CD36-Fatty Acid-Mediated Metastasis via the Bidirectional Interactions of Cancer Cells and Macrophages
Abstract
:1. Introduction
2. CD36 Receptor: Gene, Structure, Distribution and Function
2.1. CD36 Discovery and Structure-Function
2.2. CD36 Distribution and Functions
3. Metabolic Reprogramming and Metastasis
3.1. CD36 Responds to Exogenous Fatty Acids
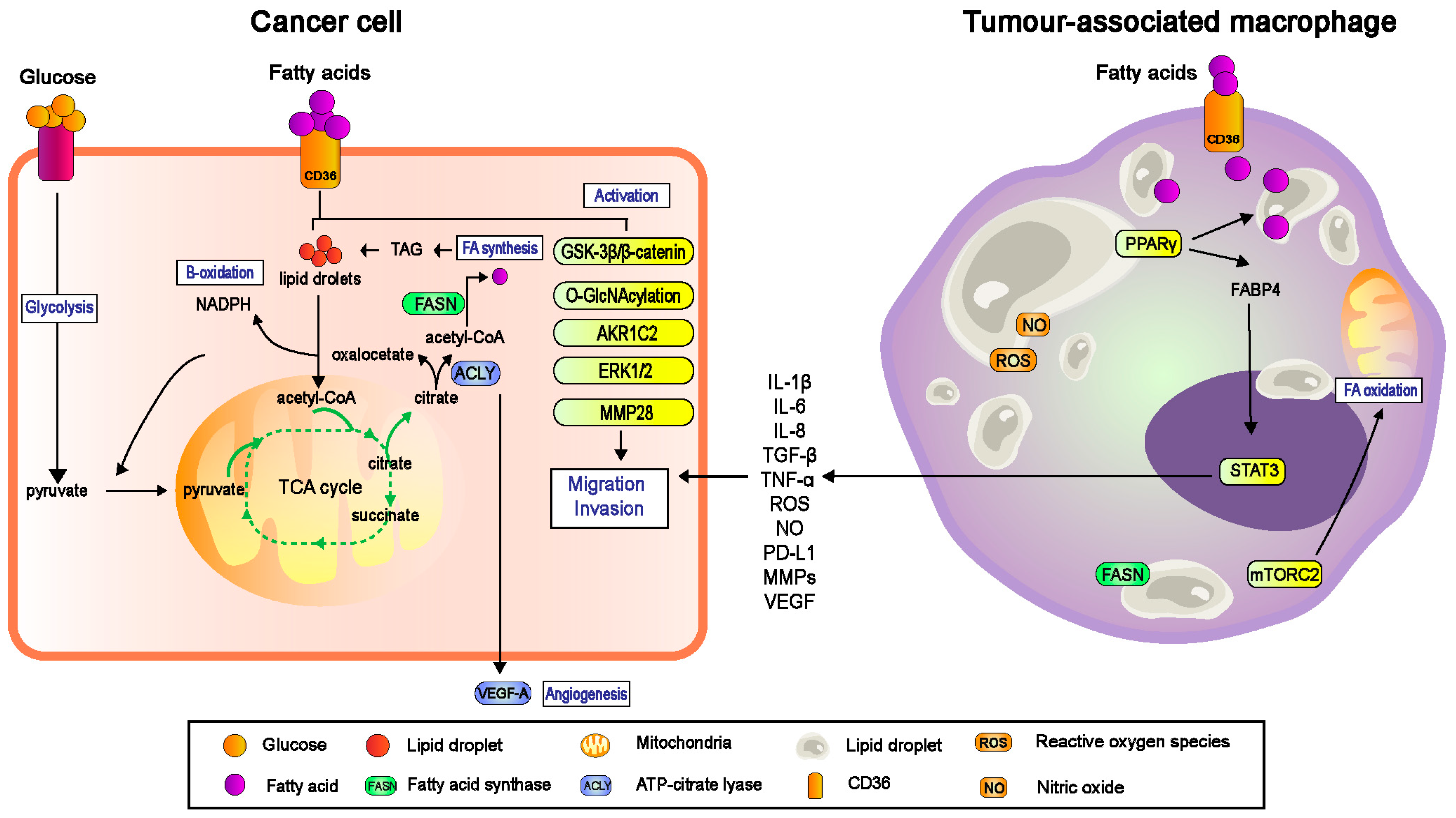
3.2. CD36 and Metabolic Symbiosis
4. Involvement of CD36 and Macrophages in Metastasis
4.1. CD36 Regulated TAMs-Facilitated Metastasis in TME
4.1.1. TAMs and Their Pro-Tumorigenic Functions
4.1.2. CD36-Mediated Lipid Droplet Accumulation in TAMs
4.1.3. TAMs-Mediated Migration and Invasion
4.2. CD36 Targeted Nano-Immunotherapy
5. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liberti, M.V.; Locasale, J.W. The Warburg Effect: How Does It Benefit Cancer Cells? Trends Biochem. Sci. 2016, 41, 211–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schiliro, C.; Firestein, B.L. Mechanisms of Metabolic Reprogramming in Cancer Cells Supporting Enhanced Growth and Proliferation. Cells 2021, 10, 1056. [Google Scholar] [CrossRef] [PubMed]
- DeBerardinis, R.J.; Chandel, N.S. Fundamentals of cancer metabolism. Sci. Adv. 2016, 2, e1600200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gouirand, V.; Guillaumond, F.; Vasseur, S. Influence of the Tumor Microenvironment on Cancer Cells Metabolic Repro-gramming. Front. Oncol. 2018, 8, 117. [Google Scholar] [CrossRef]
- Chen, Y.-J.; Liao, W.-X.; Huang, S.-Z.; Yu, Y.-F.; Wen, J.-Y.; Chen, J.; Lin, D.-G.; Wu, X.-Y.; Jiang, N.; Li, X. Prognostic and immunological role of CD36: A pan-cancer analysis. J. Cancer 2021, 12, 4762–4773. [Google Scholar] [CrossRef]
- Gharib-Naseri, K.; Heras-Saldana, S.D.L.; Kheravii, S.; Qin, L.; Wang, J.; Wu, S.-B. Necrotic enteritis challenge regulates peroxisome proliferator-1 activated receptors signaling and β-oxidation pathways in broiler chickens. Anim. Nutr. 2020, 7, 239–251. [Google Scholar] [CrossRef]
- Kobylka, D.; Carraway, K.L. Proteolytic digestion of proteins of the milk fat globule membrane. Biochim. Biophys. Acta (BBA)-Biomembr. 1973, 307, 133–140. [Google Scholar] [CrossRef]
- Tandon, N.N.; Kralisz, U.; Jamieson, G.A. Identification of glycoprotein IV (CD36) as a primary receptor for platelet-collagen adhesion. J. Biol. Chem. 1989, 264, 7576–7583. [Google Scholar] [CrossRef]
- Asch, A.S.; Barnwell, J.; Silverstein, R.L.; Nachman, R.L. Isolation of the thrombospondin membrane receptor. J. Clin. Investig. 1987, 79, 1054–1061. [Google Scholar] [CrossRef]
- Endemann, G.; Stanton, L.W.; Madden, K.S.; Bryant, C.M.; White, R.T.; Protter, A.A. CD36 is a receptor for oxidized low density lipoprotein. J. Biol. Chem. 1993, 268, 11811–11816. [Google Scholar] [CrossRef]
- Abumrad, N.; El-Maghrabi, M.; Amri, E.; Lopez, E.; Grimaldi, P. Cloning of a rat adipocyte membrane protein implicated in binding or transport of long-chain fatty acids that is induced during preadipocyte differentiation. Homology with human CD36. J. Biol. Chem. 1993, 268, 17665–17668. [Google Scholar] [CrossRef]
- Fernández-Ruiz, E.; Armesilla, A.L.; Sánchez-Madrid, F.; Vega, M.A. Gene Encoding the Collagen Type I and Thrombospondin Receptor CD36 Is Located on Chromosome 7q11.2. Genomics 1993, 17, 759–761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Enciu, A.M.; Radu, E.; Popescu, I.D.; Hinescu, M.E.; Ceafalan, L.C. Targeting CD36 as Biomarker for Metastasis Prognostic: How Far from Translation into Clinical Practice? BioMed Res. Int. 2018, 2018, 7801202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pepino, M.Y.; Kuda, O.; Samovski, D.; Abumrad, N.A. Structure-Function of CD36 and Importance of Fatty Acid Signal Transduction in Fat Metabolism. Annu. Rev. Nutr. 2014, 34, 281–303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tao, N.; Wagner, S.J.; Lublin, D.M. CD36 Is Palmitoylated on Both N- and C-terminal Cytoplasmic Tails. J. Biol. Chem. 1996, 271, 22315–22320. [Google Scholar] [CrossRef] [Green Version]
- De Filippo, E.; Namasivayam, V.; Zappe, L.; El-Tayeb, A.; Schiedel, A.C.; Müller, C.E. Role of extracellular cysteine residues in the adenosine A2A receptor. Purinergic Signal. 2016, 12, 313–329. [Google Scholar] [CrossRef] [Green Version]
- Stanchev, L.; Marek, M.; Xian, F.; Klöhn, M.; Silvestro, D.; Dittmar, G.; López-Marqués, R.; Pomorski, T.G. Functional Significance of Conserved Cysteines in the Extracellular Loops of the ATP Binding Cassette Transporter Pdr11p. J. Fungi 2021, 7, 2. [Google Scholar] [CrossRef]
- Neculai, D.; Schwake, M.; Ravichandran, M.; Zunke, F.; Collins, R.F.; Peters, J.; Neculai, M.; Plumb, J.; Loppnau, P.; Pizarro, J.C.; et al. Structure of LIMP-2 provides functional insights with implications for SR-BI and CD36. Nature 2013, 504, 172–176. [Google Scholar] [CrossRef]
- Zingg, J.-M.; Vlad, A.; Ricciarelli, R. Oxidized LDLs as Signaling Molecules. Antioxidants 2021, 10, 1184. [Google Scholar] [CrossRef]
- Syed Najmuddin, S.U.F.; Romli, M.F.; Hamid, M.; Alitheen, N.B.; Nik Abd Rahman, N.M.A. Anti-cancer effect of Annona Muricata Linn Leaves Crude Extract (AMCE) on breast cancer cell line. BMC Complement. Altern. Med. 2016, 16, 311. [Google Scholar] [CrossRef]
- Huang, T.; Sun, L.; Yuan, X.; Qiu, H. Thrombospondin-1 is a multifaceted player in tumor progression. Oncotarget 2017, 8, 84546–84558. [Google Scholar] [CrossRef] [Green Version]
- Kourtzelis, I.; Hajishengallis, G.; Chavakis, T. Phagocytosis of Apoptotic Cells in Resolution of Inflammation. Front. Immunol. 2020, 11, 553. [Google Scholar] [CrossRef] [PubMed]
- Kigerl, K.A.; de Rivero Vaccari, J.P.; Dietrich, W.D.; Popovich, P.G.; Keane, R.W. Pattern recognition receptors and central nervous system repair. Exp. Neurol. 2014, 258, 5–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woo, M.-S.; Yang, J.; Beltran, C.; Cho, S. Cell Surface CD36 Protein in Monocyte/Macrophage Contributes to Phagocytosis during the Resolution Phase of Ischemic Stroke in Mice. J. Biol. Chem. 2016, 291, 23654–23661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hao, J.-W.; Wang, J.; Guo, H.; Zhao, Y.-Y.; Sun, H.-H.; Li, Y.-F.; Lai, X.-Y.; Zhao, N.; Wang, X.; Xie, C.; et al. CD36 facilitates fatty acid uptake by dynamic palmitoylation-regulated endocytosis. Nat. Commun. 2020, 11, 4765. [Google Scholar] [CrossRef]
- Pietka, T.A.; Schappe, T.; Conte, C.; Fabbrini, E.; Patterson, B.W.; Klein, S.; Abumrad, N.A.; Love-Gregory, L. Adipose and Muscle Tissue Profile of CD36 Transcripts in Obese Subjects Highlights the Role of CD36 in Fatty Acid Homeostasis and Insulin Resistance. Diabetes Care 2014, 37, 1990–1997. [Google Scholar] [CrossRef] [Green Version]
- Glatz, J.F.C.; Luiken, J.J.F.P. Dynamic role of the transmembrane glycoprotein CD36 (SR-B2) in cellular fatty acid uptake and utilization. J. Lipid Res. 2018, 59, 1084–1093. [Google Scholar] [CrossRef] [Green Version]
- Yusuf, B.; Mukovozov, I.; Patel, S.; Huang, Y.-W.; Liu, G.Y.; Reddy, E.C.; Skrtic, M.; Glogauer, M.; Robinson, L.A. The neurorepellent, Slit2, prevents macrophage lipid loading by inhibiting CD36-dependent binding and internalization of oxidized low-density lipoprotein. Sci. Rep. 2021, 11, 3614. [Google Scholar] [CrossRef]
- Espinosa-Cueto, P.; Magallanes-Puebla, A.; Castellanos, C.; Mancilla, R. Dendritic cells that phagocytose apoptotic macrophages loaded with mycobacterial antigens activate CD8 T cells via cross-presentation. PLoS ONE 2017, 12, e0182126. [Google Scholar] [CrossRef] [Green Version]
- Zaidi, N.E.; Shazali, N.A.H.; Chor, A.L.T.; Osman, M.A.; Ibrahim, K.; Jaoi-Edward, M.; Rahman, N.M.A.N.A. Time-Lapse 2D Imaging of Phagocytic Activity in M1 Macrophage-4T1 Mouse Mammary Carcinoma Cells in Co-cultures. J. Vis. Exp. 2019, 154, e60281. [Google Scholar] [CrossRef]
- Schönfeld, P.; Wojtczak, L. Short- and Medium-Chain Fatty Acids in Energy Metabolism: The Cellular Perspective. J. Lipid Res. 2016, 57, 943–954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shu, H.; Peng, Y.; Hang, W.; Nie, J.; Zhou, N.; Wang, D.W. The role of CD36 in cardiovascular disease. Cardiovasc. Res. 2020, 118, 115–129. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.S.; Karunakaran, U.; Suma, E.; Chung, S.M.; Won, K.C. The Role of CD36 in Type 2 Diabetes Mellitus: β-Cell Dys-function and Beyond. Diabetes Metab. J. 2020, 44, 222–233. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, H.; Zhang, Z.; Lu, W.; Zhu, J.; Shi, J. IL-6 promotes chemoresistance via upregulating CD36 mediated fatty acids uptake in acute myeloid leukemia. Exp. Cell Res. 2022, 415, 113112. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Wilkins, O.; Bang, S.; Ung, M.; Li, J.; An, J.; Del Genio, C.; Canfield, K.; DiRenzo, J.; Wells, W.; et al. CD36-Mediated Metabolic Rewiring of Breast Cancer Cells Promotes Resistance to HER2-Targeted Therapies. Cell Rep. 2019, 29, 3405–3420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, Y.; Han, H.; Liu, L.; Duan, Y.; Yang, X.; Ma, C.; Zhu, Y.; Han, J.; Li, X.; Chen, Y. CD36 plays a critical role in proliferation, migration and tamoxifen-inhibited growth of ER-positive breast cancer cells. Oncogenesis 2018, 7, 98. [Google Scholar] [CrossRef] [Green Version]
- Deng, M.; Cai, X.; Long, L.; Xie, L.; Ma, H.; Zhou, Y.; Liu, S.; Zeng, C. CD36 promotes the epithelial–mesenchymal transition and metastasis in cervical cancer by interacting with TGF-β. J. Transl. Med. 2019, 17, 352. [Google Scholar] [CrossRef] [Green Version]
- Yang, P.; Su, C.; Luo, X.; Zeng, H.; Zhao, L.; Wei, L.; Zhang, X.; Varghese, Z.; Moorhead, J.F.; Chen, Y.; et al. Dietary oleic acid-induced CD36 promotes cervical cancer cell growth and metastasis via up-regulation Src/ERK pathway. Cancer Lett. 2018, 438, 76–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drury, J.; Rychahou, P.G.; Kelson, C.O.; Geisen, M.E.; Wu, Y.; He, D.; Wang, C.; Lee, E.Y.; Evers, B.M.; Zaytseva, Y.Y. Upregulation of CD36, a Fatty Acid Translocase, Promotes Colorectal Cancer Metastasis by Increasing MMP28 and Decreasing E-Cadherin Expression. Cancers 2022, 14, 252. [Google Scholar] [CrossRef]
- Drury, J.; Rychahou, P.G.; He, D.; Jafari, N.; Wang, C.; Lee, E.Y.; Weiss, H.L.; Evers, B.M.; Zaytseva, Y.Y. Inhibition of Fatty Acid Synthase Upregulates Expression of CD36 to Sustain Proliferation of Colorectal Cancer Cells. Front. Oncol. 2020, 10, 1185. [Google Scholar] [CrossRef]
- Aoki, T.; Kinoshita, J.; Munesue, S.; Hamabe-Horiike, T.; Yamaguchi, T.; Nakamura, Y.; Okamoto, K.; Moriyama, H.; Nakamura, K.; Harada, S.; et al. Hypoxia-Induced CD36 Expression in Gastric Cancer Cells Promotes Peritoneal Metastasis via Fatty Acid Uptake. Ann. Surg. Oncol. 2022, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Fan, Z.; Wang, Z.; Dai, Q.; Xiang, Z.; Yuan, F.; Yan, M.; Zhu, Z.; Liu, B.; Li, C. CD36 mediates palmitate acid-induced metastasis of gastric cancer via AKT/GSK-3β/β-catenin pathway. J. Exp. Clin. Cancer Res. 2019, 38, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hale, J.S.; Otvos, B.; Sinyuk, M.; Alvarado, A.G.; Hitomi, M.; Stoltz, K.; Wu, Q.; Flavahan, W.; Levison, B.; Johansen, M.L.; et al. Cancer Stem Cell-Specific Scavenger Receptor CD36 Drives Glioblastoma Progression. Stem Cells 2014, 32, 1746–1758. [Google Scholar] [CrossRef] [Green Version]
- Nath, A.; Li, I.; Roberts, L.R.; Chan, C. Elevated free fatty acid uptake via CD36 promotes epithelial-mesenchymal transition in hepatocellular carcinoma. Sci. Rep. 2015, 5, 14752. [Google Scholar] [CrossRef] [Green Version]
- Pascual, G.; Avgustinova, A.; Mejetta, S.; Martín, M.; Castellanos, A.; Attolini, C.S.-O.; Berenguer, A.; Prats, N.; Toll, A.; Hueto, J.A.; et al. Targeting metastasis-initiating cells through the fatty acid receptor CD36. Nature 2017, 541, 41–45. [Google Scholar] [CrossRef]
- Haidari, S.; Tröltzsch, M.; Knösel, T.; Liokatis, P.; Kasintsova, A.; Eberl, M.; Ortner, F.; Otto, S.; Fegg, F.; Boskov, M.; et al. Fatty Acid Receptor CD36 Functions as a Surrogate Parameter for Lymph Node Metastasis in Oral Squamous Cell Carcinoma. Cancers 2021, 13, 4125. [Google Scholar] [CrossRef] [PubMed]
- Ladanyi, A.; Mukherjee, A.; Kenny, H.A.; Johnson, A.; Mitra, A.K.; Sundaresan, S.; Nieman, K.M.; Pascual, G.; Benitah, S.A.; Montag, A.; et al. Adipocyte-induced CD36 expression drives ovarian cancer progression and metastasis. Oncogene 2018, 37, 2285–2301. [Google Scholar] [CrossRef]
- Sakurai, K.; Tomihara, K.; Yamazaki, M.; Heshiki, W.; Moniruzzaman, R.; Sekido, K.; Tachinami, H.; Ikeda, A.; Imaue, S.; Fujiwara, K.; et al. CD36 expression on oral squamous cell carcinoma cells correlates with enhanced proliferation and migratory activity. Oral Dis. 2020, 26, 745–755. [Google Scholar] [CrossRef]
- Jia, S.; Zhou, L.; Shen, T.; Zhou, S.; Ding, G.; Cao, L. Down-expression of CD36 in pancreatic adenocarcinoma and its correlation with clinicopathological features and prognosis. J. Cancer 2018, 9, 578–583. [Google Scholar] [CrossRef] [Green Version]
- Watt, M.J.; Clark, A.K.; Selth, L.A.; Haynes, V.R.; Lister, N.; Rebello, R.; Porter, L.H.; Niranjan, B.; Whitby, S.T.; Lo, J.; et al. Suppressing fatty acid uptake has therapeutic effects in preclinical models of prostate cancer. Sci. Transl. Med. 2019, 11, eaau5758. [Google Scholar] [CrossRef]
- Li, F.; Simon, M.C. Cancer Cells Don’t Live Alone: Metabolic Communication within Tumor Microenvironments. Dev. Cell 2020, 54, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Cucchi, D.; Camacho-Muñoz, D.; Certo, M.; Pucino, V.; Nicolaou, A.; Mauro, C. Fatty acids—From energy substrates to key regulators of cell survival, proliferation and effector function. Cell Stress 2020, 4, 9–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muscella, A.; Stefàno, E.; Lunetti, P.; Capobianco, L.; Marsigliante, S. The Regulation of Fat Metabolism during Aerobic Ex-ercise. Biomolecules 2020, 10, 1699. [Google Scholar] [CrossRef]
- Kendall, A.C.; Pilkington, S.M.; Massey, K.A.; Sassano, G.; Rhodes, L.E.; Nicolaou, A. Distribution of Bioactive Lipid Mediators in Human Skin. J. Investig. Dermatol. 2015, 135, 1510–1520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, C.-K.; Jeong, S.-H.; Jang, C.; Bae, H.; Kim, Y.H.; Park, I.; Kim, S.K.; Koh, G.Y. Tumor metastasis to lymph nodes requires YAP-dependent metabolic adaptation. Science 2019, 363, 644–649. [Google Scholar] [CrossRef] [Green Version]
- Ismail, R.; Allaudin, Z.N.; Abdullah, R.; Lila, M.-A.M.; Rahman, N.-M.N.A.; Rahman, S.-O.A. Combination of VP3 and CD147-knockdown enhance apoptosis and tumor growth delay index in colorectal tumor allograft. BMC Cancer 2016, 16, 461. [Google Scholar] [CrossRef] [Green Version]
- Nik-Mohd-Afizan, N.A.R.; Zeenathul, N.A.; Noordin, M.M.; Ruzila, I.; NorHidayah, M.; Mohd-Azmi, M.L. Apoptosis and Tumour Cell Death in Response to Pro-Apoptotic Gene. Pertanika J. Trop. Agric. Sci. 2011, 34, 163–166. [Google Scholar]
- Grabner, G.F.; Xie, H.; Schweiger, M.; Zechner, R. Lipolysis: Cellular mechanisms for lipid mobilization from fat stores. Nat. Metab. 2021, 3, 1445–1465. [Google Scholar] [CrossRef]
- Hussin, Y.; Aziz, M.N.M.; Rahim, N.F.C.; Yeap, S.K.; Mohamad, N.E.; Masarudin, M.J.; Nordin, N.; Rahman, N.M.A.-N.A.; Yong, C.Y.; Akhtar, M.N.; et al. DK1 Induces Apoptosis via Mitochondria-Dependent Signaling Pathway in Human Colon Carcinoma Cell Lines In Vitro. Int. J. Mol. Sci. 2018, 19, 1151. [Google Scholar] [CrossRef] [Green Version]
- Tang, Y.; Zhou, J.; Hooi, S.C.; Jiang, Y.M.; Lu, G.-D. Fatty acid activation in carcinogenesis and cancer development: Essential roles of long-chain acyl-CoA synthetases (Review). Oncol. Lett. 2018, 16, 1390–1396. [Google Scholar] [CrossRef] [Green Version]
- Fu, Y.; Zou, T.; Shen, X.; Nelson, P.J.; Li, J.; Wu, C.; Yang, J.; Zheng, Y.; Bruns, C.; Zhao, Y.; et al. Lipid metabolism in cancer progression and therapeutic strategies. MedComm 2021, 2, 27–59. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Wu, N.; Xu, B.; Chu, Y.; Li, X.; Su, S.; Chen, D.; Li, W.; Shi, Y.; Gao, X.; et al. Fatty acid-induced CD36 expression via O-GlcNAcylation drives gastric cancer metastasis. Theranostics 2019, 9, 5359–5373. [Google Scholar] [CrossRef] [PubMed]
- Pascual, G.; Domínguez, D.; Elosúa-Bayes, M.; Beckedorff, F.; Laudanna, C.; Bigas, C.; Douillet, D.; Greco, C.; Symeonidi, A.; Hernández, I.; et al. Dietary palmitic acid promotes a prometastatic memory via Schwann cells. Nature 2021, 599, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, D.; Lv, B.; Hou, X.; Liu, Q.; Liao, C.; Xu, R.; Zhang, Y.; Xu, F.; Zhang, P. Oleic Acid and Insulin as Key Characteristics of T2D Promote Colorectal Cancer Deterioration in Xenograft Mice Revealed by Functional Metabolomics. Front. Oncol. 2021, 11, 685059. [Google Scholar] [CrossRef] [PubMed]
- Morandi, V.; Petrik, J.; Lawler, J. Endothelial Cell Behavior Is Determined by Receptor Clustering Induced by Thrombos-pondin-1. Front. Cell Dev. Biol. 2021, 9, 721. [Google Scholar] [CrossRef] [PubMed]
- Gyamfi, J.; Yeo, J.H.; Kwon, D.; Min, B.S.; Cha, Y.J.; Koo, J.S.; Jeong, J.; Lee, J.; Choi, J. Interaction between CD36 and FABP4 modulates adipocyte-induced fatty acid import and metabolism in breast cancer. NPJ Breast Cancer 2021, 7, 129. [Google Scholar] [CrossRef] [PubMed]
- Kohandel, Z.; Farkhondeh, T.; Aschner, M.; Pourbagher-Shahri, A.M.; Samarghandian, S. STAT3 pathway as a molecular target for resveratrol in breast cancer treatment. Cancer Cell Int. 2021, 21, 468. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Chiang, C.-Y.; Daifotis, H.A.; Nieman, K.M.; Fahrmann, J.F.; Lastra, R.R.; Romero, I.L.; Fiehn, O.; Lengyel, E. Adipocyte-Induced FABP4 Expression in Ovarian Cancer Cells Promotes Metastasis and Mediates Carboplatin Resistance. Cancer Res. 2020, 80, 1748–1761. [Google Scholar] [CrossRef] [Green Version]
- Tan, Y.; Lin, K.; Zhao, Y.; Wu, Q.; Chen, D.; Wang, J.; Liang, Y.; Li, J.; Hu, J.; Wang, H.; et al. Adipocytes fuel gastric cancer omental metastasis via PITPNC1-mediated fatty acid metabolic reprogramming. Theranostics 2018, 8, 5452–5468. [Google Scholar] [CrossRef]
- Vecchione, R.; Quagliariello, V.; Giustetto, P.; Calabria, D.; Sathya, A.; Marotta, R.; Profeta, M.; Nitti, S.; Silvestri, N.; Pellegrino, T.; et al. Oil/water nano-emulsion loaded with cobalt ferrite oxide nanocubes for photo-acoustic and magnetic resonance dual imaging in cancer: In vitro and preclinical studies. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Virchow, R. As Based upon Physiological and Pathological Histology. Nutr. Rev. 2009, 47, 23–25. [Google Scholar] [CrossRef] [PubMed]
- Yahaya, M.A.F.; Lila, M.A.M.; Ismail, S.; Zainol, M.; Afizan, N. Tumour-Associated Macrophages (TAMs) in Colon Cancer and How to Reeducate Them. J. Immunol. Res. 2019, 2019, 2368249. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.; Xu, J.; Lan, H. Tumor-associated macrophages in tumor metastasis: Biological roles and clinical therapeutic applications. J. Hematol. Oncol. 2019, 12, 76. [Google Scholar] [CrossRef] [PubMed]
- Batista-Gonzalez, A.; Vidal, R.; Criollo, A.; Carreño, L.J. New Insights on the Role of Lipid Metabolism in the Metabolic Reprogramming of Macrophages. Front. Immunol. 2020, 10, 2993. [Google Scholar] [CrossRef]
- Nakamura, M.T.; Yudell, B.E.; Loor, J.J. Regulation of energy metabolism by long-chain fatty acids. Prog. Lipid Res. 2014, 53, 124–144. [Google Scholar] [CrossRef]
- Huang, S.C.-C.; Everts, B.; Ivanova, Y.; O’Sullivan, D.; Nascimento, M.; Smith, A.M.; Beatty, W.; Love-Gregory, L.; Lam, W.Y.; O’Neill, C.M.; et al. Cell-intrinsic lysosomal lipolysis is essential for alternative activation of macrophages. Nat. Immunol. 2014, 15, 846–855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, P.; Wang, Q.; Bi, E.; Ma, X.; Liu, L.; Yang, M.; Qian, J.; Yi, Q. Enhanced Lipid Accumulation and Metabolism Are Required for the Differentiation and Activation of Tumor-Associated Macrophages. Cancer Res. 2020, 80, 1438–1450. [Google Scholar] [CrossRef]
- Wu, K.; Lin, K.; Li, X.; Yuan, X.; Xu, P.; Ni, P.; Xu, D. Redefining Tumor-Associated Macrophage Subpopulations and Functions in the Tumor Microenvironment. Front. Immunol. 2020, 11, 1731. [Google Scholar] [CrossRef]
- Deng, Q.; Geng, Y.; Zhao, L.; Li, R.; Zhang, Z.; Li, K.; Liang, R.; Shao, X.; Huang, M.; Zuo, D.; et al. NLRP3 inflammasomes in macrophages drive colorectal cancer metastasis to the liver. Cancer Lett. 2018, 442, 21–30. [Google Scholar] [CrossRef]
- Xu, Y.; Li, H.; Chen, W.; Yao, X.; Xing, Y.; Wang, X.; Zhong, J.; Meng, G. Mycoplasma hyorhinis Activates the NLRP3 Inflammasome and Promotes Migration and Invasion of Gastric Cancer Cells. PLoS ONE 2013, 8, e77955. [Google Scholar] [CrossRef] [Green Version]
- Hofbauer, D.; Mougiakakos, D.; Broggini, L.; Zaiss, M.; Büttner-Herold, M.; Bach, C.; Spriewald, B.; Neumann, F.; Bisht, S.; Nolting, J.; et al. β2-microglobulin triggers NLRP3 inflammasome activation in tumor-associated macrophages to promote multiple myeloma progression. Immunity 2021, 54, 1772–1787. [Google Scholar] [CrossRef] [PubMed]
- Walther, T.C.; Farese, R.V., Jr. Lipid Droplets and Cellular Lipid Metabolism. Annu. Rev. Biochem. 2012, 81, 687–714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, H.; Han, Y.; Sillke, Y.R.; Deng, H.; Siddiqui, S.; Treese, C.; Schmidt, F.; Friedrich, M.; Keye, J.; Wan, J.; et al. Lipid droplet-dependent fatty acid metabolism controls the immune suppressive phenotype of tumor-associated macrophages. EMBO Mol. Med. 2019, 11, e10698. [Google Scholar] [CrossRef]
- van Dierendonck, X.A.M.H.; Vrieling, F.; Smeehuijzen, L.; Deng, L.; Boogaard, J.P.; Croes, C.-A.; Temmerman, L.; Wetzels, S.; Biessen, E.; Kersten, S.; et al. Triglyceride Breakdown from Lipid Droplets Regulates the Inflammatory Response in Macrophages. Proc. Natl. Acad. Sci. USA 2022, 119, e2114739119. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Sen, P. Lipid droplet: A functionally active organelle in monocyte to macrophage differentiation and its inflammatory properties. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2021, 1866, 158981. [Google Scholar] [CrossRef]
- Lee, C.-H.; Liu, S.-Y.; Chou, K.-C.; Yeh, C.-T.; Shiah, S.-G.; Huang, R.-Y.; Cheng, J.-C.; Yen, C.-Y.; Shieh, Y.-S. Tumor-Associated Macrophages Promote Oral Cancer Progression Through Activation of the Axl Signaling Pathway. Ann. Surg. Oncol. 2013, 21, 1031–1037. [Google Scholar] [CrossRef]
- Ge, Z.; Ding, S. The Crosstalk Between Tumor-Associated Macrophages (TAMs) and Tumor Cells and the Corresponding Targeted Therapy. Front. Oncol. 2020, 10, 590941. [Google Scholar] [CrossRef]
- Chen, Y.; Song, Y.; Du, W.; Gong, L.; Chang, H.; Zou, Z. Tumor-associated macrophages: An accomplice in solid tumor progression. J. Biomed. Sci. 2019, 26, 78. [Google Scholar] [CrossRef] [Green Version]
- Lu, X.; Li, M.; Wu, C.; Zhou, C.; Zhang, J.; Zhu, Q.; Shen, T. Bisphenol A promotes macrophage proinflammatory subtype polarization via upregulation of IRF5 expression in vitro. Toxicol. Vitr. 2019, 60, 97–106. [Google Scholar] [CrossRef]
- Quagliariello, V.; Coppola, C.; Mita, D.; Piscopo, G.; Iaffaioli, R.; Botti, G.; Maurea, N. Low doses of Bisphenol A have pro-inflammatory and pro-oxidant effects, stimulate lipid peroxidation and increase the cardiotoxicity of Doxorubicin in cardiomyoblasts. Environ. Toxicol. Pharmacol. 2019, 69, 1–8. [Google Scholar] [CrossRef]
- Derouiche, S.; Warnier, M.; Mariot, P.; Gosset, P.; Mauroy, B.; Bonnal, J.-L.; Slomianny, C.; Delcourt, P.; Prevarskaya, N.; Roudbaraki, M. Bisphenol A stimulates human prostate cancer cell migration via remodelling of calcium signalling. SpringerPlus 2013, 2, 54. [Google Scholar] [CrossRef] [PubMed]
- Deng, P.; Tan, M.; Zhou, W.; Chen, C.; Xi, Y.; Gao, P.; Ma, Q.; Liang, Y.; Chen, M.; Tian, L.; et al. Bisphenol A promotes breast cancer cell proliferation by driving miR-381-3p-PTTG1-dependent cell cycle progression. Chemosphere 2021, 268, 129221. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-L.; Liu, N.; Weng, S.-F.; Wang, H.-S. Bisphenol A Increases the Migration and Invasion of Triple-Negative Breast Cancer Cells via Oestrogen-related Receptor Gamma. Basic Clin. Pharmacol. Toxicol. 2016, 119, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-L.; Wang, Y.-C.; Hsu, Y.-A.; Chen, C.-S.; Weng, R.-C.; Lu, Y.-P.; Chuang, C.-Y.; Wan, L. Bisphenol A Coupled with a High-Fat Diet Promotes Hepatosteatosis through Reactive-Oxygen-Species-Induced CD36 Overexpression. Toxics 2022, 10, 208. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Huang, Q.; Liu, C.; Kwong, C.H.T.; Yue, L.; Wan, J.-B.; Lee, S.M.Y.; Wang, R. Treatment of atherosclerosis by macrophage-biomimetic nanoparticles via targeted pharmacotherapy and sequestration of proinflammatory cytokines. Nat. Commun. 2020, 11, 2622. [Google Scholar] [CrossRef]
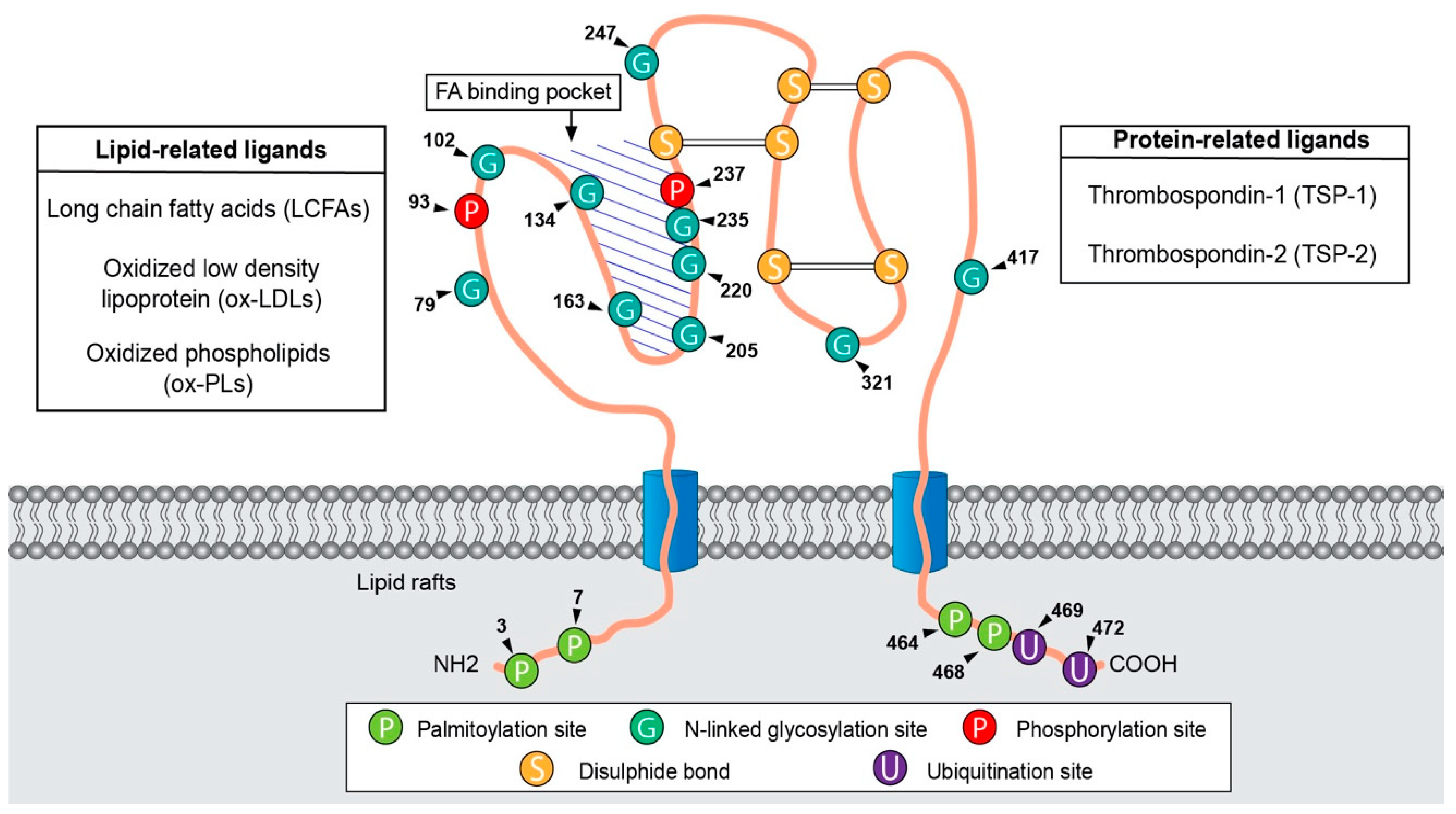
| Type of Cancer | Contribution of CD36 | References |
|---|---|---|
| Acute myeloid leukaemia | Increases leukaemia burden and shorten survival in vivo | [34] |
| Breast cancer | Essential survival mechanism in HER2-positive breast cancer Activates expression of pro-proliferation and migration genes while inhibiting expression of apoptotic genes | [35] |
| [36] | ||
| Cervical cancer | Promotes the epithelial–mesenchymal transition and metastasis in cervical cancer by interacting with TGF-β Promotes cervical cancer cell growth and metastasis via up-regulating the Src/ERK pathway | [37] |
| [38] | ||
| Colorectal cancer | Promotes metastasis by increasing MMP28 and decreasing e-cadherin expression Increases in cellular proliferation via upregulation of survivin in CRC cells | [39] |
| [40] | ||
| Gastric cancer | Promotes peritoneal metastasis via fatty acid uptake | [41] |
| Promotes metastasis of gastric cancer via AKT/GSK-3β/β-catenin pathway | [42] | |
| Glioblastoma | Increases glioblastoma progression and tumour initiation in cancer-stem cells | [43] |
| Hepatocellular carcinoma | Promotes epithelial–mesenchymal transition, enhances migration and invasion | [44] |
| Oral squamous carcinoma | Initiates and promotes metastasis and worsens prognosis Promotes lymph node metastasis | [45] |
| [46] | ||
| Ovarian cancer | Omental adipocytes reprogram tumour metabolism due to high exogenous fatty acid uptake | [47] |
| Facilitates the proliferation and migration and lymph node metastasis | [48] | |
| Pancreatic cancer | Mediates pancreatic cancer development and progression | [49] |
| Prostate cancer | Increases cancer cell proliferation and migration, and increase tumour burden in vivo | [50] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaidi, N.E.; Shazali, N.A.H.; Leow, T.-C.; Osman, M.A.; Ibrahim, K.; Cheng, W.-H.; Lai, K.-S.; Nik Abd Rahman, N.M.A. CD36-Fatty Acid-Mediated Metastasis via the Bidirectional Interactions of Cancer Cells and Macrophages. Cells 2022, 11, 3556. https://doi.org/10.3390/cells11223556
Zaidi NE, Shazali NAH, Leow T-C, Osman MA, Ibrahim K, Cheng W-H, Lai K-S, Nik Abd Rahman NMA. CD36-Fatty Acid-Mediated Metastasis via the Bidirectional Interactions of Cancer Cells and Macrophages. Cells. 2022; 11(22):3556. https://doi.org/10.3390/cells11223556
Chicago/Turabian StyleZaidi, Noorzaileen Eileena, Nur Aima Hafiza Shazali, Thean-Chor Leow, Mohd Azuraidi Osman, Kamariah Ibrahim, Wan-Hee Cheng, Kok-Song Lai, and Nik Mohd Afizan Nik Abd Rahman. 2022. "CD36-Fatty Acid-Mediated Metastasis via the Bidirectional Interactions of Cancer Cells and Macrophages" Cells 11, no. 22: 3556. https://doi.org/10.3390/cells11223556
APA StyleZaidi, N. E., Shazali, N. A. H., Leow, T.-C., Osman, M. A., Ibrahim, K., Cheng, W.-H., Lai, K.-S., & Nik Abd Rahman, N. M. A. (2022). CD36-Fatty Acid-Mediated Metastasis via the Bidirectional Interactions of Cancer Cells and Macrophages. Cells, 11(22), 3556. https://doi.org/10.3390/cells11223556






