Chlorogenic Acid Relieves the Lupus Erythematosus-like Skin Lesions and Arthritis in MRL/lpr Mice
Abstract
:1. Introduction
2. Results
2.1. CGA Relieves Incidence of Skin Damage in MRL/lpr Mice
2.2. CGA Ameliorates Epidermal Layer Damage and Dermal Mast Cells in MRL/lpr Mice
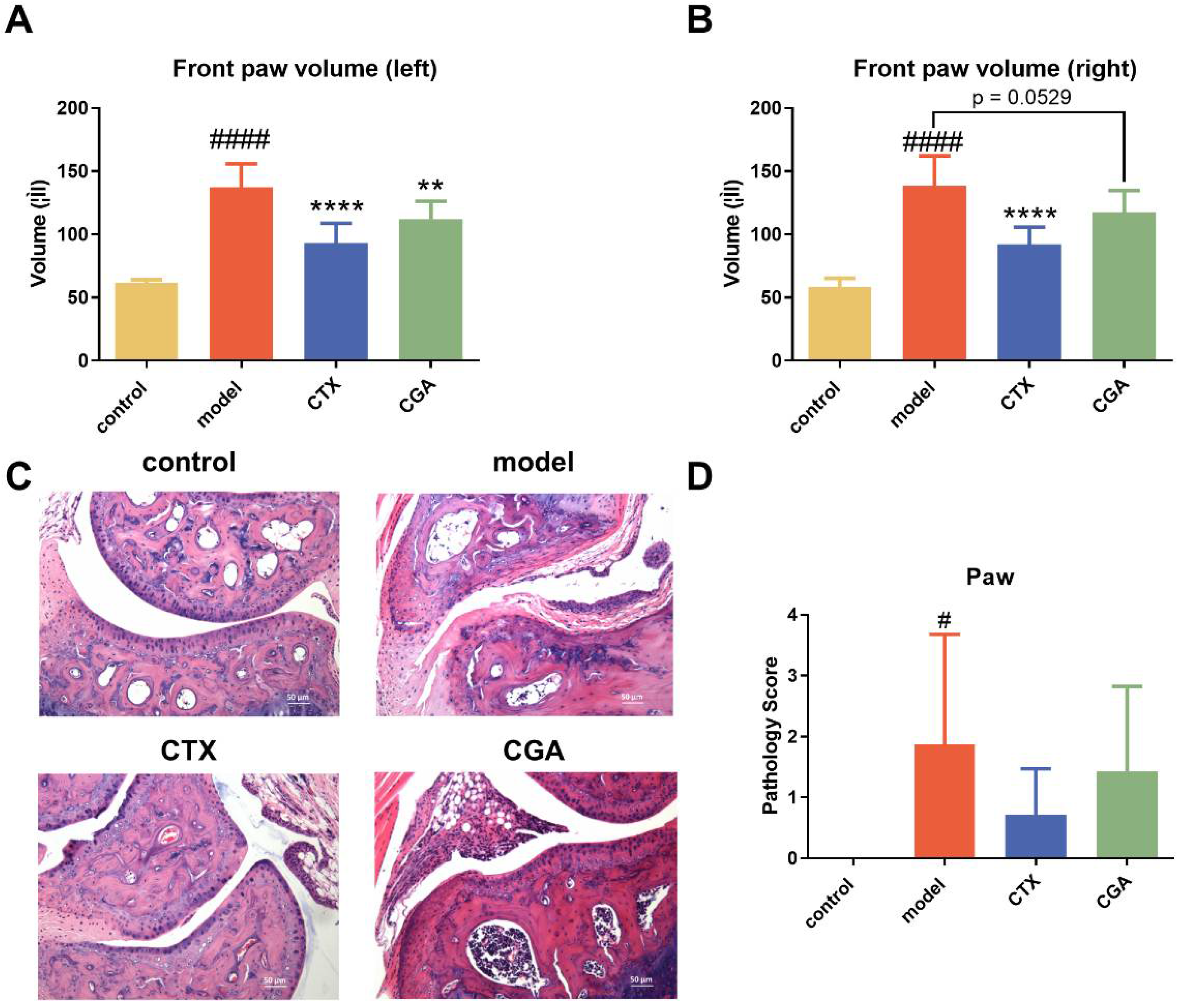
2.3. CGA Treatment Alleviates Lupus-like Arthritis in MRL/lpr Mice
2.4. CGA Decreased Serum Anti-dsDNA Antibody Concentration
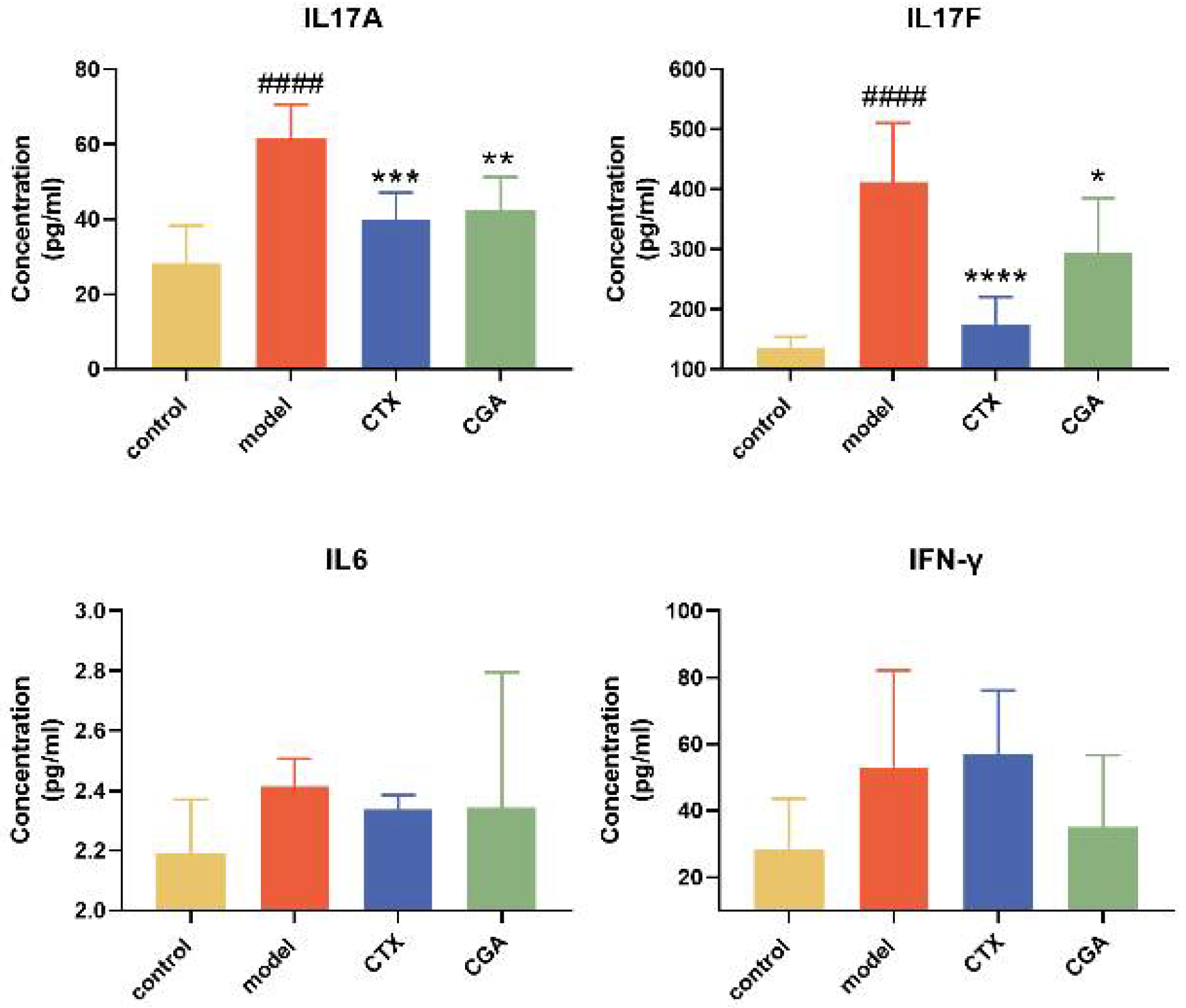
2.5. CGA Rebalances Serum Inflammatory Cytokines
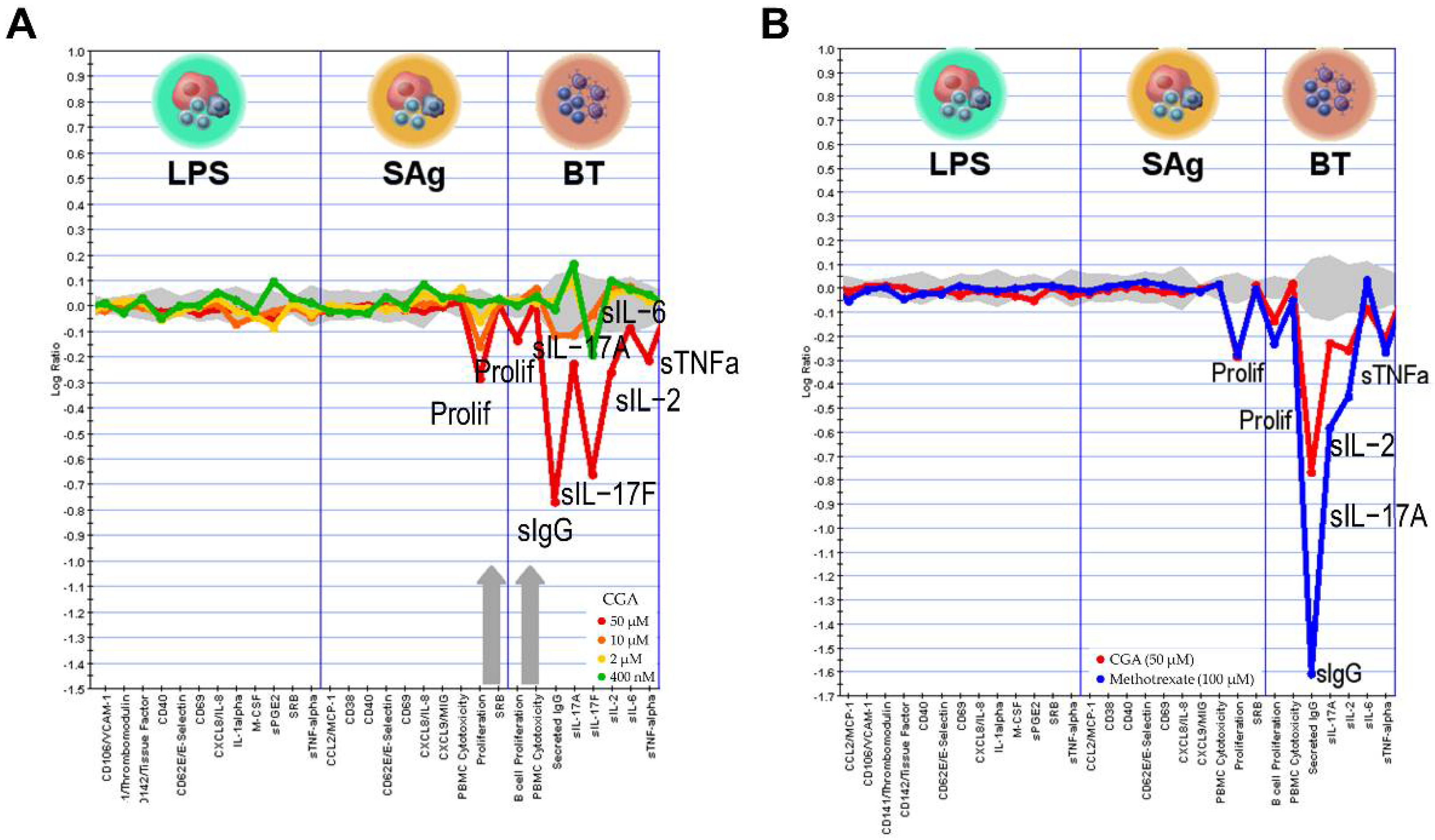
2.6. Effect of CGA on Human Primary Cell
3. Discussion
4. Materials and Methods
4.1. Mice and Treatment
4.2. Chemicals
4.3. Pathology of Skin and Joint Tissues
4.4. Measurement of Serum Antibodies
4.5. Serum ELISA for Inflammatory Cytokines
4.6. BioMAP Profiling Analysis
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Durcan, L.; O’Dwyer, T.; Petri, M. Management Strategies and Future Directions for Systemic Lupus Erythematosus in Adults. Lancet 2019, 393, 2332–2343. [Google Scholar] [CrossRef]
- Kiriakidou, M.; Ching, C.L. Systemic Lupus Erythematosus. Ann. Intern. Med. 2020, 172, ITC81–ITC96. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Zhao, M.; Chang, C.; Lu, Q. The Real Culprit in Systemic Lupus Erythematosus: Abnormal Epigenetic Regulation. Int. J. Mol. Sci. 2015, 16, 11013. [Google Scholar] [CrossRef] [PubMed]
- Pisetsky, D.S. Evolving Story of Autoantibodies in Systemic Lupus Erythematosus. J. Autoimmun. 2020, 110, 102356. [Google Scholar] [CrossRef]
- Pan, L.; Lu, M.P.; Wang, J.H.; Xu, M.; Yang, S.R. Immunological Pathogenesis and Treatment of Systemic Lupus Erythematosus. World J. Pediatr. 2020, 16, 19. [Google Scholar] [CrossRef] [Green Version]
- Barber, M.R.W.; Drenkard, C.; Falasinnu, T.; Hoi, A.; Mak, A.; Kow, N.Y.; Svenungsson, E.; Peterson, J.; Clarke, A.E.; Ramsey-Goldman, R. Global Epidemiology of Systemic Lupus Erythematosus. Nat. Rev. Rheumatol. 2021, 17, 515–532. [Google Scholar] [CrossRef]
- Allen, M.E.; Rus, V.; Szeto, G.L. Leveraging Heterogeneity in Systemic Lupus Erythematosus for New Therapies. Trends Mol. Med. 2021, 27, 152. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Tian, Z.; Cui, Y.; Liu, Z.; Ma, X. Chlorogenic Acid: A Comprehensive Review of the Dietary Sources, Processing Effects, Bioavailability, Beneficial Properties, Mechanisms of Action, and Future Directions. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3130–3158. [Google Scholar] [CrossRef]
- Xue, N.; Zhou, Q.; Ji, M.; Jin, J.; Lai, F.; Chen, J.; Zhang, M.; Jia, J.; Yang, H.; Zhang, J.; et al. Chlorogenic Acid Inhibits Glioblastoma Growth through Repolarizating Macrophage from M2 to M1 Phenotype. Sci. Rep. 2017, 7, 39011. [Google Scholar] [CrossRef] [Green Version]
- Ye, J.; Gao, Y.; Ji, M.; Yang, Y.; Wang, Z.; Wang, B.; Jin, J.; Li, L.; Wang, H.; Xu, X.; et al. Original Research: Oral SMEDDS Promotes Lymphatic Transport and Mesenteric Lymph Nodes Target of Chlorogenic Acid for Effective T-Cell Antitumor Immunity. J. Immunother. Cancer 2021, 9, e002753. [Google Scholar] [CrossRef]
- Zheng, Z.; Shi, L.; Sheng, Y.; Zhang, J.; Lu, B.; Ji, L. Chlorogenic Acid Suppresses Monocrotaline-Induced Sinusoidal Obstruction Syndrome: The Potential Contribution of NFκB, Egr1, Nrf2, MAPKs and PI3K Signals. Environ. Toxicol. Pharmacol. 2016, 46, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Hile, G.A.; Michelle Kahlenberg, J. Immunopathogenesis of Skin Injury in SLE. Curr. Opin. Rheumatol. 2021, 33, 173. [Google Scholar] [CrossRef] [PubMed]
- Shimomatsu, T.; Kanazawa, N.; Mikita, N.; Nakatani, Y.; Li, H.J.; Inaba, Y.; Ikeda, T.; Kondo, T.; Furukawa, F. The Effect of Hydroxychloroquine on Lupus Erythematosus-like Skin Lesions in MRL/Lpr Mice. Mod. Rheumatol. 2016, 26, 744–748. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, O.P.; Ryu, M.; Kantner, C.; Sárdy, M.; Naylor, D.; Lambert, D.; Brown, R.; Anders, H.J. Recombinant Chaperonin 10 Suppresses Cutaneous Lupus and Lupus Nephritis in MRL-(Fas)Lpr Mice. Nephrol. Dial. Transplant. 2012, 27, 1358–1367. [Google Scholar] [CrossRef] [Green Version]
- Kunkel, E.J.; Plavec, I.; Nguyen, D.; Melrose, J.; Rosler, E.S.; Kao, L.T.; Wang, Y.; Hytopoulos, E.; Bishop, A.C.; Bateman, R.; et al. Rapid Structure-Activity and Selectivity Analysis of Kinase Inhibitors by BioMAP Analysis in Complex Human Primary Cell-Based Models. Assay Drug Dev. Technol. 2004, 2, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Haselmayer, P.; Camps, M.; Muzerelle, M.; El Bawab, S.; Waltzinger, C.; Bruns, L.; Abla, N.; Polokoff, M.A.; Jond-Necand, C.; Gaudet, M.; et al. Characterization of Novel PI3Kδ Inhibitors as Potential Therapeutics for SLE and Lupus Nephritis in Pre-Clinical Studies. Front. Immunol. 2014, 5, 233. [Google Scholar] [CrossRef] [Green Version]
- Kuhn, A.; Landmann, A.; Bonsmann, G. The Skin in Autoimmune Diseases—Unmet Needs. Autoimmun. Rev. 2016, 15, 948–954. [Google Scholar] [CrossRef]
- Li, Q.; Wu, H.; Liao, W.; Zhao, M.; Chan, V.; Li, L.; Zheng, M.; Chen, G.; Zhang, J.; Lau, C.S.; et al. A Comprehensive Review of Immune-Mediated Dermatopathology in Systemic Lupus Erythematosus. J. Autoimmun. 2018, 93, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Gatto, M.; Zen, M.; Iaccarino, L.; Doria, A. New Therapeutic Strategies in Systemic Lupus Erythematosus Management. Nat. Rev. Rheumatol. 2018, 15, 30–48. [Google Scholar] [CrossRef]
- Deeks, E.D. Anifrolumab: First Approval. Drugs 2021, 81, 1795–1802. [Google Scholar] [CrossRef]
- Dhillon, S. Telitacicept: First Approval. Drugs 2021, 81, 1671–1675. [Google Scholar] [CrossRef] [PubMed]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Orhan, I.E.; Banach, M.; Rollinger, J.M.; Barreca, D.; Weckwerth, W.; Bauer, R.; Bayer, E.A.; et al. Natural Products in Drug Discovery: Advances and Opportunities. Nat. Rev. Drug Discov. 2021, 20, 200. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Pan, X.; Jiang, L.; Chu, Y.; Gao, S.; Jiang, X.; Zhang, Y.; Chen, Y.; Luo, S.; Peng, C. The Biological Activity Mechanism of Chlorogenic Acid and Its Applications in Food Industry: A Review. Front. Nutr. 2022, 9, 943911. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Park, J.H.; Kim, Y.S.; Han, Y. Chlorogenic Acid, a Polyphenolic Compound, Treats Mice with Septic Arthritis Caused by Candida Albicans. Int. Immunopharmacol. 2008, 8, 1681–1685. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Jiang, W.; Chen, X.; Xu, M.; Wang, J.; Lai, Y.; Zha, D. Chlorogenic Acid Ameliorated Allergic Rhinitis-Related Symptoms in Mice by Regulating Th17 Cells. Biosci. Rep. 2020, 40, 20201643. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, P.S.; Satti, N.K.; Sharma, P.; Sharma, V.K.; Suri, K.A.; Bani, S. Differential Effects of Chlorogenic Acid on Various Immunological Parameters Relevant to Rheumatoid Arthritis. Phytother. Res. 2012, 26, 1156–1165. [Google Scholar] [CrossRef]
- Wang, D.; Huang, S.; Yuan, X.; Liang, J.; Xu, R.; Yao, G.; Feng, X.; Sun, L. The Regulation of the Treg/Th17 Balance by Mesenchymal Stem Cells in Human Systemic Lupus Erythematosus. Cell. Mol. Immunol. 2017, 14, 423. [Google Scholar] [CrossRef] [Green Version]
- Shan, J.; Jin, H.; Xu, Y. T Cell Metabolism: A New Perspective on Th17/Treg Cell Imbalance in Systemic Lupus Erythematosus. Front. Immunol. 2020, 11, 1027. [Google Scholar] [CrossRef]
- Miossec, P.; Kolls, J.K. Targeting IL-17 and TH17 Cells in Chronic Inflammation. Nat. Rev. Drug Discov. 2012, 11, 763–776. [Google Scholar] [CrossRef]
- Rafael-Vidal, C.; Pérez, N.; Altabás, I.; Garcia, S.; Pego-Reigosa, J.M. Blocking IL-17: A Promising Strategy in the Treatment of Systemic Rheumatic Diseases. Int. J. Mol. Sci. 2020, 21, 7100. [Google Scholar] [CrossRef]
- King, E.M.; Mazor, R.; Çuburu, N.; Pastan, I. Low-Dose Methotrexate Prevents Primary and Secondary Humoral Immune Responses and Induces Immune Tolerance to a Recombinant Immunotoxin. J. Immunol. 2018, 200, 2038. [Google Scholar] [CrossRef] [PubMed]
- Cronstein, B.N.; Aune, T.M. Methotrexate and Its Mechanisms of Action in Inflammatory Arthritis. Nat. Rev. Rheumatol. 2020, 16, 145–154. [Google Scholar] [CrossRef]
- Brown, P.M.; Pratt, A.G.; Isaacs, J.D. Mechanism of Action of Methotrexate in Rheumatoid Arthritis, and the Search for Biomarkers. Nat. Rev. Rheumatol. 2016, 12, 731–742. [Google Scholar] [CrossRef] [PubMed]
- Berg, E.L.; Kunkel, E.J.; Hytopoulos, E.; Plavec, I. Characterization of Compound Mechanisms and Secondary Activities by BioMAP Analysis. J. Pharmacol. Toxicol. Methods 2006, 53, 67–74. [Google Scholar] [CrossRef]
- Xu, D.; Kim, Y.; Postelnek, J.; Vu, M.D.; Hu, D.Q.; Liao, C.; Bradshaw, M.; Hsu, J.; Zhang, J.; Pashine, A.; et al. RN486, a Selective Bruton’s Tyrosine Kinase Inhibitor, Abrogates Immune Hypersensitivity Responses and Arthritis in Rodents. J. Pharmacol. Exp. Ther. 2012, 341, 90–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berg, E.L.; Yang, J.; Melrose, J.; Nguyen, D.; Privat, S.; Rosler, E.; Kunkel, E.J.; Ekins, S. Chemical Target and Pathway Toxicity Mechanisms Defined in Primary Human Cell Systems. J. Pharmacol. Toxicol. Methods 2010, 61, 3–15. [Google Scholar] [CrossRef]



| Acanthosis | Hyperkeratosis | Skin Mast Cells | Mast Cell Reduction Rate (%) | |||
|---|---|---|---|---|---|---|
| Incidence * | Chi-Squared Statistic | Incidence # | Chi-Squared Statistic | |||
| model | 100% (0/8) | 63% (5/8) | 96.375 | |||
| CTX | 29% (2/7) | p = 0.007 | 29% (2/7) | p = 0.315 | 24.4286 | 75% |
| CGA | 29% (2/7) | p = 0.007 | 0 (0/3) | p = 0.026 | 47.8571 | 50% |
| System | Cell Type | Environment | Readouts |
|---|---|---|---|
LPS | Peripheral blood mononuclear cells + Venular endothelial cells | TLR4 | MCP-1, VCAM-1, TM, TF, CD40, E-selectin, CD69, IL-8, IL-1α, M-CSF, sPGE2, SRB, sTNFα |
SAg | Peripheral blood mononuclear cells + Venular endothelial cells | Superantigens (TCR) | MCP-1, CD38, CD40, E-selectin, CD69, IL-8, MIG, PBMC Cytotoxicity, Proliferation, SRB |
BT | Peripheral blood mononuclear cells + B cells | Anti-IgM, TCR | B cell Proliferation, PBMC Cytotoxicity, Secreted IgG, sIL-17A, sIL-17F, sIL-2, sIL-6, sTNFα |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, R.; Yang, X.; You, S.; Hao, M.; Li, J.; Chen, X.; Jin, J. Chlorogenic Acid Relieves the Lupus Erythematosus-like Skin Lesions and Arthritis in MRL/lpr Mice. Pharmaceuticals 2022, 15, 1327. https://doi.org/10.3390/ph15111327
Wang R, Yang X, You S, Hao M, Li J, Chen X, Jin J. Chlorogenic Acid Relieves the Lupus Erythematosus-like Skin Lesions and Arthritis in MRL/lpr Mice. Pharmaceuticals. 2022; 15(11):1327. https://doi.org/10.3390/ph15111327
Chicago/Turabian StyleWang, Ruxuan, Xiaoyi Yang, Shen You, Mengyao Hao, Jianguang Li, Xiaoguang Chen, and Jing Jin. 2022. "Chlorogenic Acid Relieves the Lupus Erythematosus-like Skin Lesions and Arthritis in MRL/lpr Mice" Pharmaceuticals 15, no. 11: 1327. https://doi.org/10.3390/ph15111327
APA StyleWang, R., Yang, X., You, S., Hao, M., Li, J., Chen, X., & Jin, J. (2022). Chlorogenic Acid Relieves the Lupus Erythematosus-like Skin Lesions and Arthritis in MRL/lpr Mice. Pharmaceuticals, 15(11), 1327. https://doi.org/10.3390/ph15111327






