Pyrrolizidine Alkaloid Extraction and Analysis: Recent Updates
Abstract
:1. Introduction
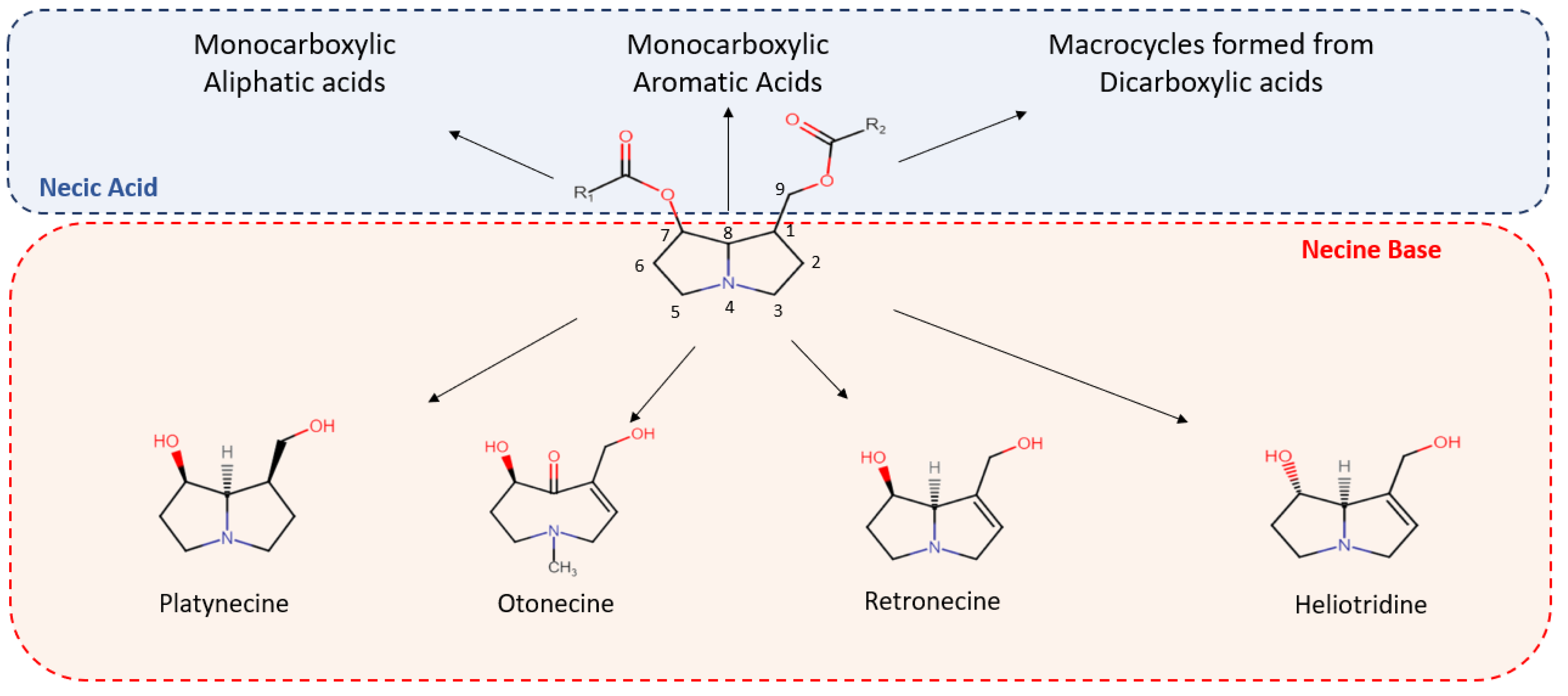
2. PA Chemistry
3. Toxicology of PAs
4. Food and Pharmaceutical Products Safety Recommendation Regarding PAs
5. Analysis of PAs
5.1. PA Extraction
5.2. PA Separation
5.2.1. PA Separation by Gas Chromatography
5.2.2. High-Performance Liquid Chromatography Separation of PAs
5.3. PA Identification
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Disclaimer
References
- Dembitsky, V.M. Naturally occurring bioactive cyclobutane-containing (CBC) alkaloids in fungi, fungal endophytes, and plants. Phytomedicine 2014, 21, 1559–1581. [Google Scholar] [CrossRef]
- Zotchev, S.B. Alkaloids from marine bacteria. Adv. Bot. Res. 2013, 68, 301–333. [Google Scholar] [CrossRef]
- Tamariz, J.; Burgueño-Tapia, E.; Vázquez, M.A.; Delgado, F. Pyrrolizidine Alkaloids. Alkaloids Chem. Biol. 2018, 80, 1–314. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.W.; Culvenor, C.C.J. Plant sources of hepatotoxic pyrrolizidine alkaloids. J. Nat. Prod. 1981, 44, 129–152. [Google Scholar] [CrossRef]
- Kaltner, F.; Rychlik, M.; Gareis, M.; Gottschalk, C. Occurrence and risk assessment of pyrrolizidine alkaloids in spices and culinary herbs from various geographical origins. Toxins 2020, 12, 155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreira, R.; Pereira, D.M.; Valentão, P.; Andrade, P.B. Pyrrolizidine alkaloids: Chemistry, pharmacology, toxicology and food safety. Int. J. Mol. Sci. 2018, 19, 1668. [Google Scholar] [CrossRef] [Green Version]
- Tamariz, J.; Burgueño-Tapia, E.; Vázquez, M.A.; Delgado, F. Pyrrolizidine Alkaloids; Knölker, H.-J., Ed.; Academic Press Inc.: Cambridge, MA, USA, 2018; Volume 80, p. 314. [Google Scholar]
- Stegelmeier, B.L. Pyrrolizidine Alkaloid–Containing Toxic Plants (Senecio, Crotalaria, Cynoglossum, Amsinckia, Heliotropium, and Echium spp.). Vet. Clin. Food Anim. Pract. 2011, 27, 419–428. [Google Scholar] [CrossRef] [Green Version]
- Schramm, S.; Köhler, N.; Rozhon, W. Pyrrolizidine alkaloids: Biosynthesis, biological activities and occurrence in crop plants. Molecules 2019, 24, 498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, Q.; He, X.; Shi, Q.; Lin, G.; Fu, P.P. Quantitation of DNA reactive pyrrolic metabolites of senecionine–A carcinogenic pyrrolizidine alkaloid by LC/MS/MS analysis. J. Food Drug Anal. 2020, 28, 167–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, S.; Agrawal, R. Toxic behaviour of naturally occurring pyrrolizidine alkaloids. Int. J. Multidiscip. Curr. Res. 2015, 3, 594–597. [Google Scholar]
- Teschke, R.; Vongdala, N.; Quan, N.V.; Quy, T.N.; Xuan, T.D. Metabolic toxification of 1, 2-unsaturated pyrrolizidine alkaloids causes human hepatic sinusoidal obstruction syndrome: The update. Int. J. Mol. Sci. 2021, 22, 10419. [Google Scholar] [CrossRef]
- Casado, N.; Morante-Zarcero, S.; Sierra, I. The concerning food safety issue of pyrrolizidine alkaloids: An overview. Trends Food Sci. Technol. 2022, 120, 123–139. [Google Scholar] [CrossRef]
- Zheng, P.; Xu, Y.; Ren, Z.; Wang, Z.; Wang, S.; Xiong, J.; Zhang, H.; Jiang, H. Toxic Prediction of Pyrrolizidine Alkaloids and Structure-Dependent Induction of Apoptosis in HepaRG Cells. Oxidative Med. Cell. Longev. 2021, 2021, 8822304. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain. Scientific opinion on pyrrolizidine alkaloids in food and feed. EFSA J. 2011, 9, 2406. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain; Knutsen, H.K.; Alexander, J.; Barregård, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Cottrill, B.; Dinovi, M.; Edler, L. Risks for human health related to the presence of pyrrolizidine alkaloids in honey, tea, herbal infusions and food supplements. EFSA J. 2017, 15, e04908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- LEYEN, U.V.D. COMMISSION REGULATION (EU) 2020/2040 of 11 December 2020 amending Regulation (EC) No 1881/2006 as regards maximum levels of pyrrolizidine alkaloids in certain foodstuffs. J. Eur. Union Off. J. Eur. Union 2020.
- Bundesinstitut für Risikobewertung. Pyrrolizidinalkaloide: Gehalte in Lebensmitteln Sollen Nach wie vor so Weit wie Möglich Gesenkt Werden; Bundesinstitut für Risikobewertung: Berlin, Germany, 2016. [Google Scholar]
- Guo, Q.; Yang, Y.; Li, J.; Shao, B.; Zhang, J. Screening for plant toxins in honey and herbal beverage by ultrahigh-performance liquid chromatography-ion mobility-quadrupole time of flight mass spectrometry. Am. J. Anal. Chem. 2022, 13, 108–134. [Google Scholar] [CrossRef]
- León, N.; Miralles, P.; Yusà, V.; Coscollà, C. A green analytical method for the simultaneous determination of 30 tropane and pyrrolizidine alkaloids and their N-oxides in teas and herbs for infusions by LC-Q-Orbitrap HRMS. J. Chromatogr. A 2022, 1666, 462835. [Google Scholar] [CrossRef]
- Izcara, S.; Casado, N.; Morante-Zarcero, S.; Pérez-Quintanilla, D.; Sierra, I. Miniaturized and modified QuEChERS method with mesostructured silica as clean-up sorbent for pyrrolizidine alkaloids determination in aromatic herbs. Food Chem. 2022, 380, 132189. [Google Scholar] [CrossRef] [PubMed]
- Martinello, M.; Manzinello, C.; Gallina, A.; Mutinelli, F. In-house validation and application of UHPLC-MS/MS method for the quantification of pyrrolizidine and tropane alkaloids in commercial honey bee-collected pollen, teas and herbal infusions purchased on Italian market in 2019–2020 referring to recent European Union regulations. Int. J. Food Sci. Technol. 2022, 57, 7505–7516. [Google Scholar]
- Han, H.; Jiang, C.; Wang, C.; Wang, Z.; Chai, Y.; Zhang, X.; Liu, X.; Lu, C.; Chen, H. Development, optimization, validation and application of ultra high performance liquid chromatography tandem mass spectrometry for the analysis of pyrrolizidine alkaloids and pyrrolizidine alkaloid N-oxides in teas and weeds. Food Control 2022, 132, 108518. [Google Scholar] [CrossRef]
- Bandini, T.B.; Spisso, B.F. Development and validation of an LC-HRMS method for the determination of pyrrolizidine alkaloids and quinolones in honey employing a simple alkaline sample dilution. J. Food Meas. Charact. 2021, 15, 4758–4770. [Google Scholar] [CrossRef]
- Jeong, S.H.; Choi, E.Y.; Kim, J.; Lee, C.; Kang, J.; Cho, S.; Ko, K.Y. LC-ESI-MS/MS simultaneous analysis method coupled with cation-exchange solid-phase extraction for determination of pyrrolizidine alkaloids on five kinds of herbal medicines. J. AOAC Int. 2021, 104, 1514–1525. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.; Koo, Y.; Jeong, Y. Determination of Pyrrolizidine Alkaloids in Teas Using Liquid Chromatography–Tandem Mass Spectrometry Combined with Rapid-Easy Extraction. Foods 2021, 10, 2250. [Google Scholar] [CrossRef]
- Chen, Y.; Li, L.; Xiong, F.; Xie, Y.; Xiong, A.; Wang, Z.; Yang, L. Rapid identification and determination of pyrrolizidine alkaloids in herbal and food samples via direct analysis in real-time mass spectrometry. Food Chem. 2021, 334, 127472. [Google Scholar] [CrossRef]
- Valese, A.C.; Daguer, H.; Muller, C.M.O.; Molognoni, L.; da Luz, C.F.P.; de Barcellos Falkenberg, D.; Gonzaga, L.V.; Brugnerotto, P.; Gorniak, S.L.; Barreto, F. Quantification of pyrrolizidine alkaloids in Senecio brasiliensis, beehive pollen, and honey by LC-MS/MS. J. Environ. Sci. Health Part B 2021, 56, 685–694. [Google Scholar] [CrossRef] [PubMed]
- Moreira, R.; Fernandes, F.; Valentão, P.; Pereira, D.M.; Andrade, P.B. Echium plantagineum L. honey: Search of pyrrolizidine alkaloids and polyphenols, anti-inflammatory potential and cytotoxicity. Food Chem. 2020, 328, 127169. [Google Scholar] [CrossRef]
- He, Y.; Zhu, L.; Ma, J.; Wong, L.; Zhao, Z.; Ye, Y.; Fu, P.P.; Lin, G. Comprehensive investigation and risk study on pyrrolizidine alkaloid contamination in Chinese retail honey. Environ. Pollut. 2020, 267, 115542. [Google Scholar] [CrossRef] [PubMed]
- Letsyo, E.; Adams, Z.S.; Dzikunoo, J.; Asante-Donyinah, D. Uptake and accumulation of pyrrolizidine alkaloids in the tissues of maize (Zea mays L.) plants from the soil of a 4-year-old Chromolaena odorata dominated fallow farmland. Chemosphere 2021, 270, 128669. [Google Scholar] [CrossRef] [PubMed]
- Prada, F.; Stashenko, E.E.; Martínez, J.R. LC/MS study of the diversity and distribution of pyrrolizidine alkaloids in Crotalaria species growing in Colombia. J. Sep. Sci. 2020, 43, 4322–4337. [Google Scholar] [CrossRef] [PubMed]
- Izcara, S.; Casado, N.; Morante-Zarcero, S.; Sierra, I. A miniaturized QuEChERS method combined with ultrahigh liquid chromatography coupled to tandem mass spectrometry for the analysis of pyrrolizidine alkaloids in oregano samples. Foods 2020, 9, 1319. [Google Scholar] [CrossRef] [PubMed]
- Kaczyński, P.; Łozowicka, B. A novel approach for fast and simple determination pyrrolizidine alkaloids in herbs by ultrasound-assisted dispersive solid phase extraction method coupled to liquid chromatography–tandem mass spectrometry. J. Pharm. Biomed. Anal. 2020, 187, 113351. [Google Scholar] [CrossRef]
- Dzuman, Z.; Jonatova, P.; Stranska-Zachariasova, M.; Prusova, N.; Brabenec, O.; Novakova, A.; Fenclova, M.; Hajslova, J. Development of a new LC-MS method for accurate and sensitive determination of 33 pyrrolizidine and 21 tropane alkaloids in plant-based food matrices. Anal. Bioanal. Chem. 2020, 412, 7155–7167. [Google Scholar] [CrossRef]
- Sixto, A.; Niell, S.; Heinzen, H. Straightforward Determination of Pyrrolizidine Alkaloids in Honey through Simplified Methanol Extraction (QuPPE) and LC-MS/MS Modes. ACS Omega 2019, 4, 22632–22637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Celano, R.; Piccinelli, A.L.; Campone, L.; Russo, M.; Rastrelli, L. Determination of selected pyrrolizidine alkaloids in honey by dispersive liquid–liquid microextraction and ultrahigh-performance liquid chromatography–tandem mass spectrometry. J. Agric. Food Chem. 2019, 67, 8689–8699. [Google Scholar] [CrossRef]
- Chen, L.; Mulder, P.P.; Peijnenburg, A.; Rietjens, I.M. Risk assessment of intake of pyrrolizidine alkaloids from herbal teas and medicines following realistic exposure scenarios. Food Chem. Toxicol. 2019, 130, 142–153. [Google Scholar] [CrossRef]
- Chmit, M.S.; Wahrig, B.; Beuerle, T. Quantitative and qualitative analysis of pyrrolizidine alkaloids in liqueurs, elixirs and herbal juices. Fitoterapia 2019, 136, 104172. [Google Scholar] [CrossRef]
- Wang, T.; Frandsen, H.L.; Christiansson, N.R.; Rosendal, S.E.; Pedersen, M.; Smedsgaard, J. Pyrrolizidine alkaloids in honey: Quantification with and without standards. Food Control 2019, 98, 227–237. [Google Scholar] [CrossRef] [Green Version]
- Selmar, D.; Wittke, C.; Beck-von Wolffersdorff, I.; Klier, B.; Lewerenz, L.; Kleinwächter, M.; Nowak, M. Transfer of pyrrolizidine alkaloids between living plants: A disregarded source of contaminations. Environ. Pollut. 2019, 248, 456–461. [Google Scholar] [CrossRef]
- Kaltner, F.; Stiglbauer, B.; Rychlik, M.; Gareis, M.; Gottschalk, C. Development of a sensitive analytical method for determining 44 pyrrolizidine alkaloids in teas and herbal teas via LC-ESI-MS/MS. Anal. Bioanal. Chem. 2019, 411, 7233–7249. [Google Scholar] [CrossRef] [PubMed]
- Mulder, P.P.; López, P.; Castelari, M.; Bodi, D.; Ronczka, S.; Preiss-Weigert, A.; These, A. Occurrence of pyrrolizidine alkaloids in animal-and plant-derived food: Results of a survey across Europe. Food Addit. Contam. Part A 2018, 35, 118–133. [Google Scholar] [CrossRef] [Green Version]
- Kowalczyk, E.; Sieradzki, Z.; Kwiatek, K. Determination of pyrrolizidine alkaloids in honey with sensitive gas chromatography-mass spectrometry method. Food Anal. Methods 2018, 11, 1345–1355. [Google Scholar] [CrossRef] [Green Version]
- Picron, J.-F.; Herman, M.; van Hoeck, E.; Goscinny, S. Analytical strategies for the determination of pyrrolizidine alkaloids in plant based food and examination of the transfer rate during the infusion process. Food Chem. 2018, 266, 514–523. [Google Scholar] [CrossRef] [PubMed]
- Kaltner, F.; Rychlik, M.; Gareis, M.; Gottschalk, C. Influence of storage on the stability of toxic pyrrolizidine alkaloids and their N-oxides in peppermint tea, hay, and honey. J. Agric. Food Chem. 2018, 66, 5221–5228. [Google Scholar] [CrossRef]
- Martinello, M.; Borin, A.; Stella, R.; Bovo, D.; Biancotto, G.; Gallina, A.; Mutinelli, F. Development and validation of a QuEChERS method coupled to liquid chromatography and high resolution mass spectrometry to determine pyrrolizidine and tropane alkaloids in honey. Food Chem. 2017, 234, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Onduso, S.O.; Ngâ, M.M.; Wanjohi, W.; Hassanali, A. Determination of pyrrolizidine alkaloids levels in Symphytum asperum. Asian J. Nat. Prod. Biochem. 2017, 15, 65–78. [Google Scholar] [CrossRef] [Green Version]
- Kowalczyk, E.; Kwiatek, K. Determination of pyrrolizidine alkaloids in selected feed materials with gas chromatography-mass spectrometry. Food Addit. Contam. Part A 2017, 34, 853–863. [Google Scholar] [CrossRef]
- Lorena, L.; Roberta, M.; Alessandra, R.; Clara, M.; Francesca, C. Evaluation of some pyrrolizidine alkaloids in honey samples from the Veneto region (Italy) by LC-MS/MS. Food Anal. Methods 2016, 9, 1825–1836. [Google Scholar] [CrossRef]
- Valese, A.C.; Molognoni, L.; de Sá Ploêncio, L.A.; de Lima, F.G.; Gonzaga, L.V.; Górniak, S.L.; Daguer, H.; Barreto, F.; Costa, A.C.O. A fast and simple LC-ESI-MS/MS method for detecting pyrrolizidine alkaloids in honey with full validation and measurement uncertainty. Food Control 2016, 67, 183–191. [Google Scholar] [CrossRef]
- Mulder, P.P.; de Witte, S.L.; Stoopen, G.M.; van der Meulen, J.; van Wikselaar, P.G.; Gruys, E.; Groot, M.J.; Hoogenboom, R.L. Transfer of pyrrolizidine alkaloids from various herbs to eggs and meat in laying hens. Food Addit. Contam. Part A 2016, 33, 1826–1839. [Google Scholar] [CrossRef] [Green Version]
- Yoon, S.H.; Kim, M.-S.; Kim, S.H.; Park, H.M.; Pyo, H.; Lee, Y.M.; Lee, K.-T.; Hong, J. Effective application of freezing lipid precipitation and SCX-SPE for determination of pyrrolizidine alkaloids in high lipid foodstuffs by LC-ESI-MS/MS. J. Chromatogr. B 2015, 992, 56–66. [Google Scholar] [CrossRef]
- Avula, B.; Sagi, S.; Wang, Y.-H.; Zweigenbaum, J.; Wang, M.; Khan, I.A. Characterization and screening of pyrrolizidine alkaloids and N-oxides from botanicals and dietary supplements using UHPLC-high resolution mass spectrometry. Food Chem. 2015, 178, 136–148. [Google Scholar] [CrossRef] [PubMed]
- Dzuman, Z.; Zachariasova, M.; Veprikova, Z.; Godula, M.; Hajslova, J. Multi-analyte high performance liquid chromatography coupled to high resolution tandem mass spectrometry method for control of pesticide residues, mycotoxins, and pyrrolizidine alkaloids. Anal. Chim. Acta 2015, 863, 29–40. [Google Scholar] [CrossRef]
- Schulz, M.; Meins, J.; Diemert, S.; Zagermann-Muncke, P.; Goebel, R.; Schrenk, D.; Schubert-Zsilavecz, M.; Abdel-Tawab, M. Detection of pyrrolizidine alkaloids in German licensed herbal medicinal teas. Phytomedicine 2015, 22, 648–656. [Google Scholar] [CrossRef]
- Griffin, C.T.; Mitrovic, S.M.; Danaher, M.; Furey, A. Development of a fast isocratic LC-MS/MS method for the high-throughput analysis of pyrrolizidine alkaloids in Australian honey. Food Addit. Contam. Part A 2015, 32, 214–228. [Google Scholar] [CrossRef] [PubMed]
- Mudge, E.M.; Jones, A.M.P.; Brown, P.N. Quantification of pyrrolizidine alkaloids in North American plants and honey by LC-MS: Single laboratory validation. Food Addit. Contam. Part A 2015, 32, 2068–2074. [Google Scholar] [CrossRef] [PubMed]
- Bolechová, M.; Čáslavský, J.; Pospíchalová, M.; Kosubová, P. UPLC–MS/MS method for determination of selected pyrrolizidine alkaloids in feed. Food Chem. 2015, 170, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Griffin, C.T.; O’Mahony, J.; Danaher, M.; Furey, A. Liquid chromatography tandem mass spectrometry detection of targeted pyrrolizidine alkaloids in honeys purchased within Ireland. Food Anal. Methods 2015, 8, 18–31. [Google Scholar] [CrossRef]
- Shimshoni, J.A.; Duebecke, A.; Mulder, P.P.; Cuneah, O.; Barel, S. Pyrrolizidine and tropane alkaloids in teas and the herbal teas peppermint, rooibos and chamomile in the Israeli market. Food Addit. Contam. Part A 2015, 32, 2058–2067. [Google Scholar] [CrossRef] [PubMed]
- Griffin, C.T.; Gosetto, F.; Danaher, M.; Sabatini, S.; Furey, A. Investigation of targeted pyrrolizidine alkaloids in traditional Chinese medicines and selected herbal teas sourced in Ireland using LC-ESI-MS/MS. Food Addit. Contam. Part A 2014, 31, 940–961. [Google Scholar] [CrossRef]
- Diaz, G.J.; Almeida, L.X.; Gardner, D.R. Effects of dietary Crotalaria pallida seeds on the health and performance of laying hens and evaluation of residues in eggs. Res. Vet. Sci. 2014, 97, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Martinello, M.; Cristofoli, C.; Gallina, A.; Mutinelli, F. Easy and rapid method for the quantitative determination of pyrrolizidine alkaloids in honey by ultra performance liquid chromatography-mass spectrometry: An evaluation in commercial honey. Food Control 2014, 37, 146–152. [Google Scholar] [CrossRef]
- Kast, C.; Dübecke, A.; Kilchenmann, V.; Bieri, K.; Böhlen, M.; Zoller, O.; Beckh, G.; Lüllmann, C. Analysis of Swiss honeys for pyrrolizidine alkaloids. J. Apic. Res. 2014, 53, 75–83. [Google Scholar] [CrossRef]
- Bodi, D.; Ronczka, S.; Gottschalk, C.; Behr, N.; Skibba, A.; Wagner, M.; Lahrssen-Wiederholt, M.; Preiss-Weigert, A.; These, A. Determination of pyrrolizidine alkaloids in tea, herbal drugs and honey. Food Addit. Contam. Part A 2014, 31, 1886–1895. [Google Scholar] [CrossRef]
- Vaclavik, L.; Krynitsky, A.J.; Rader, J.I. Targeted analysis of multiple pharmaceuticals, plant toxins and other secondary metabolites in herbal dietary supplements by ultra-high performance liquid chromatography–quadrupole-orbital ion trap mass spectrometry. Anal. Chim. Acta 2014, 810, 45–60. [Google Scholar] [CrossRef] [PubMed]
- Orantes-Bermejo, F.; Serra Bonvehí, J.; Gómez-Pajuelo, A.; Megías, M.; Torres, C. Pyrrolizidine alkaloids: Their occurrence in Spanish honey collected from purple viper’s bugloss (Echium spp.). Food Addit. Contam. Part A 2013, 30, 1799–1806. [Google Scholar] [CrossRef] [PubMed]
- Griffin, C.T.; Danaher, M.; Elliott, C.T.; Kennedy, D.G.; Furey, A. Detection of pyrrolizidine alkaloids in commercial honey using liquid chromatography–ion trap mass spectrometry. Food Chem. 2013, 136, 1577–1583. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Colegate, S.; Edgar, J. Persistence of echimidine, a hepatotoxic pyrrolizidine alkaloid, from honey into mead. J. Food Compos. Anal. 2013, 29, 106–109. [Google Scholar] [CrossRef]
- Cramer, L.; Schiebel, H.-M.; Ernst, L.; Beuerle, T. Pyrrolizidine alkaloids in the food chain: Development, validation, and application of a new HPLC-ESI-MS/MS sum parameter method. J. Agric. Food Chem. 2013, 61, 11382–11391. [Google Scholar] [CrossRef]
- Cramer, L.; Beuerle, T. Detection and quantification of pyrrolizidine alkaloids in antibacterial medical honeys. Planta Med. 2012, 78, 1976–1982. [Google Scholar] [CrossRef]
- Hoogenboom, L.; Mulder, P.P.; Zeilmaker, M.J.; van den Top, H.J.; Remmelink, G.J.; Brandon, E.F.; Klijnstra, M.; Meijer, G.A.; Schothorst, R.; van Egmond, H.P. Carry-over of pyrrolizidine alkaloids from feed to milk in dairy cows. Food Addit. Contam. Part A 2011, 28, 359–372. [Google Scholar] [CrossRef] [Green Version]
- Mol, H.; van Dam, R.; Zomer, P.; Mulder, P.P. Screening of plant toxins in food, feed and botanicals using full-scan high-resolution (Orbitrap) mass spectrometry. Food Addit. Contam. Part A 2011, 28, 1405–1423. [Google Scholar] [CrossRef] [PubMed]
- Kempf, M.; Wittig, M.; Schönfeld, K.; Cramer, L.; Schreier, P.; Beuerle, T. Pyrrolizidine alkaloids in food: Downstream contamination in the food chain caused by honey and pollen. Food Addit. Contam. Part A 2011, 28, 325–331. [Google Scholar] [CrossRef]
- Kempf, M.; Wittig, M.; Reinhard, A.; von der Ohe, K.; Blacquière, T.; Raezke, K.-P.; Michel, R.; Schreier, P.; Beuerle, T. Pyrrolizidine alkaloids in honey: Comparison of analytical methods. Food Addit. Contam. Part A 2011, 28, 332–347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dübecke, A.; Beckh, G.; Lüllmann, C. Pyrrolizidine alkaloids in honey and bee pollen. Food Addit. Contam. Part A 2011, 28, 348–358. [Google Scholar] [CrossRef]
- Crews, C.; Driffield, M.; Berthiller, F.; Krska, R. Loss of pyrrolizidine alkaloids on decomposition of ragwort (Senecio jacobaea) as measured by LC-TOF-MS. J. Agric. Food Chem. 2009, 57, 3669–3673. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Liu, F.; Goh, J.J.L.; Yu, L.; Li, S.F.Y.; Ong, E.S.; Ong, C.N. Determination of senkirkine and senecionine in Tussilago farfara using microwave-assisted extraction and pressurized hot water extraction with liquid chromatography tandem mass spectrometry. Talanta 2009, 79, 539–546. [Google Scholar] [CrossRef]
- Kempf, M.; Beuerle, T.; Bühringer, M.; Denner, M.; Trost, D.; von der Ohe, K.; Bhavanam, V.B.; Schreier, P. Pyrrolizidine alkaloids in honey: Risk analysis by gas chromatography-mass spectrometry. Mol. Nutr. Food Res. 2008, 52, 1193–1200. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, C.h.; Wang, W.; Chen, L.x.; Ma, H.y.; Zhang, C.f.; Zhang, M.; Bligh, S.A.; Wang, Z.t. Quantitative analysis by HPLC-MS2 of the pyrrolizidine alkaloid adonifoline in Senecio scandens. Phytochem. Anal. Int. J. Plant Chem. Biochem. Tech. 2008, 19, 25–31. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, C.-h.; Xiong, A.-z.; Wang, W.; Yang, L.; Branford-White, C.J.; Wang, Z.-t.; Bligh, S.A. Quantitative analysis of total retronecine esters-type pyrrolizidine alkaloids in plant by high performance liquid chromatography. Anal. Chim. Acta 2007, 605, 94–101. [Google Scholar] [CrossRef]
- Crews, C. Methods for analysis of pyrrolizidine alkaloids. In Natural Products: Phytochemistry, Botany and Metabolism of Alkaloids, Phenolics and Terpenes; Ramawat, K.G., Mérillon, J.-M., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 1049–1068. [Google Scholar] [CrossRef]
- González-Gómez, L.; Morante-Zarcero, S.; Pérez-Quintanilla, D.; Sierra, I. Occurrence and Chemistry of Tropane Alkaloids in Foods, with a Focus on Sample Analysis Methods: A Review on Recent Trends and Technological Advances. Foods 2022, 11, 407. [Google Scholar] [CrossRef] [PubMed]
- These, A.; Bodi, D.; Ronczka, S.; Lahrssen-Wiederholt, M.; Preiss-Weigert, A. Structural screening by multiple reaction monitoring as a new approach for tandem mass spectrometry: Presented for the determination of pyrrolizidine alkaloids in plants. Anal. Bioanal. Chem. 2013, 405, 9375–9383. [Google Scholar] [CrossRef]
- Herrmann, M.; Joppe, H.; Schmaus, G. Thesinine-4′-O-beta-D-glucoside the first glycosylated plant pyrrolizidine alkaloid from Borago officinalis. Phytochemistry 2002, 60, 399–402. [Google Scholar] [CrossRef] [PubMed]
- Mroczek, T.; Baj, S.; Chrobok, A.; Glowniak, K. Screening for pyrrolizidine alkaloids in plant materials by electron ionization RP-HPLC-MS with thermabeam interface. Biomed. Chromatogr. 2004, 18, 745–751. [Google Scholar] [CrossRef]
- Pietrosiuk, A.; Sykłowska-Baranek, K.; Wiedenfeld, H.; Wolinowska, R.; Furmanowa, M.; Jaroszyk, E. The shikonin derivatives and pyrrolizidine alkaloids in hairy root cultures of Lithospermum canescens (Michx.) Lehm. Plant Cell Rep. 2006, 25, 1052–1058. [Google Scholar] [CrossRef] [PubMed]
- Lang, G.; Passreiter, C.M.; Medinilla, B.; Castillo, J.; Witte, L. Non-toxic pyrrolizidine alkaloids from Eupatorium semialatum. Biochem. Syst. Ecol. 2001, 29, 143–147. [Google Scholar] [CrossRef]
- Schenk, A.; Siewert, B.; Toff, S.; Drewe, J. UPLC TOF MS for sensitive quantification of naturally occurring pyrrolizidine alkaloids in Petasites hybridus extract (Ze 339). J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2015, 997, 23–29. [Google Scholar] [CrossRef] [Green Version]
- Kopp, T.; Salzer, L.; Abdel-Tawab, M.; Mizaikoff, B. Efficient extraction of pyrrolizidine alkaloids from plants by pressurised liquid extraction—A preliminary study. Planta Med. 2020, 86, 85–90. [Google Scholar] [CrossRef]
- Mroczek, T.; Glowniak, K.; Wlaszczyk, A. Simultaneous determination of N-oxides and free bases of pyrrolizidine alkaloids by cation-exchange solid-phase extraction and ion-pair high-performance liquid chromatography. J. Chromatogr. A 2002, 949, 249–262. [Google Scholar] [CrossRef]
- El-Shazly, A.; El-Domiaty, M.; Witte, L.; Wink, M. Pyrrolizidine alkaloids in members of the Boraginaceae from Sinai (Egypt). Biochem. Syst. Ecol. 1998, 26, 619–636. [Google Scholar] [CrossRef]
- Kopp, T.; Abdel-Tawab, M.; Mizaikoff, B. Extracting and analyzing pyrrolizidine alkaloids in medicinal plants: A review. Toxins 2020, 12, 320. [Google Scholar] [CrossRef]
- Aguilera, J.M. The food matrix: Implications in processing, nutrition and health. Crit. Rev. Food Sci. Nutr. 2019, 59, 3612–3629. [Google Scholar] [CrossRef]
- Copper, R.A.; Bowers, R.J.; Beckham, C.J.; Huxtable, R.J. Preparative separation of pyrrolizidine alkaloids by high-speed counter-current chromatography. J. Chromatogr. A 1996, 732, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Aydın, A.A.; Letzel, T. Simultaneous investigation of sesquiterpenes, pyrrolizidine alkaloids and N-oxides in Butterbur (Petasites hybridus) with an offline 2D-combination of HPLC-UV and LC-MMI-ToF-MS. J. Pharm. Biomed. Anal. 2013, 85, 74–82. [Google Scholar] [CrossRef]
- Pande, J. Metabolic profiling of bioactive compounds from different medicinal plants: An overview. Int. J. Chem. Stud. 2020. [CrossRef]
- Kristensen, M. Nutri-Metabolomics: Effect and Exposure Markers of Apple and Pectin Intake; University of Copenhagen, Faculty of Life Sciences, Department of Food Science: Copenhagen, Denmark, 2010. [Google Scholar]
- Klein, L.M.; Gabler, A.M.; Rychlik, M.; Gottschalk, C.; Kaltner, F. A sensitive LC–MS/MS method for isomer separation and quantitative determination of 51 pyrrolizidine alkaloids and two tropane alkaloids in cow’s milk. Anal. Bioanal. Chem. 2022, 414, 8107–8124. [Google Scholar] [CrossRef]
- Joosten, L.; Mulder, P.P.; Vrieling, K.; van Veen, J.A.; Klinkhamer, P.G. The analysis of pyrrolizidine alkaloids in Jacobaea vulgaris; a comparison of extraction and detection methods. Phytochem. Anal. 2010, 21, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Lu, A.-J.; Lu, Y.-L.; Tan, D.-P.; Qin, L.; Ling, H.; Wang, C.-H.; He, Y.-Q. Identification of pyrrolizidine alkaloids in senecio plants by liquid chromatography-mass spectrometry. J. Anal. Methods Chem. 2021, 2021, 1957863. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Li, N.; Choi, F.; Qiao, C.-F.; Song, J.-Z.; Li, S.-L.; Liu, X.; Cai, Z.; Fu, P.; Lin, G.; et al. A new approach for simultaneous screening and quantification of toxic pyrrolizidine alkaloids in some potential pyrrolizidine alkaloid-containing plants by using ultra performance liquid chromatography-tandem quadrupole mass spectrometry. Anal. Chim. Acta 2010, 681, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Ruan, J.; Li, N.; Xia, Q.; Fu, P.P.; Peng, S.; Ye, Y.; Lin, G. Characteristic ion clusters as determinants for the identification of pyrrolizidine alkaloid N-oxides in pyrrolizidine alkaloid-containing natural products using HPLC-MS analysis. J. Mass Spectrom. 2012, 47, 331–337. [Google Scholar] [CrossRef]
- Gottschalk, C.; Ronczka, S.; Preiß-Weigert, A.; Ostertag, J.; Klaffke, H.; Schafft, H.; Lahrssen-Wiederholt, M. Pyrrolizidine alkaloids in natural and experimental grass silages and implications for feed safety. Anim. Feed Sci. Technol. 2015, 207, 253–261. [Google Scholar] [CrossRef]
- Takatsuji, Y.; Kakitani, A.; Nagatomi, Y.; Harayama, K.; Suzuki, K. A novel method for the detection of pyrrolizidine alkaloids in bottled tea and tea leaves by LC-MS/MS. Jpn. J. Food Chem. Saf. 2018, 25, 97–104. [Google Scholar] [CrossRef]
- Sixto, A.; Pérez-Parada, A.; Niell, S.; Heinzen, H. GC–MS and LC–MS/MS workflows for the identification and quantitation of pyrrolizidine alkaloids in plant extracts, a case study: Echium plantagineum. Rev. Bras. Farmacogn. 2019, 29, 500–503. [Google Scholar] [CrossRef]
| PA | LD50 (g/kg) |
|---|---|
| Monocrotaline * | 0.731 |
| Echimidine | 0.616 |
| Senkirkine | 0.275 |
| Trichodesmine | 0.324 |
| Acetyllycopsamine | 0.356 |
| Seneciphylline | 0.264 |
| Retrorsine * | 0.320 |
| Senecionine | 0.127 |
| Heliosupine | 0.708 |
| Riddelliine | 0.616 |
| Clivorine | 0.386 |
| Usaramine | 0.264 |
| Jacobine | 0.461 |
| Echiumine | 0.122 |
| Lycopsamine | 0.239 |
| Heliotrine | 0.056 |
| Heliocoromandaline | 0.246 |
| Otosenine | 0.106 |
| Foodstuffs | Max Sum Level of PAs (µg/kg) |
|---|---|
| Herbal infusions (dried product) | 200 |
| Herbal infusions of rooibos, anise (Pimpinella anisum), lemon balm, chamomile, thyme, peppermint, lemon verbena (dried product), and mixtures exclusively composed of these dried herbs | 400 |
| Tea (Camellia sinensis) and flavored tea (Camellia sinensis) (dried product) | 150 |
| Tea (Camellia sinensis), flavored tea (Camellia sinensis), and herbal infusions for infants and young children (dried product) | 75 |
| Tea (Camellia sinensis), flavored tea (Camellia sinensis), and herbal infusions for infants and young children (liquid) | 1.0 |
| Food supplements containing herbal ingredients including extracts | 400 |
| Pollen-based food supplements, pollen, and pollen products | 500 |
| Borage leaves (fresh, frozen) placed on the market for the final consumer | 750 |
| Cumin seeds (seed spice) | 400 |
| Borage, lovage, marjoram, and oregano (dried) and mixtures exclusively composed of these dried herbs | 1000 |
| Sample | Analysis | |||||
|---|---|---|---|---|---|---|
| Sample Type | Sample Preparation | Instrument | Analytes | Recovery (%) | LOD/LOQ | Ref. |
| Honey and herbal beverage | Prepare using QuEChERs
| UPLC-IM-QTOF-MS/MS
| 7 PAs | 61–120 | LOQ: 1–20 µg/kg | [19] |
| Teas and herbs | Prepare using QuEChERs
| HPLC-Q-Orbitrap-MS/MS
| 28 Pas/PA N-Oxides | 87–111 | LOQ: 5 µg/kg | [20] |
| Aromatic herbs | Prepare using QuEChERs
| UHPLC-IT-MS/MS
| 21 PAs/PA N-Oxides | 73–105 | LOQ: 1.2–9.9 µg/kg | [21] |
| Pollen | Prepare using QuEChERs
| UHPLC-TQ-MS/MS
| 20 PAs | 73–106 | LOQ: 4.0–9.0 µg/kg | [22] |
| Teas and Weeds |
| UHPLC-MS/MS
| 14 PAs/PA N-Oxides | 68–110 | LOD: 0.001–0.4 μg/kg LOQ: 1–5 μg/kg | [23] |
| Honey |
| UHPLC-QTOF-MS/MS
| 26 PAs/PA N-Oxides | 75–120 | LOD: 1–7 µg/kg LOQ: 10–20 µg/kg | [24] |
| Herbal Medicines |
| LC-MS/MS
| 28 PAs | 67–151 | LOD: 0.03–2.1 µg/kg LOQ: 0.1–6.5 µg/kg | [25] |
| Black tea and Herbal tea |
| UPLC-MS/MS
| 21 PAs | 86–101 | LOD: 0.1–3 µg/kg LOQ: 0.3–9 µg/kg | [26] |
| Milk |
| DART-IT-MS
| 6 PAs | 89–112 | LOD: 0.5–8 µg/kg LOQ: 1.8–2.8 µg/kg | [27] |
| Dried Plant, Pollen, and Honey | Plant and pollen:
| LC-Q-TRAP-MS/MS Mode: ESI, MRM
| 8 PAs/PA N-Oxides | - | - | [28] |
| Honey |
| HPLC-DAD (wavelength: 223 nm)
| 2 PAs | - | - | [29] |
| Honey |
| HPLC-TQ-MS/MS
| 17 PAs | - | - | [30] |
| Maize |
| HPLC-QTRAP-MS/MS
| Sum of 1, 2- unsaturated retronecine/ heliotridine- PAs | - | - | [31] |
| Plant and Seeds |
| UHPLC-MS/MS
| 45 PAs/PA N-oxides | LOD: 0.05 ng/mL LOQ: - | [32] | |
| Oregano | Prepare using QuEChERs
| UHPLC-IT MS/MS
| 21 PAs/PA N-oxides | 77–96 | LOD: 0.1–7.5 µg/kg LOQ: 0.5–25.0 µg/kg | [33] |
| Spices and Herbs |
| HPLC-TQ-MS/MS
| 44 PAs/PA N-oxides | 50–119 | LOD: Less than 0.1–2.6 µg/kg LOQ: - | [5] |
| Herbs | Prepare using QuEChERs
| HPLC-QTRAP MS/MS
| 30 PAs/PA N-oxides | 61–128 | LOD: - LOQ: 1 µg/kg | [34] |
| Herbs |
| UHPLC-QTRAP-MS/MS
| 33 PAs/PA N-oxides | 78–117 | LOD: - LOQ: 0.5–10 µg/kg | [35] |
| Honey | Prepare using QuEChERs
| LC-QTRAP MS/MS
| 5 PAs/PA N-oxides | 86–111 | LOD: - LOQ: 8–18 µg/kg | [36] |
| Honey |
| UHPLC-QTRAP-MS/MS
| 9 PAs/PA N-oxides | 63–103 | LOD: - LOQ: 0.03–0.06 µg/kg | [37] |
| Herbal teas |
| UHPLC-TQ-MS/MS
| 70 PAs/PA N-oxides | 73–107 | LOD: 0.01–0.02 µg/L LOQ: 0.05 µg/L | [38] |
| Herbal juices |
| HPLC-QTRAP-MS/MS
| 30 PAs/PA N-oxides | - | - | [39] |
| Honey |
| HPLC-Q-TOF-MS/MS
| 12 PAs/PA N-oxides | 79–104 | LOD: 0.2–0.6 µg/kg LOQ: 0.5–1.3 µg/kg | [40] |
| Herbs |
| HPLC-TQ-MS/MS
| 12 PAs/PA N-oxides | - | - | [41] |
| Teas and Herbs |
| HPLC-TQ-MS/MS
| 44 PAs/PA N-oxides | 52–152 | LOD: 0.1–7.0 µg/kg LOQ: 0.1–27.9 µg/kg | [42] |
| Milk, Dairy products, eggs, meat, meat products, Herbs and Food supplements | Animal-derived samples:
| UHPLC-TQ-MS/MS
| 38 PAs/PA N-oxides | 30–122 | LODs: Milk and yoghurt 0.03–0.05 µg/L egg, cheese, chicken, and pork meat: 0.05–0.15 µg/kg red meat: 0.1–0.25 µg/kg Teas and supplements: 0.2–3.8 µg/kg | [43] |
| Honey |
| GC-Q-MS EI
| 4 PAs/PA N-oxides | 73–94 | LOD: - LOQ: 1 µg/kg | [44] |
| Herbs, Spices, Teas, and ice-tea drinks | Herbs:
| UHPLC-TQ-MS/MS
| 31 PAs/PA N-oxides | 86–125 | No LODs for all LOQs: 0.1–1 ng/g Infusion extracts: 0.01 ng/mL | [45] |
| Peppermint tea and Honey |
| HPLC-TQ-MS/MS
| 25 PAs/PA N-oxides | 49–121 | LOD: 0.01–1.60 µg/kg LOQ: 0.03–5.40 µg/kg | [46] |
| Honey | Extract using QuEChERS
| HPLC-Q-Orbitrap-MS/MS
| 9 PAs/PA N-oxides | 92–115 | LOD: 0.04–0.2 µg/kg LOQ: 0.1–0.7 µg/kg | [47] |
| Plants |
| GC-MS
| 5 PAs | - | - | [48] |
| Feed (Silage and hay) |
| GC-MS
| 2 (sum of retronecine derivative and heliotridine derivative) | 72.7–94.4 | LOD: - LOQ: 10 µg/kg | [49] |
| Honey |
| LC-IT-MS/MS
| 6 PAs/PA N-oxides | 74–108 | LOD: - LOQ: 0.25 µg/kg | [50] |
| Honey |
| HPLC-QTRAP-MS/MS
| 8 PAs/PA N-oxides | 93–110 | LOD: 0.1–1 µg/kg LOQ: 0.2–1.5 | [51] |
| Eggs and Meat |
| UHPLC-TQ-MS/MS
| 51 PAs/PA N-oxides | - | LOD: - LOQ: 0.1–1 µg/kg | [52] |
| Milks, Soybean, Seed oils, and Margarines | Milk and Soy:
| HPLC-TQ-MS/MS
| 9 PAs/PA N-oxides | 82–105 | LOD: 0.07–0.59 µg/kg LOQ: 0.20–1.43 ng/mL | [53] |
| Herbal supplements |
| UHPLC-Q-TOF-MS/MS
| 25 PAs/PA N-oxides | - | LOD: 0.05–5 ng/mL LOQ: - | [54] |
| Teas, Wheat, and Leek | Prepare using QuEChERs
| HPLC-Q-Orbitrap-MS/MS
| 11 PAs/PA N-oxides | 71–93 | LOD: - µg/kg LOQ: 1–100 µg/kg | [55] |
| Herbal teas | Dry samples:
| HPLC-TQ-MS/MS
| 23 PAs/PA N-oxides | 76–125 | LOD: - LOQ: 10 µg/kg | [56] |
| Honey |
| HPLC-TQ-MS/MS
| 14 PAs/PA N-oxides | 82–112 | LOD: 0.4–3.3 µg/kg LOQ: 1.4–10.9 µg/kg | [57] |
| Honey |
| HPLC-TQ-MS/MS
| 5 PAs/PA N-oxides | 40–106 | LOD: 0.45–0.67 ng/mL LOQ: 1.21–1.79 ng/mL | [58] |
| Feed | Prepare using QuEChERs
| UHPLC-TQ-MS/MS
| 5 PAs | 72–98 | LOD: - LOQ: 5 µg/kg | [59] |
| Honey |
| HPLC-TQ-MS/MS
| 14 PAs/PA N-oxides | 70–125 | LOD: 0.5–3.9 µg/kg LOQ: 2.3–12.9 µg/kg | [60] |
| Herbal teas |
| HPLC-QTRAP-MS/MS
| 28 PAs/PA N-oxides | 80–95 | LOD: - LOQ: 10–50 µg/kg | [61] |
| Herbal teas |
| HPLC-TQ-MS/MS
| 14 PAs/PA N-oxides | 93–127 | LOD: 0.4–1.9 µg/kg LOQ: 1.3–6.3 µg/kg | [62] |
| Eggs |
| HPLC-IT-MS/MS
| 2 PAs/PA N-oxides | - | LOD: - µg/kg LOQ: 2 ng/g | [63] |
| Honey | Prepare using QuEChERs
| UHPLC-Q-MS
| 9 PAs | 67–122 | LOD: - LOQ: 0.08–4.3 µg/kg | [64] |
| Honey |
| HPLC-QTRAP-MS/MS
| 18 PAs/PA N-oxides | - | LOD: - LOQ: 1–3 µg/kg | [65] |
| Honey and Herbal teas | Honey Samples
| HPLC-TQ-MS/MS
| 17 PAs/PA N-oxides | 45–122 | LOD: 0.06–2.0 µg/kg LOQ: 0.18–6.4 µg/kg | [66] |
| Herbal supplement in form of tablets, capsules, soft gels, and liquids | Prepare using QuEChERs
| UHPLC-Q-Orbitrap-MS/MS
| 11 PAs/PA N-oxides | 70–120 | LOD: - LOQ: 50–2500 µg/kg | [67] |
| Honey |
| HPLC-TQ-MS/MS
| 17 PAs/PA N-oxides | More than 80% | LOD: - LOQ: 1–3 µg/kg | [68] |
| Honey |
| HPLC-IT-MS/MS
| 11 PAs/PA N-oxides | 87 | LOD: 0.01–0.03 µg/mL LOQ: 0.04–0.10 µg/kg | [69] |
| Honey and mead | Honey:
| HPLC-IT-MS/MS
| 7 PAs/PA N-oxides | - | LOD: 50 ng/kg LOQ: - | [70] |
| Herbs and Honey |
| HPLC-QTRAP-MS/MS
| 3 PAs/PA N-oxides | 69–104 | LOD: 0.1–1 µg/kg LOQ: 0.3–3 µg/kg | [71] |
| Honey |
| HRGC-Q-MS
| 2 PAs/PA N-oxides | - | LOD: 2 µg/kg LOQ: 6 µg/kg | [72] |
| Milk |
| UHPLC-QHQ-MS/MS
| 21 PAs/PA N-oxides | 44–67 | LOD: - LOQ: 0.05–0.2 µg/L | [73] |
| Honey, Food supplements, and feed | Prepare using QuEChERs
| HPLC-Orbitrap-MS
| 14 PAs/PA N-oxides | - | - | [74] |
| Honey, pollen, and honey- products | Mead and fennel honey:
| HRGC-Q-MS
| 6 PAs/PA N-oxides | 74–88 | LOD: - LOQ: 10 µg/kg | [75] |
| Honey | Prepare using QuEChERs
| HPLC-TQ-MS/MS
| 16 PAs/PA N-oxides | 97–105 | LOD: - LOQ: HPLC MS/MS: 1–50 µg/kg GC-MS 10 µg/kg | [76] |
| Honey |
| HPLC-QTRAP-MS/MS
| 17 PAs/PA N-oxides | 60–110 | LOD: - LOQ: 1–3 µg/kg | [77] |
| Plant |
| LC-TOF-MS
| 342 PAs/PA N-oxides | - | - | [78] |
| Plant |
|
HPLC-diode array
| 2 PAs/PA N-oxides | 99–107 | LOD MAE: 0.26 PHWE: 1.32 µg/g LOQ: MAE: 1.04 PHWE: 5.29 µg/g | [79] |
| Honey |
| HRGC-MS
| 2 (sum of retronecine and heliotridine) | 80–86 | LOD: - LOQ: 0.01 ppm | [80] |
| Plant |
| HPLC-IT-MS
| 1 PAs | - | LOD: 0.5 ng/mL LOQ: 1 ng/mL | [81] |
| Plant |
| HPLC-IT-MS
| 13 PAs/PA N-oxides | 91–102 | LOD: 0.26 nmol/mL LOQ: - | [82] |
| No. | Compound | MS1 a | MS2 b | DP c | EP d | CE e | CXP f | Reference |
|---|---|---|---|---|---|---|---|---|
| (m/z) | (m/z) | (V) | (eV) | (V) | ||||
| 1 | Monocrotaline | 326.2 | 121 | 53 | 10 | 28 | 45 | [34] |
| 326.3 | 121.2 | 106 | 10 | 39 | 10 | [105] | ||
| 121 | 131 | 10 | 41 | 10 | [106] | |||
| 94.0 | 106 | 10 | 73 | 18 | [105] | |||
| 326.1 | 120.1 | 161 | 10 | 43 | 8 | [51] | ||
| 94.1 | 161 | 10 | 73 | 12 | [51] | |||
| 194.1 | 161 | 10 | 39 | 12 | [51] | |||
| 2 | Erucifoline | 350.2 | 138 | 42 | 10 | 33 | 64 | [34] |
| 350.2 | 94.0 | 40 | [101] | |||||
| 350.3 | 67.2 | 121 | 10 | 73 | 12 | [106] | ||
| 3 | Monocrotaline NOs | 342.2 | 137 | 38 | 10 | 34 | 53 | [34] |
| 137.0 | 136 | 10 | 41 | 6 | [105] | |||
| 120.1 | 136 | 10 | 51 | 6 | [105] | |||
| 342.2 | 146 | 10 | 15 | 22 | [106] | |||
| 4 | Europine | 330.2 | 138 | 43 | 10 | 22 | 68 | [34] |
| 330.4 | 138.1 | 66 | 10 | 31 | 10 | [106] | ||
| 5 | Intermedine | 300.1 | 94.1 | 96 | 10 | 33 | 12 | [34] |
| 138.1 | 96 | 10 | 27 | 8 | [51] | |||
| 156.0 | 96 | 10 | 37 | 10 | [51] | |||
| 300.2 | 94.1 | 81 | 10 | 37 | 6 | [51] | ||
| 138.1 | 81 | 10 | 31 | 6 | [105] | |||
| 300.4 | 94.0 | 96 | 10 | 37 | 8 | [105] | ||
| [106] | ||||||||
| 6 | Indicine | 300.1 | 156 | 42 | 10 | 24 | 48 | [34] |
| 300.5 | 94.1 | 91 | 10 | 37 | 8 | [106] | ||
| 7 | Lycopsamine | 300.2 | 156 | 52 | 10 | 39 | 48 | [34] |
| 300.1 | 94.1 | 96 | 10 | 33 | 12 | [51] | ||
| 138.1 | 96 | 10 | 27 | 8 | [51] | |||
| 156.0 | 96 | 10 | 37 | 10 | [51] | |||
| 300.2 | 138.2 | 60 | 10 | 30 | [107] | |||
| 120.3 | 60 | 10 | 32 | [107] | ||||
| 138.1 | 91 | 10 | 29 | 8 | [105] | |||
| 94.1 | 91 | 10 | 37 | 16 | [105] | |||
| 300.5 | 94.0 | 86 | 10 | 37 | 8 | [106] | ||
| 8 | Erucifoline NOs | 366.2 | 118 | 16 | 10 | 33 | 48 | [34] |
| 366.1 | 94.1 | 111 | 10 | 65 | 10 | [106] | ||
| 9 | Europine NOs | 346.2 | 256 | 25 | 10 | 25 | 75 | [34] |
| 172.2 | 126 | 10 | 43 | 6 | [106] | |||
| 10 | Intermedine NOs | 316.3 | 172.2 | 56 | 10 | 37 | 14 | [106] |
| 11 | Indicine NOs | 316.2 | 172 | 28 | 10 | 31 | 68 | [34] |
| 316.4 | 172.2 | 71 | 10 | 39 | 12 | [106] | ||
| 12 | Lycopsamine NOs | 316.2 | 172 | 42 | 10 | 37 | 47 | [34] |
| 316.3 | 138.2 | 118 | 10 | 29 | [107] | |||
| 94.0 | 118 | 10 | 44 | [107] | ||||
| 316.4 | 172.3 | 66 | 10 | 43 | 14 | [106] | ||
| 13 | Retrorsine | 352.3 | 120.1 | 116 | 10 | 43 | 8 | [105] |
| 138.1 | 116 | 10 | 43 | 8 | [105] | |||
| 352.2 | 120.0 | 30 | [101] | |||||
| 352.1 | 138.1 | 161 | 10 | 43 | 8 | [51] | ||
| 119.2 | 161 | 10 | 73 | 12 | [51] | |||
| 94.0 | 161 | 10 | 39 | 12 | [51] | |||
| 352.2 | 138 | 45 | 10 | 47 | 41 | [34] | ||
| 352.4 | 120.1 | 121 | 10 | 41 | 10 | [106] | ||
| 14 | Trichodesmine | 354.3 | 222.0 | 111 | 10 | 41 | 12 | [105] |
| 120.1 | 111 | 10 | 53 | 6 | [105] | |||
| 354.2 | 222 | 28 | 10 | 33 | 47 | [34] | ||
| 354.3 | 222.1 | 121 | 10 | 39 | 14 | [106] | ||
| 15 | Retrorsine NOs | 368.3 | 94.1 | 111 | 10 | 73 | 16 | [105] |
| 120.1 | 111 | 10 | 49 | 6 | [105] | |||
| 94.0 | 40 | [101] | ||||||
| 368.1 | 119.0 | 121 | 10 | 39 | 8 | [51] | ||
| 94.1 | 121 | 10 | 71 | 6 | [51] | |||
| 84.0 | 121 | 10 | 41 | 8 | [51] | |||
| 368.2 | 118 | 38 | 10 | 37 | 64 | [34] | ||
| 368.3 | 94.0 | 60 | 10 | 30 | 12 | [106] | ||
| 16 | Seneciphylline | 334.2 | 138 | 43 | 10 | 31 | 75 | [34] |
| 120.0 | 39 | [101] | ||||||
| 138.1 | 106 | 10 | 30 | 8 | [105] | |||
| 120.1 | 106 | 10 | 39 | 10 | [105] | |||
| 334.3 | 120.1 | 106 | 10 | 37 | 10 | [106] | ||
| 17 | Heliotrine | 314.2 | 156 | 35 | 10 | 26 | 48 | [34] |
| 138.0 | 25 | [101] | ||||||
| 314.3 | 138.1 | 76 | 10 | 31 | 8 | [105] | ||
| 156.1 | 76 | 10 | 39 | 8 | [105] | |||
| 314.2 | 138.2 | 86 | 10 | 29 | 10 | [106] | ||
| 18 | Seneciphylline NOs | 350.2 | 118 | 37 | 10 | 28 | 75 | [34] |
| 120.0 | 30 | [101] | ||||||
| 94.1 | 86 | 10 | 67 | 16 | [105] | |||
| 118.1 | 86 | 10 | 45 | 6 | [105] | |||
| 350.4 | 94.1 | 121 | 10 | 63 | 8 | [106] | ||
| 19 | Heliotrine NOs | 330.2 | 172 | 45 | 10 | 26 | 53 | [34] |
| 330.3 | 172.2 | 71 | 10 | 39 | 12 | [106] | ||
| 20 | Senecionine | 336.2 | 120.0 | 121 | 10 | 41 | 20 | [105] |
| 138.0 | 121 | 10 | 41 | 8 | [105] | |||
| 120 | 27 | 10 | 33 | 42 | [34] | |||
| 120 | 30 | [101] | ||||||
| 336.1 | 120.1 | 136 | 10 | 37 | 8 | [51] | ||
| 93.9 | 136 | 10 | 39 | 12 | [51] | |||
| 91.1 | 136 | 10 | 77 | 14 | [51] | |||
| 336.3 | 120.0 | 136 | 10 | 43 | 10 | [106] | ||
| 21 | Senecivernine | 336.2 | 120 | 43 | 10 | 28 | 46 | [34] |
| 336.3 | 120.1 | 136 | 10 | 41 | 10 | [106] | ||
| 22 | Senecionine NOs | 352.3 | 94.2 | 91 | 10 | 67 | 6 | [105] |
| 136.0 | 91 | 10 | 51 | 12 | [105] | |||
| 352.2 | 120.0 | 30 | [101] | |||||
| 136 | 35 | 10 | 37 | 47 | [34] | |||
| 352.1 | 120.1 | 156 | 10 | 39 | 6 | [51] | ||
| 324.3 | 156 | 10 | 37 | 14 | [51] | |||
| 93.9 | 156 | 10 | 41 | 12 | [51] | |||
| 352.4 | 94.0 | 126 | 10 | 65 | 8 | [106] | ||
| 23 | Senecivernine NOs | 352.2 | 136 | 43 | 10 | 36 | 48 | [34] |
| 352.4 | 94.0 | 131 | 10 | 63 | 8 | [106] | ||
| 24 | Echimidine | 398.2 | 220 | 23 | 10 | 24 | 54 | [34] |
| 120.2 | 131 | 10 | 31 | 8 | [51] | |||
| 220.1 | 131 | 10 | 23 | 10 | [51] | |||
| 83.0 | 131 | 10 | 29 | 6 | [51] | |||
| 120.3 | 75 | 10 | 35 | [107] | ||||
| 398.3 | 220.3 | 75 | 10 | 22 | [107] | |||
| 120.0 | 76 | 10 | 35 | 8 | [105] | |||
| 220.1 | 76 | 10 | 25 | 12 | [105] | |||
| 398.2 | 120.0 | 111 | 10 | 33 | 10 | [106] | ||
| 25 | Senkirkine | 366.3 | 168.0 | 86 | 10 | 43 | 8 | [105] |
| 150.0 | 86 | 10 | 39 | 8 | [105] | |||
| 366.2 | 168 | 44 | 10 | 24 | 54 | [34] | ||
| 366.1 | 168.2 | 96 | 10 | 39 | 12 | [106] | ||
| 26 | Lasiocarpine | 412.2 | 220 | 53 | 10 | 22 | 67 | [34] |
| 412.3 | 120.1 | 96 | 10 | 39 | 10 | [106] | ||
| 27 | Lasiocarpine NOs | 428.2 | 254 | 75 | 10 | 30 | 38 | [34] |
| 428.4 | 94.1 | 111 | 10 | 69 | 6 | [106] | ||
| 28 | Jacobine | 352.2 | 155 | 47 | 10 | 34 | 47 | [34] |
| 29 | Jacobine NOs | 368.2 | 296 | 36 | 10 | 26 | 45 | [34] |
| 30 | Spartioidine | 334.2 | 120.0 | 30 | [101] | |||
| 31 | Integerrimine | 336.2 | 120.0 | 30 | [101] | |||
| 32 | Integerrimine NOs | 352.2 | 120.0 | 30 | [101] | |||
| 33 | Jacozine | 350.2 | 94.0 | 40 | [101] | |||
| 34 | Riddelliine | 350.2 | 94.0 | 40 | [101] | |||
| 35 | Riddelliine NOs | 366.2 | 94.0 | 40 | [101] | |||
| 36 | Jacobine | 352.2 | 120.0 | 30 | [101] | |||
| 37 | Jacobine NOs | 368.2 | 94.0 | 40 | [101] | |||
| 38 | Jacoline | 370.2 | 120.0 | 30 | [101] | |||
| 39 | Jacoline NOs | 386 | 94.0 | 40 | [101] | |||
| 40 | Acetylseneciphylline | 376.2 | 120.0 | 30 | [101] | |||
| 41 | Acetylseneciphylline NOs | 392.2 | 120.0 | 30 | [101] | |||
| 42 | Jaconine | 388.2 | 120.0 | 30 | [101] | |||
| 43 | Jaconine NOs | 404.2 | 94.0 | 40 | [101] | |||
| 44 | Acetylerucifoline | 392.2 | 120.0 | 40 | [101] | |||
| 45 | Acetylerucifoline NOs | 408.2 | 94.0 | 40 | [101] | |||
| 46 | Acetyllycopsamine | 342.3 | 198.4 | 53 | 10 | 38 | [107] | |
| 138.3 | 53 | 10 | 36 | [107] | ||||
| 120.2 | 53 | 10 | 36 | [107] | ||||
| 94.2 | 53 | 10 | 60 | [107] | ||||
| 47 | Echimidine NOs | 414.2 | 352 | 42 | 10 | 21 | 75 | [34] |
| 414.4 | 396.4 | 80 | 10 | 35 | [107] | |||
| 254.0 | 80 | 10 | 41 | [107] | ||||
| 48 | Echiumine | 382.5 | 220.3 | 51 | 10 | 25 | [107] | |
| 120.3 | 51 | 10 | 38 | [107] | ||||
| 49 | Echiumine NOs | 398.3 | 220.4 | 80 | 10 | 22 | [107] | |
| 120.2 | 80 | 10 | 35 | [107] | ||||
| 50 | 7,9-Ditigloylretronecine NOs | 336.0 | 138.2 | 60 | 10 | 42 | [107] | |
| 120.2 | 60 | 10 | 42 | [107] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Subaie, S.F.; Alowaifeer, A.M.; Mohamed, M.E. Pyrrolizidine Alkaloid Extraction and Analysis: Recent Updates. Foods 2022, 11, 3873. https://doi.org/10.3390/foods11233873
Al-Subaie SF, Alowaifeer AM, Mohamed ME. Pyrrolizidine Alkaloid Extraction and Analysis: Recent Updates. Foods. 2022; 11(23):3873. https://doi.org/10.3390/foods11233873
Chicago/Turabian StyleAl-Subaie, Sarah F., Abdullah M. Alowaifeer, and Maged E. Mohamed. 2022. "Pyrrolizidine Alkaloid Extraction and Analysis: Recent Updates" Foods 11, no. 23: 3873. https://doi.org/10.3390/foods11233873







