Monkeypox Infection 2022: An Updated Narrative Review Focusing on the Neonatal and Pediatric Population
Highlights
- Monkeypox disease has recently attracted attention due to a multi-country outbreak in 2022, raising serious public health concerns and being considered a public health emergency by the World Health Organization.
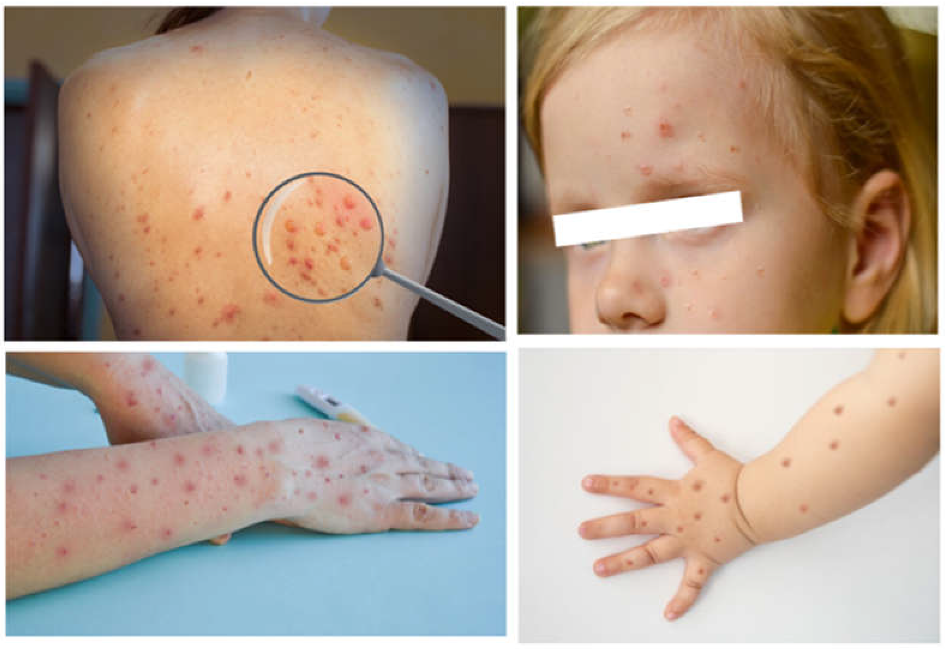
- Although it can affect anyone outside of endemic areas, the disease has been observed to be more common in men who have sex with men. Symptoms include fever, lymphadenopathy, and a vesicular rash.
- Monkeypox disease can cause severe illness, especially in individuals with compromised immune systems, children, and pregnant women.
- Pre- and post-exposure prophylaxis with available smallpox vaccine formulations offer protection against the infection. However, undertreatment and underdiagnosis in under-resourced countries remain relevant issues that need urgent attention.
- Maintaining a high level of clinical suspicion is needed to accurately diagnose, report, and isolate cases and dispel public misconceptions and fears. In the event of additional outbreaks, this will aid clinic management and therapy.
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Monkeypox Virus: Generalities
3.2. Clinical Presentation, Transmission and Prevention
3.2.1. Monkeypox in the Pediatric Population
3.2.2. Monkeypox in Pregnancy and in Newborns
3.3. Diagnosis
3.4. Treatment
3.4.1. Antiviral Medicines
- (a)
- Cidofovir (Vistide) is primarily used as a treatment for retinitis, encephalitis and oesophagitis caused by cytomegalovirus, especially in people with HIV. It is the phosphorylated active metabolite of brincidovir. In-vitro and preclinical studies showed that is effective against poxviruses [4];
- (b)
- Brincidofovir (Tembexa) is available as oral suspension/tablets, approved by FDA for smallpox disease [58]. Both drugs are inhibitors of DNA replication with a broad spectrum of activity against multiple families of double-stranded DNA viruses.
- (c)
- Tecovirimat (ST-246): is an antiviral medication which impairs the function of the VP37 envelope protein necessary for the formation of the extracellular enveloped virus required for cell-to-cell transmission; it has more specific activity against orthopoxviruses [58]. It has been approved by Food and Drug Administration (FDA), used to treat human smallpox disease but can be used against MPV. Tecovirimat is given orally (TPOXX®: 200 mg capsule) or as an injectable formulation [52]. Capsules should be taken within 30 min after a full meal with moderate to high fat. Per CDC guidelines, for those who cannot swallow they can be opened and mixed with liquids/soft food [59] Because it is an inducer of cytochrome P450 (CYP) 3A and CYP2B6, co-administration with this drug may lead to reduced plasma exposures of sensitive substrates of CYP3A4 or CYP2B6, reducing the effects. Because of the presence in its IV formulation of a potentially nephrotoxic substance (hydroxypropyl-β-cyclodextrin), it is advisable to dose creatinine clearance (CrCl) and liver function before starting treatment. Intravenous therapy is safe in mild/moderate renal impairment but is contraindicated in severe nephropathies (CrCl < 30 mL/min), both in adults and children [39]. Dose adjustments for oral therapy instead are not required in the case of mild, moderate, severe nephropathy or even in patients requiring hemodialysis in end-stage renal disease [60]. Although reduced fertility due to testicular toxicity was found in mouse models, no human data are available [60].
3.4.2. Children’s Treatment
3.4.3. Treatment during Pregnancy and Breast-Feeding
3.5. Vaccines: Pre- and Post-Exposure Prophylaxis
3.5.1. Vaccine in Adults
3.5.2. Vaccines in the Pediatric Population
3.5.3. Vaccines during Pregnancy
3.6. Post-Exposure Measures
3.7. Population, Ethics and Risk of Discrimination
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moore, M.J.; Rathish, B.; Zahra, F. Monkeypox; Stat Pearls Publishing LLC: Treasure Island, FL, USA, 2022. Available online: www.ncbi.nlm.nih.gov/books/NBK574519/ (accessed on 18 November 2022).
- Ladnyj, I.D.; Ziegler, P.; Kima, E. A human infection caused by monkeypox virus in Basankusu Territory, Democratic Republic of the Congo. Bull. World Health Organ. 1972, 46, 593–597. [Google Scholar] [PubMed]
- Reynolds, M.G.; Yorita, K.L.; Kuehnert, M.J.; Davidson, W.B.; Huhn, G.D.; Holman, R.C.; Damon, I.K. Clinical Manifestations of Human Monkeypox Influenced by Route of Infection. J. Infect. Dis. 2006, 194, 773–780. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Acharya, A.; Gendelman, H.E.; Byrareddy, S.N. The 2022 outbreak and the pathobiology of the monkeypox virus. J. Autoimmun. 2022, 131, 102855. [Google Scholar] [CrossRef] [PubMed]
- CDC. 2022 Monkeypox Outbreak Global Map. Available online: https://www.cdc.gov/poxvirus/monkeypox/response/2022/world-map.html (accessed on 18 November 2022).
- ECDC. 2022 Monkeypox Situation Update. Available online: https://www.ecdc.europa.eu/en/news-events/monkeypox-situation-update (accessed on 18 November 2022).
- WHO. Monkeypox Outbreak 2022. Available online: https://www.who.int/emergencies/situation-reports (accessed on 18 November 2022).
- Hennessee, I.; Shelus, V.; McArdle, C.E.; Wolf, M.; Schatzman, S.; Carpenter, A.; Minhaj, F.S.; Petras, J.K.; Cash-Goldwasser, S.; Maloney, M.; et al. Epidemiologic and Clinical Features of Children and Adolescents Aged < 18 Years with Monkeypox—United States, 17 May–24 September 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 1407–1411. [Google Scholar] [CrossRef] [PubMed]
- WHO Director-General Declares the Ongoing Monkeypox Outbreak a Public Health Emergency of International Concern. Available online: https://www.who.int/europe/news/item/23-07-2022-who-director-general-declares-the-ongoing-monkeypox-outbreak-a-public-health-event-of-international-concern (accessed on 18 November 2022).
- Alakunle, E.; Moens, U.; Nchinda, G.; Okeke, M. Monkeypox Virus in Nigeria: Infection Biology, Epidemiology, and Evolution. Viruses 2020, 12, 1257. [Google Scholar] [CrossRef] [PubMed]
- Likos, A.M.; Sammons, S.A.; Olson, V.A.; Frace, A.M.; Li, Y.; Olsen-Rasmussen, M.; Davidson, W.; Galloway, R.; Khristova, M.L.; Reynolds, M.G.; et al. A tale of two clades: Monkeypox viruses. J. Gen. Virol. 2005, 86 Pt 10, 2661–2672. [Google Scholar] [CrossRef] [PubMed]
- WHO. Variants Names. Available online: https://www.who.int/news/item/12-08-2022-monkeypox--experts-give-virus-variants-new-names (accessed on 18 November 2022).
- Agrati, C.; Cossarizza, A.; Mazzotta, V.; Grassi, G.; Casetti, R.; De Biasi, S.; Pinnetti, C.; Gili, S.; Mondi, A.; Cristofanelli, F.; et al. Immunological Signature in Human Cases of Monkeypox Infection in 2022 Outbreak. Lancet Infect. Dis. 2022. [Google Scholar] [CrossRef]
- Johnston, S.C.; Johnson, J.C.; Stonier, S.W.; Lin, K.L.; Kisalu, N.K.; Hensley, L.E.; Rimoin, A.W. Cytokine modulation correlates with severity of monkeypox disease in humans. J. Clin. Virol. 2015, 63, 42–45. [Google Scholar] [CrossRef]
- Sanjuán, R.; Domingo-Calap, P. Mechanisms of viral mutation. Cell. Mol. Life Sci. 2016, 73, 4433–4448. [Google Scholar] [CrossRef] [Green Version]
- Gigante, C.M.; Plumb, M.; Ruprecht, A.; Zhao, H.; Wicker, V.; Wilkins, K.; Matheny, A. Genomic deletions and rearrangements in monkeypox virus from the 2022 outbreak, USA. bioRxiv 2022. [Google Scholar] [CrossRef]
- Wassenaar, T.M.; Wanchai, V.; Ussery, D.W. Comparison of Monkeypox virus genomes from the 2017 Nigeria outbreak and the 2022 outbreak. J. Appl. Microbiol. 2022, 133, 3690–3698. [Google Scholar] [CrossRef] [PubMed]
- Kozlov, M. The monkeypox virus is mutating. Are scientists worried? Nature 2022. epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Angelo, K.M.; Petersen, B.W.; Hamer, D.H.; Schwartz, E.; Brunette, G. Monkeypox transmission among international travellers—Serious monkey business? J. Travel Med. 2019, 26, taz002. [Google Scholar] [CrossRef] [PubMed]
- York, A. The bodily distribution of monkeypox virus. Nat. Rev. Genet. 2022, 20, 703. [Google Scholar] [CrossRef] [PubMed]
- Palich, R.; Burrel, S.; Monsel, G.; Nouchi, A.; Bleibtreu, A.; Seang, S.; Bérot, V.; Brin, C.; Gavaud, C.; Wakim, Y.; et al. Viral loads in clinical samples of men with monkeypox virus infection: A French case series. Lancet Infect. Dis. 2022. [Google Scholar] [CrossRef]
- Lum, F.-M.; Torres-Ruesta, A.; Tay, M.Z.; Lin, R.T.P.; Lye, D.C.; Rénia, L.; Ng, L.F.P. Monkeypox: Disease epidemiology, host immunity and clinical interventions. Nat. Rev. Immunol. 2022, 22, 597–613. [Google Scholar] [CrossRef]
- Di Gennaro, F.; Veronese, N.; Marotta, C.; Shin, J.I.; Koyanagi, A.; Silenzi, A.; Antunes, M.; Saracino, A.; Bavaro, D.F.; Soysal, P.; et al. Human Monkeypox: A Comprehensive Narrative Review and Analysis of the Public Health Implications. Microorganisms 2022, 10, 1633. [Google Scholar] [CrossRef]
- Vouga, M.; Nielsen-Saines, K.; Dashraath, P.; Baud, D. The monkeypox outbreak: Risks to children and pregnant women. Lancet Child Adolesc. Health 2022, 6, 751–753. [Google Scholar] [CrossRef]
- D’Antonio, F.; Pagani, G.; Buca, D.; Khalil, A. Monkeypox infection in pregnancy: A systematic review and metaanalysis. Am. J. Obstet. Gynecol. MFM 2022, 5, 100747. [Google Scholar] [CrossRef]
- CDC. Monkeypox in Animals. Available online: https://www.cdc.gov/poxvirus/monkeypox/veterinarian/monkeypox-in-animals.html (accessed on 18 November 2022).
- Grant, R.; Nguyen, L.-B.L.; Breban, R. Modelling human-to-human transmission of monkeypox. Bull. World Health Organ. 2020, 98, 638–640. [Google Scholar] [CrossRef]
- WHO. Disease Outbreak News. Available online: https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON396 (accessed on 18 November 2022).
- Pastula, D.M.; Tyler, K.L. An Overview of Monkeypox Virus and Its Neuroinvasive Potential. Ann. Neurol. 2022, 92, 527–531. [Google Scholar] [CrossRef]
- CDC. Clinical Recognition. Available online: https://www.cdc.gov/poxvirus/monkeypox/clinicians/clinical-recognition.html (accessed on 18 November 2022).
- Sejvar, J.J.; Chowdary, Y.; Schomogyi, M.; Stevens, J.; Patel, J.; Karem, K.; Fischer, M.; Kuehnert, M.J.; Zaki, S.R.; Paddock, C.D.; et al. Human Monkeypox Infection: A Family Cluster in the Midwestern United States. J. Infect. Dis. 2004, 190, 1833–1840. [Google Scholar] [CrossRef]
- Kaler, J.; Hussain, A.; Flores, G.; Kheiri, S.; Desrosiers, D. Monkeypox: A Comprehensive Review of Transmission, Pathogenesis, and Manifestation. Cureus 2022, 14, e26531. [Google Scholar] [CrossRef]
- Adalja, A.; Inglesby, T. A Novel International Monkeypox Outbreak. Ann. Intern. Med. 2022, 175, 1175–1176. [Google Scholar] [CrossRef]
- Nörz, D.; Pfefferle, S.; Brehm, T.T.; Franke, G.; Grewe, I.; Knobling, B.; Aepfelbacher, M.; Huber, S.; Klupp, E.M.; Jordan, S.; et al. Evidence of surface contamination in hospital rooms occupied by patients infected with monkeypox, Germany, June 2022. Eurosurveillance 2022, 27, 2200477. [Google Scholar] [CrossRef]
- CDC. Isolation and Infection Control at Home. Available online: https://www.cdc.gov/poxvirus/monkeypox/clinicians/infection-control-home.html (accessed on 18 November 2022).
- Dashraath, P.; Nielsen-Saines, K.; Mattar, C.; Musso, D.; Tambyah, P.; Baud, D. Guidelines for pregnant individuals with monkeypox virus exposure. Lancet 2022, 400, 21–22. [Google Scholar] [CrossRef]
- Bunge, E.M.; Hoet, B.; Chen, L.; Lienert, F.; Weidenthaler, H.; Baer, L.R.; Steffen, R. The changing epidemiology of human monkeypox—A potential threat? A systematic review. PLOS Negl. Trop. Dis. 2022, 16, e0010141. [Google Scholar] [CrossRef]
- Clinical Considerations for Monkeypox in Children and Adolescents. Available online: https://www.cdc.gov/poxvirus/monkeypox/clinicians/pediatric.html (accessed on 18 November 2022).
- Monkeypox—AAP Book American Academy of Pediatrics. Available online: https://www.aap.org/en/patient-care/monkeypox/ (accessed on 18 November 2022).
- Van Furth, A.M.T.; van der Kuip, M.; van Els, A.L.; Fievez, L.C.; van Rijckevorsel, G.G.; van den Ouden, A.; Jonges, M.; Welkers, M.R. Paediatric monkeypox patient with unknown source of infection, the Netherlands, June 2022. Eurosurveillance 2022, 27, 2200552. [Google Scholar] [CrossRef]
- CDC Schools, Early Care and Education Programs, and Other Settings Serving Children or Adolescents. Available online: www.cdc.gov/poxvirus/monkeypox/schools/faq.html (accessed on 18 November 2022).
- Ramnarayan, P.; Mitting, R.; Whittaker, E.; Marcolin, M.; O’Regan, C.; Sinha, R.; Bennett, A.; Moustafa, M.; Tickner, N.; Gilchrist, M.; et al. Neonatal Monkeypox Virus Infection. N. Engl. J. Med. 2022, 387, 1618–1620. [Google Scholar] [CrossRef]
- Obstetric Care Considerations for Monkeypox. Available online: https://www.acog.org/clinical-information/physician-faqs/obstetric-care-considerations-monkeypox (accessed on 18 November 2022).
- Wisner, K. Monkeypox: A Brief for Perinatal Nurses. MCN Am. J. Matern. Child Nurs. 2022. [Google Scholar] [CrossRef]
- Khalil, A.; Samara, A.; O’Brien, P.; Morris, E.; Draycott, T.; Lees, C.; Ladhani, S. Monkeypox and pregnancy: What do obstetricians need to know? Ultrasound Obstet. Gynecol. 2022, 60, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Khalil, A.; Samara, A.; O’Brien, P.; Ladhani, S. Call for a unified approach to Monkeypox infection in pregnancy: Lessons from the COVID-19 pandemic. Nat. Commun. 2022, 13, 5038. [Google Scholar] [CrossRef] [PubMed]
- Khalil, A.; Samara, A.; O’Brien, P.; Coutinho, C.M.; Duarte, G.; Quintana, S.M.; Ladhani, S.N. Monkeypox in pregnancy: Update on current outbreak. Lancet Infect. Dis. 2022, 22, 1534–1535. [Google Scholar] [CrossRef] [PubMed]
- Rash Illness Testing Protocol. Acute, Generalized Vesicular or Pustular Rash Illness Testing Protocol in the United States. Available online: https://www.cdc.gov/smallpox/lab-personnel/laboratory-procedures/rash-testing.html (accessed on 15 October 2022).
- Mccollum, A.M.; Damon, I.K. Human Monkeypox. Clin. Infect. Dis. 2014, 58, 260–267. [Google Scholar] [CrossRef] [Green Version]
- Thornhill, J.P.; Barkati, S.; Walmsley, S.; Rockstroh, J.; Antinori, A.; Harrison, L.B.; Palich, R.; Nori, A.; Reeves, I.; Habibi, M.S.; et al. Monkeypox Virus Infection in Humans across 16 Countries—April–June 2022. N. Engl. J. Med. 2022, 387, 679–691. [Google Scholar] [CrossRef] [PubMed]
- WHO. Laboratory Testing for the Monkeypox Virus: Interim Guidance 23 May 2022. Available online: https://www.who.int/publications/i/item/WHO-MPX-laboratory-2022.1 (accessed on 18 November 2022).
- Singhal, T.; Kabra, S.K.; Lodha, R. Monkeypox: A Review. Indian J. Pediatr. 2022, 89, 955–960. [Google Scholar] [CrossRef] [PubMed]
- MacNeil, A.; Reynolds, M.; Braden, Z.; Carroll, D.S.; Bostik, V.; Karem, K.; Smith, S.K.; Davidson, W.; Li, Y.; Moundeli, A.; et al. Transmission of Atypical Varicella-Zoster Virus Infections Involving Palm and Sole Manifestations in an Area with Monkeypox Endemicity. Clin. Infect. Dis. 2009, 48, e6–e8. [Google Scholar] [CrossRef] [Green Version]
- Baud, D.; Nielsen-Saines, K.; Dashraath, P. Approach to monkeypox in pregnancy: Conjecture is best guided by evidence. Am. J. Obstet. Gynecol. 2022. epub ahead of print. [Google Scholar] [CrossRef]
- Webb, E.; Rigby, I.; Michelen, M.; Dagens, A.; Cheng, V.; Rojek, A.M.; Dahmash, D.; Khader, S.; Gedela, K.; Norton, A.; et al. Availability, scope and quality of monkeypox clinical management guidelines globally: A systematic review. BMJ Glob. Health 2022, 7, e009838. [Google Scholar] [CrossRef]
- CDC. Monkeypox. Available online: https://www.cdc.gov/poxvirus/monkeypox/if-sick/treatment.html#:~:text=There%20are%20no%20treatments%20specifically,and%20treat%20monkeypox%20virus%20infections (accessed on 18 November 2022).
- Adler, H.; Gould, S.; Hine, P.; Snell, L.B.; Wong, W.; Houlihan, C.F.; Osborne, J.C.; Rampling, T.; Beadsworth, M.B.; Duncan, C.J.; et al. NHS England High Consequence Infectious Diseases (Airborne) Network. Clinical features and management of human monkeypox: A retrospective observational study in the UK. Lancet Infect. Dis. 2022, 22, 1153–1162, Erratum in Lancet Infect. Dis. 2022, 22, e177. [Google Scholar] [CrossRef]
- Siegrist, A.E.; Sassine, J. Antivirals with Activity Against Monkeypox: A Clinically Oriented Review. Clin. Infect. Dis. 2022. epub ahead of print. [Google Scholar] [CrossRef]
- CDC. Guidance for Tecovirimat Use. Available online: https://www.cdc.gov/poxvirus/monkeypox/pdf/tecovirimat-ind-protocol-cdc-irb.pdf (accessed on 18 November 2022).
- Expanded Access IND Protocol: Use of Tecovirimat (TPOXX®) for Treatment of Human Non-Variola Orthopoxvirus Infections in Adults and Children IND No. 116,039 CDC IRB No. 6402 Version 6.1 10 August 2022. Available online: https://solanocounty.com/documents/TecovirimatINDProtocol_CDCIRB6402_v5.105.20.22_versionfor2022MPXOutbreak.pdf (accessed on 18 November 2022).
- O’Laughlin, K.; Tobolowsky, F.A.; Elmor, R.; Overton, R.; O’Connor, S.M.; Damon, I.K.; Petersen, B.W.; Rao, A.K.; Chatham-Stephens, K.; Yu, P.; et al. Clinical Use of Tecovirimat (Tpoxx) for Treatment of Monkeypox Under an Investigational New Drug Protocol—United States, May–August 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 1190–1195. [Google Scholar] [CrossRef]
- Rabaan, A.A.; Abas, A.H.; Tallei, T.E.; Al-Zaher, M.A.; Al-Sheef, N.M.; Fatimawali; Al-Nass, E.Z.; Al-Ebrahim, E.A.; Effendi, Y.; Idroes, R.; et al. Monkeypox Outbreak 2022: What We Know So Far and Its Potential Drug Targets and Management Strategies. J. Med. Virol. 2022. epub ahead of print. [Google Scholar] [CrossRef]
- Red Book Online Outbreak: Monkeypox Virus Outbreak. Available online: https://publications.aap.org/redbook/resources/20705?autologincheck=redirected?nfToken=00000000-0000-0000-0000-000000000000 (accessed on 18 November 2022).
- Medscape Drug and Disease. Tecovirimat. Available online: https://reference.medscape.com/drug/tpoxx-tecovirimat-1000237 (accessed on 18 November 2022).
- EMA European Medicine Agency—Tecovirimat. Available online: www.ema.europa.eu/en/medicines/human/EPAR/tecovirimat-siga (accessed on 18 November 2022).
- Grosenbach, D.W.; Jordan, R.; Hruby, D.E. Development of the small-molecule antiviral ST-246® as a smallpox therapeutic. Futur. Virol. 2011, 6, 653–671. [Google Scholar] [CrossRef] [Green Version]
- Rizk, J.G.; Lippi, G.; Henry, B.M.; Forthal, D.N.; Rizk, Y. Prevention and Treatment of Monkeypox. Drugs 2022, 82, 957–963. [Google Scholar] [CrossRef]
- Priyamvada, L.; Carson, W.C.; Ortega, E.; Navarra, T.; Tran, S.; Smith, T.G.; Pukuta, E.; Muyamuna, E.; Kabamba, J.; Nguete, B.U.; et al. Serological responses to the MVA-based JYNNEOS monkeypox vaccine in a cohort of participants from the Democratic Republic of Congo. Vaccine 2022, 40, 7321–7327. [Google Scholar] [CrossRef]
- Islam, M.R.; Hossain, M.J.; Roy, A.; Hasan, A.H.M.N.; Rahman, M.A.; Shahriar, M.; Bhuiyan, M.A. Repositioning potentials of smallpox vaccines and antiviral agents in monkeypox outbreak: A rapid review on comparative benefits and risks. Health Sci. Rep. 2022, 5, e798. [Google Scholar] [CrossRef]
- Kozlov, M. Monkeypox vaccination begins—Can the global outbreaks be contained? Nature 2022, 606, 444–445. [Google Scholar] [CrossRef]
- US Centers for Disease Control and Prevention (CDC). Vaccination. Timing of Post-exposure Prophylaxis. Available online: https://www.cdc.gov/poxvirus/monkeypox/interim-considerations/overview.html (accessed on 18 November 2022).
- Overton, E.T.; Lawrence, S.J.; Stapleton, J.T.; Weidenthaler, H.; Schmidt, D.; Koenen, B.; Silbernagl, G.; Nopora, K.; Chaplin, P. A randomized phase II trial to compare safety and immunogenicity of the MVA-BN smallpox vaccine at various doses in adults with a history of AIDS. Vaccine 2020, 38, 2600–2607. [Google Scholar] [CrossRef]
- CDC—Interim Clinical Considerations for Use of JYNNEOS and ACAM2000 Vaccines during the 2022 U.S. Monkeypox Outbreak. Available online: https://www.cdc.gov/poxvirus/monkeypox/health-departments/vaccine-considerations.html (accessed on 18 November 2022).
- Afolabi, M.O.; Ishola, D.; Manno, D.; Keshinro, B.; Bockstal, V.; Rogers, B.; Owusu-Kyei, K.; Serry-Bangura, A.; Swaray, I.; Lowe, B.; et al. Safety and immunogenicity of the two-dose heterologous Ad26.ZEBOV and MVA-BN-Filo Ebola vaccine regimen in children in Sierra Leone: A randomised, double-blind, controlled trial. Lancet Infect. Dis. 2022, 22, 110–122. [Google Scholar] [CrossRef]
- O’Shea, J.; Filardo, T.D.; Morris, S.B.; Weiser, J.; Petersen, B.; Brooks, J.T. Interim Guidance for Prevention and Treatment of Monkeypox in Persons with HIV Infection—United States, August 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 1023–1028. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.M.; Bamford, A.; Eisen, S.; Emonts, M.; Ho, D.; Kadambari, S.; Kenny, J.; Lyall, H.; Owens, S.; Porter, D.; et al. Royal College of Paediatrics and Child Health Monkeypox Working Group. Comment Title: Care of children exposed to monkeypox. Lancet Reg. Health Eur. 2022, 21, 100514. [Google Scholar] [CrossRef] [PubMed]
- FDA Highlights of Prescribing Information. CNJ-016, Vaccinia Immune Globulin Intravenous (Human). Available online: www.fda.gov/media/78174/download (accessed on 18 November 2022).
- Shrewsbury, D. Blame and shame are harming our response to monkeypox. BMJ 2022, 378, o2134. [Google Scholar] [CrossRef]
- Isaacs, D. Monkeypox. J. Paediatr. Child Health 2022, 58, 1290–1292. [Google Scholar] [CrossRef]
- JYNNEOS® (MVA-BN) Smallpox (Monkeypox) Vaccine Description. Available online: https://www.precisionvaccinations.com/vaccines/jynneos-smallpox-monkeypox-vaccine (accessed on 18 November 2022).
- Forni, D.; Cagliani, R.; Molteni, C.; Clerici, M.; Sironi, M. Monkeypox virus: The changing facets of a zoonotic pathogen. Infect. Genet. Evol. 2022, 105, 105372. [Google Scholar] [CrossRef]
- Mukherjee, A.G.; Wanjari, U.R.; Kannampuzha, S.; Das, S.; Murali, R.; Namachivayam, A.; Renu, K.; Ramanathan, G.; Doss, C.; Vellingiri, B.; et al. The pathophysiological and immunological background of the monkeypox virus infection: An update. J. Med. Virol. 2022. epub ahead of print. [Google Scholar] [CrossRef]
- Technical Report 3. Available online: https://www.cdc.gov/poxvirus/monkeypox/cases-data/technical-report/report-3.html (accessed on 18 November 2022).
- Yao, K. The diversity of clinical manifestations of human monkeypox should be emphasized in practice. Pediatr. Investig. 2022, 6, 224–225. [Google Scholar] [CrossRef]
| Oral ** | Intravenous |
|---|---|
The latest update published by the CDC (version 6.2 24 October 2022) on the use of Tecovirimat in children [60] provides additional indications of its use in children from birth:
| <35 kg: 6 mg/kg by IV infusion in <6 h. Volume of infusion depends on the weight. In children < 2 years of age,
For children <3 kg the drug is allowed according to the CDC report on treatment of 24 October 2022, but dose adjustments may be necessary in infants based on the general condition and weight [60]. |
| ACAM2000 | MVA-BN ** | LC16m8 |
|---|---|---|
| (Live vaccinia virus) Second-generation vaccine | (Attenuated, non-replicating vaccinia virus) | (Modified vaccinia virus) |
| Administration by multiple percutaneous puncture device of the skin surface. Single dose with lesion at the site of inoculation. Not recommended in A. Individuals with
Possible adverse cardiac reactions. | Administration in two subcutaneous doses, 4 weeks apart, no lesions at the inoculum site. Safe for immunocompromised patients because the virus does not replicate. If not large available, a single dose may be administered. | Single dose administration. Licensed in Japan Safer then ACAM2000, given the lower replication capacity. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaeta, F.; De Caro, F.; Franci, G.; Pagliano, P.; Vajro, P.; Mandato, C. Monkeypox Infection 2022: An Updated Narrative Review Focusing on the Neonatal and Pediatric Population. Children 2022, 9, 1832. https://doi.org/10.3390/children9121832
Gaeta F, De Caro F, Franci G, Pagliano P, Vajro P, Mandato C. Monkeypox Infection 2022: An Updated Narrative Review Focusing on the Neonatal and Pediatric Population. Children. 2022; 9(12):1832. https://doi.org/10.3390/children9121832
Chicago/Turabian StyleGaeta, Francesca, Francesco De Caro, Gianluigi Franci, Pasquale Pagliano, Pietro Vajro, and Claudia Mandato. 2022. "Monkeypox Infection 2022: An Updated Narrative Review Focusing on the Neonatal and Pediatric Population" Children 9, no. 12: 1832. https://doi.org/10.3390/children9121832
APA StyleGaeta, F., De Caro, F., Franci, G., Pagliano, P., Vajro, P., & Mandato, C. (2022). Monkeypox Infection 2022: An Updated Narrative Review Focusing on the Neonatal and Pediatric Population. Children, 9(12), 1832. https://doi.org/10.3390/children9121832







