Abstract
A Primary Healthcare-System-Strengthening Project (PSSP) is implemented by the Ministry of Health, Sri Lanka, with funding support from the World Bank for providing quality care through primary medical care institutions (PMCIs). We used an explanatory mixed-methods study to assess progress and challenges in human resources, drug availability, laboratory services and the health management information system (HMIS) at PMCIs. We conducted a checklist-based assessment followed by in-depth interviews of healthcare workers in one PMCI each in all nine provinces. All PMCIs had medical/nursing officers, but data entry operators (44%) and laboratory technicians (33%) were mostly not available. Existing staff were assigned additional responsibilities in PSSP, decreasing their motivation and efficiency. While 11/18 (61%) essential drugs were available in all PMCIs, buffer stocks were not maintained in >50% due to poor supply chain management and storage infrastructure. Only 6/14 (43%) essential laboratory investigations were available in >50% of PMCIs, non-availability was due to shortages of reagents/consumables and lack of sample collection–transportation system. The HMIS was installed in PMCIs but its usage was sub-optimal due to perceived lack of utility, few trained operators and poor internet connectivity. The PSSP needs to address these bottlenecks as a priority to ensure sustainability and successful scale-up.
1. Introduction
Noncommunicable diseases (NCDs) are the leading cause of morbidity and mortality, accounting for 74% of global deaths, of which more than one-third were ‘premature’ deaths [1,2,3]. NCDs alone are estimated to cause an economic loss of about USD 47 trillion between 2011 and 2030 due to disability and premature deaths [4]. NCDs pose additional challenges to healthcare systems in low- and middle-income countries (LMICs) that are still battling communicable diseases, maternal, neonatal and nutritional disorders [5].
The World Health Organization’s (WHO) Global Action Plan for the prevention and control of NCDs (2013–2020) aims to achieve a 25% reduction in ‘premature’ NCD mortality by 2025 [6]. The Sustainable Development Goals (2015) and the United Nations High-Level meeting on NCDs (2018) suggested universal health coverage packages, including prevention, diagnosis and management of NCDs, for achieving the global targets of reducing ‘premature’ deaths [7]. This requires a strengthening of primary healthcare (PHC) with infrastructure, trained human resources, health management information systems (HMISs) and an uninterrupted availability of drugs and laboratory investigations for quality NCD care provision [8,9].
To guide the integration of NCD care with PHC, the WHO published a “Package of Essential NCD interventions for primary care in low-resource settings” (WHO PEN) in 2010, which provided a prioritized set of cost-effective interventions suitable for LMICs [10]. However, studies from LMICs assessing the integration of NCD care in PHC have shown critical gaps in trained manpower, drug availability, laboratory services and HMISs [11,12,13,14,15,16,17,18]. The reasons for such gaps are generally context specific, requiring detailed enquiry for action [19,20].
In Sri Lanka, an LMIC in the South Asia region, NCDs account for 81% of total deaths and 77% of disability-adjusted life years (DALYs) [21]. However, NCD care provision has remained suboptimal as existing primary medical care institutions (PMCIs) in the country have had a low capacity to provide optimal curative care services [22,23]. The Ministry of Health (MoH) constituted a Technical Expert Committee in 2017 to provide policy directions for reorganising PHC for provision of NCD care in PMCIs [24,25,26]. The committee advised that PHC be reorganised with improved infrastructure and processes to attain the goals of universal health coverage with a focus on NCD care.
Accordingly, the MoH initiated the ‘Primary Healthcare-System-Strengthening Project (PSSP)’ in 2018, with technical and financial support from the World Bank [27]. The project focuses on three thematic areas: first, reorganisation of the PHC by defining the catchment area and population for each PMCI (empanelment); second, strengthening the PMCIs with trained manpower, optimizing drug supply chain management systems and expanding laboratory service capacity; and third, establishing a technology-based HMIS to provide electronic personal health records (PHRs) [28,29]. By 2023, the PSSP plans to strengthen 550 of the 990 PMCIs in the country [29].
In the first phase of PSSP (2019), 63 selected PMCIs across the nine provinces of the country were strengthened. However, there was no prior systematic assessment as to whether the PMCIs under PSSP had attained expected standards in terms of trained manpower, drug availability, laboratory services and HMIS. Such an assessment, using a combination of quantitative and qualitative techniques, could provide an in-depth understanding of operational realities and help programme managers to address bottlenecks in reorganisation [15,16,19,30]. The insights from the early experiences of strengthening PMCIs would also enable the MoH and the provincial health authorities, jointly with the project management unit (PMU), to optimise the PSSP implementation in the PMCIs in future. In 2021, we, therefore, conducted a study in nine selected PMCIs to assess if they were reorganised according to the standards endorsed by the MoH and to explore the challenges perceived by the healthcare workers (HCWs) implementing this project.
2. Materials and Methods
2.1. Study Design
This was an explanatory mixed-methods study [30]. The quantitative component was a cross-sectional descriptive study using a self-designed checklist. This was followed by the qualitative component, which was a descriptive study with in-depth interviews among healthcare workers.
2.2. Study Setting
2.2.1. General Setting
Sri Lanka is an island country with a population of 21.8 million in 2019 [31]. Administratively, the country is divided into nine provinces, each governed by an autonomous provincial council [32]. The provinces are further divided into districts, administered by a district secretariat. The most peripheral local administrative units are Grama Niladhari (GN) divisions [32].
2.2.2. Specific Setting
Healthcare System in Sri Lanka
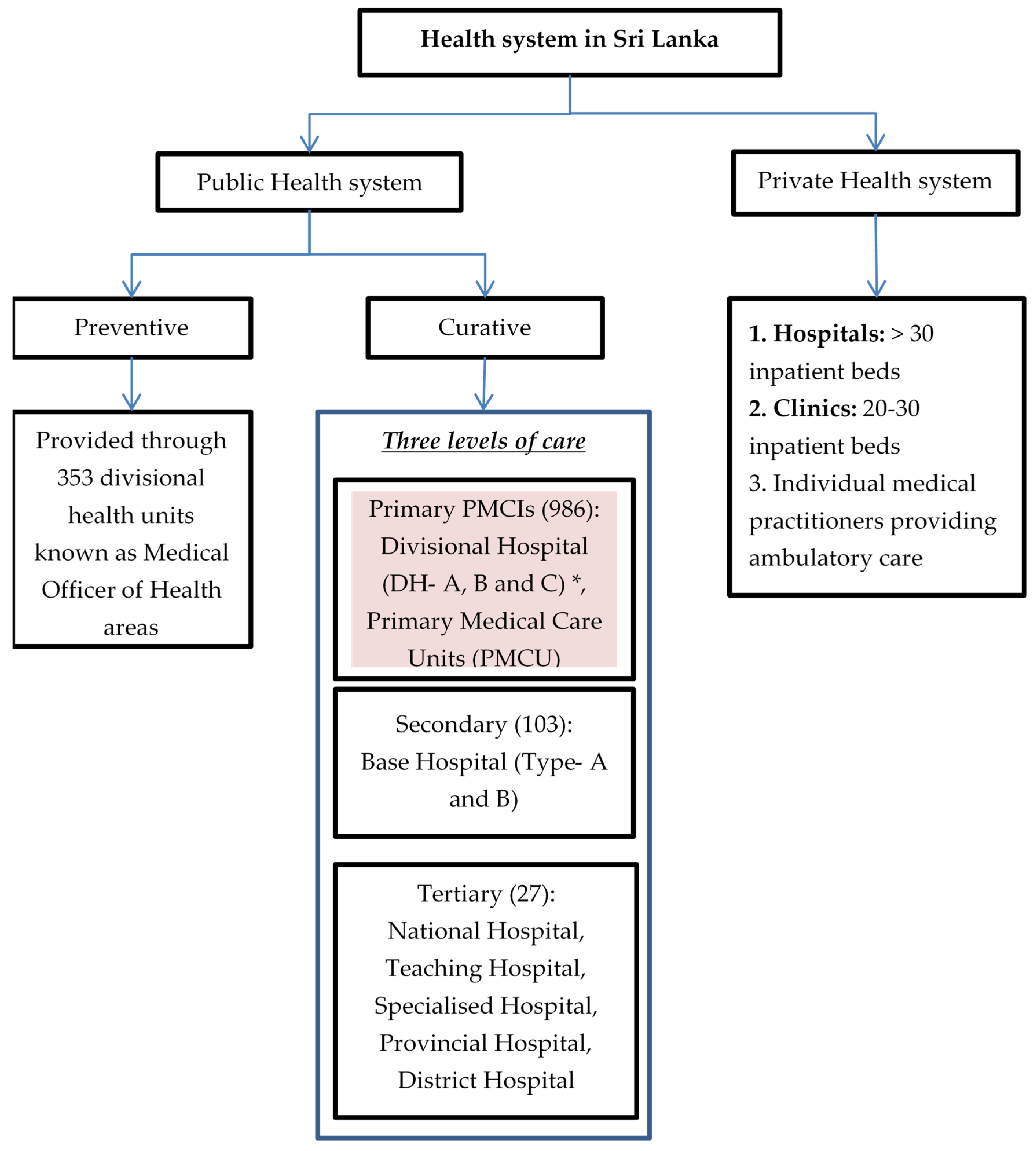
The health system in Sri Lanka is a mix of both public and private care providers (Figure 1). The public-health system provides both preventive and curative services free of cost. The former focuses mainly on maternal and child health services delivered through divisional health units known as Medical Officer of Health (MOH) areas with a catchment population of 50,000 to 100,000 [33]. Curative services are divided into three levels: primary, secondary and tertiary. Patients can avail services at any level of care and need not necessarily move sequentially from primary to tertiary level [24]. Primary medical care institutions (PMCIs), which are the focus of PSSP, include primary medical care units (PMCUs) and divisional hospitals (DHs). The PMCUs provide only outpatient services while DHs provide inpatient and delivery services as well [33]. The PMCIs are governed by provincial health departments [34].
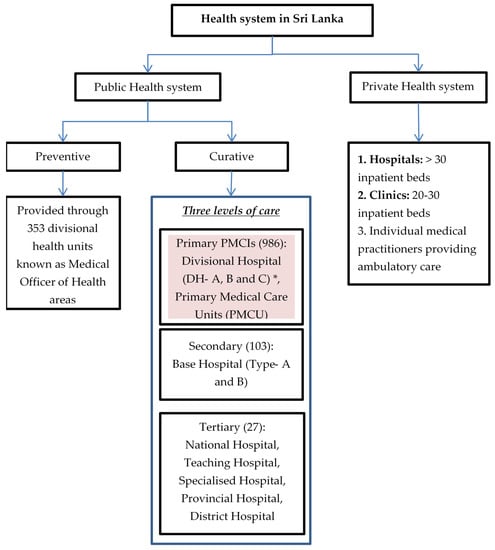
Figure 1.
Health system in Sri Lanka, 2021. * DH-A has >100 inpatient beds, DH-B has 50–100 inpatient beds and DH-C has <50 beds.
PSSP Project
The PSSP is managed and monitored by a PMU established within the MoH. The personnel in the PMU includes full-time staff deputed from MoH and the State Ministry of Provincial Councils and Local Government Affairs, along with a few others employed on a contractual basis. The PSSP receives financial aid from the World Bank, dependent on producing required results against nine agreed Disbursement-Linked Indicators (DLIs) on an annual basis [28]. The PSSP project was initiated in 63 PMCIs (4 PMCUs and 59 DHs) in the first year (June 2019) and the key activities are described below.
Empanelment of the Population
Process of empanelment was limited to the primary preventive healthcare institutions in Sri Lanka. With the PSSP, this concept was expended to the PMCIs in 2018. Empanelment refers to the process of identifying, assigning, actively reviewing and updating people’s health data. The PMCI is primarily responsible for the empanelment of residents of the GN division assigned to it by registering them, providing them unique personal health numbers (PHN) and creating their PHR. Once created, the PHR can be accessed and updated at any public-health facility.
Trained Human Resources
In accordance with guidelines of MoH, a minimum of two medical officers, one nursing officer and a dental surgeon constitute a core team and were supposed to be present in all the PMCIs [27]. Additionally, a public-health nursing officer (PHNO), health promotion officer, medical laboratory technician (MLT) and data entry operators (DEO) have been proposed to facilitate PSSP activities. These HCWs are expected to be trained on guidelines for screening and management of major NCDs, the process of empanelment and other aspects of PSSP.
Essential Drugs and Drug Supply Chain Management
The PMCIs are supposed to ensure availability of all essential drugs in blister packs and dispense drugs for a period of one month for patients with NCDs. A list of 18 essential drugs for PMCI was prepared in accordance with the guidelines of MoH (Annex S1). The pharmacist or dispenser forecasts requirements based on the prevailing morbidity and drug utilization patterns in the previous year and indents drugs online through the medical supplies management information system (MSMIS). The drugs indented are expected to be delivered within a month. Adequate space and optimum storage conditions for maintaining a three-month stock of drugs are supposed to be present in each PMCI. A detailed inventory and supply chain register is maintained in paper-based registers currently at the PMCI.
Laboratory Services
The PSSP has developed a basic investigation package that needs to be made available through all PMCIs (Annex S2). The project supports the establishment of a functional laboratory service network with apex laboratories in selected DH and secondary hospitals. All PMCIs are supposed to have certain point-of-care tests (POCT), such as blood glucose and serum cholesterol. For essential investigations not available at a PMCI, the providers are supposed to establish a system/network for sample collection and transportation (SCT) to apex laboratories and retuning the investigation reports back to the respective PMCIs.
Health Management Information System (HMIS)
All the PMCIs are supposed to have an online web-based HMIS platform managed by the HMIS unit of the MoH and used for the empanelment process. The said system has to support the information management for NCD surveillance at healthy lifestyle centres (HLCs). The health staff at the PMCI are supposed to register individuals from the assigned GN divisions by generating unique PHN on HMIS and creating PHRs. The clinical details are supposed to be updated on the HMIS during each health facility visit. The PHRs can be accessed on the HMIS using the PHN and updated across any health facility by trained DEOs, medical officers and nursing staff.
Paper-based PHRs (books) are handed over to registered individuals and they are expected to carry these while visiting any health facility, so that the management details can be documented. However, prior to the introduction of PHR books, the NCD patients had ‘clinic books’ in which follow-up details were documented.
2.3. Study Population
2.3.1. Quantitative Component
The assessments were conducted in nine selected PMCIs, one from each of the nine provinces of Sri Lanka. Using a stratified random sampling method, six rural and three urban PMCIs were selected to represent the population distribution of the country. All the nine PMCIs included in the study were selected for implementation of PSSP by the PMU in June 2019. In eight of the PMCIs, implementation started immediately but in one it started in February 2020. Therefore, by the time of assessment, all the PMCIs had at least one year of implementation of PSSP.
2.3.2. Qualitative Component
Purposive (extreme variation) sampling was used to select the facilities based on performance according to the quantitative assessment. The healthcare providers of the best and worst performing urban PMCIs and of the best and poor performing rural PMCIs were included. In total, we conducted 18 in-depth interviews among medical officers (4), nursing officers (3), drug dispensers/pharmacists (3), DEO (1) and programme managers at the provincial/district level (7). Sample size was guided by saturation of findings.
2.4. Data Collection, Study Variables, Data Source and Study Tools
2.4.1. Quantitative Component
Trained research staff completed a pretested facility assessment checklist (Annex S3) through non-participatory observation and review of records maintained at the PMCI. The checklist included details of trained human resources, laboratory services, essential drugs and supply chain and HMIS.
2.4.2. Qualitative Component
In-depth interviews were used as a method of qualitative data collection. In-depth interviews are a form of semi-structured interview conducted to gather information from key informants who have personal experiences, attitudes, perceptions and beliefs related to the topic of interest [35]. Typically, the interviewer is guided by an interview guide which lists the key issues to be explored. However, the sequencing and wording of the questions are modified based on the responses of the participant and flow of conversation. The interview guides used to explore challenges in human resources, laboratory services, essential drugs and supply chain and HMIS are provided in Annex S4.
In-depth interviews were conducted by research consultants, who were medical doctors familiar with the health system (one male and one female), fluent in the local languages (Sinhala and Tamil) and experienced in qualitative research. The interviews were conducted approximately ten days after the checklist-based assessment and the findings of the quantitative study were used to contextualise the respondent-specific interview guide for the qualitative exploration. The interviews were audio recorded and were used to prepare the transcripts. The average duration of the interviews was 32 min (range 8 to 80).
2.5. Data Analysis
2.5.1. Quantitative Component
The data collected on the checklist were entered using an EpiCollect5 application (Wellcome Sanger Institute, Cambridge, UK), a mobile-phone-based data-capture tool. The data were downloaded and analysed using Stata version 12 (StataCrop LP, College Station, TX, USA). Frequencies and percentages were used to summarise the total number of PMCIs with available adequately trained manpower, essential drugs and MSMIS, buffer stocks of essential drugs, laboratory/diagnostic services and functional HMIS.
2.5.2. Qualitative Component
The transcripts were prepared in English on the same day of interview. Thematic analysis was performed by the PI (PTK) using Atlas-Ti software to identify themes on the challenges in strengthening and reorganisation of PMCIs. The second investigator (AMVK) reviewed the analysis and decisions on coding/categories and theme generation were decided via consensus. Similar codes were combined into categories to describe certain themes. To ensure that the results reflected the data, the codes/categories were related back to the original data. The findings were reported as per ‘Consolidated Criteria for Reporting Qualitative Research’ guidelines [36].
3. Results
Of the nine PMCIs selected, three each were DH-A and DH-C, two were PMCUs and one was DH-B. Seven (DH) PMCIs functioned 24 h a day, while the rest (PMCUs) functioned for only 8 hours during the day. Outpatient attendance numbers in the month preceding data collection ranged from 50 to 196.
3.1. Human Resource and Training
Checklist-based assessments showed that a minimum of two medical officers and one nursing officer was available in all the nine PMCIs. Among additional proposed staff, DEOs were present in four (44%) PMCIs, MLTs in three (33%) and PHNO in two (22%). There was no information regarding the total number of ‘approved positions’, other than for medical officers and nursing officers. At least one medical officer trained in empanelment and NCD guidelines was present in eight (89%) PMCIs and at least one trained nurse was available in seven PMCIs (78%). Of the 38 medical officers and 82 nursing officers available in the nine PMCIs, 19 (50%) and 22 (27%) were trained, respectively.
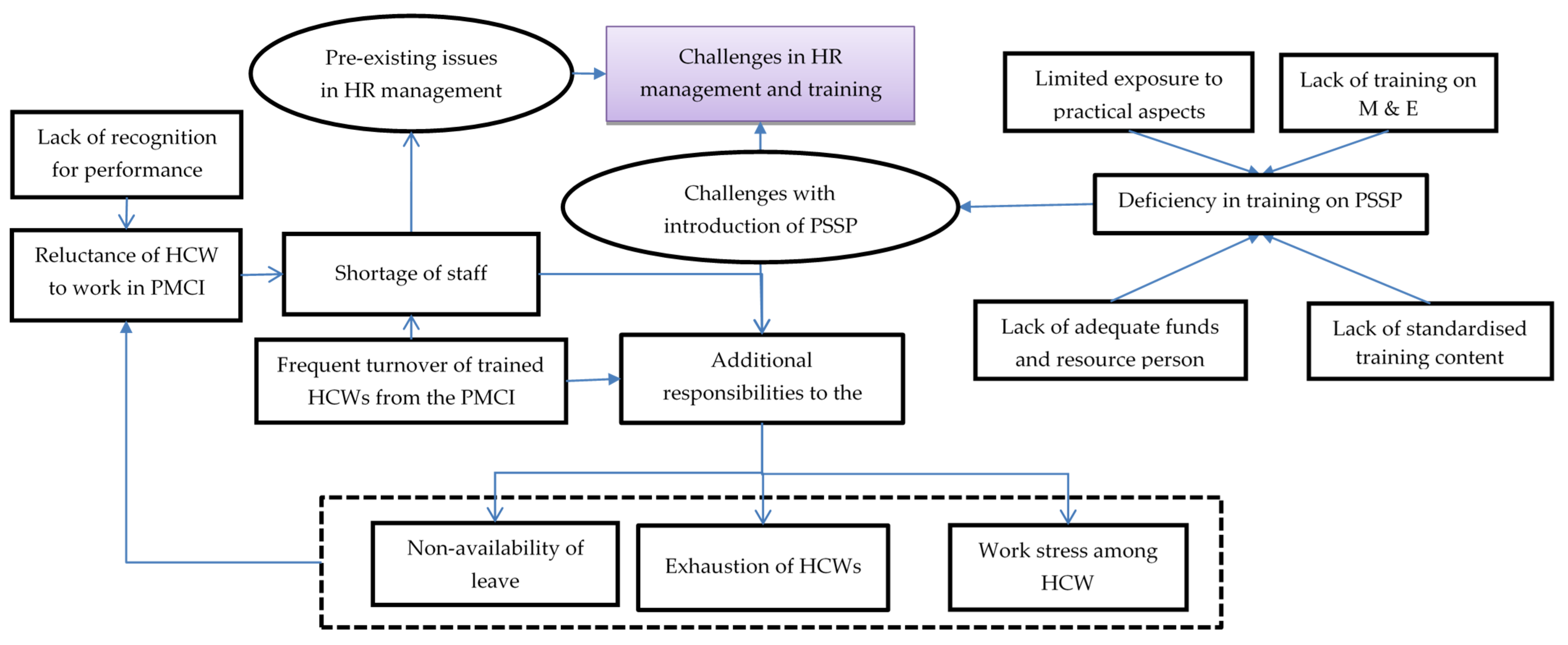
In total, 46 codes related to challenges with human resources and training were identified from the transcripts of HCW interviews, which were reduced to 11 categories describing the challenges and their impact on the HCWs. The categories highlighted in Figure 2 are explained in detail with relevant verbatim quotes in Supplementary Table S1.
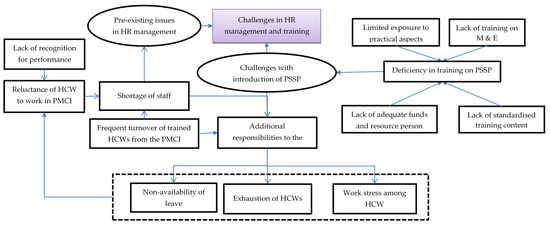
Figure 2.
Challenges with human resource management and training in PMCIs supported by PSSP in Sri Lanka, 2021. Abbreviation: HCWs: Healthcare Workers; PMCI: Primary Medical Care Institution; PSSP: Primary Healthcare-System-Strengthening Project (PSSP); M and E: Monitoring and Evaluation.
There was reluctance among HCWs to work in the rural PMCIs due to their preference for urban neighbourhoods and lack of recognition for their hard work. The HCWs felt that annual transfers and on-demand transfers of staff and the temporary recruitment of DEOs led to frequent turnover of trained staff. The HCWs felt that the PMCIs were usually understaffed, especially with respect to PHNOs, MLTs and DEOs. With a lack of PHNOs for carrying out community activities, the DEO for online registration and the MLT for sample collection and processing, these responsibilities were placed on the existing HCWs (medical and nursing officers), leading to exhaustion and work stress.
These issues were compounded by deficiencies in training in the newer activities introduced after PSSP. Without adequate funds and experienced resource persons, the training was not frequently conducted, especially with the onset of the COVID-19 pandemic. Even when conducted, some HCWs were unable to participate in the training due to a lack of spare staff to conduct their work at the PMCI and restrictions from supervisors. The HCWs felt there was no standardised training content, with various programme units supporting PSSP activities delivering different messages, and this led to confusion among trainees. Further, those who were trained did not make much effort to train the rest of the staff in the PMCIs.
Despite these challenges, the HCWs appreciated the constant push and monetary support from the PSSP to recruit DEOs. They felt that the PMCIs under the PSSP received preference during allocation of staff from the common pool because of efforts from the PMU of PSSP. Some HCWs felt that there were substantial efforts to train them on empanelment and registration. Further, the well-performing PMCIs acted as a resource centre for training the HCWs from the second set of PMCIs included under the project.
3.2. Essential Drugs and Supply Chain Management
During the assessment, 11 (61%) of the 18 essential drugs were available in all the PMCIs. Salbutamol respiratory solution, Enalapril maleate, Gliclazide and Theophylline tablets were not available in one of the PMCIs. More than 50% of the PMCIs had less than three months of buffer stock for 12 of the 18 drugs. Stock outs for Gliclazide and Enalapril tablets were reported in three (33%) PMCIs during 2020. During the previous indent, all except two PMCIs received the drugs within one month of indenting (Table 1).

Table 1.
Availability of essential drugs, stock outs and receipt of drugs within a month of indenting in the nine selected PMCIs supported by the Primary Healthcare-System-Strengthening Project (PSSP) in Sri Lanka in 2021.
Eight (89%) PMCIs disbursed drugs for more than one month but none in blister packs. Adequate drug storage space was available in seven (78%) PMCIs, with the majority (67%) not having air conditioning in the drug stores. Seven (78%) PMCIs had an online MSMIS for drug indenting. The drug indenting and dispensing registers in the PMCIs were incomplete in four PMCIs with dates of indenting and receipt of drugs missing.
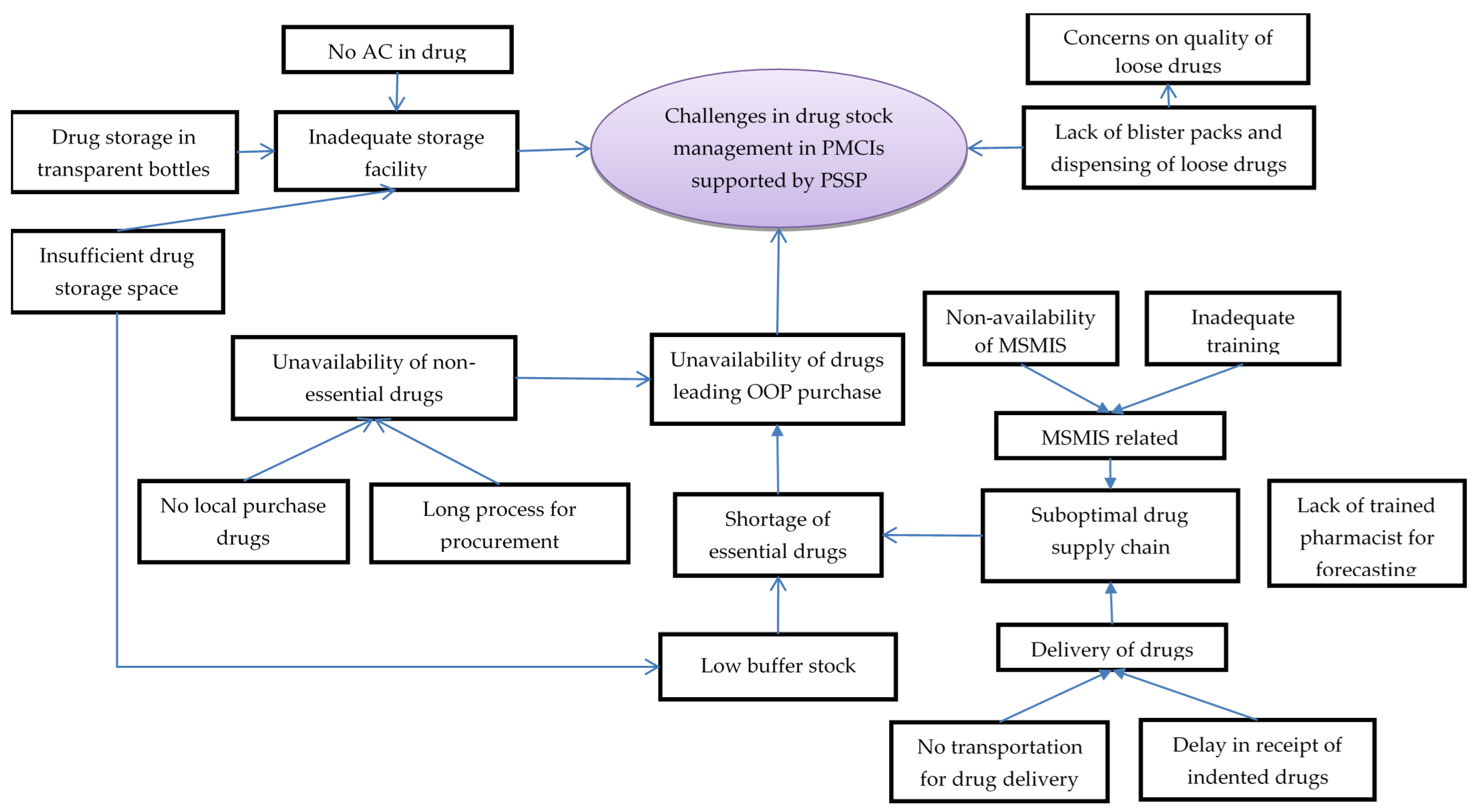
In total, 32 codes related to challenges with essential drugs and supply chain management were deduced during analysis of interviews with HCWs. The codes were summarised under five categories (Figure 3, Supplementary Table S2).
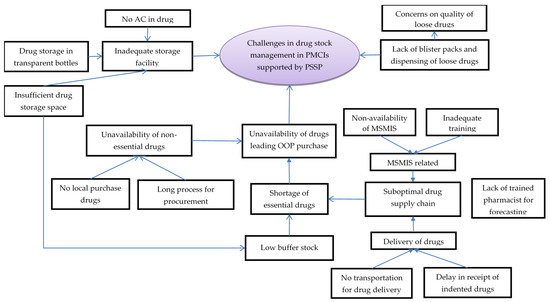
Figure 3.
Challenges in drug stock management in PMCIs supported by PSSP in Sri Lanka, 2021. Abbreviation: PMCI: Primary Medical Care Institution; PSSP: Primary Healthcare-System-Strengthening Project (PSSP); AC: Air Conditioner; MSMIS: Medical Supplies Management System.
The HCWs perceived a suboptimal drug supply chain management as one of the reasons for shortages of essential drugs. The pharmacists or dispensers were either not available or were not trained in timely drug forecasting and ordering. The MSMIS platform designed for this purpose was not functional due to a lack of trained personnel to handle it. Once ordered, delivery of drugs was often delayed due to a lack of dedicated vehicles for transporting drugs to some of the PMCIs. The HCWs complained that insufficient drug storage space, lack of air conditioning and storage of drugs in transparent pill bottles contributed to suboptimal drug storage, leading to apprehensions regarding quality of drugs and inability to store adequate buffer stocks.
The HCWs reported unavailability of non-essential drugs, especially with an increase in demand from patients with specialised needs being referred to PMCIs for follow-up from secondary/tertiary hospitals. Such drugs were not easily available, as they could not be procured locally and had to go through a long process for procurement. Ultimately, stock outs in drugs meant that the patients had to purchase drugs from private pharmacies, incurring out-of-pocket expenditures.
Another challenge related to drugs was non-availability of blister packs, resulting in dispensing of loose drugs in plastic covers, which could not be stored safely for a month and which were also perceived to be of low quality among the patients. This was also reported as a challenge for inventory management, as counting individual tablets was a tedious and error-prone exercise.
The HCWs felt there had been a positive change in availability of the essential drugs since the introduction of PSSP. Under the PSSP, PMCIs had to prioritise the supply of drugs and there was less chance of a stock-out situation. Similarly, there was less chance of having drugs expire in the PMCIs, as there was an increase in service utilization. Though there were lacunae in training and establishment of MSMIS, the HCWs perceived that MSMIS brought efficiency in supply chain management.
3.3. Laboratory Services
In-house laboratory facilities were present in seven (78%) PMCIs. All PMCIs had functional glucometers and seven (78%) had functional cholesterol meters. Out of 14 essential investigations reported, 6 (43%) were available in more than 50% of the PMCIs. Blood glucose and total cholesterol estimations were available through all the nine PMCIs (seven in-house and two in the apex laboratory). Serum creatinine estimation was available through seven (78%) PMCIs (four in-house and three in the apex laboratory). In one of the PMCIs utilizing services from an apex laboratory, SCT was not established (Table 2). In other places, the staff collected blood and patients dropped their samples at the apex laboratory. For tests that were not available through the PMCI, patients had to approach private laboratories.

Table 2.
Availability of laboratory/diagnostic services in the nine selected PMCIs supported by the PSSP in Sri Lanka in 2021.
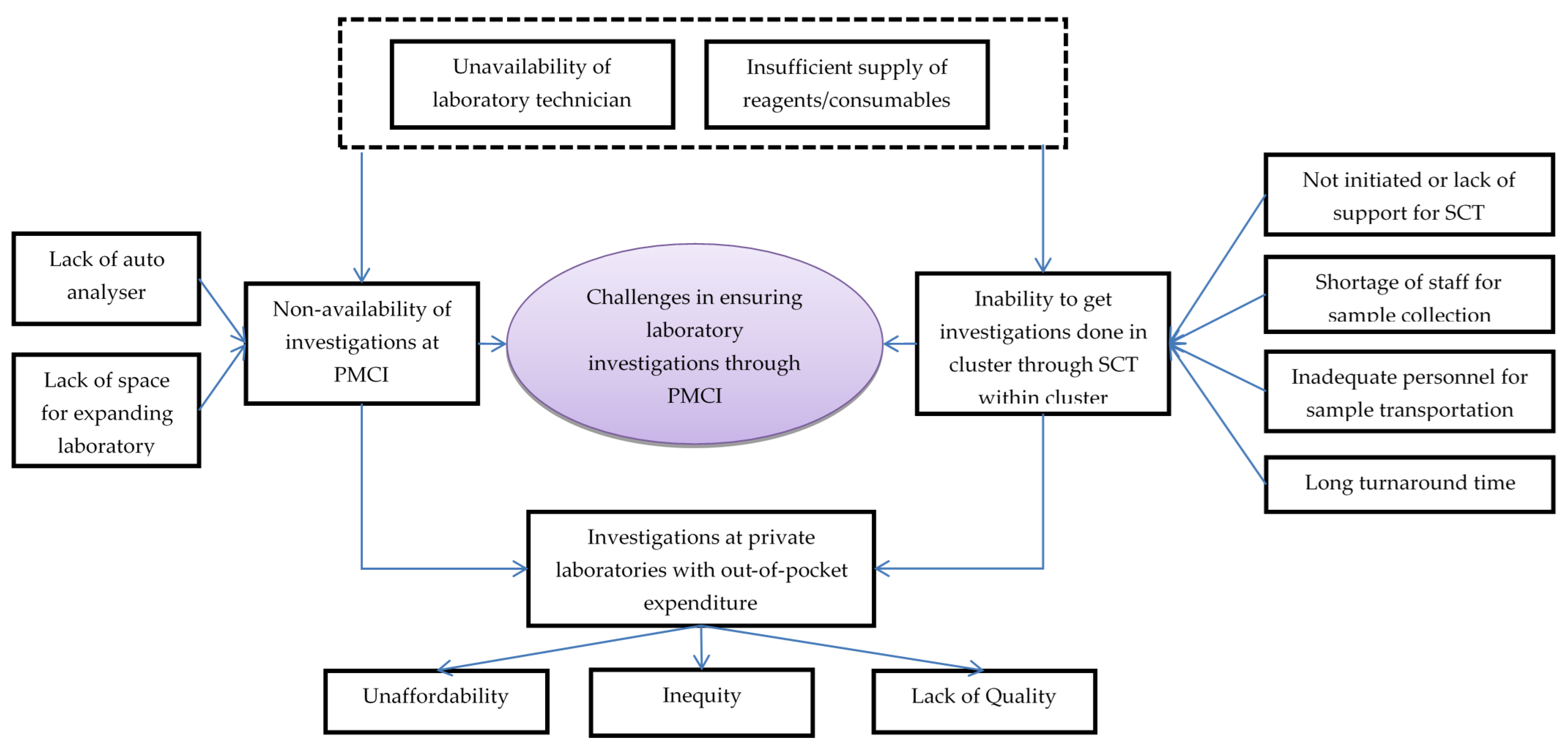
In total, 25 codes related to challenges with laboratory services in PMCIs were deduced from interviews with HCWs, which were reduced to 11 categories (Figure 4, Supplementary Table S3).
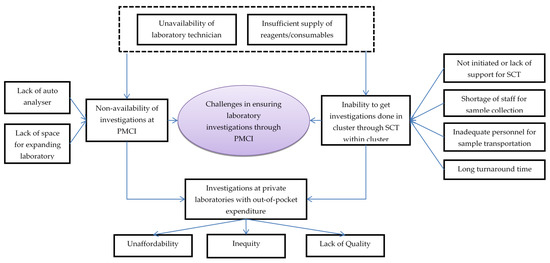
Figure 4.
Challenges in laboratory investigations conducted through PMCI supported by PSSP in Sri Lanka, 2021. Abbreviation: PMCI: Primary Medical Care Institution; PSSP: Primary Healthcare-System-Strengthening Project (PSSP); SCT: Sample collection and transportation.
The HCWs felt that some of the reasons for non-availability of investigations in the PMCI were lack of laboratory space, poor maintenance of the auto analyser and lack of reagents and consumables for POCTs. Though SCT to apex laboratories for investigations unavailable in PMCI had been suggested, the HCWs noted that the system was not well established due to a lack of programmatic support. There were no dedicated personnel for SCT from PMCI to apex laboratories. The turnaround time for investigations through SCT was generally high and these delays increased further during COVID-19 times.
The HCWs felt that the shortage of trained MLTs was a major challenge in ensuring laboratory investigations through PMCIs. Cancellations of certain investigations were frequent in apex laboratories due to a lack of trained MLTs and shortage of reagents. Investigations in private laboratories were unaffordable for most patients. As private laboratories were not stringently regulated, there were concerns about the quality of their services.
The HCWs felt that with the PSSP project, there was increased availability of POCT devices, consumables and their utilization in the PMCIs. Though there were deficiencies, the HCWs were appreciative of the SCT system established after PSSP, which had enabled them to access the follow-up investigations for better management of their NCD patients.
3.4. Health Management Information System (HMIS)
All the PMCIs had at least one computer with internet connection. The HMIS was available in all the PMCIs and was used for registering individuals and issuing PHN (empanelment). All PMCIs had hard copies of the PHR books. However, the PHR books had not been issued to all registered individuals in seven (78%) PMCIs. Electronic PHR was available in six (67%) PMCIs but only three (33%) updated clinical details in it during follow-up visits. In other PMCIs, follow-up details were documented in either ‘clinic books’ or PHR books.
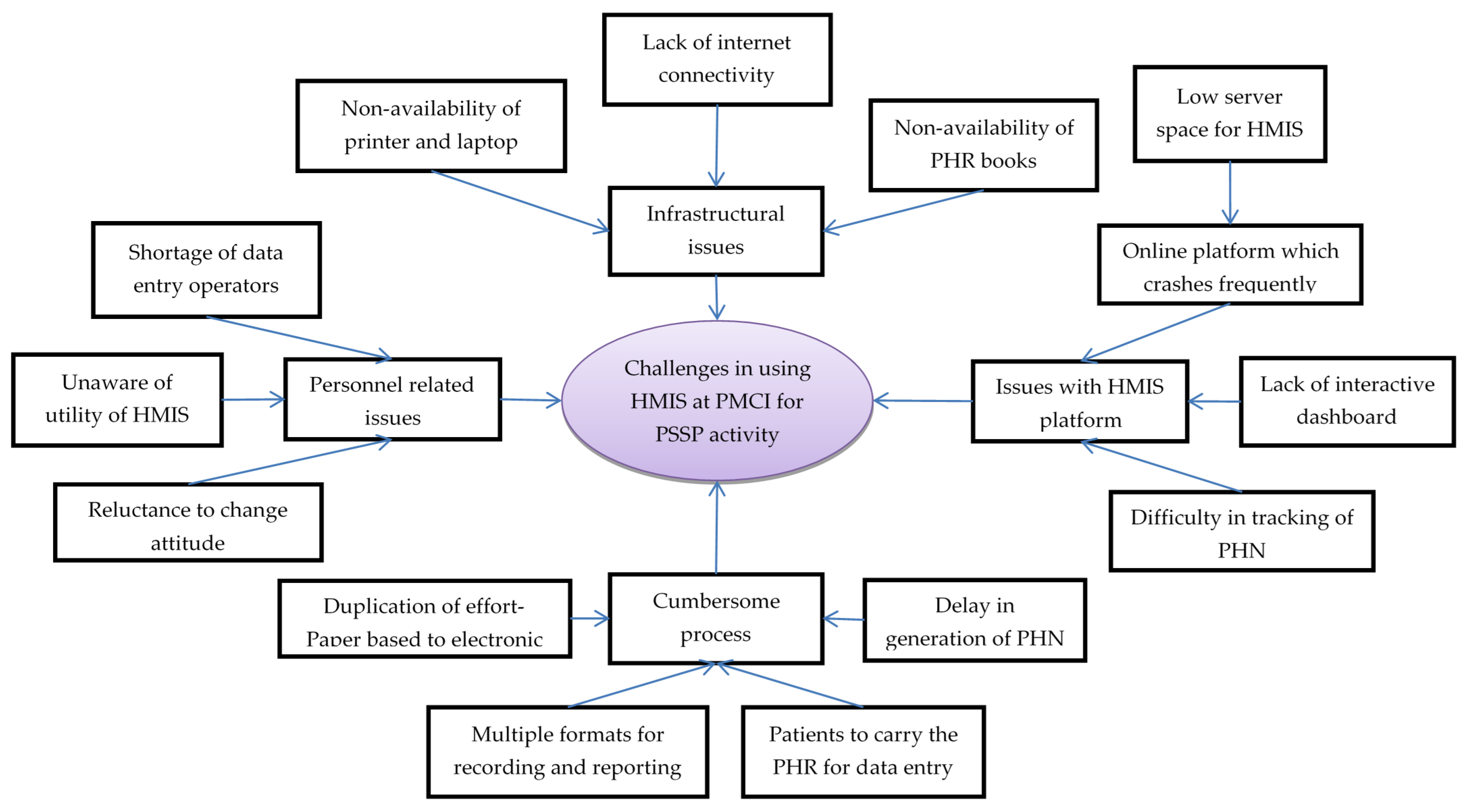
In total, 25 codes related to challenges in using HMIS were deduced from the transcripts of interviews with HCWs, which were reduced to 13 categories (Figure 5, Supplementary Table S4).
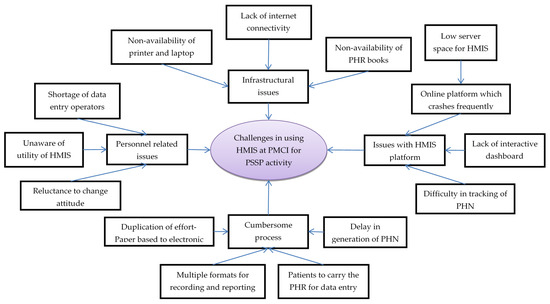
Figure 5.
Challenges in using Health Management Information System established in PMCI supported by PSSP in Sri Lanka, 2021. Abbreviation: PMCI: Primary Medical Care Institution; PSSP: Primary Healthcare-System-Strengthening Project (PSSP); PHN: Personal Health Number; PHR: Personal Health Record; HMIS: Health Management Information System.
Personnel-related issues: HCWs across all cadres complained about shortage of DEOs, as this required either the nursing officer or the doctor to enter data in the HMIS. They regarded data entry as an additional task with no relevance to routine care provision. The programme managers also mentioned that systematic collection and digitization of data was not perceived to be useful by HCWs. These challenges resulted in poor utilization of HMIS, even though the required equipment had been provided.
Infrastructural issues: The HCWs complained that the HMIS module and PHR books were not available in the initial days of project and, therefore, PHN and PHR books could not be issued to all the registered individuals. They felt that the current supply of HMIS-enabled laptops (one or two laptops per PMCI) was not sufficient for simultaneous data entry from multiple service delivery points. Although internet connection was provided, poor connectivity made the use of the online HMIS portal for real-time registration impossible, especially during outreach clinics.
The HCWs mentioned that the online HMIS platform crashed frequently, especially during working hours when multiple people used it simultaneously. The HMIS programme attributed this deficiency to allocation of low server space (16 GB instead of 64 GB RAM) from the government cloud. The HCWs perceived lack of an interactive dashboard for summary reports as a major challenge, which prevented them from monitoring their performance. They also mentioned that it was difficult to locate digital PHRs on HMIS because manual entry of the 10-character alphanumeric PHN (identifier) for tracing was prone to transcription errors.
The programme managers mentioned that HCWs in some of the PMCIs duplicated their efforts by documenting demographic details in the paper-based register first and then entering these data into online HMIS for generating PHN. The HCWs might have missed entering the data into HMIS in a timely manner, as there were no standard timelines for transcribing data. The HCWs complained that, despite issuing the PHR books, the patients failed to bring them during follow-up visits. Thus, the PHR books and electronic PHR remained incomplete, defeating the purpose of issuing the PHR.
Notwithstanding the challenges, HCWs appreciated both the efforts by the PSSP to establish the HMIS and initiatives taken by programme managers for effectively communicating changes in the module.
4. Discussion
This was the first assessment on how well PSSP had worked in strengthening the PMCIs and what were the challenges in ensuring that trained manpower, functional HMIS with intra- and inter-operability of services within the PMCIs and between different levels; uninterrupted availability of drugs and investigations was provided for quality NCD care. Although commendable progress has been made, there were several challenges which require urgent attention if the standards defined by the MoH or PSSP are to be met.
The study had several strengths. First, the mixed-methods design helped in elucidating and understanding the real-world situation of PMCI reorganisation, gaps and challenges in reorganisation. Second, the checklists and interview schedules used for facility assessments were validated in consultation with the programme managers and were amended before use. Third, all the research staff were trained on how to fill in the checklist-based assessment and dummy data extraction was practiced during the training, minimizing inter-observer bias. Finally, the quality of the completed checklists was reviewed by the PI and research consultants on a daily basis and was re-validated during in-depth interviews at the PMCI.
The study had a few limitations. First, only nine PMCIs were included in the quantitative assessment due to financial constraints and the COVID-19 situation and they, therefore, may not be representative of all the PMCIs. However, efforts were made to select one PMCI each from the nine provinces to accommodate variation across the provinces. Second, the non-availability of baseline (prior to implementation of PSSP) data of the PMCIs selected in the study limited our ability to quantitatively assess the impact of PSSP on human resource, HMIS, laboratory services and drug availability. Third, records and registers were not well maintained at the PMCIs with lots of missing data, making it difficult to extract some of the data from a few PMCIs. Fourth, the PMU staff were not interviewed to understand the challenges at the central level for reorganising the PMCIs.
All the PMCIs had the minimum required number of medical and nursing officers. The implementation of PSSP brought in additional work, but PHNOs, DEOs and MLTs needed for executing this work were not available in most of the PMCIs. Therefore, the additional tasks of PSSP were shifted to existing staff, who felt overburdened and stressed. Although ‘task shifting’ is recommended as an important intervention in LMICs, if it is implemented irrationally, it can lead to inefficiency [37]. The PMCIs did not have documents on the number of ‘approved’ positions for cadres other than medical and nursing officers, highlighting the need for developing and conducting staffing establishment assessments for PMCI based on service utilization and size of the catchment areas [38,39,40].
The non-availability of DEOs was largely due to high turnover because of the contractual nature of the DEO position. Studies from other LMICs also reported similar inefficiencies with contractual arrangements for the health workforce [41,42]. We recommend immediate deployment of DEOs and creation of a permanent cadre of DEOs in future for maintaining and updating the PHR. Like other countries, the shortage of MLTs in PMCIs was due to a lack of MLT training programs in Sri Lanka [43,44]. Therefore, training of MLTs through bachelor programs or short-term training programs has to be prioritized [45,46]. As a stop-gap measure, one of the existing nursing officers can be deployed as PHNO after being relieved from curative care services and trained in community mobilisation.
Pre-implementation training on PSSP was conducted for key personnel from the PMCIs. The training coverage was sub-optimal due to poor attendance and inability to ensure “trickle-down” training of the remaining personnel in PMCI. The training was not effective due to a lack of standardised training materials and insufficient emphasis on practical issues. Comprehensive training modules need to be developed by the PMU in consultation with major stakeholders, such as the NCD programme unit, planning unit and directorate of health services. Involvement of pro-active HCWs with experience in implementing PSSP in the first phase and use of case studies on early implementation experiences could help to orient trainees on practical aspects. Online training modules and short video lectures could be developed to serve as ready references and to enable the conduct of training during situations, such as the COVID-19 pandemic.
Blood glucose and blood cholesterol estimations with POCTs were generally available, which is appreciable given the utility of the POCTs in NCD care provision [47,48,49,50]. However, there were instances of shortages of consumables (strips), leading to underutilization of POCTs, as reported from Ghana [51]. Unavailability of other essential investigations was largely due to shortages of reagents and MLTs. Many samples had to be transported to the apex laboratories but the SCT system performed sub-optimally, mimicking the operational challenges in SCT reported elsewhere [52,53,54,55].
The laboratory services need to be strengthened as a priority, either by improving the availability of essential investigations at the PMCI or by establishing robust SCT to ensure quality NCD care. The reagents and consumables have to be supplied in sufficient quantities and in an uninterrupted manner based on previous utilization statistics. There is a need for further research to find better models to improve the efficiency of SCT, so that there is timely availability of investigations and reduced turnaround times for results [56,57,58,59].
More than 50% of the drugs on the essential drug list were available at the PMCIs. However, some PMCIs had instances of stock outs of essential drugs. The stock outs were largely attributed to inefficiencies in drug forecasting and use of MSMIS for indenting, which are in line with similar findings that have been reported from other countries [60,61]. The drugs were not available in blister packs and were disbursed as loose drugs, leading to poor perception on the quality of drugs in the PMCI [62]. There was limited storage space, poor maintenance of drug registers and no air conditioning in most sites. Furthermore, the drugs required for patients back-referred from the secondary or tertiary hospitals were not available at the PMCIs and had to be purchased from private pharmacies.
There is a need for uninterrupted supplies of essential NCD drugs in order to reduce out-of-pocket expenditures. The pharmacists and dispensers need to be trained in drug forecasting, indenting using MSMIS, drug-storage practices and record maintenance in order to bring more efficiency to the supply chain management. Blister packs need to be introduced in order to overcome patients’ concerns about the quality of drugs. Physical infrastructure for safe long-term storage of drugs, including buffer stocks, needs to be strengthened. Moving forward, the essential drugs list of PMCIs could be rationalised, to accommodate some of the oral NCD drugs that are currently available only at secondary and tertiary hospitals.
An online HMIS was available in all the PMCIs. Similar to findings from other countries, the HMIS was not utilised optimally to manage PHRs due to poor internet connectivity, frequent computer crashes because of inadequate server space, glitches in some modules and other ill-defined processes [63,64,65]. Shortages of DEOs and reluctance of HCWs to use the HMIS, because they failed to appreciate the relevance of the HMIS data, led to underutilization of the system.
As the HMIS is an online platform, there is an urgent need to improve the internet connectivity at the PMCIs. The server capacity of the HMIS should be augmented for smooth functioning, which becomes more relevant in the context of expansion of the PSSP to newer PMCIs. Moving forward, there is a need to develop an offline HMIS module to limit the dependence on continuous internet connectivity, especially in remote areas. Development of dashboards, which can aid HCWs in monitoring their progress and developing their work plans, would add significant value to the current HMIS.
5. Conclusions
This study conducted among PMCIs, strengthened through the first phase of PSSP, showed that significant efforts have been made in improving population screening for NCDs at PMCIs/HLCs, the availability of trained manpower, essential drugs, laboratory services and HMIS at HLCs and PMCIs. However, there were several facility-specific challenges that need to be addressed as a priority to ensure sustainability and successful scale-up of PSSP.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/healthcare10112251/s1, Annex S1: Standard essential drug list to be available in PMCI; Annex S2: List of laboratory services under basic laboratory package of PSSP; Annex S3: Facility assessment Checklist; Annex S4: Interview guide used for in-depth interview; Supplementary Table S1: Qualitative data on challenges with human resource management and training in PMCIs supported by PSSP in Sri Lanka, 2021; Supplementary Table S2: Qualitative data on challenges in drug stock management in PMCIs supported by PSSP in Sri Lanka, 2021; Supplementary Table S3: Qualitative data on challenges in laboratory investigations conducted through PMCI supported by PSSP in Sri Lanka, 2021; Supplementary Table S4: Qualitative data on challenges in using Health Management Information System (HMIS) established in PMCI supported by PSSP in Sri Lanka, 2021.
Author Contributions
Conceptualization, P.T., M.F., A.M.V.K., S.S., D.E.A., H.H., J.B., S.D.B. and A.D.H.; methodology, P.T., M.F., A.M.V.K., D.E.A., H.H., J.B. and A.D.H.; software, P.T.; validation, P.T., M.F., D.N., N.C., A.C. and A.M.V.K.; formal analysis, P.T., D.N. and A.M.V.K.; investigation, M.F., N.C. and A.C.; resources, D.E.A., H.H. and J.B.; data curation, P.T., M.F., N.C. and A.C.; writing—original draft preparation, P.T. and D.N.; writing—review and editing, M.F., A.M.V.K., S.S., D.E.A., H.H., J.B., S.D.B. and A.D.H.; visualization, P.T.; supervision, A.M.V.K., D.E.A., H.H., J.B. and A.D.H.; project administration, S.D.B.; funding acquisition, P.T., A.M.V.K., A.D.H., S.D.B. and A.D.H. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the Global Fund to Fight AIDS, Tuberculosis and Malaria through the “Advance Universal Health Coverage: Multi-Donor Trust Fund for Integrating Externally Financed Health Programs” administered by the World Bank (Contract number- 7197811).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Advisory Group (EAG Number: 01/21) of the International Union Against Tuberculosis and Lung disease, Paris, France, and Ethics Review Committee (ERC/01/21) of Rajarata University of Sri Lanka for studies involving humans.
Informed Consent Statement
Written informed consent was obtained from all healthcare workers involved and interviewed in the study.
Data Availability Statement
Requests to access these data should be sent to the corresponding author.
Acknowledgments
We thank the Project Management Unit of Primary Healthcare-System-Strengthening Project (PSSP) and the World Bank task team for their support with necessary administrative approvals and guidance in carrying out this study. We thank all the programme managers who attended the ‘operational research capacity building programme’ and helped in fine tuning the facility assessment checklist. We thank all the programme managers at provincial and district level, medical officers in charge and other staff at the selected PMCIs for their support in quantitative data extraction and participation in in-depth interviews. We also thank Palanivel Chinnakali (JIPMER, Puduchery, India), Hemant Deepak Shewade (ICMR-National Institute of Epidemiology, Chennai, India), Amol Dongre (Pramukhswami Medical College, Karamsad, India) and Kalaiselvi Selvaraj (AIIMS, Nagpur, India) for their support in capacity-building activities and guidance during the conduct of the study. We acknowledge the contribution of the Foundation for Health Promotion (FHP) of Sri Lanka in managing the field-level implementation of this operational research study. We thank our research staff: M.R. Fathima Sajeeda, R. Minali Yoshitha Ritigahapola, W. Nimali Hansika Rodrigo, T.H. Sulakshi Shashikala Ariyarathne, U.L.W. Yashodha Srimali Lindakumbura, H.M. Shashila Harshani Dharmapala, L.G. Tharindu Madhushan, D.M. Nuwani Buddhika Dissanayaka and B.P. Chamodi Kalpana Bulugahamulla.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results. The findings, interpretations, and conclu-sions in this paper are entirely those of the authors and should not be attributed in any manner to the World Bank, to its affiliated organizations or to members of its Board of Executive Directors or the countries they represent.
List of Abbreviation
| Abbreviation | Definition |
| DALY | Disability Adjusted Life Year |
| DH | Divisional Hospital |
| DLI | Disbursement-Linked Indicator |
| GIS | Geographic Information System |
| GN | Grama Niladhari |
| HIMS | Health Information Management System |
| HLC | Health Lifestyle Centre |
| LMIC | Low-and-middle income country |
| MoH | Medical Office of Health |
| MOH | Ministry of Health |
| NCD | Non-communicable disease |
| OOPE | Out-of-Pocket Expenditure |
| PEN | Package of Essential NCD interventions for primary care |
| PHC | Primary Health Care |
| PHN | Personal Health Number |
| PHR | Personal Health Records |
| PMCI | Primary Medical Care Institution |
| PMCU | Primary Medical Care Units |
| PMU | Project Monitoring Unit |
| POCT | Point Of Care Test |
| PSSP | Primary Health Care System Strengthening Project |
| UHC | Universal Health Coverage |
| UN | United Nations |
| WHO | World Health Organization |
References
- World Health Organization (WHO). Noncommunicable Diseases—Fact Sheet. Available online: https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases (accessed on 6 January 2021).
- World Health Organization (WHO). The Top 10 Causes of Death. Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 6 January 2021).
- GBD 2017 Causes of Death Collaborators. Global, Regional, and National Age-Sex-Specific Mortality for 282 Causes of Death in 195 Countries and Territories, 1980–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1736–1788. [Google Scholar] [CrossRef]
- World Economic Programme; World Health Organization (WHO). From Burden to “Best Buys”: Reducing the Economic Impact of Non-Communicable Diseases in Low- and Middle-Income Countries; World Health Organization (WHO): Geneva, Switzerland, 2011. [Google Scholar]
- Samb, B.; Desai, N.; Nishtar, S.; Mendis, S.; Bekedam, H.; Wright, A.; Hsu, J.; Martiniuk, A.; Celletti, F.; Patel, K.; et al. Prevention and Management of Chronic Disease: A Litmus Test for Health-Systems Strengthening in Low-Income and Middle-Income Countries. Lancet 2010, 376, 1785–1797. [Google Scholar] [CrossRef]
- World Health Organization. Global Action Plan for the Prevention and Control of NCDs 2013–2020; World Health Organization: Geneva, Switzerland, 2013. [Google Scholar]
- Pan American Health Organization (PAHO), World Health Organization (WHO). Third United Nations High-Level Meeting on NCDs. Available online: http://www3.paho.org/hq/index.php?option=com_content&view=article&id=14416:un-general-assembly-third-high-level-meeting-ncds-2018&Itemid=0&lang=en#gsc.tab=0 (accessed on 6 January 2021).
- Beaglehole, R.; Bonita, R.; Horton, R.; Adams, C.; Alleyne, G.; Asaria, P.; Baugh, V.; Bekedam, H.; Billo, N.; Casswell, S.; et al. Priority Actions for the Non-Communicable Disease Crisis. Lancet 2011, 377, 1438–1447. [Google Scholar] [CrossRef]
- Varghese, C.; Nongkynrih, B.; Onakpoya, I.; McCall, M.; Barkley, S.; Collins, T.E. Better Health and Wellbeing for Billion More People: Integrating Non-Communicable Diseases in Primary Care. BMJ 2019, 364, l327. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Package of Essential Noncommunicable (PEN) Disease Interventions for Primary Health Care in Low-Resource Settings; World Health Organization (WHO): Geneva, Switzerland, 2010. [Google Scholar]
- Leslie, H.H.; Spiegelman, D.; Zhoub, X.; Kruka, M.E. Service Readiness of Health Facilities in Bangladesh, Haiti, Kenya, Malawi, Namibia, Nepal, Rwanda, Senegal, Uganda and the United Republic of Tanzania. Bull. World Health Organ. 2017, 95, 738–748. [Google Scholar] [CrossRef]
- Huque, R.; Nasreen, S.; Ahmed, F.; Hicks, J.P.; Walley, J.; Newell, J.N.; Elsey, H. Integrating a Diabetes and Hypertension Case Management Package within Primary Health Care: A Mixed Methods Feasibility Study in Bangladesh. BMC Health Serv. Res. 2018, 18, 811. [Google Scholar] [CrossRef]
- Hyon, C.S.; Nam, K.Y.; Sun, H.C.; Garg, R.; Shrestha, S.M.; Ok, K.U.; Kumar, R. Package of Essential Noncommunicable Disease (PEN) Interventions in Primary Health-Care Settings in the Democratic People’s Republic of Korea: A Feasibility Study. WHO South-East Asia J. Public Health 2017, 6, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Wangchuk, D.; Virdi, N.; Garg, R.; Mendis, S.; Nair, N.; Wangchuk, D.; Kumar, R. Package of Essential Noncommunicable Disease (PEN) Interventions in Primary Health-Care Settings of Bhutan: A Performance Assessment Study. WHO South-East Asia J. Public Health 2014, 3, 154. [Google Scholar] [CrossRef]
- Aye, L.L.; Tripathy, J.P.; Maung Maung, T.; Oo, M.M.; Nwe, M.L.; Thu, H.M.M.; Ko, K.; Kaung, K.K. Experiences from the Pilot Implementation of the Package of Essential Non-Communicable Disease Interventions (PEN) in Myanmar, 2017–2018: A Mixed Methods Study. PLoS ONE 2020, 15, e0229081. [Google Scholar] [CrossRef]
- Kontsevaya, A.; Farrington, J. Implementation of a Package of Essential Noncommunicable (PEN) Disease Interventions in Kyrgyzstan: Evaluation of Effects and Costs in Bishkek after One Year; World Health Organization (WHO): Copenhagen, Denmark, 2017. [Google Scholar]
- Tesema, A.G.; Ajisegiri, W.S.; Abimbola, S.; Balane, C.; Kengne, A.P.; Shiferaw, F.; Dangou, J.-M.; Narasimhan, P.; Joshi, R.; Peiris, D. How Well Are Non-Communicable Disease Services Being Integrated into Primary Health Care in Africa: A Review of Progress against World Health Organization’s African Regional Targets. PLoS ONE 2020, 15, e0240984. [Google Scholar] [CrossRef]
- Mendis, S.; Al Bashir, I.; Dissanayake, L.; Varghese, C.; Fadhil, I.; Marhe, E. Gaps in Capacity in Primary Care in Low-Resource Settings for Implementation of Essential Noncommunicable Disease Interventions. Int. J. Hypertens. 2012, 2012, 584041. [Google Scholar] [CrossRef] [PubMed]
- Allen, L.; Nicholson, B.; Yeung, B.; Goiana-da-Silva, F. Implementation of Non-Communicable Disease Policies: A Geopolitical Analysis of 151 Countries. Lancet Glob. Health 2020, 8, e50–e58. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Service Availability and Readiness Assessment (SARA)—An Annual Monitoring System for Service Delivery Reference Manual; World Health Organization (WHO): Geneva, Switzerland, 2015. [Google Scholar]
- Institute for Health Metrics and Evaluation. GBD Results Tool—GHDx. Available online: http://ghdx.healthdata.org/gbd-results-tool (accessed on 6 January 2021).
- Senaratne, R.; Mendis, S. Prevention and Control of Noncommunicable Diseases: Think Globally—Act Locally; Lessons from Sri Lanka; Ministry of Health, Nutrition and Indigenous Medicine: Colombo, Sri Lanka, 2018.
- Ministry of Health Nutrition and Indigenous Medicine Department of Census and Statistics. Service Availability and Readiness Assessment Sri Lanka 2017; Ministry of Health, Nutrition and Indigenous Medicine: Colombo, Sri Lanka, 2018.
- Ministry of Health Nutrition and Indigenous Medicine. Reorganising Primary Health Care in Sri Lanka—Preserving Our Progress, Preparing Our Future; Ministry of Health, Nutrition and Indigenous Medicine: Colombo, Sri Lanka, 2017.
- World Health Organization (WHO). Colombo Declaration: Strengthening Health Systems to Accelerate Delivery of Noncommunicable Diseases Services at the Primary Health Care Level. In Proceedings of the Sixty-Ninth Session of the WHO Regional Committee for South-East Asia 2016, Colombo, Sri Lanka, 5–9 September 2016; World Health Organization (WHO): New Delhi, India, 2016. [Google Scholar]
- World Health Organization (WHO). Strengthening Health Systems to Accelerate Delivery of Noncommunicable Diseases Services at the Primary Health Care Leve—A One-Year Progress Review of the Implementation of the 2016 Colombo Declaration on NCDs; World Health Organization (WHO): New Delhi, India, 2017. [Google Scholar]
- Ministry of Health Nutrition and Indigenous Medicine. Road Map for the Primary Healthcare System Strengthening Project (PSSP); Ministry of Health, Nutrition and Indigenous Medicine: Colombo, Sri Lanka, 2019.
- The World Bank; Health Nutrition and Population Global Practice. Project Appraisal Document: Primary Health Care System Strengthening Project; The World Bank: Washington, DC, USA, 2018. [Google Scholar]
- Ministry of Health Nutrition and Indigenous Medicine. Guidelines for Operationalizing Primary Medical Care Services in Sri Lanka; Ministry of Health, Nutrition and Indigenous Medicine: Colombo, Sri Lanka, 2019.
- Creswell, J.; Plano Clark, V. Designing and Conducting Mixed Methods Research, 2nd ed.; Sage Publications Ltd.: London, UK, 2010. [Google Scholar]
- The World Bank. Sri Lanka—Data. Available online: https://data.worldbank.org/country/sri-lanka?view=chart (accessed on 6 January 2021).
- Government of Sri Lanka. List of Codes for the Administrative Divisions of Sri Lanka. 2001. Available online: https://web.archive.org/web/20110519151751/http://www.statistics.gov.lk/GNcode/introduction.pdf (accessed on 6 January 2021).
- Govindaraj, R.; Navaratne, K.; Cavagnero, E.; Seshadri, S.R. Health Care in Sri Lanka: What Can the Private Health Sector Offer; The World Bank: Washington, DC, USA, 2014. [Google Scholar]
- Ministry of Health. Summary of Government Hospitals. Available online: http://www.health.gov.lk/moh_final/english/others.php?pid=92 (accessed on 7 January 2021).
- DeJonckheere, M.; Vaughn, L.M. Semistructured Interviewing in Primary Care Research: A Balance of Relationship and Rigour. Fam. Med. Community Health 2019, 7, e000057. [Google Scholar] [CrossRef] [PubMed]
- Tong, A.; Sainsbury, P.; Craig, J. Consolidated Criteria for Reporting Qualitative Research (COREQ): A 32-Item Checklist for Interviews and Focus Groups. Int. J. Qual. Health Care 2007, 19, 349–357. [Google Scholar] [CrossRef]
- Mijovic, H.; McKnight, J.; English, M. What Does the Literature Tell Us about Health Workers’ Experiences of Task-Shifting Projects in Sub-Saharan Africa? A Systematic, Qualitative Review. J. Clin. Nurs. 2016, 25, 2083–2100. [Google Scholar] [CrossRef]
- Hurst, K. Primary and Community Care Workforce Planning and Development. J. Adv. Nurs. 2006, 55, 757–769. [Google Scholar] [CrossRef]
- Bourgeault, I.L.; Chamberland-Rowe, C.; Simkin, S. Co-Developing an Integrated Primary Care Workforce Planning Approach at a Regional Level: Overarching Framework and Guiding Principles. Hum. Resour. Health 2021, 19, 87. [Google Scholar] [CrossRef]
- Aytona, M.G.; Politico, M.R.; McManus, L.; Ronquillo, K.; Okech, M. Determining Staffing Standards for Primary Care Services Using Workload Indicators of Staffing Needs in the Philippines. Hum. Resour. Health 2022, 19, 129. [Google Scholar] [CrossRef]
- Maleki, M.; Mardani, A.; Vaismoradi, M. Insecure Employment Contracts during the COVID-19 Pandemic and the Need for Participation in Policy Making. Int. J. Environ. Res. Public Health 2021, 18, 12548. [Google Scholar] [CrossRef]
- Steinmetz, S.; Vries, D.H.D.; Tijdens, K.G. Should I Stay or Should I Go? The Impact of Working Time and Wages on Retention in the Health Workforce. Hum. Resour. Health 2014, 12, 23. [Google Scholar] [CrossRef]
- Kaplan, R.L.; Burgess, T.E. The Impending Crisis. J. Microbiol. Biol. Educ. 2010, 11, 140. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Scott, K. The Laboratory Workforce Shortage Demands New Solutions—AACC.Org. Available online: https://www.aacc.org/cln/articles/2015/november/the-laboratory-workforce-shortage-demands-new-solutions (accessed on 19 April 2022).
- Strain, A.K.; Sullivan, M.M. Strengthening Laboratory Partnerships, Enhancing Recruitment, and Improving Through Training and Outreach Activities: The Minnesota. Public Health Rep. 2019, 134, 11S–15S. [Google Scholar] [CrossRef] [PubMed]
- Harb, R.; Kachimanga, C.; Bangura, M.; Kanawa, S.; Stratton, C.W.; Milner, D.A.; Eichbaum, Q. Providing Laboratory Medicine Training in a Low-Resource Setting: Lessons Learned From a Recent Experience in Sierra Leone. Am. J. Clin. Pathol. 2021, 155, 473. [Google Scholar] [CrossRef] [PubMed]
- Motta, L.A.; Shephard, M.D.S.; Brink, J.; Lawson, S.; Rheeder, P. Point-of-Care Testing Improves Diabetes Management in a Primary Care Clinic in South Africa. Prim. Care Diabetes 2017, 11, 248–253. [Google Scholar] [CrossRef] [PubMed]
- El-Osta, A.; Woringer, M.; Pizzo, E.; Verhoef, T.; Dickie, C.; Ni, M.Z.; Huddy, J.R.; Soljak, M.; Hanna, G.B.; Majeed, A. Does Use of Point-of-Care Testing Improve Cost-Effectiveness of the NHS Health Check Programme in the Primary Care Setting? A Cost-Minimisation Analysis. BMJ Open 2017, 7, e015494. [Google Scholar] [CrossRef]
- Laurence, C.O.; Gialamas, A.; Bubner, T.; Yelland, L.; Willson, K.; Ryan, P.; Beilby, J. Patient Satisfaction with Point-of-Care Testing in General Practice. Br. J. Gen. Pract. 2010, 60, 166–171. [Google Scholar] [CrossRef]
- Weigl, B.H.; Neogi, T.; McGuire, H. Point-of-Care Diagnostics in Low-Resource Settings and Their Impact on Care in the Age of the Noncommunicable and Chronic Disease Epidemic. J. Lab. Autom. 2014, 19, 248–257. [Google Scholar] [CrossRef][Green Version]
- Kuupiel, D.; Tlou, B.; Bawontuo, V.; Drain, P.K.; Mashamba-Thompson, T.P. Poor Supply Chain Management and Stock-Outs of Point-of-Care Diagnostic Tests in Upper East Region’s Primary Healthcare Clinics, Ghana. PLoS ONE 2019, 14, e0211498. [Google Scholar] [CrossRef]
- Nybo, M.; Cadamuro, J.; Cornes, M.P.; Gómez Rioja, R.; Grankvist, K. Sample Transportation—An Overview. Diagnosis 2019, 6, 39–43. [Google Scholar] [CrossRef]
- Cureau, F.V.; Bloch, K.V.; Henz, A.; Schaan, C.W.; Klein, C.H.; De Oliveira, C.L.; Giannini, D.T.; De Leon, E.B.; de Azevedo Abreu, G.; Telo, G.H.; et al. Challenges for Conducting Blood Collection and Biochemical Analysis in a Large Multicenter School-Based Study with Adolescents: Lessons from ERICA in Brazil. Cad. Saude Publica 2017, 33, e00122816. [Google Scholar] [CrossRef]
- Gebregergs, G.B.; Sinishaw, M.A.; Shiferaw, M.B.; Antife, T.; Assefa, M.; Fiseha, D.; Klinkenberg, E. Evaluation of the Postal Service for Referral of Specimen of Drug Resistance Tuberculosis in Amhara Region, Ethiopia; Mixed Method. Afr. Health Sci. 2021, 21, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Zaman, F.A.; Aggarwal, S.; Pal, R.; Chatterjee, P.K.; Kiran, K.A.; Panda, S.; Sharma, U.; Bhattacharya, T. Exploratory Study on the Operational Issues Faced in Collection, Transportation, and Laboratory Testing Related to COVID-19 in Remote Areas of Selected EAG States of North East and East India. J. Fam. Med. Prim. Care 2021, 10, 1452. [Google Scholar] [CrossRef]
- Kebede, Y.; Fonjungo, P.N.; Tibesso, G.; Shrivastava, R.; Nkengasong, J.N.; Kenyon, T.; Kebede, A.; Gadde, R.; Ayana, G. Improved Specimen-Referral System and Increased Access to Quality Laboratory Services in Ethiopia: The Role of the Public-Private Partnership. J. Infect. Dis. 2016, 213 (Suppl. 2), S59–S64. [Google Scholar] [CrossRef] [PubMed]
- Kassambara, H.; Nana, M.L.; Samassa, F.; Traoré, M.D. Sample Transport Optimization: Mali Pilot Study. Health Secur. 2020, 18, S92–S97. [Google Scholar] [CrossRef]
- Dama, E.; Nikiema, A.; Nichols, K.; Bicaba, B.W.; Porgho, S.; Greco Koné, R.; Tarnagda, Z.; Cissé, A.; Ngendakumana, I.; Adjami, A.; et al. Designing and Piloting a Specimen Transport System in Burkina Faso. Health Secur. 2020, 18, S98–S104. [Google Scholar] [CrossRef]
- Semret, M.; Ndao, M.; Jacobs, J.; Yansouni, C.P. Point-of-Care and Point-of-“can”: Leveraging Reference-Laboratory Capacity for Integrated Diagnosis of Fever Syndromes in the Tropics. Clin. Microbiol. Infect. 2018, 24, 836–844. [Google Scholar] [CrossRef]
- Tran, D.N.; Bero, L.A. Barriers and Facilitators to the Quality Use of Essential Medicines for Maternal Health in Low–Resource Countries: An Ishikawa Framework. J. Glob. Health 2015, 5, 010406. [Google Scholar] [CrossRef]
- Shukar, S.; Zahoor, F.; Hayat, K.; Saeed, A.; Gillani, A.H.; Omer, S.; Hu, S.; Babar, Z.U.D.; Fang, Y.; Yang, C. Drug Shortage: Causes, Impact, and Mitigation Strategies. Front. Pharmacol. 2021, 12, 1772. [Google Scholar] [CrossRef]
- Conn, V.S.; Ruppar, T.M.; Chan, K.C.; Dunbar-Jacob, J.; Pepper, G.A.; De Geest, S. Packaging Interventions to Increase Medication Adherence: Systematic Review and Meta-Analysis. Curr. Med. Res. Opin. 2015, 31, 145. [Google Scholar] [CrossRef]
- Tummers, J.; Tekinerdogan, B.; Tobi, H.; Catal, C.; Schalk, B. Obstacles and Features of Health Information Systems: A Systematic Literature Review. Comput. Biol. Med. 2021, 137, 104785. [Google Scholar] [CrossRef]
- Afrizal, S.H.; Handayani, P.W.; Hidayanto, A.N.; Eryando, T.; Budiharsana, M.; Martha, E. Barriers and Challenges to Primary Health Care Information System (PHCIS) Adoption from Health Management Perspective: A Qualitative Study. Inform. Med. Unlocked 2019, 17, 100198. [Google Scholar] [CrossRef]
- Feyzabadi, V.Y.; Emami, M.; Mehrolhassani, M.H. Health Information System in Primary Health Care: The Challenges and Barriers from Local Providers’ Perspective of an Area in Iran. Int. J. Prev. Med. 2015, 6, 57. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).