Fine Mapping and Identification of a Candidate Gene of Downy Mildew Resistance, RPF2, in Spinach (Spinacia oleracea L.)
Abstract
:1. Introduction
2. Results
2.1. Phenotypic and Genetic Analysis
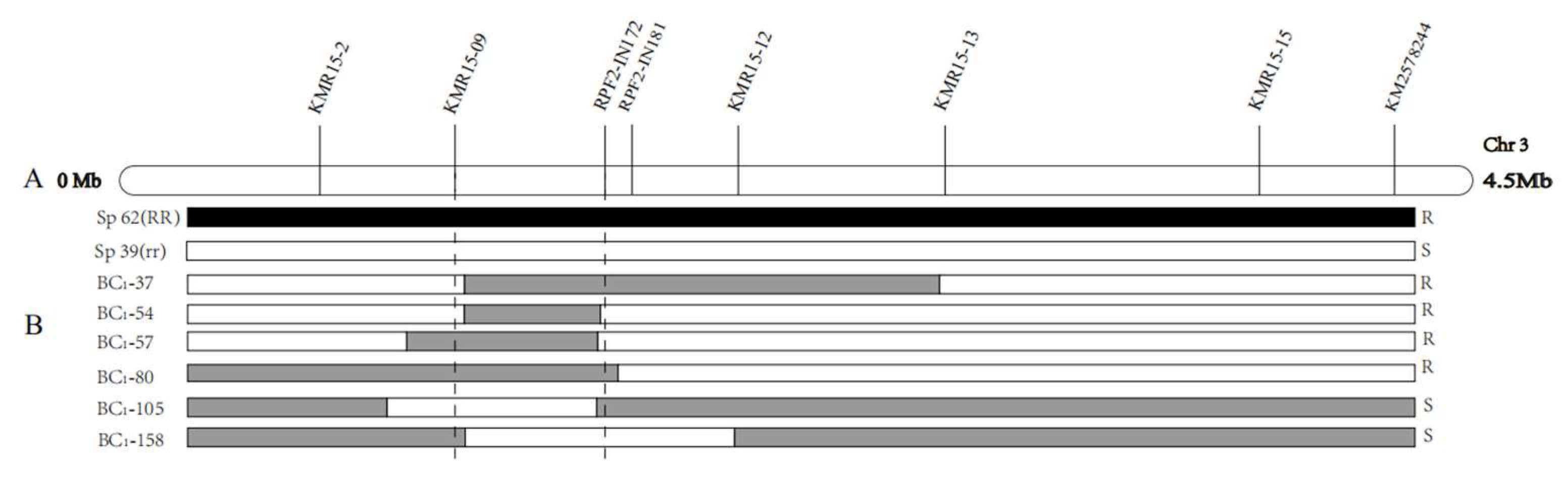
2.2. Fine Mapping of RPF2
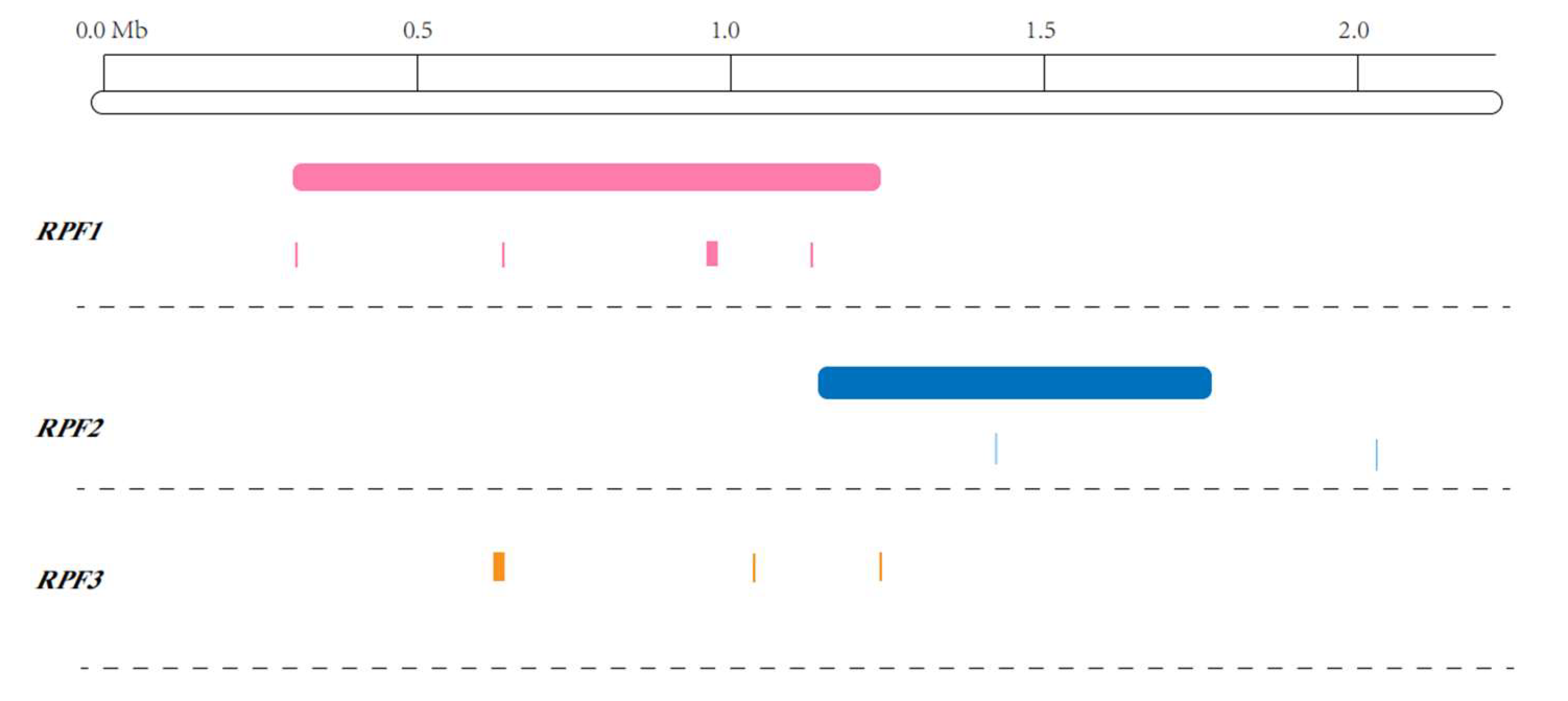
2.3. Screening of Candidate Genes
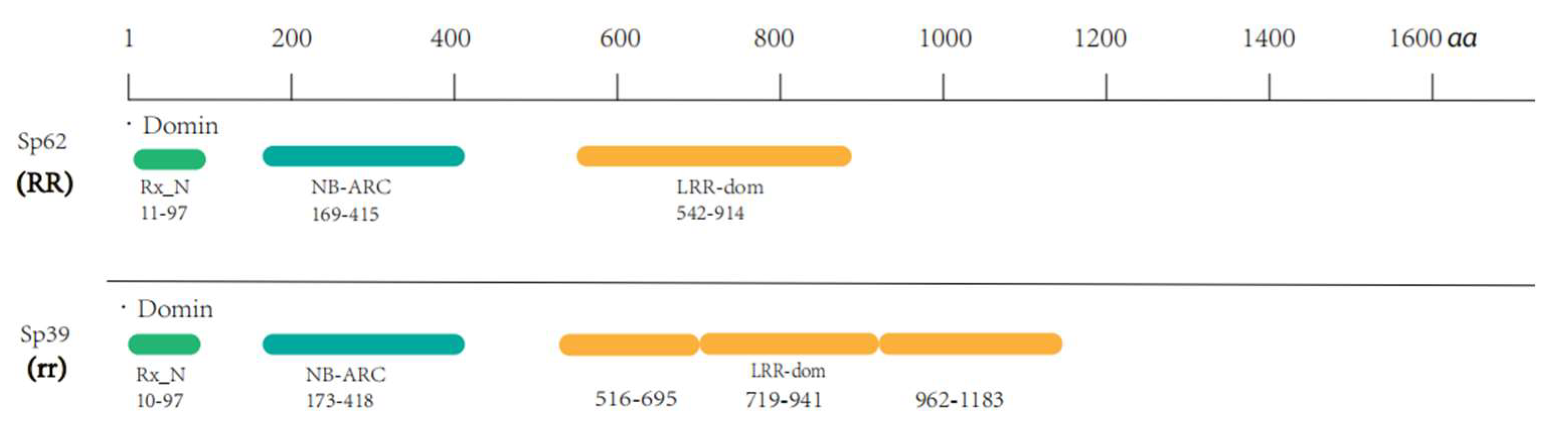
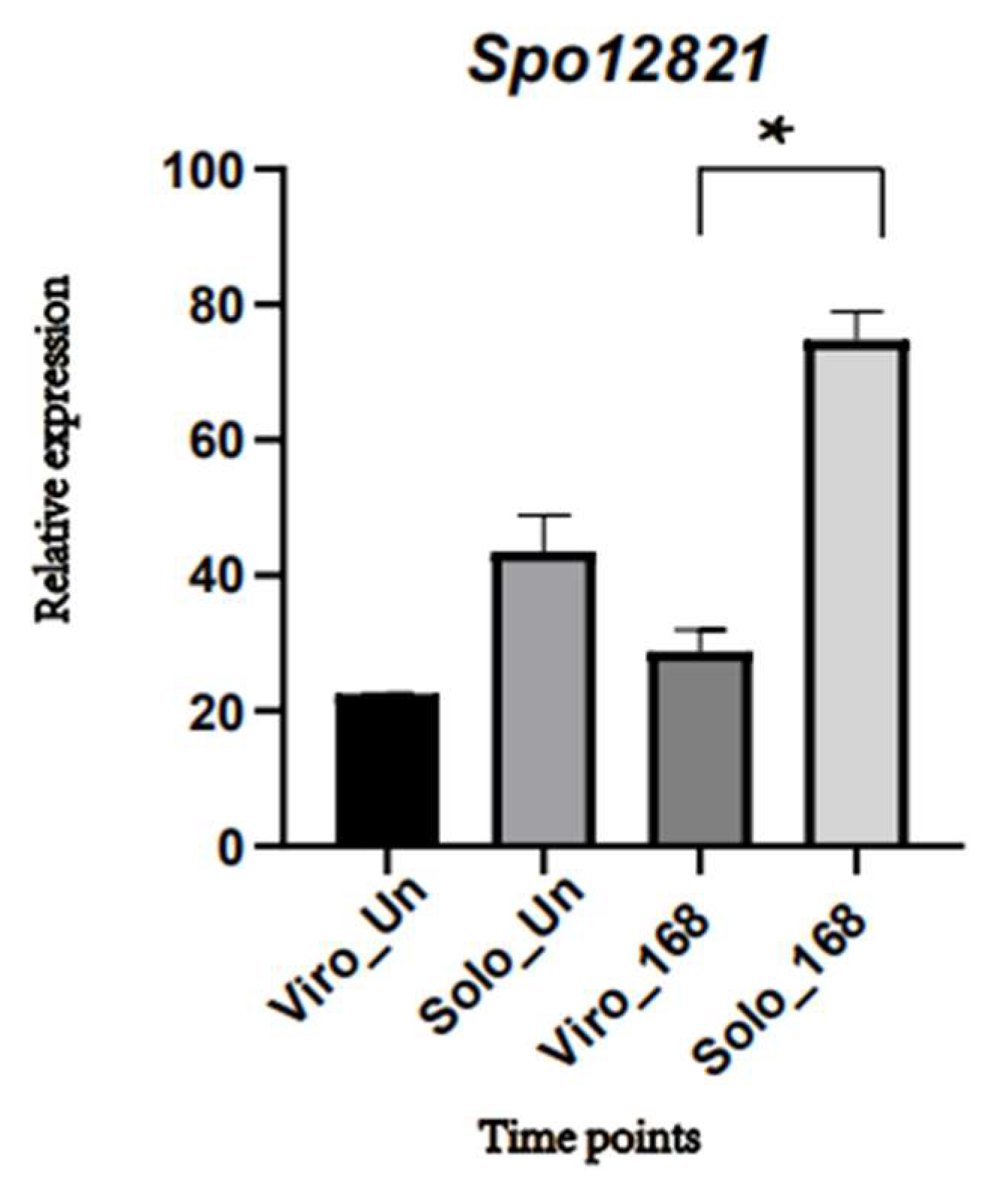
2.4. Structural Difference Analysis of the Candidate Gene Protein
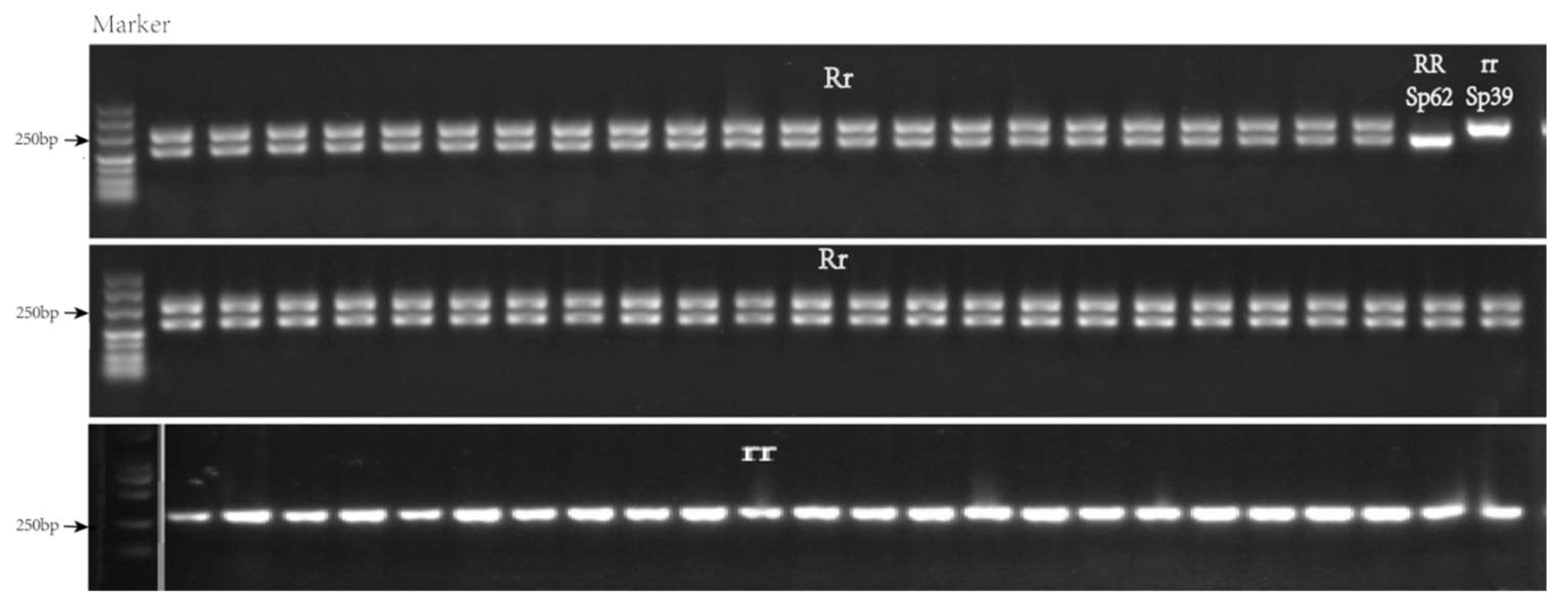
2.5. Development and Validation of Molecular Markers for Candidate Genes
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Inoculation and Genetic Analysis
4.3. DNA Extraction
4.4. The Development of InDel and KASP Markers
4.5. InDel and KASP Assays
4.6. Fine Mapping of the RPF2 Locus
4.7. The Sequence and Structural Analysis of Candidate Genes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shi, A.; Mou, B.; Correll, J.; Koike, S.T.; Motes, D.; Qin, J.; Weng, Y.; Yang, W. Association Analysis and Identification of SNP Markers for Stemphylium Leaf Spot (Stemphylium botryosum f. sp. spinacia) Resistance in Spinach (Spinacia oleracea). Am. J. Plant Sci. 2016, 7, 1600–1611. [Google Scholar] [CrossRef] [Green Version]
- Bhattarai, G.; Shi, A. Research advances and prospects of spinach breeding, genetics, and genomics. Veg. Res. 2021, 1, 9. [Google Scholar] [CrossRef]
- Correll, J.C.; Morelock, T.E.; Black, M.C.; Koike, S.T.; Brandenberger, L.P.; Dainello, F.J. Economically important diseases of spinach. Plant Dis. 1994, 78, 653–660. [Google Scholar] [CrossRef]
- Feng, C.; Bluhm, B.; Shi, A.; Correll, J.C. Development of molecular markers linked to three spinach downy mildew resistance loci. Euphytica 2018, 214, 174. [Google Scholar] [CrossRef]
- Greville, R.K. Flora Edinensis: Or, a Description of Plants Growing Near Edinburgh, Arranged According to the Linnean System, with a Concise Introduction to the Natural Orders of the Class Cryptogamia, and Illustrative Plates; W. Blackwood: Edingburgh, UK, 1824. [Google Scholar]
- Brandenberger, L.; Correll, J.; Morelock, T. Identification of and cultivar reactions to a new race (race 4) of Peronospora farinosa f. sp. spinaciae on spinach in the United States. Plant Dis. 1991, 75, 630–634. [Google Scholar] [CrossRef]
- Irish, B.; Correll, J.; Koike, S.; Schafer, J.; Morelock, T. Identification and cultivar reaction to three new races of the spinach downy mildew pathogen from the United States and Europe. Plant Dis. 2003, 87, 567–572. [Google Scholar] [CrossRef] [Green Version]
- Irish, B.; Correll, J.; Koike, S.; Morelock, T. Three new races of the spinach downy mildew pathogen identified by a modified set of spinach differentials. Plant Dis. 2007, 91, 1392–1396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, C.; Correll, J.C.; Kammeijer, K.E.; Koike, S.T. Identification of New Races and Deviating Strains of the Spinach Downy Mildew Pathogen Peronospora farinosa f. sp. spinaciae. Plant Dis. 2014, 98, 145–152. [Google Scholar] [CrossRef] [Green Version]
- Feng, C.; Saito, K.; Liu, B.; Manley, A.; Kammeijer, K.; Mauzey, S.J.; Koike, S.; Correll, J.C. New Races and Novel Strains of the Spinach Downy Mildew Pathogen Peronospora effusa. Plant Dis. 2018, 102, 613–618. [Google Scholar] [CrossRef] [Green Version]
- Feng, C.; Lamour, K.; Dhillon, B.D.S.; Villarroel-Zeballos, M.I.; Castroagudin, V.L.; Bluhm, B.H.; Shi, A.; Rojas, A.; Correll, J.C. Genetic diversity of the spinach downy mildew pathogen based on hierarchical sampling. bioRxiv 2020. [Google Scholar] [CrossRef]
- Plantum, Denomination of Pe 18 and 19, two new races of downy mildew in spinach, 2021. Available online: https://plantum.nl/denomination-of-pe-18-and-19-twonew-races-of-downy-mildew-in-spinach/ (accessed on 25 May 2021).
- Correll, J.; Bluhm, B.; Feng, C.; Lamour, K.; Du Toit, L.; Koike, S. Spinach: Better management of downy mildew and white rust through genomics. Eur. J. Plant Pathol. 2011, 129, 193–205. [Google Scholar] [CrossRef]
- Irish, B.; Correll, J.; Feng, C.; Bentley, T.; de Los Reyes, B. Characterization of a resistance locus (Pfs-1) to the spinach downy mildew pathogen (Peronospora farinosa f. sp. spinaciae) and development of a molecular marker linked to Pfs-1. Phytopathology 2008, 98, 894–900. [Google Scholar] [CrossRef] [Green Version]
- Feng, C.; Bluhm, B.H.; Correll, J.C. Construction of a Spinach Bacterial Artificial Chromosome (BAC) Library as a Resource for Gene Identification and Marker Development. Plant Mol. Biol. Rep. 2015, 33, 1996–2005. [Google Scholar] [CrossRef]
- Xu, C.; Jiao, C.; Sun, H.; Cai, X.; Wang, X.; Ge, C.; Zheng, Y.; Liu, W.; Sun, X.; Xu, Y.; et al. Draft genome of spinach and transcriptome diversity of 120 Spinacia accessions. Nat. Commun. 2017, 8, 15275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- She, H.; Qian, W.; Zhang, H.; Liu, Z.; Wang, X.; Wu, J.; Feng, C.; Correll, J.C.; Xu, Z. Fine mapping and candidate gene screening of the downy mildew resistance gene RPF1 in Spinach. Appl. Genet. 2018, 131, 2529–2541. [Google Scholar] [CrossRef]
- Bhattarai, G.; Shi, A.; Feng, C.; Dhillon, B.; Mou, B.; Correll, J.C. Genome Wide Association Studies in Multiple Spinach Breeding Populations Refine Downy Mildew Race 13 Resistance Genes. Front. Plant Sci. 2020, 11, 563187. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, G.; Yang, W.; Shi, A.; Feng, C.; Dhillon, B.; Correll, J.C.; Mou, B. High resolution mapping and candidate gene identification of downy mildew race 16 resistance in spinach. BMC Genom. 2021, 22, 478. [Google Scholar] [CrossRef]
- Mauricio, R. Mapping quantitative trait loci in plants: Uses and caveats for evolutionary biology. Nat. Rev. Genet. 2001, 2, 370–381. [Google Scholar] [CrossRef]
- Collard, B.C.; Jahufer, M.; Brouwer, J.; Pang, E.C.K. An introduction to markers, quantitative trait loci (QTL) mapping and marker-assisted selection for crop improvement: The basic concepts. Euphytica 2005, 142, 169–196. [Google Scholar] [CrossRef]
- Xiao, G.; Wang, W.; Liu, M.; Li, Y.; Liu, J.; Franceschetti, M.; Yi, Z.; Zhu, X.; Zhang, Z.; Lu, G. The Piks allele of the NLR immune receptor Pik breaks the recognition of AvrPik effectors of the rice blast fungus. J. Integr. Plant Biol. 2022. [Google Scholar] [CrossRef]
- Li, T.G.; Wang, B.L.; Yin, C.M.; Zhang, D.D.; Wang, D.; Song, J.; Zhou, L.; Kong, Z.Q.; Klosterman, S.J.; Li, J.J. The Gossypium hirsutum TIR-NBS-LRR gene GhDSC1 mediates resistance against Verticillium wilt. Mol. Plant Pathol. 2019, 20, 857–876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, H.; Wang, H.; Jiang, J.; Du, M.; Li, J. The Sm Gene Conferring Resistance to Gray Leaf Spot Disease Encodes a NBS-LRR Plant Resistance Protein in Tomato. Theor. Appl. Genet. 2021; under review. [Google Scholar]
- Gururani, M.A.; Venkatesh, J.; Upadhyaya, C.P.; Nookaraju, A.; Pandey, S.K.; Park, S.W. Plant disease resistance genes: Current status and future directions. Physiol. Mol. Plant Pathol. 2012, 78, 51–65. [Google Scholar] [CrossRef]
- Dangl, J.L.; Jones, J.D. Plant pathogens and integrated defence responses to infection. Nature 2001, 411, 826–833. [Google Scholar] [CrossRef]
- Meyers, B.C.; Kaushik, S.; Nandety, R.S. Evolving disease resistance genes. Curr. Opin. Plant Biol. 2005, 8, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Dodds, P.N.; Rathjen, J.P. Plant immunity: Towards an integrated view of plant-pathogen interactions. Nat. Rev. Genet. 2010, 11, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Tameling, W.I.; Elzinga, S.D.; Darmin, P.S.; Vossen, J.H.; Takken, F.L.; Haring, M.A.; Cornelissen, B.J. The tomato R gene products I-2 and MI-1 are functional ATP binding proteins with ATPase activity. Plant Cell 2002, 14, 2929–2939. [Google Scholar] [CrossRef]
- Liu, Y.; Li, D.; Yang, N.; Zhu, X.; Han, K.; Gu, R.; Bai, J.; Wang, A.; Zhang, Y. Genome-wide identification and analysis of CC-NBS-LRR family in response to downy mildew and black rot in Chinese cabbage. Int. J. Mol. Sci. 2021, 22, 4266. [Google Scholar] [CrossRef]
- Shao, Z.Q.; Xue, J.Y.; Wang, Q.; Wang, B.; Chen, J.Q. Revisiting the Origin of Plant NBS-LRR Genes. Trends Plant Sci. 2019, 24, 9–12. [Google Scholar] [CrossRef]
- Hwang, C.-F.; Bhakta, A.V.; Truesdell, G.M.; Pudlo, W.M.; Williamson, V.M. Evidence for a role of the N terminus and leucine-rich repeat region of the Mi gene product in regulation of localized cell death. Plant Cell 2000, 12, 1319–1329. [Google Scholar] [CrossRef] [Green Version]
- Hulbert, S.H.; Webb, C.A.; Smith, S.M.; Sun, Q. Resistance gene complexes: Evolution and utilization. Annu. Rev. Phytopathol. 2001, 39, 285. [Google Scholar] [CrossRef]
- Martin, G.B.; Brommonschenkel, S.H.; Chunwongse, J.; Frary, A.; Ganal, M.W.; Spivey, R.; Wu, T.; Earle, E.D.; Tanksley, S.D. Map-based cloning of a protein kinase gene conferring disease resistance in tomato. Science 1993, 262, 1432–1436. [Google Scholar] [CrossRef] [PubMed]
- Kandel, S.L.; Hulse-Kemp, A.M.; Stoffel, K.; Koike, S.T.; Shi, A.; Mou, B.; Van Deynze, A.; Klosterman, S.J. Transcriptional analyses of differential cultivars during resistant and susceptible interactions with Peronospora effusa, the causal agent of spinach downy mildew. Sci. Rep. 2020, 10, 6719. [Google Scholar] [CrossRef] [Green Version]
- Wakchaure, R.; Ganguly, S. Marker Assisted Selection (MAS) in Animal Breeding: A Review. J. Drug Metab. Toxicol. 2015, 6, 5. [Google Scholar] [CrossRef]
- Collard, B.C.; Mackill, D.J. Marker-assisted selection: An approach for precision plant breeding in the twenty-first century. Philos. Trans. R. Soc. B Biol. Sci. 2008, 363, 557–572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giovannelli, J.L.; Farnham, M.W.; Wang, M.; Strand, A.E. Development of sequence characterized amplified region markers linked to downy mildew resistance in broccoli. J. Am. Soc. Hortic. Sci. 2002, 127, 597–601. [Google Scholar] [CrossRef] [Green Version]
- Farinhó, M.; Coelho, P.; Monteiro, A.; Leitão, J. SCAR and CAPS markers flanking the Brassica oleracea L. Pp523 downy mildew resistance locus demarcate a genomic region syntenic to the top arm end of Arabidopsis thaliana L. chromosome 1. Euphytica 2007, 157, 215–221. [Google Scholar] [CrossRef]
- Qian, W.; Feng, C.; Zhang, H.; Liu, W.; Xu, D.; Correll, J.; Xu, Z. First report of race diversity of the spinach downy mildew pathogen, Peronospora effusa, in China. Plant Dis. 2016, 100, 1248. [Google Scholar] [CrossRef]
- Murray, M.; Thompson, W. Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res. 1980, 8, 4321–4326. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Li, H.; Durbin, R. Fast and accurate short read alignment with Burrows–Wheeler transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R. The sequence alignment/map format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voorrips, R. MapChart: Software for the graphical presentation of linkage maps and QTLs. J. Hered. 2002, 93, 77–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Song, F.; Zhu, J.; Zhang, S.; Yang, Y.; Chen, T.; Tang, B.; Dong, L.; Ding, N.; Zhao, W.; et al. GSA: Genome sequence archive. Genom. Proteom. Bioinformatic 2017, 15, 14–18. [Google Scholar] [CrossRef] [PubMed]
| Population | Total Plants Number | Resistant Plant | Susceptible Plant | ||
|---|---|---|---|---|---|
| BC1 | 226 | 110 | 116 | 0.175 | 3.841 |
| Chr ID | Markers | Start (bp) | End (bp) |
|---|---|---|---|
| Chr3 | KM2578244 | 4,333,499 | 4,333,699 |
| KMR15-15 | 3,957,426 | 3,957,481 | |
| KMR15-13 | 2,859,499 | 2,859,570 | |
| KMR15-12 | 2,225,045 | 2,225,105 | |
| RPF2-IN181 | 1,819,801 | 1,820,000 | |
| RPF2-IN172 | 1,728,147 | 1,728,346 | |
| KMR15-09 | 1,110,252 | 1,110,330 | |
| KMR15-2 | 607,940 | 607,636 |
| Chr ID | Markers |
|---|---|
| 21-003F | GCACGTTCAGAGAAGACAG |
| 21-003R | GGCCTTTTAGGGCTTTCAG |
| 21-841F | GTCAAGGGGGAAGCAAGGTT |
| 21-841R | CCGGCAGATACAGATTAAAATGG |
| ID | Sequence (5′–3′) |
|---|---|
| RPF2-IN12821F | CTACTGATCGCCAATCTGTG |
| RPF2-IN12821R | CAGTCAGAAGATTTACGGCAC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, S.; Lu, T.; She, H.; Xu, Z.; Zhang, H.; Liu, Z.; Qian, W. Fine Mapping and Identification of a Candidate Gene of Downy Mildew Resistance, RPF2, in Spinach (Spinacia oleracea L.). Int. J. Mol. Sci. 2022, 23, 14872. https://doi.org/10.3390/ijms232314872
Gao S, Lu T, She H, Xu Z, Zhang H, Liu Z, Qian W. Fine Mapping and Identification of a Candidate Gene of Downy Mildew Resistance, RPF2, in Spinach (Spinacia oleracea L.). International Journal of Molecular Sciences. 2022; 23(23):14872. https://doi.org/10.3390/ijms232314872
Chicago/Turabian StyleGao, Shuo, Tiantian Lu, Hongbing She, Zhaosheng Xu, Helong Zhang, Zhiyuan Liu, and Wei Qian. 2022. "Fine Mapping and Identification of a Candidate Gene of Downy Mildew Resistance, RPF2, in Spinach (Spinacia oleracea L.)" International Journal of Molecular Sciences 23, no. 23: 14872. https://doi.org/10.3390/ijms232314872





