstocks. Besides, the role of nanotechnology in enhancing biohydrogen production is examined. Under anaerobic conditions, hydrogen is produced during the conversion of organic substrate into organic acids using fermentative bacteria and during the conversion of organic acids into hydrogen and carbon dioxide using photofermentative bacteria. Different factors that enhance the biohydrogen production of these organisms, either combined or sequentially, using dark and photofermentation processes, are examined, and the effect of each factor on biohydrogen production efficiency is reported. A comparison of hydrogen production efficiency between dark fermentation, photofermentation, and two-stage processes is also presented.
1. Introduction
1.1. Global Energy Scenario and Global Warming
All the social, physical, and economic activities of human life are sustained by energy. The continual energy supply needed for increasing global demand creates a substantial challenge for our societies. According to the IEO reference case [1], the world energy need is expected to rise by 50% from 2018 to 2050. This energy requirement has been satisfied primarily by exploiting massive fossil fuels [1].
Carbon-rich energy carriers (fossil fuels) are produced in two steps. The first step consists of photosynthesis, while the second consists of decomposing organic matter, which has been compacted for millions of years under high pressure and temperature. The ability of fossil fuels to act as efficient energy carriers and their easy transferability into different types of energy has made them the motor of the industrial revolution [2]. However, the other side of the picture shows that burning these fossil fuels creates a major drawback in the form of greenhouse gas emissions, contributing to global warming and climate change.
Global warming is the central issue of today’s world because it continuously raises the Earth’s temperature and drastically affects agriculture and food security [3,4]. The developing regions of the world are principally affected. The expected results may include social and political uncertainty, mass migration, and military conflicts. Sea level rise and ocean acidification are the other significant problems [5,6]. In addition, greenhouse gas emissions and climate change affect the globe and require solutions for clean and sustainable energy usage [7,8,9]. Considering the danger of global warming to future generations, the leaders of most countries have united to put forward their efforts to decrease the emissions of greenhouse gases and minimize average global warming to 2 °C above the preindustrial average world temperatures during the United Nations Climate Change Conference (UNFCCC) and the Conference of the Parties (COP) 2015 in Paris [2].
1.2. Sustainable Energy Economy
The states and governments of the world consider the possibility of sustainable development not only in the context of industrial advancements but also for social, economic, and environmental concerns. According to Davidson, the definition of sustainable energy is as follows [10].
“Sustainable energy is defined as energy providing affordable, accessible and reliable energy services that meet the economic, social and environmental needs within the overall developmental context of the society for which the services are intended while recognising equitable distribution in meeting those needs.”
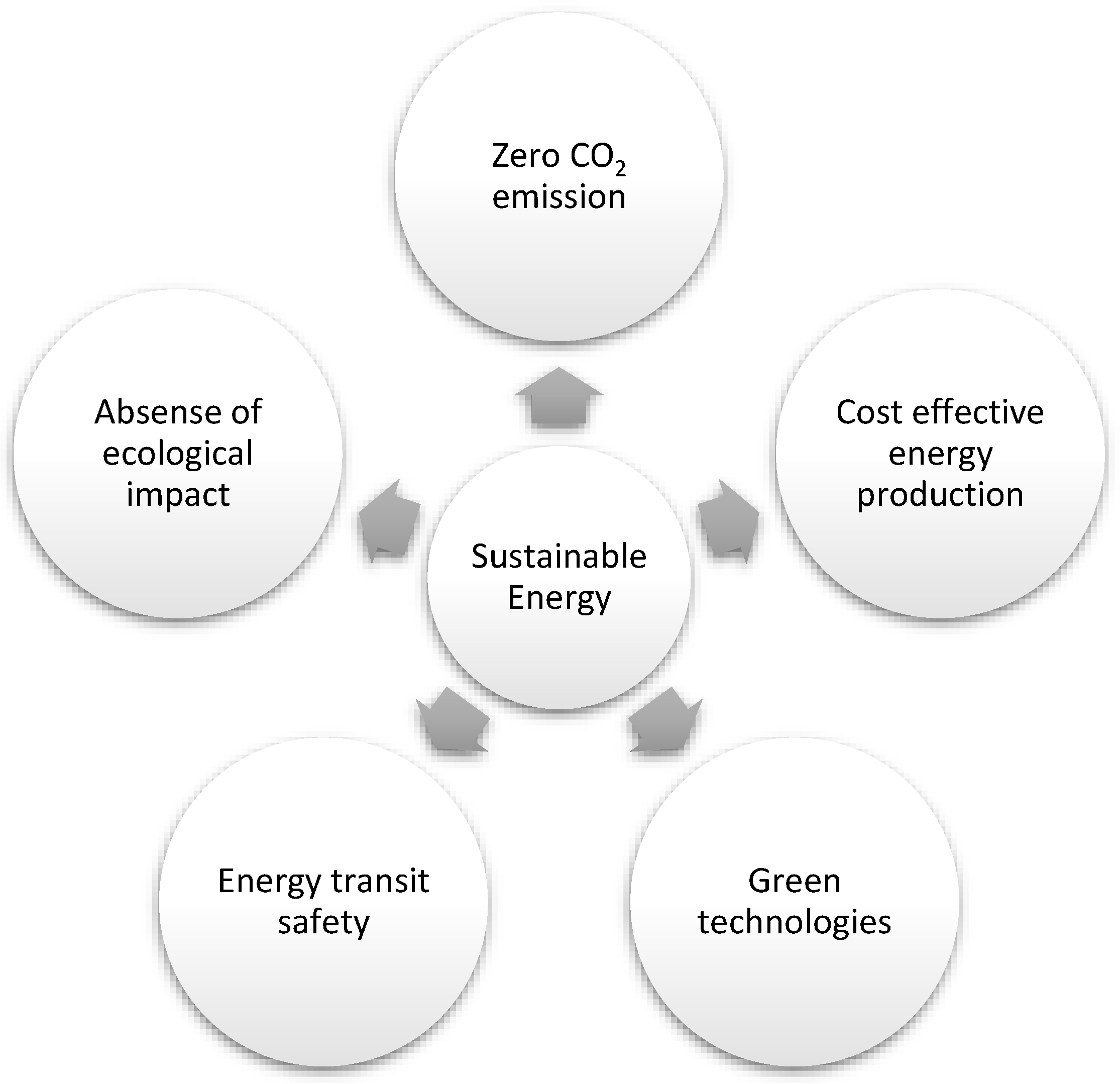
Energy, economy, and environment are the three pillars of sustainable development that join in the form of a triangle. Although renewable energy is considered the critical component of sustainable energy, it does not fulfill the requirements of sustainable development without knowing its significance to the economy and environment [11] and fulfilling the five targets mentioned in Figure 1 [11].
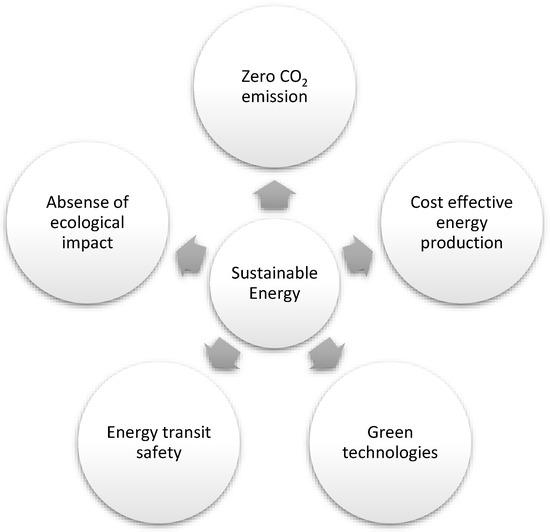
Figure 1.
Five significant targets of sustainable energy.
Second-generation biofuels covered about 3% of the world’s energy requirements in 2013 [12]. They are considered more desirable fuels as there is no competition for land and food. Nevertheless, technical problems, such as low energy conversion efficiencies, are the foremost hurdle to their use. Microalgae are used as feedstock for the third generation of biofuels [13,14]. Microalgae are vigorous unicellular organisms that can be grown in wastewater or seawater and have higher energy efficiency than other fuels. However, a considerable amount of carbon dioxide is released during the burning and production of these biofuels, which is not compensated for by carbon dioxide fixation [2].
1.3. Sustainable Hydrogen Economy
Biohydrogen has become a promising biofuel in the modern era as it is a clean and efficient energy carrier. Hydrogen (H2) has many benefits since it has the highest energy per unit mass (142 kJ g−1). Moreover, H2 produces only water as a byproduct and is called zero-carbon fuel. It is also tasteless, odorless, colorless, and the lightest gas [15]. Therefore, H2 gas is used in many applications, such as fuel cells for electricity generation and as fuel in rocket engines, including transportation applications [16].
Furthermore, H2 is a significant industrial gas and raw material in various applications and processes [17]. Nevertheless, the high cost of production, storage problems, transportation, and an immature hydrogen infrastructure are the primary hurdles to economic sustainability using H2 as a fuel source [17,18,19,20,21].
Biohydrogen can be produced in thermochemical [22,23,24,25] and biological ways [26,27,28]. The biological technologies for H2 production are preferred due to their ecological benefits. These biological processes are also less energy intensive and more environmentally friendly concerning the global reduction of carbon dioxide [18,20,29]. Furthermore, renewable hydrogen production techniques can potentially become cost-effective because they can use raw biomass as feedstock, e.g., agricultural, municipal, and industrial wastewater and organic waste [18,20]. Biohydrogen production is classified into (i) the biophotolysis of water using algae, (ii) dark fermentation using anaerobic bacteria, (iii) photofermentation using photosynthetic bacteria, and (iv) microbial electrolysis cells (MECs). Dark fermentation has several advantages as compared to others. The process is less energy-intensive because it can be carried out at an ambient temperature and pressure [30].
2. Biological Routes of Hydrogen Production
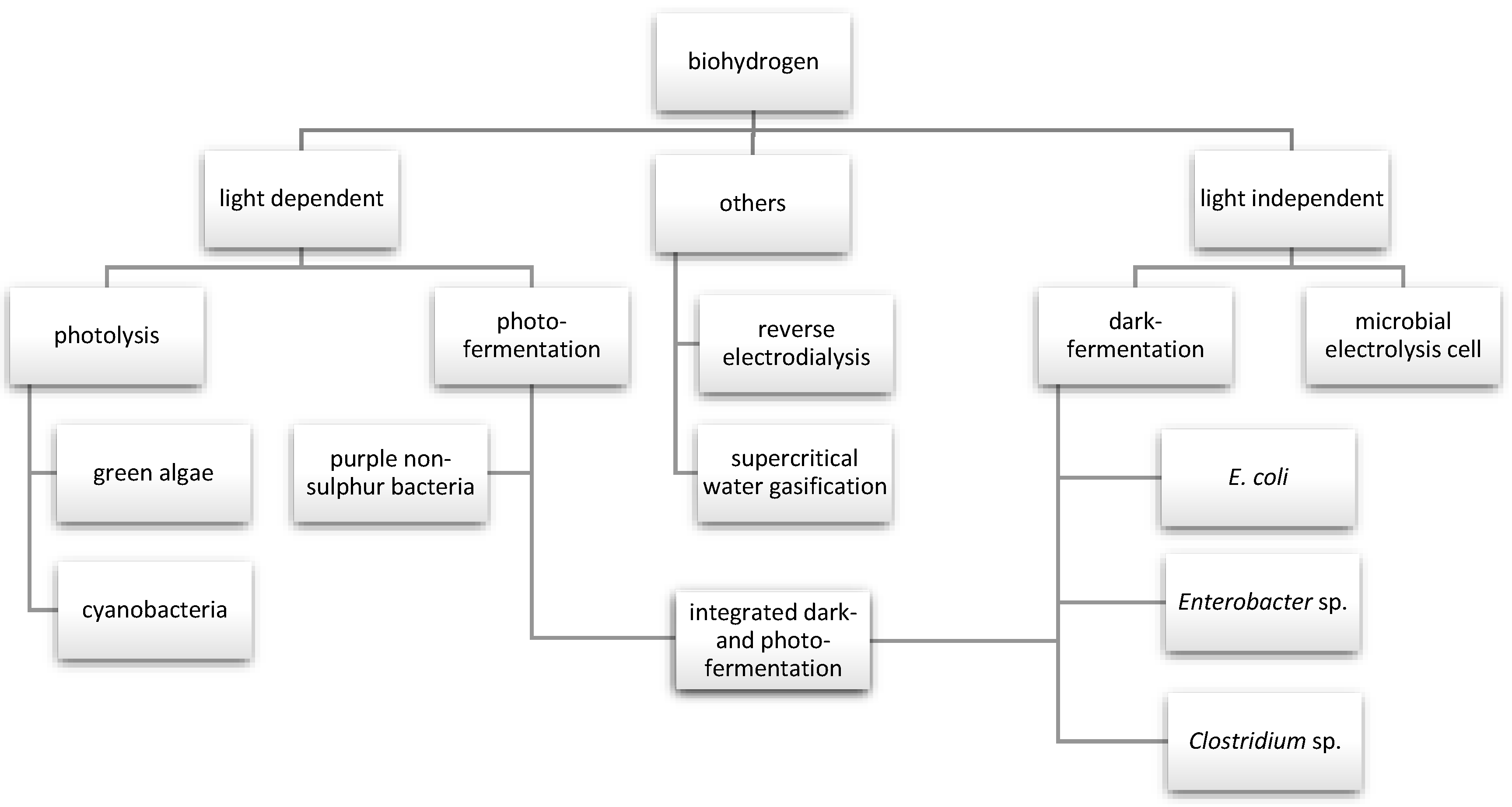
The synthesis of hydrogen by biological methods involves the active use of different microbes under various environmental conditions (Figure 2). However, they have in common the presence of hydrogenase or nitrogenase enzymes, which play a significant role in metabolic pathways in the synthesis of hydrogen [31].

Figure 2.
Methods of biological hydrogen production.
2.1. Biophotolysis
Hydrogen can be produced by photosynthetic microorganisms, such as green algae, cyanobacteria, and diatoms. These organisms integrate and store light energy as H-H bonds. Carbon dioxide (CO2) and water are used in this process, thus reducing greenhouse gas emissions [26]. Biophotolysis can be divided into direct and indirect forms.
For direct biophotolysis, several green algae, such as Chlamydomonas reinhardtii, Scenedesmus obliguus, Chlorella vulgaris, and Tetraspora sp., have been extensively studied for their efficiency in hydrogen production [27,28]. During the process of direct photolysis, light radiation causes hydrogen evolution. Unique pigments in photosynthetic microorganisms absorb light energy, which is mediated by nitrogenase and hydrogenase enzyme activity. The absorbed energy is then used to elevate the energy level of the electrons of water molecules. Some atoms attain sufficient energy to split water molecules into H and OH atoms.
Consequently, the free hydrogen atoms combine to form hydrogen molecules and are released as hydrogen gas. Green algae were used to achieve 6–24% energy efficiency under low light intensities under laboratory scans during direct photolysis [16,26]. The sensitivity of the hydrogenase enzyme to oxygen generated during photosynthesis is the major drawback of this process. The evolved oxygen constrains the enzyme’s activity, producing low H2 yields [16,26]. Another limiting factor for microalgal cultures is the low biomass concentration because of reduced light penetration. The drying of algal biomass also makes it an energy-consuming process. The higher capital cost and the intensive care of microalgal farms are significant barriers to executing this process on a commercial scale [32].
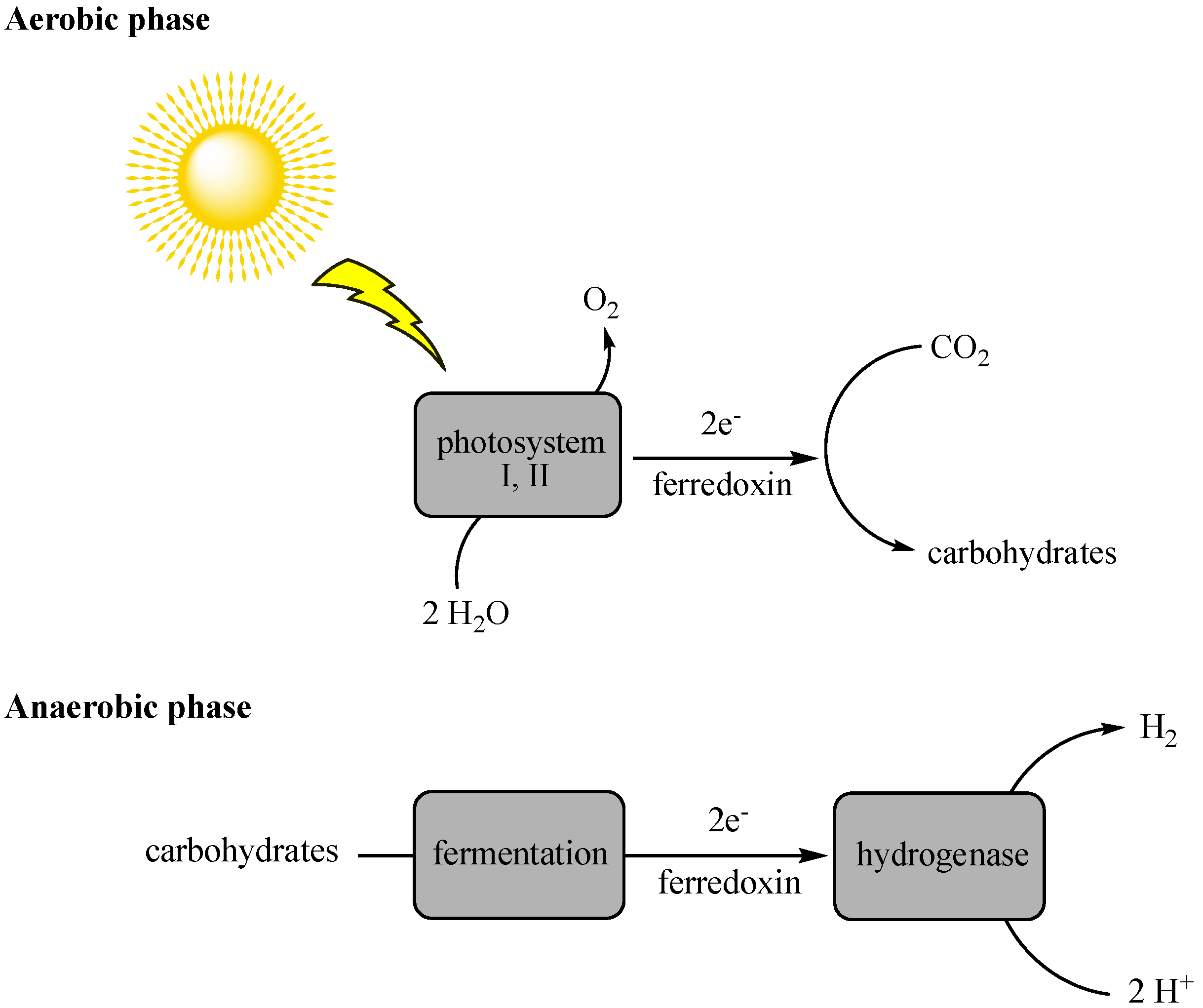
Indirect biophotolysis needs photosynthetic bacteria (e.g., blue-green algae) to utilize CO2 in the presence of light to produce carbohydrates, which are fermented in the presence of light to produce energy and, thus, liberate high yields of hydrogen molecules as byproducts (Figure 3) [33,34]. The cyanobacteria, Anabaena variabilis, was reported as a probable candidate for hydrogen production via indirect photolysis [35]. In this method, oxygen is the inhibiting factor for carbon dioxide-consuming bacteria. Besides, the requirement for a continuous light source is another limiting factor.
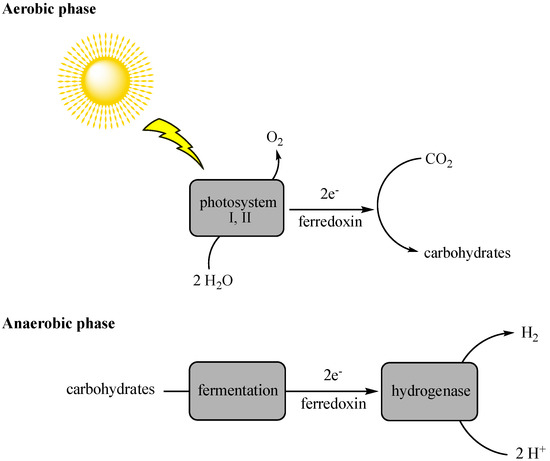
Figure 3.
Process of hydrogen production via indirect photolysis.
2.2. Photofermentation Process
The process of photofermentation utilizes purple non-Sulfur bacteria to produce H2 in the presence of light. These bacteria use organic acids (lactic acid, acetic acid, and butyric acid) as a carbon source and produce H2 by nitrogenase enzyme action in N2-deficient conditions. The purple non-Sulfur bacteria utilize organic acids as a source of electrons for photosynthesis, while the external source of light energy is utilized to oxidize the volatile organic acids to generate electrons for the synthesis of H2 [36]. The released electrons are driven via electron carriers, and the protons are pushed across the membrane. A gradient is formed due to high and low concentrations of hydrogen ions. The energy produced by the protons is used to make adenosine triphosphate (ATP) from adenosine diphosphate (ADP) by the ATP synthase enzyme. The electrons are then transferred to the ferredoxin by utilizing surplus ATP energy. The nitrogenase enzyme takes electrons derived from ferredoxin and accelerates the reduced protons into H2. All the reactions occur in the absence of nitrogen and the availability of excess energy of ATP [26]. This way, the organic compounds are entirely converted into H2 and CO2.
The significant advantage of photofermentation is the 100% release of electrons from organic acids by photosynthetic bacteria to synthesize carbon dioxide and hydrogen. There is no inhibitory effect of oxygen on the performance of the nitrogenase enzyme, as this process occurs in the absence of oxygen. This technology uses a wide range of feedstock and generates fewer byproducts in the waste stream. The feedback on cost-effective photofermentation technology benefits waste management and the H2 economy. Some studies have reported using industrial wastewater as an effective raw material [37]. Although much research has been reported on this process, some technical obstacles avoid its large-scale usage, including low light conversion efficiency, hydrogen production by nitrogenase, and low photosynthetic conversion efficiency [26]. A study using Rhodobacter sphaeroides with various volatile acids as the carbon source reported a maximum hydrogen yield of 17.8 L H2/mol-substrate for 2 g/L lactate and 2 g/L succinate [38]. The reactor design for photofermentation and the selection of biohydrogen specialists are the most critical factors for improving production yields [39]. Akroum et al. observed an optimal operational pH of 7.5 for hydrogen production in a column bioreactor using Rhodobacter sphaeroides [40]. The study reported a maximum hydrogen production rate of 0.04 L/L/h compared to 0.03 L H2/h studied in the dark fermentation process [41]. However, recent research shows that the combination of photofermentation with the dark fermentation process can boost hydrogen yield. An integrated photo and dark fermentation study investigated by Ghameri et al. resulted in a 40% rise in H2 production when compared with the singular processes of photo and dark fermentation [20]. It was reported that different fermentation parameters, such as substrate concentration, pH, and temperature, are automatically controlled by the microbial cultures and help to enhance hydrogen yields [42]. A study conducted by Policastro et al. used the ethanol-rich wastewater produced during dark fermentation as a substrate for light-fermentative hydrogen production with a 0.31 L H2/g COD yield [43].
2.3. Dark Fermentation Process
Dark fermentation works with microorganisms that use various organic materials, such as industrial and agricultural wastes, to generate H2 without oxygen and light [44,45]. These bacteria are either facultative or obligate anaerobes, but the process occurs without light when organic carbon is used as energy. The fermentation process involves the production of volatile or intermediate chemicals, including butyrate or propionate, ethanol, and acetate, depending on the mechanism used. The way of acetate byproduct generation is the most promising when compared to butyrate because it can give maximum yield. This is because, theoretically, four molecules of H2 are produced per hexose in the acetate pathway, called the ‘Thauer limit,’ while two molecules of H2 are formed in the butyrate pathway. The accumulation of H2 as a metabolic inhibitor is a major drawback for the commercialization of bio-H2 production [46,47,48]. The oxygen sensitivity of the hydrogenate enzyme in dark-fermentative bacteria is another limiting factor that reduces the H2 yield [49].
The research on molasses wastewater gave a dominant pathway of acetate and ethanol with a maximum yield of 0.20 L H2/g-COD sucrose. The final gas composition from a reactor depends on the fermentation substrate and operating conditions [35,50].
Biohydrogen production involves the ethanol-type fermentation of molasses in an expanded granular sludge bed reactor. Another study showing a process dominated by the butyrate pathway observed a general decrease in butyrate concentration for reduced HRT, also marked by increased hydrogen productivity. Another study of the glucose substrate in a batch reactor observed a maximum conversion of 0.01 L of H2 per g-COD/L, while the side products of hydrogen generation were 14–63% butyrate, 10–45% formate, and 16–40% ethanol [51].
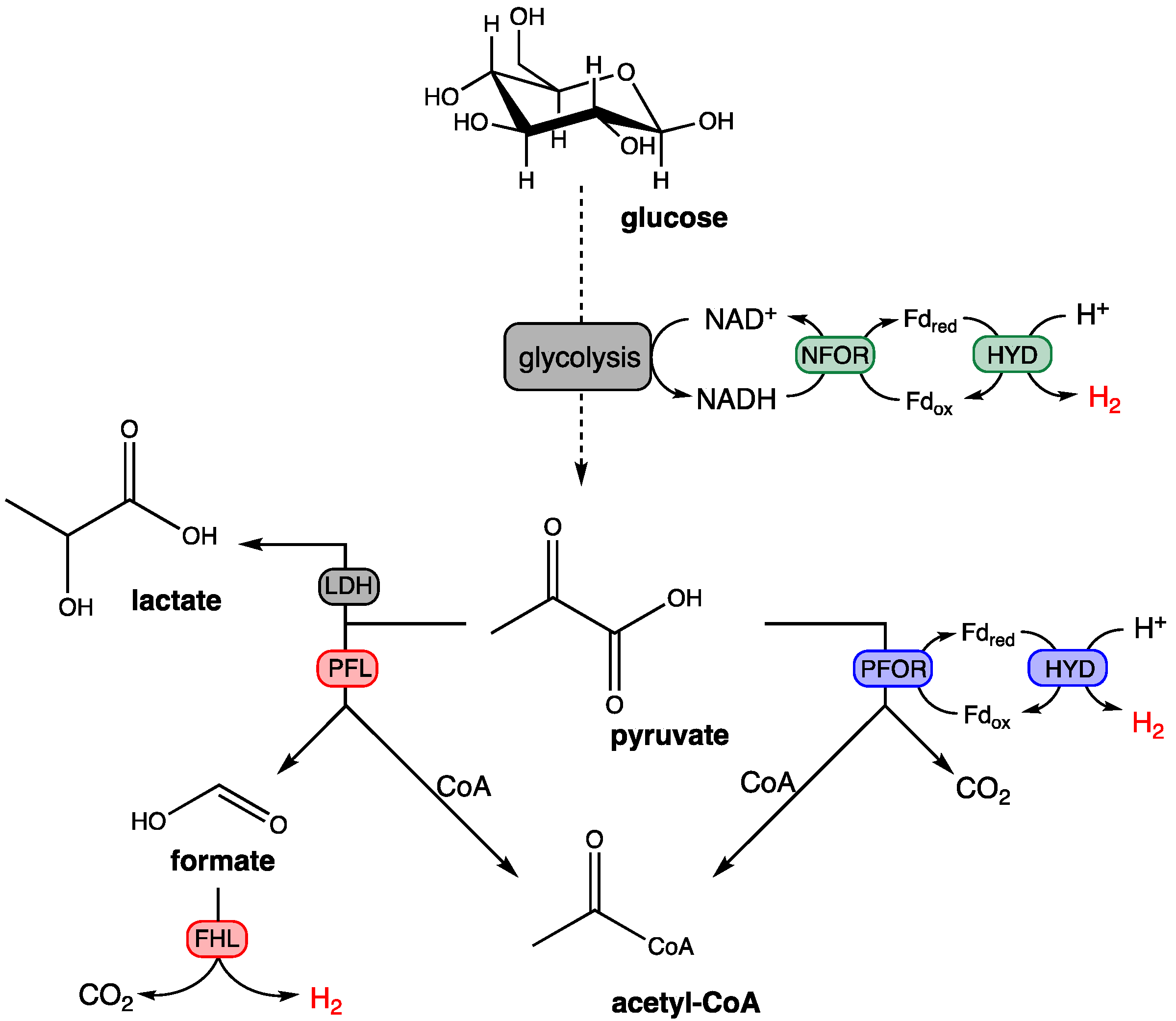
In fermentative hydrogen production, three biochemical pathways may be used: (i) the utilization of pyruvate-formate lyase (PFL) and formate-hydrogen lyase (FHL), (ii) pyruvate-ferredoxin oxidoreductase (PFOR) and Ferredoxin (Fd)-dependent hydrogenase (HYD) under strict anaerobic conditions, and (iii) the utilization of NADH-ferredoxin oxidoreductase (NFOR) and HYD (Figure 4) [52].

Figure 4.
Dark fermentation metabolic pathways for hydrogen production. (red pathway) E. coli and Enterobacteriaceae. (blue pathway) Clostridium sp. and (green pathway) thermophilic bacteria). Abbreviations: NFOR = NADH-ferredoxin oxidoreductase, HYD = ferredoxin-dependent hydrogenase, PFOR = pyruvate-ferredoxin oxidoreductase, LDH = Lactate dehydrogenase, PFL = pyruvate-formate lyase, FHL = formate-hydrogen lyase.
Different strategies have emerged to overcome the limitations of the dark fermentation process. The single-stage fermentative hydrogen process produced large amounts of acids and alcohols as byproducts. These liquids can be used as feedstock in photofermentation and MECs to enhance H2 generation. The integration of dark fermentation with other techniques has evolved to enhance the production of H2 through the complete conversion of the substrate [53]. Metabolically or genetically engineered bacteria are other ways of producing H2 [54]. However, this method is not economical at an industrial scale.
2.4. Microbial Electrolysis Cell
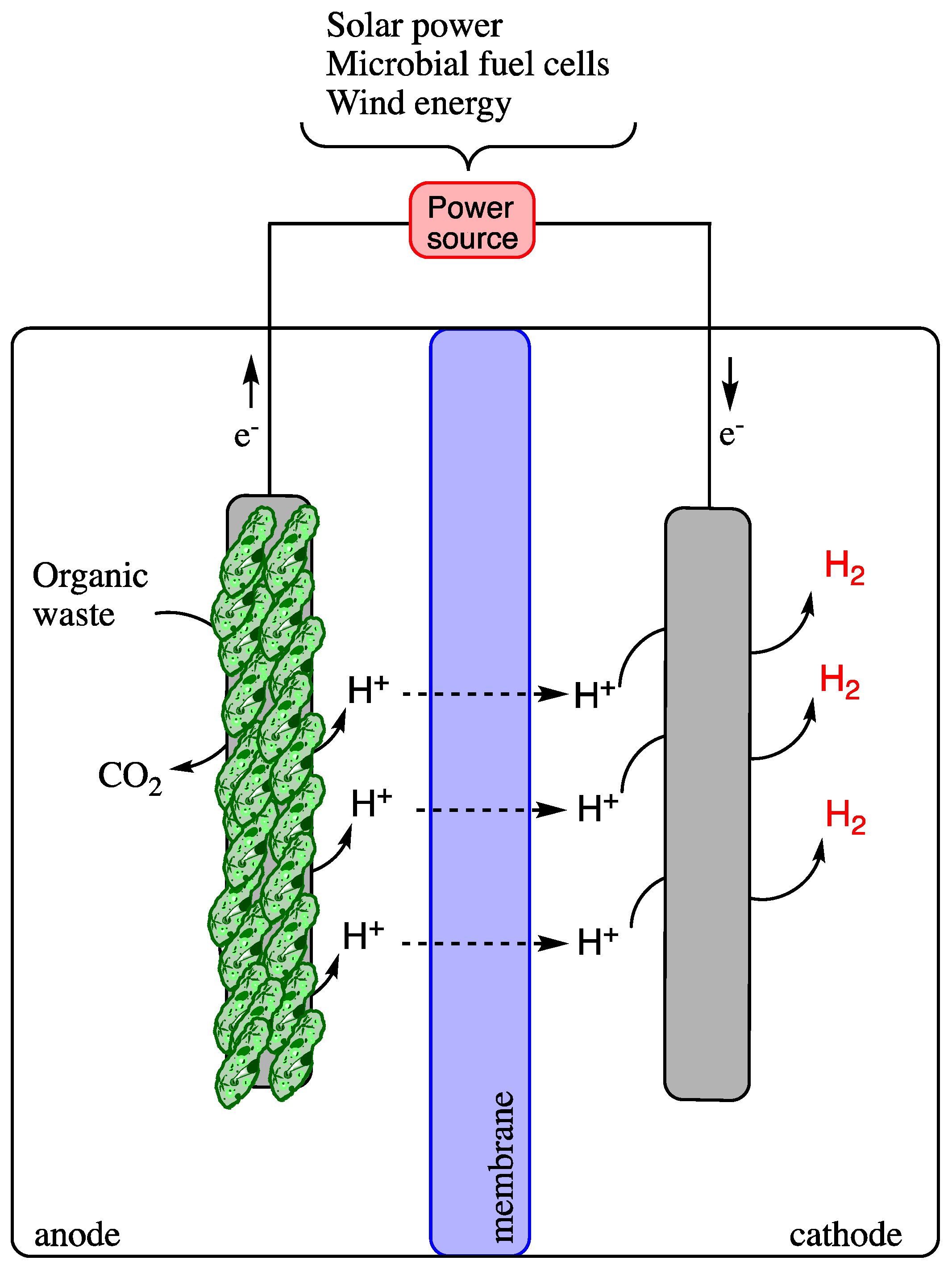
Microbially catalyzed electrolysis cells produce H2 during the fermentation of soluble organic matter found in wastewater when an electric current is passed through a small amount [55,56,57]. The simplest carbon source for H2 generation in MECs is acetate. However, the presence of volatile fatty acids in the effluent of biohydrogen production via the dark process is considered favorable for producing hydrogen via microbial electrolysis cells [44,58]. The MEC consists of four main parts: the cathode and anode, the external electrical connection, and a cationic exchange membrane between the electrodes (Figure 5).
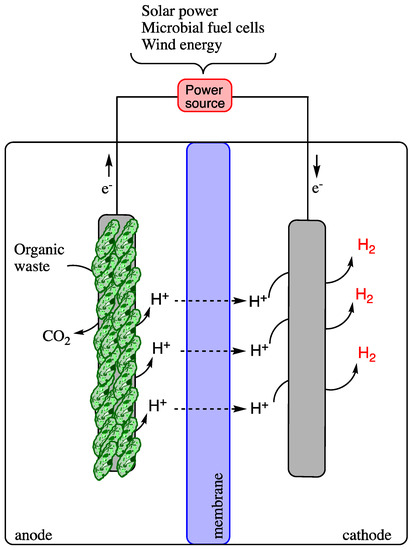
Figure 5.
Schematic diagram of a microbial electrolysis cell for H2 production.
In the cell, bacteria inhabit the anode surface and oxidize the carbon sources of the waste to carbon dioxide, electrons, and protons over a series of redox reactions. The produced electrons are transferred to the surface of the anode by the bacteria. At the same time, the proton diffuses freely into the solution and migrates via the cation exchange membrane to the cathode compartment, contributing to the reduction reactions occurring on the cathode electrode surface. At the same time, the electrons are transported via an external power source to the cathode electrode, which couples with the free proton in the electrolyte solution to produce H2 gas [59]. An AC/DC electricity source is required to energize the MEC. The energy required by MEC is only 0.11V, which is lower than water electrolysis (1.8–2.0) [60]. Therefore, this technology is considered to have high potential and to surge, having few limitations, such as reduced mass transfer and energy loss. The five major challenges faced by MECs are the loss of methanogenic electrons, metabolic diversity, and electrode resistance (anode), complex wiring in cell design, power supply, membrane problems (high cost, biofouling, substrate, and gas crossover and long-term stability) and cathode related obstacles (long-term stability, high catalyst cost, side reaction, and electrode resistance). Future research in this field is based on the improvements in the challenges mentioned above [49]. Microbial electrolysis cells work efficiently when combined with dark fermentation techniques. The MEC–DF combined process produces about a 98% H2 yield as compared to the single-stage process [61].
3. Feedstock for Biohydrogen Production
Biological hydrogen production has been carried out using several waste materials and lignocellulosic materials, depending upon their availability and suitability in particular geographic situations. Table 1 shows the utilization of different feedstocks for hydrogen production. Numerous raw materials such as sugarcane and sugar beet molasses [62,63,64,65,66,67], cheese whey powder [68], coffee drink manufacturing wastewater [69], corn stalk [70], crude glycerol [71], rice slurry [72], starch wastewater [67], paper and pulp industry effluent [30], baggase [73], dairy wastewater [37], vegetable waste [74], palm oil mill waste [75], distillery wastewater, and waste barley [76,77] have been reported.

Table 1.
Utilization of different feedstocks for hydrogen production.
4. Diversity of Biohydrogen-Producing Bacteria
There are numerous types of fermentative hydrogen-producing bacteria. Clostridium sp. is one of the most common anaerobic bacteria. Different species of Clostridium, such as Clostridium butyricum, Clostridium beijerienckii, Clostridium amygdalinum, Clostridium cellolosi, and Clostridium acetobutylicum, have been reported for fermentative hydrogen production [100,101,102,103,104]. Anaerobic bacteria utilize glucose to produce hydrogen, while butyric acid or acetic acid is produced as the product—Chong et al. isolated Clostridium butyricum from POME [105]. The optimum hydrogen production was obtained at pH 5.5.
Some facultative anaerobic bacteria (e.g., Enterobacter aerogenes) have also been recognized as H2 producers since the hydrogenase enzyme was found in these bacteria [106]. The critical parameters, such as substrate concentration, temperature, pH, inoculum size, and yeast extract, were optimized to obtain a maximum H2 yield of 0.21 L H2 /g glucose. The culture and maintenance of facultative anaerobes are more feasible than obligate anaerobes.
These microorganisms are further classified into mesophylls and thermophiles based on their growth temperatures. Although thermophiles are cultivated at elevated temperatures, with highly intensive energy requirements, their H2 production can be closer to the theoretical yield than mesophylls by overwhelming the thermodynamic barrier [107,108]. Some photofermentative bacteria require light energy to produce H2 in anoxygenic conditions. Without O2, these photoautotrophs, including cyanobacteria and green algae, produce H2 through biophotolysis using their specific metabolic routes advantageously under defined conditions [107]. Mixed cultures are also considered the best choice for maximum H2 yield. A study by Nicolau shows hydrogen production using heat-treated mesophilic anaerobic sludge inoculum instead of pure culture [109]. The hydrogen yield at pH 5.5 was 0.37 mol H2/mol of carbohydrate, equal to 18.14 L H2/kg of dry solid.
5. Enzymes
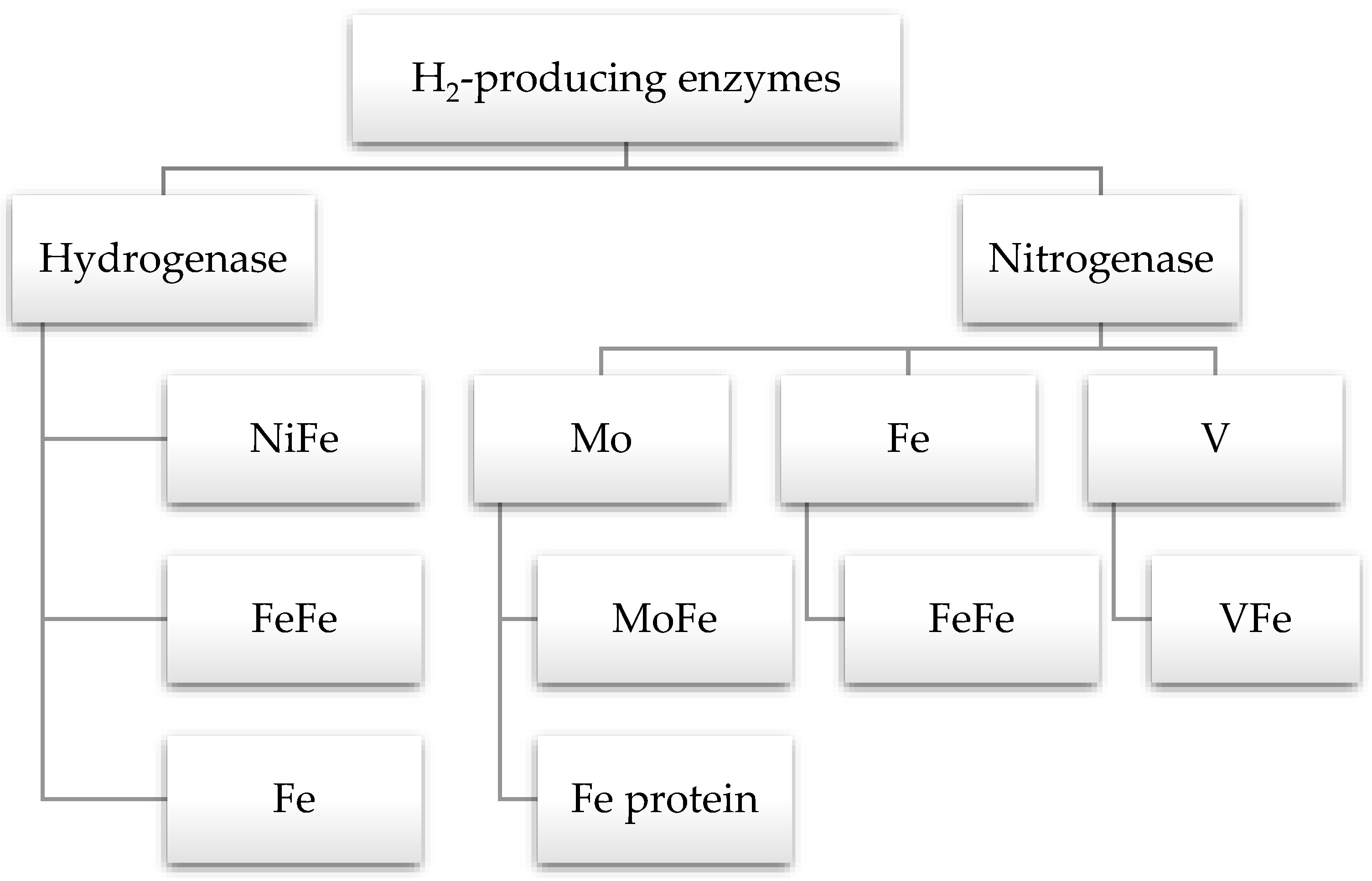
Life depends on several chemical reactions, most of which are slow. Therefore, enzymes are naturally occurring catalysts to speed up biochemical reactions. The two enzymes involved in hydrogen production are hydrogenase and nitrogenase (Figure 6). The enzyme hydrogenase catalyzes the consumption and generation of H2. After the discovery of this enzyme in 1930 by Stephenson and Stickland, numerous experiments have been conducted to learn more about it. Despite this, its crystal structure was elucidated approximately 20 years ago [110]. It is present in dark fermentative hydrogen-producing bacteria, green algae, and cyanobacteria. The hydrogenase enzyme is classified into three types based on the structure of active sites: NiFe-, Fe-Fe-, and Fe-hydrogenase. The NiFe-hydrogenase is only present in bacteria and archaea, while algae and bacteria have FeFe-hydrogenase. Hence, Fe-hydrogenase is a homodimer and is only present in methanogenic archaea [16,110,111].
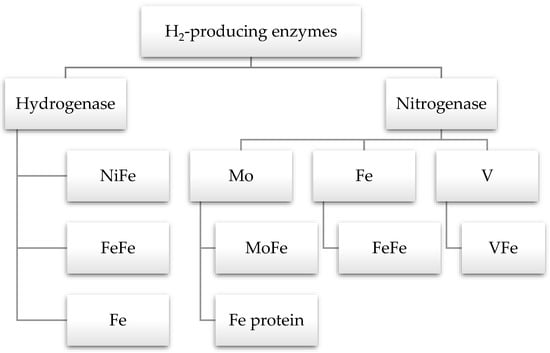
Figure 6.
Enzymes involved in hydrogen production.
Nitrogenase is another enzyme responsible for the production of H2. It is found in purple non-Sulfur bacteria, archaea, and cyanobacteria [111]. Most atmospheric nitrogen is fixed by cyanobacteria and generates H2 as a byproduct. There are three forms of nitrogenase enzyme: Molybdenum, iron, and vanadium. They are located at the active sites of nitrogen reduction and bind with rare metal centers. Mo-nitrogenase consists of two proteins: dinitrogenase (MoFe protein) and dinitrogenase reductase (Fe protein). The nitrogenase helps to generate ammonium from nitrogen, but in nitrogen-deficient conditions, it starts producing hydrogen in an anaerobic environment [16]. The structure of iron and vanadium nitrogenases are similar to the structure of the Mo form, but they have FeFe and VFe cofactors, respectively. The FeFe and VFe nitrogenases enhance hydrogen production compared to Mo nitrogenase. Only one type of photofermentative bacteria, R. palustris, has been reported to have all three types of these nitrogenases [111].
6. Factors Affecting the Production of Hydrogen
The production rate and yield of hydrogen depend on many factors during dark and photofermentation processes, including the following.
6.1. Pretreatment Methods
The use of food waste and food processing wastewater as feedstock provides several organic compounds and nutrients with enhanced hydrogen production, but some inhibitory compounds affect the production and yield of hydrogen [112]. In addition, different pretreatment methods have been reported to increase the utilization of raw materials for successive hydrogen generation.
Among the pretreatment methods, hydrolysis and preheating are the most preferred methods. Hydrolysis can be acid/alkaline or ultrasound-assisted. The six-hour alkaline hydrolysis increases H2 generation 206 times at a pH level of 12 [113]. Meanwhile, acid 12 h hydrolysis enhances the production of H2 three-fold at a pH of 2. Hence, the main disadvantages are the utilization of chemicals in large quantities and the requirement of some other processes to neutralize the pH [114]. The ultrasonication of food waste, assisted with hydrolysis, increases H2 yield by 75–88% [115,116,117,118], but investment in equipment and energy cost are the major hurdles to commercializing this method.
The preheating of food waste is another pretreatment method [119]. The results depicted that, prior to starting fermentation, heating waste for at least 20 min at 90 °C could increase the H2 yield.
6.2. Effect of Substrate Concentration
The substrate concentration plays a vital role in H2 production by dark fermentation. When substrate concentration increases, it creates unfavorable conditions and consequently changes the pH, H2 partial pressure, and the concentration of volatile fatty acids. Therefore, substrate inhibition may be minimized by arranging the optimum initial concentration of the substrate [120]. Many researchers have reported inhibition by substrate concentration, but the main focus was on the sources of carbohydrates. The use of wastewater and organic waste as a substrate has rarely been reported in the literature [121]. The fed-batch reactors can be used to avoid substrate inhibition. Some bacteria, such as Enterobacter aerogens, can reduce substrate inhibition by stimulating the microbial activity of H2 production [120]. The effect of substrate concentration on hydrogen production by Lactobacillus casei and Clostridium butyricum was also evaluated [122]. Glucose and galactose were used as carbon sources during the batch process. The results were based on the inoculum utilization of a single species or a mixture. It was observed that L. casei could not utilize galactose properly when used alone, while C. butyricum gave a fast response to galactose usage as a carbon source. On the other hand, the response for glucose utilization was faster by L. casei than C. butyricum under low concentrations of glucose, and, in turn, low hydrogen production was observed because Lactobacillus outcompeted the most significant H2-generating bacteria.
6.3. Effect of Initial pH
Initial pH is another essential factor to be considered in the dark fermentation process, and it is noted that each microbe can function effectively in different conditions. For example, the effective pH for hydrogen production is 5–8. When the initial pH becomes lower than 5, hydrogen production reduces by half [123]. A pH range of 5–9 also has been used during the batch fermentation process, but a PH range of 5–6 has been reported as the initial optimum pH [124]. During fermentation, volatile fatty acids reduce the pH of the medium. Therefore, the initial pH was set from 6-7 to compensate for the end of the process [114].
The effect of pH on hydrogen production by green algae was evaluated [125]. The results showed that the pH of the medium affects the activity of the hydrogenase enzyme. They controlled the pH by adding NaOH and HCl over the range of 6.5–9.0, which does not affect algae growth. It was observed that an increased yield of 2.4% was obtained at a pH of 6.5. A considerable pH value is also required for Purple non-Sulfur bacteria (PNSB) to produce hydrogen via photofermentation. According to studies on hydrogen production during the photo-biological fermentation process, a pH of 7 is best for transporting electrons to the nitrogenase enzymes for H2 generation in the media [126].
6.4. Effect of Operational Temperature
The operational temperature significantly affects the production of hydrogen and the activity of enzymes involved in hydrogen generation [127,128]. Thermophilic bacteria observed an enhanced biosynthesis of hydrogen at high temperatures compared to mesophilic bacteria during dark fermentation. The temperature range of 30–55 °C has been reported as optimal for enhanced biohydrogen production [128,129]. Hence, it has been reported that the activity of H2 producers is inhibited at a very extreme temperature of more than 60 °C. Only hyperthermophilic bacteria (Pyrococcus furiosus and Thermotoga maritime) can produce H2 at extreme temperatures. These bacteria can produce H2 at temperatures greater than 80 °C [130].
PNSBs are also sensitive to different ranges of temperature. For example, a study conducted to show the effect of cultural conditions on H2 production by photofermentation described that the growth rate and the rate of H2 production initially increased with an increase in temperature up to 30 °C. However, after 30 °C, the production rate of H2 gas decreased rapidly because the higher temperature above 30 °C inhibits the activity of the enzyme nitrogenase [126].
6.5. Effect of Nutrients
The macronutrients also play a vital role in the growth of bacteria to produce H2. The essential nutrients are sulfur, phosphorus, and nitrogen. The common form of inorganic sulfur in many organic wastes is sulfate (SO42−) [131]. The sulfate-reducing bacteria reduce sulfate into sulfide during the process of fermentation. The sulfur-containing proteins also produce sulfide in the fermentation medium. Studies have been reported about the toxic effects of high sulfide levels in a medium, which inhibit the activity of microorganisms from producing H2. The increased sulfide concentration also decreases the bioavailability of some trace elements [132].
Another essential nutrient for the growth of anaerobic bacteria is nitrogen. The high concentration of ammonia hydrogen decreases the activity of fermentative bacteria and the rate of H2 production [133,134]. The degradation of proteins and amino acids also produces a high amount of ammonia in the fermentation media. The high concentration of nitrogen also interferes with the intracellular pH and affects the performance of microbes responsible for H2 production. The inhibition of nitrogen can be overcome by diluting the feedstock [135]. Besides nitrogen and sulfur, phosphorus is another nutrient required to enhance hydrogen production [136]. It was observed that a high rate of H2 can be obtained in the presence of 600 mg L−1 K2HPO4 [137]. A 40% increase in the production of H2 was observed at a 30% increase or decline in the respective chemical compound.
6.6. Effect of Light Intensity
Light intensity plays a significant role in producing H2 by PNSB. It was shown that the performance of PNSB increases with an increase in light intensity from 2500–5000 lx, but a further increase in light intensity reduces the growth and production of hydrogen [138]. It was also observed that the photosynthetic system of PNSB demanded more ATP and reduced power with increasing illumination intensity [138]. The enzyme nitrogenase also requires high ATP to sensitize the cells and produce H2. Hence, the high intensity can become a limiting factor in photohydrogen production.
Photoinhibition in PNSB was also investigated [139]. They suggested that hydrogen production decreased when light intensity was increased above 200 Wm−2, while a study conducted by Cai and Wang found that the H2 production decreased at an illumination intensity of 6000 lx. The favorable light source is LED because it has a wavelength range of 770–920 nm, which is considered best for the activity of PNSB. Furthermore, LED light is cost-effective in terms of heat generation, energy consumption, and life expectancy [40].
6.7. Effect of Metal Ions
Different metal ions are used for microbes’ growth and to optimize the activity of the enzyme during dark- and photofermentation. These metal ions are required only in a moderate amount. When they are used in high quantities, they inhibit the fermentation process by inhibiting the growth of the bacteria. The effects of using higher concentrations of metals include destroying membrane function and eliminating the transmission of valuable ions and nutrients to the cell and intracellular accumulation of metals [140,141]. A study was conducted to describe the importance of Fe for metabolic changes and its involvement in the expression of non-Fe-S and Fe-S proteins in hydrogenase enzymes. However, when the concentration of Fe increases in the medium, it makes cell clumps and reduces the mass transfer activity. It has been reported that the pure culture requires very little Fe, while mixed culture can tolerate high doses of Fe without an inhibitory effect [124].
Trace metal ions, such as sodium, magnesium, and calcium, are also needed for the growth of bacteria. High amounts of these trace metals slow down the growth of microbes and become toxic at higher concentrations. The authors of [142] observed a high hydrogen yield in the absence of sodium. The higher concentrations of sodium raise the osmotic pressure, affect the activity of bacteria, and sometimes cause bacterial death. It has been recommended that the sodium concentration should be kept under 20 g L−1 to achieve a maximum level of hydrogen production [143]. Ca2+n is another trace element required for the growth of bacteria and H2 production [144]. Mg2+ is also responsible for cell function and reaction. It is the most demanding ion as a cofactor for 10 types of enzymes involved in the glycolysis process. The Ni2+ has no inhibitory effect on the yield of H2 at a level of 0.1 mg L−1. Hence, no significant measure has been taken to control metal inhibition during fermentation, but a few pretreatment techniques, such as biosorption, electrodialysis, and cofermentation, can effectively overcome metal inhibition problems [145].
7. Nanotechnology and Biohydrogen
The vast and newly emerging field of nanotechnology deals with nm-sized particles. The nanoparticles (NPs) have been utilized in several fields, such as biosensors, medicines, immobilization, and the production of biofuels [146,147,148]. The NPs also help produce biohydrogen by influencing the metabolic activities of microbes under aerobic conditions [149]. Nanoparticles prepared by different methods (biological, physical, and chemical) have been reported for H2 production. The NPs of gold, silver, copper, nickel, iron, zinc oxide, palladium, titanium, silica, carbon nanotubes, and activated carbon have been used to enhance H2 production [146,150,151,152]. These nanoparticles provide a larger surface area to adsorb electrons and, hence, enhance the production rate of H2 by stimulating the hydrogen-producing enzymes [153].
Enhancement of Biohydrogen by Metallic Nanoparticles
Zhang’s group was the first to use gold nanoparticles to enhance the biosynthesis of H2 [153]. They used artificial wastewater for H2 production via dark fermentation. The preheated and non-heat-treated cultures were used as inoculum. It was observed that the gold nanoparticles successfully increased the metabolic activity of the microbes, enhancing the rate of H2 compared to the control. The cumulative hydrogen yield was maximum when 5 nm gold particles were used [153]. A study evaluating silver NPs for hydrogen production has also been conducted [154]. They used mixed culture and Ag-NPs to produce H2 from glucose. When the concentration of the Ag-NPs was increased up to 20 nM, it affected the activity of the bacteria for enhancing H2 generation. However, there was no increase in hydrogen production rate at more significant concentrations. The higher yield of H2 observed at 20 nM Ag-NPs was 67.6%. The Ag-NPs also increased cell biomass and decreased the lag phase for H2 production.
Han and colleagues investigated the effect of hematite NPs and initial pH on hydrogen production in mixed bacteria in an anaerobic fed-batch process. The maximum observed H2 yield was 3.21 mol H2/mol−1 sucrose. A transmission electron microscope was used to check the slow discharge of hematite nanoparticles and their effect on the shape of bacteria. Furthermore, a study was conducted utilizing biogenic palladium nanoparticles and palladium ions [150]. The leaf extract of Cortandrum sattvum was used to synthesize palladium nanoparticles. They obtained a maximum H2 yield of 1.48 mol H2/mol−1 glucose using a 5 mg L−1 palladium nanoparticles concentration because of the higher activity of the hydrogenase enzyme. On the other hand, palladium ions showed a negative impact on the yield and lag phase of hydrogen.
Many bacterial cultures have been investigated for producing H2 via iron NPs. For example, Fe-NPs and iron ions were used to investigate their possible enhancement effect on the production of H2 [155]. Both showed a positive impact on the hydrogen yield compared to the control. However, Fe+2 ions and Fe-NPs illustrated different behavior towards the generation of intermediate metabolites. The propionate production declined by 75% with Fe-NPs compared to a 35% reduction by the Fe+2 ions. The enhancement effect of phytogenic iron nanoparticles and iron ions was also investigated. The green Fe-NPs were prepared using the extract of leaves and bark of Syzygium cumini and FeSO4. The mesophilic bacterial strain of Enterobacter cloacae DH-89 was isolated from the soil and used to produce hydrogen. A 100% increase in hydrogen production (1.9 mol H2/mol−1 hexose) was observed under 100 mg L−1 Fe-NPs compared to the control (0.95 mol H2/mol −1 glucose). Meanwhile, Fe+2 ions helped to raise the yield of hydrogen to 1.45 mol/mol−1 glucose) [156]. Similarly, some other researchers have also reported Fe, Fe2O3, and Fe3O4 NPs prepared by different physical, chemical, and biological methods for the enhanced biosynthesis of hydrogen [101,157,158,159,160,161,162].
The effect of ZnO nanoparticles on hydrogen production was also reported [163]. The ZnO-NPs were synthesized by the typical precipitation method. The pretreated biomass of water hyacinth was saccharified by the enzyme activity and used for the fermentative production of H2. It was observed that the ZnO-NPs reduced the hydrogen yield compared to the control. On the other hand, metallic NPs (copper, nickel, silicon dioxide, and titanium dioxide) positively affected the rate of generation and yield of H2 [164,165,166,167,168].
Many studies have reported an enhancement of H2 production via metallic NPs using the dark fermentation process, but few studies have been found in the literature for enhanced photofermentative H2 production by nanoparticles. Zhao et al. investigated the effect of TiO2-NPs on photofermentative H2 production using the effluent of the dark fermentation process as a feedstock [165]. It was observed from the results that the TiO2-NPs enhanced the activity of PNSB for the production of H2 and reduced the activity of the uptake of hydrogenase enzyme. Another study by Pandey et al. reflected a similar enhancement effect of TiO2-NPs for photofermentative H2 production [164]. Meanwhile, Kanwal and colleagues investigated the effect of a phytofabricated nanoscale iron complex for H2 production using photofermentative PNSB [169]. The use of carbon nanotubes (CNTs) has also been reported for improved hydrogen generation by H2-producing bacteria [170].
8. Future Perspective of H2 Production
The selection of favorable biological H2-producing technology for future research is based on different factors. Hossienzadeh et al. conducted technoeconomic and life cycle assessments of H2-producing techniques [49]. From the technological perspective, dark fermentation was chosen as a better process than others. The combination of dark fermentation with MECs and photofermentation generated around 1 L H2/g organic waste. Among biological H2-producing techniques, the dark-fermentative process (2.3 US $/g) is the most cost-effective, followed by MECs (2.8 US $/g) and photofermentation (3.5 US $/g). According to an environmental impact assessment, low greenhouse gas emissions were observed from both fermentation processes compared to MECs [49].
According to EIA, the hybrid dark fermentation and MEC technology can biosynthesize 105 million tons of H2 gas from 1.3 billion metric food waste. This represents a 120% higher potential than the actual demand in 2020 (90 million metric tons) [1].
9. Conclusions
Using biomass to harvest hydrogen gas is a promising and sustainable method for producing clean energy. Even though there are several renewable energy options, no single energy source can fully replace fossil fuels. However, hydrogen production, in this way, might be highly beneficial as it can reduce greenhouse gas emissions while future energy demands are met. Different routes of biological hydrogen production, such as photolysis, photofermentation, dark fermentation, and microbial electrolysis cells (MECs), represent the different categories of solid and liquid waste feedstock, microorganisms, and enzymes involved in biohydrogen production, which were discussed. In addition, various factors affecting the production rate (e.g., substrate concentration, temperature, and pH) were also examined.
Furthermore, several studies regarding the use of nanomaterials for the enhanced production of hydrogen were investigated. Of course, all techniques have some pros and cons. However, after analyzing various studies, dark fermentation was found to be the most suitable method when integrated with MECs. Besides, the utilization of engineered bacterial cultures and the role of nanotechnology have also enhanced hydrogen biosynthesis. However, the practical implication of these processes still requires further efforts from engineers and researchers.
Author Contributions
All authors reviewed the results and approved the final version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- U.S. Energy Information Administration. International Energy Outlook. Washington, DC 20585: 2021. Available online: https://www.eia.gov/ieo (accessed on 20 September 2022).
- Bayro-Kaiser, V.; Nelson, N. Microalgal hydrogen production: Prospects of an essential technology for a clean and sustainable energy economy. Photosynth. Res. 2017, 133, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, T.; von Braun, J. Climate change impacts on global food security. Science 2013, 341, 508–513. [Google Scholar] [CrossRef] [PubMed]
- Tai, A.P.; Martin, M.V.; Heald, C.L. Threat to future global food security from climate change and ozone air pollution. Nat. Clim. Chang. 2014, 4, 817. [Google Scholar] [CrossRef]
- Schaeffer, M.; Hare, W.; Rahmstorf, S.; Vermeer, M. Long-term sea-level rise implied by 1.5 c and 2 c warming levels. Nat. Clim. Chang. 2012, 2, 867. [Google Scholar] [CrossRef]
- Kroeker, K.J.; Kordas, R.L.; Crim, R.; Hendriks, I.E.; Ramajo, L.; Singh, G.S.; Duarte, C.M.; Gattuso, J.P. Impacts of ocean acidification on marine organisms: Quantifying sensitivities and interaction with warming. Glob. Chang. Biol. 2013, 19, 1884–1896. [Google Scholar] [CrossRef] [PubMed]
- Corlett, R.T.; Westcott, D.A. Will plant movements keep up with climate change? Trends Ecol. Evol. 2013, 28, 482–488. [Google Scholar] [CrossRef]
- Franks, S.J.; Weber, J.J.; Aitken, S.N. Evolutionary and plastic responses to climate change in terrestrial plant populations. Evol. Appl. 2014, 7, 123–139. [Google Scholar] [CrossRef]
- Moritz, C.; Agudo, R. The future of species under climate change: Resilience or decline? Science 2013, 341, 504–508. [Google Scholar] [CrossRef]
- Vidadili, N.; Suleymanov, E.; Bulut, C.; Mahmudlu, C. Transition to renewable energy and sustainable energy development in azerbaijan. Renew. Sustain. Energy Rev. 2017, 80, 1153–1161. [Google Scholar] [CrossRef]
- Ozturk, M.; Yuksel, Y.E. Energy structure of turkey for sustainable development. Renew. Sustain. Energy Rev. 2016, 53, 1259–1272. [Google Scholar] [CrossRef]
- Ho, D.P.; Ngo, H.H.; Guo, W. A mini review on renewable sources for biofuel. Bioresour. Technol. 2014, 169, 742–749. [Google Scholar] [CrossRef] [PubMed]
- Rodionova, M.; Poudyal, R.; Tiwari, I.; Voloshin, R.; Zharmukhamedov, S.; Nam, H.; Zayadan, B.; Bruce, B.; Hou, H.; Allakhverdiev, S. Biofuel production: Challenges and opportunities. Int. J. Hydrog. Energy 2017, 42, 8450–8461. [Google Scholar] [CrossRef]
- Voloshin, R.A.; Rodionova, M.V.; Zharmukhamedov, S.K.; Veziroglu, T.N.; Allakhverdiev, S.I. Biofuel production from plant and algal biomass. Int. J. Hydrog. Energy 2016, 41, 17257–17273. [Google Scholar] [CrossRef]
- Mitra, R.; Balachandar, G.; Singh, V.; Sinha, P.; Das, D. Improvement in energy recovery by dark fermentative biohydrogen followed by biobutanol production process using obligate anaerobes. Int. J. Hydrog. Energy 2017, 42, 4880–4892. [Google Scholar] [CrossRef]
- Khetkorn, W.; Rastogi, R.P.; Incharoensakdi, A.; Lindblad, P.; Madamwar, D.; Pandey, A.; Larroche, C. Microalgal hydrogen production–a review. Bioresour. Technol. 2017, 243, 1194–1206. [Google Scholar] [CrossRef]
- Oey, M.; Sawyer, A.L.; Ross, I.L.; Hankamer, B. Challenges and opportunities for hydrogen production from microalgae. Plant Biotechnol. J. 2016, 14, 1487–1499. [Google Scholar] [CrossRef]
- Bundhoo, M.Z.; Mohee, R. Inhibition of dark fermentative bio-hydrogen production: A review. Int. J. Hydrog. Energy 2016, 41, 6713–6733. [Google Scholar] [CrossRef]
- Chu, S.; Majumdar, A. Opportunities and challenges for a sustainable energy future. Nature 2012, 488, 294. [Google Scholar] [CrossRef]
- Ghimire, A.; Frunzo, L.; Pirozzi, F.; Trably, E.; Escudie, R.; Lens, P.N.; Esposito, G. A review on dark fermentative biohydrogen production from organic biomass: Process parameters and use of by-products. Appl. Energy 2015, 144, 73–95. [Google Scholar] [CrossRef]
- Rashid, N.; Rehman, M.S.U.; Memon, S.; Rahman, Z.U.; Lee, K.; Han, J.-I. Current status, barriers and developments in biohydrogen production by microalgae. Renew. Sustain. Energy Rev. 2013, 22, 571–579. [Google Scholar] [CrossRef]
- Demirbas, A. Hydrogen-rich gas from fruit shells via supercritical water extraction. Int. J. Hydrog. Energy 2004, 29, 1237–1243. [Google Scholar] [CrossRef]
- Pipitone, G.; Zoppi, G.; Pirone, R.; Bensaid, S. A critical review on catalyst design for aqueous phase reforming. Int. J. Hydrog. Energy 2022, 47, 151–180. [Google Scholar] [CrossRef]
- Tak, S.S.; Shetye, O.; Muley, O.; Jaiswal, H.; Malik, S.N. Emerging technologies for hydrogen production from wastewater. Int. J. Hydrog. Energy 2022. [Google Scholar] [CrossRef]
- Zoppi, G.; Pipitone, G.; Galletti, C.; Rizzo, A.M.; Chiaramonti, D.; Pirone, R.; Bensaid, S. Aqueous phase reforming of lignin-rich hydrothermal liquefaction by-products: A study on catalyst deactivation. Catal. Today 2021, 365, 206–213. [Google Scholar] [CrossRef]
- Rahman, S.; Masdar, M.; Rosli, M.; Majlan, E.; Husaini, T.; Kamarudin, S.; Daud, W. Overview biohydrogen technologies and application in fuel cell technology. Renew. Sustain. Energy Rev. 2016, 66, 137–162. [Google Scholar] [CrossRef]
- Torzillo, G.; Scoma, A.; Faraloni, C.; Giannelli, L. Advances in the biotechnology of hydrogen production with the microalga chlamydomonas reinhardtii. Crit. Rev. Biotechnol. 2015, 35, 485–496. [Google Scholar] [CrossRef]
- Rumpel, S.; Siebel, J.F.; Farès, C.; Duan, J.; Reijerse, E.; Happe, T.; Lubitz, W.; Winkler, M. Enhancing hydrogen production of microalgae by redirecting electrons from photosystem i to hydrogenase. Energy Environ. Sci. 2014, 7, 3296–3301. [Google Scholar] [CrossRef]
- Liu, H.; Hu, H.; Chignell, J.; Fan, Y. Microbial electrolysis: Novel technology for hydrogen production from biomass. Biofuels 2010, 1, 129–142. [Google Scholar] [CrossRef]
- Lakshmidevi, R.; Muthukumar, K. Enzymatic saccharification and fermentation of paper and pulp industry effluent for biohydrogen production. Int. J. Hydrog. Energy 2010, 35, 3389–3400. [Google Scholar] [CrossRef]
- Silva, J.S.; Mendes, J.S.; Correia, J.A.C.; Rocha, M.V.P.; Micoli, L. Cashew apple bagasse as new feedstock for the hydrogen production using dark fermentation process. J. Biotechnol. 2018, 286, 71–78. [Google Scholar] [CrossRef]
- Wang, J.; Yin, Y. Fermentative hydrogen production using pretreated microalgal biomass as feedstock. Microb. Cell Factories 2018, 17, 22. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Arya, S.K. Hydrogen from algal biomass: A review of production process. Biotechnol. Rep. 2017, 15, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Hitam, C.N.C.; Jalil, A.A. A review on biohydrogen production through photo fermentation of lignocellulosic biomass. Biomass Convers. Biorefinery 2020. [Google Scholar] [CrossRef]
- Arimi, M.M.; Knodel, J.; Kiprop, A.; Namango, S.S.; Zhang, Y.; Geißen, S.-U. Strategies for improvement of biohydrogen production from organic-rich wastewater: A review. Biomass Bioenergy 2015, 75, 101–118. [Google Scholar] [CrossRef]
- Park, J.-Y.; Kim, B.-N.; Kim, Y.-H.; Min, J. Whole-genome sequence of purple non-sulfur bacteria, rhodobacter sphaeroides strain mbtlj-8 with improved co2 reduction capacity. J. Biotechnol. 2018, 288, 9–14. [Google Scholar] [CrossRef]
- Seifert, K.; Waligorska, M.; Laniecki, M. Brewery wastewaters in photobiological hydrogen generation in presence of rhodobacter sphaeroides O.U. 001. Int. J. Hydrog. Energy 2010, 35, 4085–4091. [Google Scholar] [CrossRef]
- Han, H.; Liu, B.; Yang, H.; Shen, J. Effect of carbon sources on the photobiological production of hydrogen using rhodobacter sphaeroides rv. Int. J. Hydrog. Energy 2012, 37, 12167–12174. [Google Scholar] [CrossRef]
- Basak, N.; Das, D. Photofermentative hydrogen production using purple non-sulfur bacteria rhodobacter sphaeroides ou 001 in an annular photobioreactor: A case study. Biomass Bioenergy 2009, 33, 911–919. [Google Scholar] [CrossRef]
- Akroum-Amrouche, D.; Abdi, N.; Lounici, H.; Mameri, N. Effect of physico-chemical parameters on biohydrogen production and growth characteristics by batch culture of rhodobacter sphaeroides cip 60.6. Appl. Energy 2011, 88, 2130–2135. [Google Scholar] [CrossRef]
- Yang, H.; Shao, P.; Lu, T.; Shen, J.; Wang, D.; Xu, Z.; Yuan, X. Continuous bio-hydrogen production from citric acid wastewater via facultative anaerobic bacteria. Int. J. Hydrog. Energy 2006, 31, 1306–1313. [Google Scholar] [CrossRef]
- Rai, P.K.; Singh, S.P.; Asthana, R.K.; Singh, S. Biohydrogen production from sugarcane bagasse by integrating dark- and photo fermentation. Bioresour. Technol. 2014, 152, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Policastro, G.; Giugliano, M.; Luongo, V.; Napolitano, R.; Fabbricino, M. Enhancing photo fermentative hydrogen production using ethanol rich dark fermentation effluents. Int. J. Hydrog. Energy 2022, 47, 117–126. [Google Scholar] [CrossRef]
- Kumar, G.; Sivagurunathan, P.; Pugazhendhi, A.; Thi, N.B.D.; Zhen, G.; Chandrasekhar, K.; Kadier, A. A comprehensive overview on light independent fermentative hydrogen production from wastewater feedstock and possible integrative options. Energy Convers. Manag. 2017, 141, 390–402. [Google Scholar] [CrossRef]
- Hallenbeck, P.C. Fermentative hydrogen production: Principles, progress, and prognosis. Int. J. Hydrog. Energy 2009, 34, 7379–7389. [Google Scholar] [CrossRef]
- Sambusiti, C.; Bellucci, M.; Zabaniotou, A.; Beneduce, L.; Monlau, F. Algae as promising feedstocks for fermentative biohydrogen production according to a biorefinery approach: A comprehensive review. Renew. Sustain. Energy Rev. 2015, 44, 20–36. [Google Scholar] [CrossRef]
- Singh, R.; White, D.; Demirel, Y.; Kelly, R.; Noll, K.; Blum, P. Uncoupling fermentative synthesis of molecular hydrogen from biomass formation in thermotoga maritima. Appl. Environ. Microbiol. 2018, 84, e00998-18. [Google Scholar] [CrossRef]
- Ergal, İ.; Gräf, O.; Hasibar, B.; Steiner, M.; Vukotić, S.; Bochmann, G.; Fuchs, W.; Rittmann, S.K.M.R. Biohydrogen production beyond the thauer limit by precision design of artificial microbial consortia. Commun. Biol. 2020, 3, 443. [Google Scholar] [CrossRef]
- Hosseinzadeh, A.; Zhou, J.L.; Li, X.; Afsari, M.; Altaee, A. Techno-economic and environmental impact assessment of hydrogen production processes using bio-waste as renewable energy resource. Renew. Sustain. Energy Rev. 2022, 156, 111991. [Google Scholar] [CrossRef]
- Guo, W.-Q.; Ren, N.-Q.; Wang, X.-J.; Xiang, W.-S.; Meng, Z.-H.; Ding, J.; Qu, Y.-Y.; Zhang, L.-S. Biohydrogen production from ethanol-type fermentation of molasses in an expanded granular sludge bed (egsb) reactor. Int. J. Hydrog. Energy 2008, 33, 4981–4988. [Google Scholar] [CrossRef]
- Skonieczny, M.T.; Yargeau, V. Biohydrogen production from wastewater by clostridium beijerinckii: Effect of ph and substrate concentration. Int. J. Hydrog. Energy 2009, 34, 3288–3294. [Google Scholar] [CrossRef]
- Cao, Y.; Liu, H.; Liu, W.; Guo, J.; Xian, M. Debottlenecking the biological hydrogen production pathway of dark fermentation: Insight into the impact of strain improvement. Microb. Cell Factories 2022, 21, 166. [Google Scholar] [CrossRef] [PubMed]
- Ramprakash, B.; Lindblad, P.; Eaton-Rye, J.J.; Incharoensakdi, A. Current strategies and future perspectives in biological hydrogen production: A review. Renew. Sustain. Energy Rev. 2022, 168, 112773. [Google Scholar] [CrossRef]
- Pontrelli, S.; Chiu, T.-Y.; Lan, E.I.; Chen, F.Y.H.; Chang, P.; Liao, J.C. Escherichia coli as a host for metabolic engineering. Metab. Eng. 2018, 50, 16–46. [Google Scholar] [CrossRef] [PubMed]
- Modestra, J.A.; Babu, M.L.; Mohan, S.V. Electro-fermentation of real-field acidogenic spent wash effluents for additional biohydrogen production with simultaneous treatment in a microbial electrolysis cell. Sep. Purif. Technol. 2015, 150, 308–315. [Google Scholar] [CrossRef]
- Zhang, Y.; Angelidaki, I. Microbial electrochemical systems and technologies: It is time to report the capital costs. Environ. Sci. Technol. 2016, 50, 5432–5433. [Google Scholar] [CrossRef]
- Butti, S.K.; Velvizhi, G.; Sulonen, M.L.K.; Haavisto, J.M.; Koroglu, E.O.; Cetinkaya, A.Y.; Singh, S.; Arya, D.; Modestra, J.A.; Krishna, K.V.; et al. Microbial electrochemical technologies with the perspective of harnessing bioenergy: Maneuvering towards upscaling. Renew. Sustain. Energy Rev. 2016, 53, 462–476. [Google Scholar] [CrossRef]
- Rivera, I.; Buitrón, G.; Bakonyi, P.; Nemestóthy, N.; Bélafi-Bakó, K. Hydrogen production in a microbial electrolysis cell fed with a dark fermentation effluent. J. Appl. Electrochem. 2015, 45, 1223–1229. [Google Scholar] [CrossRef]
- Kadier, A.; Jain, P.; Lai, B.; Kalil, M.S.; Kondaveeti, S.; Alabbosh, K.F.S.; Abu-Reesh, I.M.; Mohanakrishna, G. Biorefinery perspectives of microbial electrolysis cells (mecs) for hydrogen and valuable chemicals production through wastewater treatment. Biofuel Res. J. 2020, 7, 1128–1142. [Google Scholar] [CrossRef]
- Kadier, A.; Singh, R.; Song, D.; Ghanbari, F.; Zaidi, N.S.; Aryanti, P.T.P.; Jadhav, D.A.; Islam, M.A.; Kalil, M.S.; Nabgan, W.; et al. A novel pico-hydro power (php)-microbial electrolysis cell (mec) coupled system for sustainable hydrogen production during palm oil mill effluent (pome) wastewater treatment. Int. J. Hydrog. Energy 2022. [Google Scholar] [CrossRef]
- Lee, H.-S.; Xin, W.; Katakojwala, R.; Mohan, S.V.; Tabish, N.M.D. Microbial electrolysis cells for the production of biohydrogen in dark fermentation—A review. Bioresour. Technol. 2022, 363, 127934. [Google Scholar] [CrossRef]
- Li, W.; Cheng, C.; Cao, G.; Ren, N. Enhanced biohydrogen production from sugarcane molasses by adding ginkgo biloba leaves. Bioresour. Technol. 2020, 298, 122523. [Google Scholar] [CrossRef] [PubMed]
- Oceguera-Contreras, E.; Aguilar-Juarez, O.; Oseguera-Galindo, D.; Macías-Barragán, J.; Ortiz-Torres, G.; Pita-López, M.L.; Domínguez, J.; Titov, I.; Kamen, A. Establishment of the upstream processing for renewable production of hydrogen using vermicomposting-tea and molasses as substrate. Waste Manag. 2022, 139, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.A.; Fuess, L.T.; Soares, L.A.; Damianovic, M.H.R.Z. Thermophilic biohydrogen production from sugarcane molasses under low ph: Metabolic and microbial aspects. Int. J. Hydrog. Energy 2020, 45, 4182–4192. [Google Scholar] [CrossRef]
- Kars, G.; Alparslan, Ü. Valorization of sugar beet molasses for the production of biohydrogen and 5-aminolevulinic acid by rhodobacter sphaeroides o.U.001 in a biorefinery concept. Int. J. Hydrog. Energy 2013, 38, 14488–14494. [Google Scholar] [CrossRef]
- Sagir, E.; Ozgur, E.; Gunduz, U.; Eroglu, I.; Yucel, M. Single-stage photofermentative biohydrogen production from sugar beet molasses by different purple non-sulfur bacteria. Bioprocess Biosyst. Eng. 2017, 40, 1589–1601. [Google Scholar] [CrossRef]
- Wei, J.; Liu, Z.-T.; Zhang, X. Biohydrogen production from starch wastewater and application in fuel cell. Int. J. Hydrog. Energy 2010, 35, 2949–2952. [Google Scholar] [CrossRef]
- Cota-Navarro, C.B.; Carrillo-Reyes, J.; Davila-Vazquez, G.; Alatriste-Mondragón, F.; Razo-Flores, E. Continuous hydrogen and methane production in a two-stage cheese whey fermentation system. Water Sci. Technol. 2011, 64, 367–374. [Google Scholar] [CrossRef]
- Jung, K.-W.; Kim, D.-H.; Lee, M.-Y.; Shin, H.-S. Two-stage uasb reactor converting coffee drink manufacturing wastewater to hydrogen and methane. Int. J. Hydrog. Energy 2012, 37, 7473–7481. [Google Scholar] [CrossRef]
- Bala-Amutha, K.; Murugesan, A.G. Biohydrogen production using corn stalk employing bacillus licheniformis msu agm 2 strain. Renew. Energy 2013, 50, 621–627. [Google Scholar] [CrossRef]
- Chookaew, T.; O-Thong, S.; Prasertsan, P. Fermentative production of hydrogen and soluble metabolites from crude glycerol of biodiesel plant by the newly isolated thermotolerant klebsiella pneumoniae tr17. Int. J. Hydrog. Energy 2012, 37, 13314–13322. [Google Scholar] [CrossRef]
- Fang, H.H.P.; Li, C.; Zhang, T. Acidophilic biohydrogen production from rice slurry. Int. J. Hydrog. Energy 2006, 31, 683–692. [Google Scholar] [CrossRef]
- Anam, K.; Habibi, M.S.; Harwati, T.U.; Susilaningsih, D. Photofermentative hydrogen production using rhodobium marinum from bagasse and soy sauce wastewater. Int. J. Hydrog. Energy 2012, 37, 15436–15442. [Google Scholar] [CrossRef]
- Adessi, A.; McKinlay, J.B.; Harwood, C.S.; de Philippis, R. A rhodopseudomonas palustris nifa* mutant produces h2 from nh4+-containing vegetable wastes. Int. J. Hydrog. Energy 2012, 37, 15893–15900. [Google Scholar] [CrossRef]
- Alam, M.Z.; Jamal, P.; Nadzir, M.M. Bioconversion of palm oil mill effluent for citric acid production: Statistical optimization of fermentation media and time by central composite design. World J. Microbiol. Biotechnol. 2008, 24, 1177–1185. [Google Scholar] [CrossRef]
- Akil, K.; Jayanthi, S. The biohydrogen potential of distillery wastewater by dark fermentation in an anaerobic sequencing batch reactor. Int. J. Green Energy 2014, 11, 28–39. [Google Scholar] [CrossRef]
- Kars, G.; Ceylan, A. Biohydrogen and 5-aminolevulinic acid production from waste barley by rhodobacter sphaeroides o.U.001 in a biorefinery concept. Int. J. Hydrog. Energy 2013, 38, 5573–5579. [Google Scholar] [CrossRef]
- Pan, C.; Fan, Y.; Hou, H. Fermentative production of hydrogen from wheat bran by mixed anaerobic cultures. Ind. Eng. Chem. Res. 2008, 47, 5812–5818. [Google Scholar] [CrossRef]
- Han, W.; Wang, X.; Ye, L.; Huang, J.; Tang, J.; Li, Y.; Ren, N. Fermentative hydrogen production using wheat flour hydrolysate by mixed culture. Int. J. Hydrog. Energy 2015, 40, 4474–4480. [Google Scholar] [CrossRef]
- Kapdan, I.K.; Kargi, F.; Oztekin, R.; Argun, H. Bio-hydrogen production from acid hydrolyzed wheat starch by photo fermentation using different rhodobacter sp. Int. J. Hydrog. Energy 2009, 34, 2201–2207. [Google Scholar] [CrossRef]
- Kargi, F.; Eren, N.S.; Ozmihci, S. Bio-hydrogen production from cheese whey powder (cwp) solution: Comparison of thermophilic and mesophilic dark fermentations. Int. J. Hydrog. Energy 2012, 37, 8338–8342. [Google Scholar] [CrossRef]
- Pattanamanee, W.; Choorit, W.; Deesan, C.; Sirisansaneeyakul, S.; Chisti, Y. Photofermentive production of biohydrogen from oil palm waste hydrolysate. Int. J. Hydrog. Energy 2012, 37, 4077–4087. [Google Scholar] [CrossRef]
- Hu, B.-B.; Li, M.-Y.; Wang, Y.-T.; Zhu, M.-J. High-yield biohydrogen production from non-detoxified sugarcane bagasse: Fermentation strategy and mechanism. Chem. Eng. J. 2018, 335, 979–987. [Google Scholar] [CrossRef]
- Sangyoka, S.; Reungsang, A.; Lin, C.-Y. Optimization of biohydrogen production from sugarcane bagasse by mixed cultures using a statistical method. Sustain. Environ. Res. 2016, 26, 235–242. [Google Scholar] [CrossRef]
- Lu, C.; Zhang, Z.; Ge, X.; Wang, Y.; Zhou, X.; You, X.; Liu, H.; Zhang, Q. Bio-hydrogen production from apple waste by photosynthetic bacteria hau-m1. Int. J. Hydrog. Energy 2016, 41, 13399–13407. [Google Scholar] [CrossRef]
- Akinbomi, J.; Taherzadeh, M.J. Evaluation of fermentative hydrogen production from single and mixed fruit wastes. Energies 2015, 8, 4253–4272. [Google Scholar] [CrossRef]
- Argun, H.; Dao, S. Bio-hydrogen production from waste peach pulp by dark fermentation: Effect of inoculum addition. Int. J. Hydrog. Energy 2017, 42, 2569–2574. [Google Scholar] [CrossRef]
- Shi, X.-Y.; Jin, D.-W.; Sun, Q.-Y.; Li, W.-W. Optimization of conditions for hydrogen production from brewery wastewater by anaerobic sludge using desirability function approach. Renew. Energy 2010, 35, 1493–1498. [Google Scholar] [CrossRef]
- Cappelletti, M.; Bucchi, G.; Mendes, J.D.; Alberini, A.; Fedi, S.; Bertin, L.; Frascari, D. Biohydrogen production from glucose, molasses and cheese whey by suspended and attached cells of four hyperthermophilic thermotoga strains. J. Chem. Technol. Biotechnol. 2012, 87, 1291–1301. [Google Scholar] [CrossRef]
- Han, W.; Wang, B.; Zhou, Y.; Wang, D.-X.; Wang, Y.; Yue, L.-R.; Li, Y.-F.; Ren, N.-Q. Fermentative hydrogen production from molasses wastewater in a continuous mixed immobilized sludge reactor. Bioresour. Technol. 2012, 110, 219–223. [Google Scholar] [CrossRef]
- Kothandapani, S.; Preetha, B. Effect of initial ph on biohydrogen production using distillery wastewater by batch process. J. Chem. Pharm. Sci. 2015, 8, 148–151. [Google Scholar]
- Moreno-Dávila, I.M.M.; Ríos-González, L.J.; Garza-García, Y.; Garza, J.A.R.-D.L.; Rodríguez-Martínez, J. Biohydrogen production from diary processing wastewater by anaerobic biofilm reactors. Afr. J. Biotechnol. 2011, 10, 5320. [Google Scholar]
- Zhu, H.; Ueda, S.; Asada, Y.; Miyake, J. Hydrogen production as a novel process of wastewater treatment—Studies on tofu wastewater with entrapped r. Sphaeroides and mutagenesis. Int. J. Hydrog. Energy 2002, 27, 1349–1357. [Google Scholar] [CrossRef]
- Zheng, G.H.; Wang, L.; Kang, Z.H. Feasibility of biohydrogen production from tofu wastewater with glutamine auxotrophic mutant of rhodobacter sphaeroides. Renew. Energy 2010, 35, 2910–2913. [Google Scholar] [CrossRef]
- Lay, C.-H.; Sen, B.; Huang, S.-C.; Chen, C.-C.; Lin, C.-Y. Sustainable bioenergy production from tofu-processing wastewater by anaerobic hydrogen fermentation for onsite energy recovery. Renew. Energy 2013, 58, 60–67. [Google Scholar] [CrossRef]
- Jamil, Z.; Annuar, M.S.M.; Ibrahim, S.; Vikineswary, S. Optimization of phototrophic hydrogen production by rhodopseudomonas palustris pbum001 via statistical experimental design. Int. J. Hydrog. Energy 2009, 34, 7502–7512. [Google Scholar] [CrossRef]
- Garritano, A.D.N.; de Sá, L.R.V.; Aguieiras, É.C.G.; Freire, D.M.G.; Ferreira-Leitão, V.S. Efficient biohydrogen production via dark fermentation from hydrolized palm oil mill effluent by non-commercial enzyme preparation. Int. J. Hydrog. Energy 2017, 42, 29166–29174. [Google Scholar] [CrossRef]
- Park, J.-I.; Lee, J.; Sim, S.J.; Lee, J.-H. Production of hydrogen from marine macro-algae biomass using anaerobic sewage sludge microflora. Biotechnol. Bioprocess Eng. 2009, 14, 307. [Google Scholar] [CrossRef]
- Nguyen, T.-A.D.; Kim, K.-R.; Nguyen, M.-T.; Kim, M.S.; Kim, D.; Sim, S.J. Enhancement of fermentative hydrogen production from green algal biomass of thermotoga neapolitana by various pretreatment methods. Int. J. Hydrog. Energy 2010, 35, 13035–13040. [Google Scholar] [CrossRef]
- Pattra, S.; Lay, C.-H.; Lin, C.-Y.; O-Thong, S.; Reungsang, A. Performance and population analysis of hydrogen production from sugarcane juice by non-sterile continuous stirred tank reactor augmented with clostridium butyricum. Int. J. Hydrog. Energy 2011, 36, 8697–8703. [Google Scholar] [CrossRef]
- Zhao, W.; Zhao, J.; Chen, G.D.; Feng, R.; Yang, J.; Zhao, Y.F.; Wei, Q.; Du, B.; Zhang, Y.F. Anaerobic biohydrogen production by the mixed culture with mesoporous Fe3O4 nanoparticles activation. Presented at Advanced Materials Research. Trans. Tech. Publ. 2011, 306, 1528–1531. [Google Scholar]
- Jayasinghearachchi, H.S.; Singh, S.; Sarma, P.M.; Aginihotri, A.; Lal, B. Fermentative hydrogen production by new marine clostridium amygdalinum strain c9 isolated from offshore crude oil pipeline. Int. J. Hydrog. Energy 2010, 35, 6665–6673. [Google Scholar] [CrossRef]
- Cai, J.; Wang, Y.; Liu, J.; Zhang, X.; Li, F. Pretreatment enhanced structural disruption, enzymatic hydrolysis, fermentative hydrogen production from rice straw. Int. J. Hydrog. Energy 2022, 47, 11778–11786. [Google Scholar] [CrossRef]
- Zhang, H.; Bruns, M.A.; Logan, B.E. Biological hydrogen production by clostridium acetobutylicum in an unsaturated flow reactor. Water Res. 2006, 40, 728–734. [Google Scholar] [CrossRef] [PubMed]
- Chong, M.-L.; Rahim, R.A.; Shirai, Y.; Hassan, M.A. Biohydrogen production by clostridium butyricum eb6 from palm oil mill effluent. Int. J. Hydrog. Energy 2009, 34, 764–771. [Google Scholar] [CrossRef]
- Rao, R.; Basak, N. Optimization and modelling of dark fermentative hydrogen production from cheese whey by enterobacter aerogenes 2822. Int. J. Hydrog. Energy 2021, 46, 1777–1800. [Google Scholar] [CrossRef]
- Chandrasekhar, K.; Lee, Y.-J.; Lee, D.-W. Biohydrogen production: Strategies to improve process efficiency through microbial routes. Int. J. Mol. Sci. 2015, 16, 8266–8293. [Google Scholar] [CrossRef]
- Singh, V.; Das, D. Chapter 3—Potential of hydrogen production from biomass. In Science and Engineering of Hydrogen-Based Energy Technologies; de Miranda, P.E.V., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 123–164. [Google Scholar]
- Massanet-Nicolau, J.; Dinsdale, R.; Guwy, A. Hydrogen production from sewage sludge using mixed microflora inoculum: Effect of ph and enzymatic pretreatment. Bioresour. Technol. 2008, 99, 6325–6331. [Google Scholar] [CrossRef]
- Xu, T.; Chen, D.; Hu, X. Hydrogen-activating models of hydrogenases. Coord. Chem. Rev. 2015, 303, 32–41. [Google Scholar] [CrossRef]
- Kim, D.-H.; Kim, M.-S. Hydrogenases for biological hydrogen production. Bioresour. Technol. 2011, 102, 8423–8431. [Google Scholar] [CrossRef]
- Ghosh, S.; Dairkee, U.K.; Chowdhury, R.; Bhattacharya, P. Hydrogen from food processing wastes via photofermentation using purple non-sulfur bacteria (pnsb)–a review. Energy Convers. Manag. 2017, 141, 299–314. [Google Scholar] [CrossRef]
- Jang, S.; Kim, D.-H.; Yun, Y.-M.; Lee, M.-K.; Moon, C.; Kang, W.-S.; Kwak, S.-S.; Kim, M.-S. Hydrogen fermentation of food waste by alkali-shock pretreatment: Microbial community analysis and limitation of continuous operation. Bioresour. Technol. 2015, 186, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Jarunglumlert, T.; Prommuak, C.; Putmai, N.; Pavasant, P. Scaling-up bio-hydrogen production from food waste: Feasibilities and challenges. Int. J. Hydrog. Energy 2018, 43, 634–648. [Google Scholar] [CrossRef]
- Gadhe, A.; Sonawane, S.S.; Varma, M.N. Ultrasonic pretreatment for an enhancement of biohydrogen production from complex food waste. Int. J. Hydrog. Energy 2014, 39, 7721–7729. [Google Scholar] [CrossRef]
- Elbeshbishy, E.; Nakhla, G. Comparative study of the effect of ultrasonication on the anaerobic biodegradability of food waste in single and two-stage systems. Bioresour. Technol. 2011, 102, 6449–6457. [Google Scholar] [CrossRef]
- Elbeshbishy, E.; Hafez, H.; Dhar, B.R.; Nakhla, G. Single and combined effect of various pretreatment methods for biohydrogen production from food waste. Int. J. Hydrog. Energy 2011, 36, 11379–11387. [Google Scholar] [CrossRef]
- Elbeshbishy, E.; Hafez, H.; Nakhla, G. Viability of ultrasonication of food waste for hydrogen production. Int. J. Hydrog. Energy 2012, 37, 2960–2964. [Google Scholar] [CrossRef]
- Kim, D.-H.; Kim, S.-H.; Shin, H.-S. Hydrogen fermentation of food waste without inoculum addition. Enzym. Microb. Technol. 2009, 45, 181–187. [Google Scholar] [CrossRef]
- Kothari, R.; Kumar, V.; Pathak, V.V.; Ahmad, S.; Aoyi, O.; Tyagi, V. A critical review on factors influencing fermentative hydrogen production. Front. Biosci. 2017, 22, 1195–1220. [Google Scholar] [CrossRef]
- Kim, E.-J.; Kim, M.-S.; Lee, J.K. Hydrogen evolution under photoheterotrophic and dark fermentative conditions by recombinant rhodobacter sphaeroides containing the genes for fermentative pyruvate metabolism of rhodospirillum rubrum. Int. J. Hydrog. Energy 2008, 33, 5131–5136. [Google Scholar] [CrossRef]
- Park, J.-H.; Kim, D.-H.; Kim, S.-H.; Yoon, J.-J.; Park, H.-D. Effect of substrate concentration on the competition between clostridium and lactobacillus during biohydrogen production. Int. J. Hydrog. Energy 2017, 43, 11460–11469. [Google Scholar] [CrossRef]
- Dong-Jie, N.; Jing-Yuan, W.; Bao-Ying, W.; You-Cai, Z. Effect of mo-containing additives on biohydrogen fermentation from cassava’s stillage. Int. J. Hydrog. Energy 2011, 36, 5289–5295. [Google Scholar] [CrossRef]
- Elbeshbishy, E.; Dhar, B.R.; Nakhla, G.; Lee, H.-S. A critical review on inhibition of dark biohydrogen fermentation. Renew. Sustain. Energy Rev. 2017, 79, 656–668. [Google Scholar] [CrossRef]
- Maneeruttanarungroj, C.; Phunpruch, S. Effect of ph on biohydrogen production in green alga tetraspora sp. Cu2551. Energy Procedia 2017, 138, 1085–1092. [Google Scholar] [CrossRef]
- Wang, B.-N.; Yang, C.-F.; Lee, C.-M. The factors influencing direct photohydrogen production and anaerobic fermentation hydrogen production combination bioreactors. Int. J. Hydrog. Energy 2011, 36, 14069–14077. [Google Scholar] [CrossRef]
- Guo, X.M.; Trably, E.; Latrille, E.; Carrere, H.; Steyer, J.-P. Hydrogen production from agricultural waste by dark fermentation: A review. Int. J. Hydrog. Energy 2010, 35, 10660–10673. [Google Scholar] [CrossRef]
- Sinha, P.; Pandey, A. An evaluative report and challenges for fermentative biohydrogen production. Int. J. Hydrog. Energy 2011, 36, 7460–7478. [Google Scholar] [CrossRef]
- Saady, N.M.C. Homoacetogenesis during hydrogen production by mixed cultures dark fermentation: Unresolved challenge. Int. J. Hydrog. Energy 2013, 38, 13172–13191. [Google Scholar] [CrossRef]
- Pawar, S.S.; van Niel, E.W. Thermophilic biohydrogen production: How far are we? Appl. Microbiol. Biotechnol. 2013, 97, 7999–8009. [Google Scholar] [CrossRef]
- Hwang, J.-H.; Choi, J.-A.; Oh, Y.-K.; Abou-Shanab, R.A.; Song, H.; Min, B.; Cho, Y.; Na, J.-G.; Koo, J.; Jeon, B.-H. Hydrogen production from sulfate-and ferrous-enriched wastewater. Int. J. Hydrog. Energy 2011, 36, 13984–13990. [Google Scholar] [CrossRef]
- Dhar, B.R.; Elbeshbishy, E.; Nakhla, G. Influence of iron on sulfide inhibition in dark biohydrogen fermentation. Bioresour. Technol. 2012, 126, 123–130. [Google Scholar] [CrossRef]
- Ahmadi-Pirlou, M.; Ebrahimi-Nik, M.; Khojastehpour, M.; Ebrahimi, S.H. Mesophilic co-digestion of municipal solid waste and sewage sludge: Effect of mixing ratio, total solids, and alkaline pretreatment. Int. Biodeterior. Biodegrad. 2017, 125, 97–104. [Google Scholar] [CrossRef]
- del Pilar Anzola-Rojas, M.; da Fonseca, S.G.; da Silva, C.C.; de Oliveira, V.M.; Zaiat, M. The use of the carbon/nitrogen ratio and specific organic loading rate as tools for improving biohydrogen production in fixed-bed reactors. Biotechnol. Rep. 2015, 5, 46–54. [Google Scholar] [CrossRef]
- Salerno, M.B.; Park, W.; Zuo, Y.; Logan, B.E. Inhibition of biohydrogen production by ammonia. Water Res. 2006, 40, 1167–1172. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Elbeshbishy, E.; Nakhla, G. Optimization of biological hydrogen production for anaerobic co-digestion of food waste and wastewater biosolids. Bioresour. Technol. 2013, 130, 710–718. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Lay, C. Carbon/nitrogen-ratio effect on fermentative hydrogen production by mixed microflora. Int. J. Hydrog. Energy 2004, 29, 41–45. [Google Scholar] [CrossRef]
- Assawamongkholsiri, T.; Reungsang, A. Photo fermentational hydrogen production of rhodobacter sp. Kku-ps1 isolated from an uasb reactor. Electron. J. Biotechnol. 2015, 18, 221–230. [Google Scholar] [CrossRef]
- Kim, M.-S.; Baek, J.-S.; Lee, J.K. Comparison of h2 accumulation by rhodobacter sphaeroides kd131 and its uptake hydrogenase and phb synthase deficient mutant. Int. J. Hydrog. Energy 2006, 31, 121–127. [Google Scholar] [CrossRef]
- Lemire, J.A.; Harrison, J.J.; Turner, R.J. Antimicrobial activity of metals: Mechanisms, molecular targets and applications. Nat. Rev. Microbiol. 2013, 11, 371. [Google Scholar] [CrossRef]
- Le, D.T.H.; Nitisoravut, R. Modified hydrotalcites for enhancement of biohydrogen production. Int. J. Hydrog. Energy 2015, 40, 12169–12176. [Google Scholar] [CrossRef]
- Junghare, M.; Subudhi, S.; Lal, B. Improvement of hydrogen production under decreased partial pressure by newly isolated alkaline tolerant anaerobe, clostridium butyricum tm-9a: Optimization of process parameters. Int. J. Hydrog. Energy 2012, 37, 3160–3168. [Google Scholar] [CrossRef]
- Cao, X.; Zhao, Y. The influence of sodium on biohydrogen production from food waste by anaerobic fermentation. J. Mater. Cycles Waste Manag. 2009, 11, 244–250. [Google Scholar] [CrossRef]
- Yu, H.-Q.; Tay, J.-H.; Fang, H.H. The roles of calcium in sludge granulation during uasb reactor start-up. Water Res. 2001, 35, 1052–1060. [Google Scholar] [CrossRef]
- Singhania, R.R.; Patel, A.K.; Christophe, G.; Fontanille, P.; Larroche, C. Biological upgrading of volatile fatty acids, key intermediates for the valorization of biowaste through dark anaerobic fermentation. Bioresour. Technol. 2013, 145, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Elreedy, A.; Ibrahim, E.; Hassan, N.; El-Dissouky, A.; Fujii, M.; Yoshimura, C.; Tawfik, A. Nickel-graphene nanocomposite as a novel supplement for enhancement of biohydrogen production from industrial wastewater containing mono-ethylene glycol. Energy Convers. Manag. 2017, 140, 133–144. [Google Scholar] [CrossRef]
- Patel, S.K.; Choi, S.H.; Kang, Y.C.; Lee, J.-K. Large-scale aerosol-assisted synthesis of biofriendly fe 2 o 3 yolk–shell particles: A promising support for enzyme immobilization. Nanoscale 2016, 8, 6728–6738. [Google Scholar] [CrossRef]
- Otari, S.; Pawar, S.; Patel, S.K.; Singh, R.K.; Kim, S.-Y.; Lee, J.H.; Zhang, L.; Lee, J.-K. Canna edulis leaf extract-mediated preparation of stabilized silver nanoparticles: Characterization, antimicrobial activity, and toxicity studies. J. Microbiol. Biotechnol. 2017, 27, 731–738. [Google Scholar] [CrossRef]
- Beckers, L.; Hiligsmann, S.; Lambert, S.D.; Heinrichs, B.; Thonart, P. Improving effect of metal and oxide nanoparticles encapsulated in porous silica on fermentative biohydrogen production by clostridium butyricum. Bioresour. Technol. 2013, 133, 109–117. [Google Scholar] [CrossRef]
- Mohanraj, S.; Anbalagan, K.; Kodhaiyolii, S.; Pugalenthi, V. Comparative evaluation of fermentative hydrogen production using enterobacter cloacae and mixed culture: Effect of Pd(ii) ion and phytogenic palladium nanoparticles. J. Biotechnol. 2014, 192, 87–95. [Google Scholar] [CrossRef]
- Mohanraj, S.; Anbalagan, K.; Rajaguru, P.; Pugalenthi, V. Effects of phytogenic copper nanoparticles on fermentative hydrogen production by enterobacter cloacae and clostridium acetobutylicum. Int. J. Hydrog. Energy 2016, 41, 10639–10645. [Google Scholar] [CrossRef]
- Gadhe, A.; Sonawane, S.S.; Varma, M.N. Influence of nickel and hematite nanoparticle powder on the production of biohydrogen from complex distillery wastewater in batch fermentation. Int. J. Hydrog. Energy 2015, 40, 10734–10743. [Google Scholar] [CrossRef]
- Zhang, Y.; Shen, J. Enhancement effect of gold nanoparticles on biohydrogen production from artificial wastewater. Int. J. Hydrog. Energy 2007, 32, 17–23. [Google Scholar] [CrossRef]
- Zhao, W.; Zhang, Y.; Du, B.; Wei, D.; Wei, Q.; Zhao, Y. Enhancement effect of silver nanoparticles on fermentative biohydrogen production using mixed bacteria. Bioresour. Technol. 2013, 142, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Taherdanak, M.; Zilouei, H.; Karimi, K. The effects of fe0 and ni0 nanoparticles versus fe2+ and ni2+ ions on dark hydrogen fermentation. Int. J. Hydrog. Energy 2016, 41, 167–173. [Google Scholar] [CrossRef]
- Nath, D.; Manhar, A.K.; Gupta, K.; Saikia, D.; Das, S.K.; Mandal, M. Phytosynthesized iron nanoparticles: Effects on fermentative hydrogen production by enterobacter cloacae dh-89. Bull. Mater. Sci. 2015, 38, 1533–1538. [Google Scholar] [CrossRef]
- Mahmood, T. Effect of iron nanoparticles on hyacinth’ s fermentation. Int. J. Sci. 2013, 2, 106–121. [Google Scholar]
- Zhang, L.; Zhang, L.; Li, D. Enhanced dark fermentative hydrogen production by zero-valent iron activated carbon micro-electrolysis. Int. J. Hydrog. Energy 2015, 40, 12201–12208. [Google Scholar] [CrossRef]
- Lin, R.; Cheng, J.; Ding, L.; Song, W.; Liu, M.; Zhou, J.; Cen, K. Enhanced dark hydrogen fermentation by addition of ferric oxide nanoparticles using enterobacter aerogenes. Bioresour. Technol. 2016, 207, 213–219. [Google Scholar] [CrossRef]
- Nasr, M.; Tawfik, A.; Ookawara, S.; Suzuki, M.; Kumari, S.; Bux, F. Continuous biohydrogen production from starch wastewater via sequential dark-photo fermentation with emphasize on maghemite nanoparticles. J. Ind. Eng. Chem. 2015, 21, 500–506. [Google Scholar] [CrossRef]
- Reddy, K.; Nasr, M.; Kumari, S.; Kumar, S.; Gupta, S.K.; Enitan, A.M.; Bux, F. Biohydrogen production from sugarcane bagasse hydrolysate: Effects of ph, s/x, Fe2+, and magnetite nanoparticles. Environ. Sci. Pollut. Res. 2017, 24, 8790–8804. [Google Scholar] [CrossRef]
- Dolly, S.; Pandey, A.; Pandey, B.K.; Gopal, R. Process parameter optimization and enhancement of photo-biohydrogen production by mixed culture of rhodobacter sphaeroides nmbl-02 and escherichia coli nmbl-04 using fe-nanoparticle. Int. J. Hydrog. Energy 2015, 40, 16010–16020. [Google Scholar] [CrossRef]
- Zada, B.; Mahmood, T.; Malik, S.A. Effect of zinc oxide nanoparticles on hyacinth’s fermentation. Int. J. Enhanc. Res. Sci. Technol. Eng. 2014, 3, 78–92. [Google Scholar]
- Pandey, A.; Gupta, K.; Pandey, A. Effect of nanosized tio2 on photofermentation by rhodobacter sphaeroides nmbl-02. Biomass Bioenergy 2015, 72, 273–279. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, Y. Nano-tio2 enhanced photofermentative hydrogen produced from the dark fermentation liquid of waste activated sludge. Environ. Sci. Technol. 2011, 45, 8589–8595. [Google Scholar] [CrossRef] [PubMed]
- Giannelli, L.; Torzillo, G. Hydrogen production with the microalga chlamydomonas reinhardtii grown in a compact tubular photobioreactor immersed in a scattering light nanoparticle suspension. Int. J. Hydrog. Energy 2012, 37, 16951–16961. [Google Scholar] [CrossRef]
- Mullai, P.; Yogeswari, M.K.; Sridevi, K. Optimisation and enhancement of biohydrogen production using nickel nanoparticles—A novel approach. Bioresour. Technol. 2013, 141, 212–219. [Google Scholar] [CrossRef]
- Patel, S.K.S.; Lee, J.-K.; Kalia, V.C. Nanoparticles in biological hydrogen production: An overview. Indian J. Microbiol. 2018, 58, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Kanwal, F.; Tahir, A.; Shah, S.A.Q.; Tsuzuki, T.; Nisbet, D.; Chen, J.; Rehman, Y. Effect of phyto-fabricated nanoscale organic-iron complex on photo-fermentative hydrogen production by rhodopseudomonas palustris mp2 and rhodopseudomonas palustris mp4. Biomass Bioenergy 2020, 140, 105667. [Google Scholar] [CrossRef]
- Liu, Z.; Lv, F.; Zheng, H.; Zhang, C.; Wei, F.; Xing, X.-H. Enhanced hydrogen production in a uasb reactor by retaining microbial consortium onto carbon nanotubes (cnts). Int. J. Hydrog. Energy 2012, 37, 10619–10626. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).