Relationship between Intervertebral Disc Compression Force and Sagittal Spinopelvic Lower Limb Alignment in Elderly Women in Standing Position with Patient-Specific Whole Body Musculoskeletal Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient-Specific Data
2.2. Image Acquisition and Anatomical Parameters Extraction
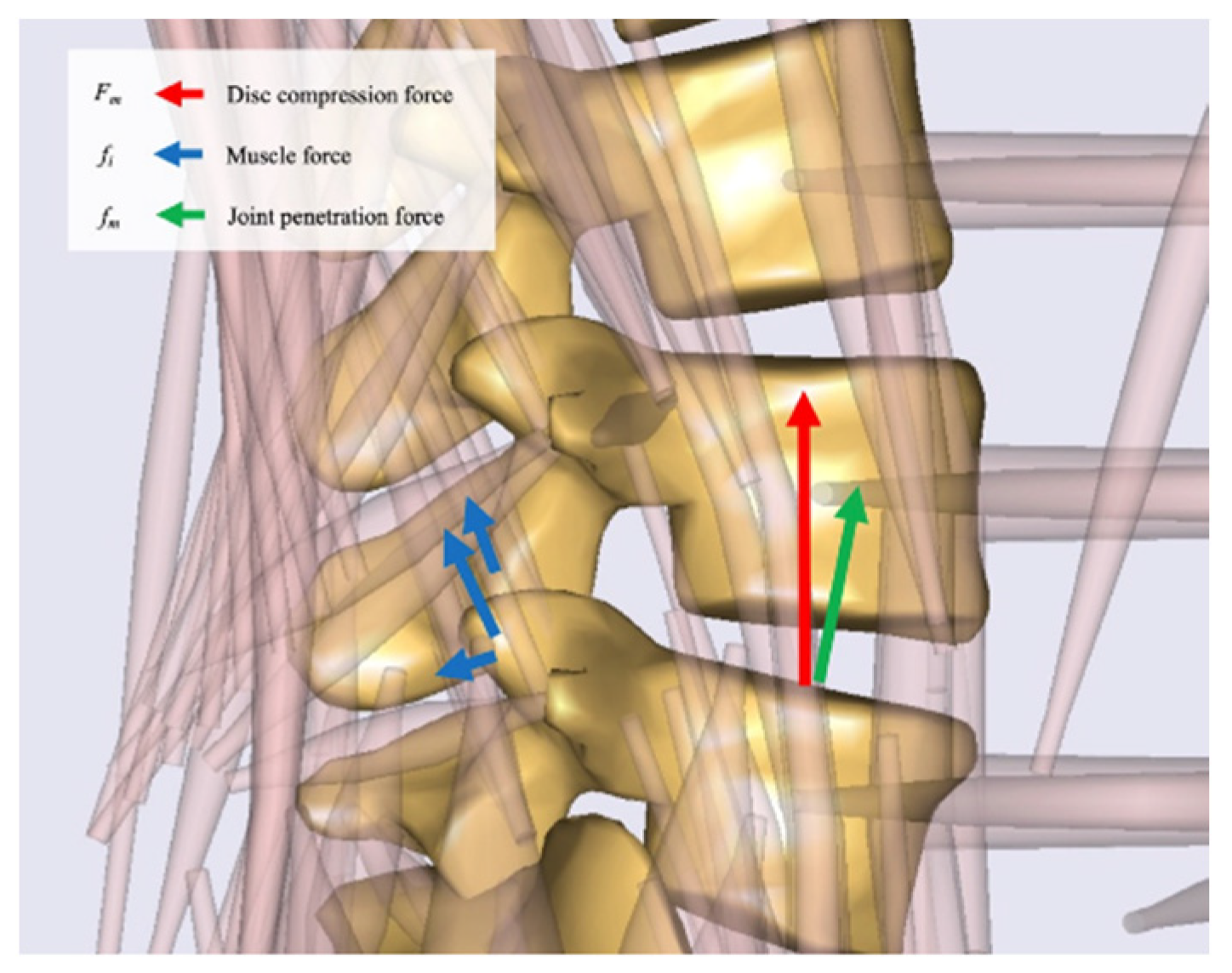
2.3. Musculoskeletal Model
2.4. Spinopelvic and Lower-Extremity Sagittal Alignment Input and Simulation Process
2.5. Model Outputs
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schwab, F.; Ungar, B.; Blondel, B. Scoliosis research society—schwab adult spinal deformity classification. Spine 2012, 37, 1077–1082. [Google Scholar] [CrossRef] [PubMed]
- Bess, S.; Schwab, F.; Lafage, V.; Shaffrey, C.I.; Ames, C.P. Classifications for adult spinal deformity and use of the scoliosis research society -schwab adult spinal deformity classification. Neurosurg. Clin. N. Am. 2013, 24, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Miyakoshi, N.; Itoi, E.; Kobayashi, M.; Kodama, H. Impact of postural deformities and spinal mobility on quality of life in postmenopausal osteoporosis. Osteoporos Int. 2003, 14, 1007–1012. [Google Scholar] [CrossRef] [PubMed]
- Bess, S.; Protopsaltis, T.S.; Lafage, V.; Lafage, R.; Ames, C.P.; Errico, T.; Smith, J. Clinical and Radiographic Evaluation of Adult Spinal Deformity [Internet]. 2015. Available online: www.clinicalspinesurgery.com (accessed on 12 July 2022).
- Glassman, S.D.; Berven, S.; Bridwell, K.; Horton, W.; Dimar, J.R. Correlation of radiographic parameters and clinical symptoms in adult scoliosis. Spine 2005, 30, 682–688. [Google Scholar] [CrossRef] [Green Version]
- Barrey, C.; Roussouly, P.; Perrin, G. Sagittal balance disorders in severe degenerative spine. Can we identify the compensatory mechanisms? Eur. Spine J. 2011, 20, 626–633. [Google Scholar] [CrossRef] [Green Version]
- Roussouly, P.; Huec, J. Compensatory mechanisms contributing to keep the sagittal balance of the spine. Eur. Spine J. 2013, 22, 834–841. [Google Scholar]
- Yagi, M.; Takeda, K.; Machida, M.; Asazuma, T. Discordance of gravity line and C7PL in patient with adult spinal deformity-Factors affecting the occiput-trunk sagittal discordance. Spine J. 2015, 15, 213–221. [Google Scholar] [CrossRef]
- Day, L.M.; Ramchandran, S.; Jalai, C.M.; Diebo, B.G.; Liabaud, B.; Lafage, R.; Protopsaltis, T.; Passias, P.G.; Schwab, F.J.; Bess, S.; et al. Thoracolumbar realignment surgery results in simultaneous reciprocal changes in lower extremities and cervical spine. Spine 2017, 42, 799–807. [Google Scholar] [CrossRef]
- Schwab, F.J.; Lafage, V.; Shaffrey, C.I.; Smith, J.S.; Moal, B.; Klineberg, E.O.; Ames, C.P.; Hostin, R.; Fu, K.-M.G.; Kebaish, K.M.; et al. The SRS-schwab adult spinal deformity classification: Assessment and clinical correlations based on a prospective operative and nonoperative cohort. Neurosurgery 2013, 73, 559–568. [Google Scholar] [CrossRef]
- Diebo, B.G.; Shah, N.V.; Boachie-Adjei, O.; Zhu, F.; Rothenfluh, D.A.; Paulino, C.B.; Schwab, F.J.; Lafage, V. Adult spinal deformity. Lancet 2019, 394, 160–172. [Google Scholar] [CrossRef]
- Sparrey, C.J.; Bailey, J.F.; Safaee, M.; Clark, A.J.; Lafage, V.; Schwab, F.; Smith, J.S.; Ames, C.P. Etiology of lumbar lordosis and its pathophysiology: A review of the evolution of lumbar lordosis, and the mechanics and biology of lumbar degeneration. Neurosurg. Focus 2014, 36, E1. [Google Scholar] [CrossRef] [PubMed]
- Sugrue, P.A.; McClendon, J., Jr.; Smith, T.R.; Halpin, R.J.; Nasr, F.F.; O’Shaughnessy, B.A.; Koski, T.R. Redefining global spinal balance. Spine 2013, 38, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.A.; Scheer, J.K.; Smith, J.S.; Deviren, V.; Bess, S.; Hart, R.A.; Lafage, V.; Shaffrey, C.I.; Schwab, F.; Ames, C.P. The impact of standing regional cervical sagittal alignment on outcomes in posterior cervical fusion surgery. Neurosurgery 2012, 71, 662–669. [Google Scholar] [CrossRef] [PubMed]
- Schwab, F.; Lafage, V.; Boyce, R.; Skalli, W.; Farcy, J. Gravity line analysis in adult volunteers age-related correlation with spinal parameters, pelvic parameters, and foot position. Spine 2006, 31, 959–967. [Google Scholar] [CrossRef] [PubMed]
- Lafage, V.; Schwab, F.; Skalli, W.; Hawkinson, N.; Gagey, P.-M.; Ondra, S.; Farcy, J.-P. Standing balance and sagittal plane spinal deformity. Spine 2008, 33, 1572–1578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polga, D.J.; Beaubien, B.P.; Kallemeier, P.M.; Schellhas, K.P.; Lew, W.D.; Buttermann, G.R.; Wood, K.B. Measurement of in vivo intradiscal pressure in healthy thoracic intervertebral discs. Spine 2004, 29, 1320–1324. [Google Scholar] [CrossRef]
- Schultz, A.; Andersson, G.; Ortengren, R.; Haderspeck, K.; Nachemson, A. Loads on the lumbar spine. Validation of a biomechanical analysis by measurements of intradiscal pressures and myoelectric signals. J. Bone Jt. Surg. Am. 1982, 64, 713–720. [Google Scholar] [CrossRef]
- Nachemson, A.; Elfström, G. Intravital dynamic pressure measurements in lumbar discs. A study of common movements, maneuvers and exercises. Scand. J. Rehabil. Med. Suppl. 1970, 1, 1–40. [Google Scholar]
- Takahashi, I.; Kikuchi, S.I.; Sato, K.; Sato, N. Mechanical load of the lumbar spine during forward bending motion of the trunk-a biomechanical study. Spine 2006, 31, 18–23. [Google Scholar] [CrossRef]
- Bayoglu, R.; Galibarov, P.E.; Verdonschot, N.; Koopman, B.; Homminga, J. Twente spine model: A thorough investigation of the spinal loads in a complete and coherent musculoskeletal model of the human spine. Med. Eng. Phys. 2019, 68, 35–45. [Google Scholar] [CrossRef]
- Bayoglu, R.; Geeraedts, L.; Groenen, K.H.J.; Verdonschot, N.; Koopman, B.; Homminga, J. Twente spine model: A complete and coherent dataset for musculo-skeletal modeling of the thoracic and cervical regions of the human spine. J. Biomech. 2017, 58, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Arshad, R.; Schmidt, H.; El-Rich, M.; Moglo, K. Sensitivity of the cervical disc loads, translations, intradiscal pressure, and muscle activity due to segmental mass, disc stiffness, and muscle strength in an upright neutral posture. Front. Bioeng. Biotechnol. 2022, 27, 10. [Google Scholar] [CrossRef] [PubMed]
- Bassani, T.; Stucovitz, E.; Qian, Z.; Briguglio, M.; Galbusera, F. Validation of the AnyBody full body musculoskeletal model in computing lumbar spine loads at L4L5 level. J. Biomech. 2017, 58, 89–96. [Google Scholar] [CrossRef] [PubMed]
- De Zee, M.; Falla, D.; Farina, D.; Rasmussen, J. A detailed rigid-body cervical spine model based on inverse dynamics. J. Biomech. 2007, 40, S284. [Google Scholar] [CrossRef]
- Ignasiak, D.; Dendorfer, S.; Ferguson, S.J. Thoracolumbar spine model with articulated ribcage for the prediction of dynamic spinal loading. J. Biomech. 2016, 49, 959–966. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, R.; Komatsu, A.; Iida, J.; Iwami, T.; Shimada, Y. Construction and validation under dynamic conditions of a novel thoracolumbar spine model with defined muscle paths using the wrapping method. J. Biomech. Sci. Eng. 2019, 14, 18-00432. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, K.; Komatsu, A.; Hatakeyama, K.; Iida, J.; Iwami, T.; Shimada, Y. Validation of the hip contact force for a novel lower extremity muscloskeletal model. In Proceedings of the 2019 International Symposium on Micro-NanoMechatronics and Human Science (MHS), Nagoya, Japan, 1–4 December 2019; IEEE: Piscataway, NJ, USA, 2019; pp. 1–5. Available online: https://ieeexplore.ieee.org/document/9249302/ (accessed on 23 November 2020).
- Iida, J.; Miyakoshi, N.; Hongo, M.; Iwami, T.; Higuchi, R.; Komatsu, A.; Matsunaga, T.; Shimada, Y. Calculation of intervertebral disc pressure in the thoracic and lumbar spine in elderly women with kyphosis using a novel musculoskeletal model with isolated thoracic vertebrae and rib cage. Open J. Orthop. 2019, 9, 241–253. [Google Scholar] [CrossRef] [Green Version]
- Bassani, T.; Casaroli, G.; Galbusera, F. Dependence of lumbar loads on spinopelvic sagittal alignment: An evaluation based on musculoskeletal modeling. PLoS ONE 2019, 14, e0207997. [Google Scholar] [CrossRef] [Green Version]
- Miura, T.; Miyakoshi, N.; Saito, K.; Kijima, H.; Iida, J.; Hatakeyama, K.; Suzuki, K.; Komatsu, A.; Iwami, T.; Matsunaga, T.; et al. Association between global sagittal malalignment and increasing hip joint contact force, analyzed by a novel musculoskeletal modeling system. PLoS ONE 2021, 16, e0259049. [Google Scholar] [CrossRef]
- Ota, M.; Neo, M.; Aoyama, T.; Ishizaki, T.; Fujibayashi, S.; Takemoto, M.; Nakayama, T.; Nakamura, T. Impact of the O-C2 angle on the oropharyngeal space in normal patients. Spine 2011, 36, E720–E726. [Google Scholar] [CrossRef]
- Ohara, A.; Miyamoto, K.; Naganawa, T.; Matsumoto, K.; Shimizu, K. Reliabilities of and correlations among five standard methods of assessing the sagittal alignment of the cervical spine. Spine 2006, 31, 2585–2591. [Google Scholar] [CrossRef] [PubMed]
- Ames, C.P.; Smith, J.S.; Eastlack, R.; Blaskiewicz, D.J.; Shaffrey, C.I.; Schwab, F.; Bess, S.; Kim, H.J.; Mundis, G.M.; Klineberg, E.; et al. Reliability assessment of a novel cervical spine deformity classification system. J. Neurosurg. Spine 2015, 23, 673–683. [Google Scholar] [CrossRef] [PubMed]
- Mizutani, J.; Verma, K.; Endo, K.; Ishii, K.; Abumi, K.; Yagi, M.; Hosogane, N.; Yang, J.; Tay, B.; Deviren, V.; et al. Global spinal alignment in cervical kyphotic deformity: The importance of head position and thoracolumbar alignment in the compensatory mechanism. Neurosurgery 2018, 82, 686–694. [Google Scholar] [CrossRef] [PubMed]
- Knott, P.T.; Mardjetko, S.M.; Techy, F. The use of the T1 sagittal angle in predicting overall sagittal balance of the spine. Spine J. 2010, 10, 994–998. [Google Scholar] [CrossRef] [PubMed]
- Huec, J.C.; Leijssen, P.; Duarte, M.; Aunoble, S. Thoracolumbar imbalance analysis for osteotomy planification using a new method: FBI technique. Eur. Spine J. 2011, 20, 669–680. [Google Scholar] [CrossRef] [Green Version]
- Hasegawa, K.; Okamoto, M.; Hatsushikano, S. Normative values of spino-pelvic sagittal alignment, balance, age, and health-related quality of life in a cohort of healthy adult subjects. Eur. Spine J. 2016, 25, 3675–3686. [Google Scholar] [CrossRef] [Green Version]
- Martini, M.L.; Neifert, S.N.; Chapman, E.K.; Mroz, T.E.; Rasouli, J.J. Cervical spine alignment in the sagittal axis: A review of the best validated measures in clinical practice. Glob. Spine J. 2021, 11, 1307–1312. [Google Scholar] [CrossRef]
- Schwab, F.; Patel, A.; Ungar, B.; Farcy, J.P.; Lafage, V. Adult spinal deformity—postoperative standing imbalance: How much can you tolerate? An overview of key parameters in assessing alignment and planning corrective surgery. Spine 2010, 35, 2224–2231. [Google Scholar] [CrossRef]
- Ergun, T.; Lakadamyali, H.; Şahin, M.Ş. The relation between sagittal morphology of the lumbosacral spine and the degree of lumbar intervertebral disc degeneration. Acta Orthop. Traumatol. Turc. 2010, 44, 293–299. [Google Scholar] [CrossRef]
- Barrey, C.; Jund, J.; Noseda, O.; Roussouly, P. Sagittal balance of the pelvis-spine complex and lumbar degenerative diseases. A comparative study about 85 cases. Eur. Spine J. 2007, 16, 1459–1467. [Google Scholar] [CrossRef] [Green Version]
- Sato, K.; Kikuchi, S.; Yonezawa, T. In vivo intradiscal pressure measurement in healthy individuals and in patients with ongoing back problems. Spine 1999, 24, 2468–2474. [Google Scholar] [CrossRef] [PubMed]
- Wilke, H.J.; Neef, P.; Caimi, M.; Hoogland, T.; Claes, L.E. New in vivo measurements of pressures in the intervertebral disc in daily life. Spine 1999, 24, 755–762. [Google Scholar] [CrossRef]
- Keller, T.S.; Colloca, C.J.; Harrison, D.E.; Harrison, D.D.; Janik, T.J. Influence of spine morphology on intervertebral disc loads and stresses in asymptomatic adults: Implications for the ideal spine. Spine J. 2005, 5, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Harrison, D.E.; Colloca, C.J.; Harrison, D.D.; Janik, T.J.; Haas, J.W.; Keller, T.S. Anterior thoracic posture increases thoracolumbar disc loading. Eur. Spine J. 2005, 14, 234–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruno, A.G.; Burkhart, K.; Allaire, B.; Anderson, D.E.; Bouxsein, M.L. Spinal loading patterns from biomechanical modeling explain the high incidence of vertebral fractures in the thoracolumbar region. J. Bone Miner. Res. 2017, 32, 1282–1290. [Google Scholar] [CrossRef] [Green Version]
- Bruno, A.G.; Anderson, D.E.; D’Agostino, J.; Bouxsein, M.L. The effect of thoracic kyphosis and sagittal plane alignment on vertebral compressive loading. J. Bone Miner. Res. 2012, 27, 2144–2151. [Google Scholar] [CrossRef] [Green Version]
- Dubousset, J. Three-Dimensional Analysis of the Scoliotic Deformity; Raven Press: New York, NY, USA, 1994; pp. 479–483. Available online: https://cir.nii.ac.jp/crid/1570572700207103744 (accessed on 20 June 2022).
- Glassman, S.D.; Bridwell, K.; Dimar, J.R.; Horton, W.; Berven, S.; Schwab, F. The impact of positive sagittal balance in adult spinal deformity. Spine 2005, 30, 2024–2029. [Google Scholar] [CrossRef]
- Hasegawa, K.; Okamoto, M.; Hatsushikano, S.; Shimoda, H.; Ono, M.; Homma, T.; Watanabe, K. Standing sagittal alignment of the whole axial skeleton with reference to the gravity line in humans. J. Anat. 2017, 230, 619–630. [Google Scholar] [CrossRef]
- Manoharan, S.R.; Joshi, D.; Owen, M.; Theiss, S.M.; Deinlein, D. Relationship of cervical sagittal vertical alignment after sagittal balance correction in adult spinal deformity: A retrospective radiographic study. Int. J. Spine Surg. 2018, 12, 269. [Google Scholar] [CrossRef]
- Weng, C.; Wang, J.; Tuchman, A.; Wang, J.; Fu, C.; Hsieh, P.C.; Buser, Z.; Wang, J.C. Influence of T1 slope on the cervical sagittal balance in degenerative cervical spine. Spine 2016, 41, 185–190. [Google Scholar] [CrossRef]
- Staub, B.N.; Lafage, R.; Kim, H.J.; Shaffrey, C.I.; Mundis, G.M.; Hostin, R.; Burton, D.; Lenke, L.; Gupta, M.C.; Ames, C.; et al. Cervical mismatch: The normative value of T1 slope minus cervical lordosis and its ability to predict ideal cervical lordosis. J. Neurosurg. Spine 2018, 30, 31–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.J.; Lenke, L.G.; Oshima, Y.; Chuntarapas, T.; Mesfin, A.; Hershman, S.; Fogelson, J.L.; Riew, K.D. Cervical lordosis actually increases with aging and progressive degeneration in spinal deformity patients. Spine Deform. 2014, 2, 410–414. [Google Scholar] [CrossRef]
- Fujimori, T.; Le, H.; Schairer, W.; Inoue, S.; Iwasaki, M.; Oda, T.; Hu, S.S. The relationship between cervical degeneration and global spinal alignment in patients with adult spinal deformity. Clin. Spine Surg. A Spine Publ. 2017, 30, E423–E429. [Google Scholar] [CrossRef] [PubMed]
- Schairer, W.W.; Carrer, A.; Lu, M.; Hu, S.S. The increased prevalence of cervical spondylosis in patients with adult thoracolumbar spinal deformity. J. Spinal Disord. Tech. 2014, 27, E305–E308. [Google Scholar] [CrossRef] [PubMed]
- Okada, E.; Matsumoto, M.; Ichihara, D.; Chiba, K.; Toyama, Y.; Fujiwara, H.; Momoshima, S.; Nishiwaki, Y.; Hashimoto, T.; Ogawa, J.; et al. Does the sagittal alignment of the cervical spine have an impact on disk degeneration? Minimum 10-year follow-up of asymptomatic volunteers. Eur. Spine J. 2009, 18, 1644–1651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, K.; Zhang, J.; Lai, J.; Liu, W.; Lyu, H.; Wu, Y.; Lin, Z.; Cao, Y. Correlation between cervical lordosis and cervical disc herniation in young patients with neck pain. Medicine 2019, 98, e16545. [Google Scholar] [CrossRef] [PubMed]






| Variable | Value |
|---|---|
| Total patients (n) | 14 |
| Age (years) | 78.8 ± 7.0 (68–92) |
| BMI (kg/m2) | 22.9 ± 4.3 (16.4–32.8) |
| O–C2 angle (°) | 12.3 ± 7.6 (0–28) |
| C2–C7 angle (°) | 21.2 ± 11.0 (6–42) |
| C2–C7 SVA (mm) | 21.0 ± 8.0 (7.6–38.7) |
| COG–SVA (mm) | 86.3 ± 55.6 (15–224.7) |
| T1 slope (°) | 29.4 ± 11.6 (12–58) |
| TK (°) | 36.4 ± 15.2 (12–67) |
| LL (°) | 38.2 ± 15.8 (15–69) |
| PT (°) | 24.5 ± 8.7 (12–47) |
| SS (°) | 27.8 ± 6.7 (13–38) |
| PI (°) | 52.9 ± 9.5 (30–65) |
| PI−LL (°) | 10.9 ± 19.5 (−23–41) |
| SVA (mm) | 66.1 ± 52.5 (4–162) |
| FOA (°) | 5.8 ± 3.0 (0–13) |
| KFA (°) | 8.5 ± 4.5 (0.3–18) |
| Variables | Low SVA Group (n = 7) | High SVA Group (n = 7) | p-Value |
|---|---|---|---|
| Age (years) | 74.0 ± 6.7 | 82.1 ± 6.0 | 0.046 * |
| BMI (kg/m2) | 25.4 ± 3.5 | 22.0 ± 3.8 | 0.142 |
| O–C2 angle (°) | 12.7 ± 7.9 | 12.8 ± 7.3 | 0.980 |
| C2–C7 angle (°) | 18.4 ± 12.3 | 21.3 ± 10.7 | 0.685 |
| C2–C7 SVA (mm) | 17.1 ± 8.1 | 24.7 ± 7.0 | 0.089 |
| COG–SVA (mm) | 45.5 ± 15.5 | 134.0 ± 47.4 | <0.001 * |
| T1 slope (°) | 24.2 ± 5.7 | 35.5 ± 13.7 | 0.070 |
| TK (°) | 33.7 ± 8.4 | 35.7 ± 19.0 | 0.820 |
| LL (°) | 50.6 ± 11.7 | 27.9 ± 12.5 | <0.001 * |
| PT (°) | 23.6 ± 7.0 | 24.7 ± 9.7 | 0.819 |
| SS (°) | 32.2 ± 4.5 | 24.6 ± 6.8 | 0.041 * |
| PI (°) | 55.9 ± 7.6 | 50.0 ± 10.9 | 0.338 |
| PI−LL (°) | 5.3 ± 13.0 | 15.0 ± 23.4 | 0.396 |
| SVA (mm) | 21.1 ± 13.7 | 109.9 ± 39.5 | 0.001 * |
| FOA (°) | 4.3 ± 3.1 | 6.7 ± 2.5 | 0.166 |
| KFA (°) | 7.4 ± 4.6 | 9.4 ± 4.6 | 0.463 |
| Cervical (%BW) | 23.9 ± 6.5 | 24.3 ± 7.9 | 0.912 |
| Upper thoracic (%BW) | 29.3 ± 4.5 | 33.6 ± 6.9 | 0.225 |
| Lower thoracic (%BW) | 70.7 ± 10.4 | 82.5 ± 9.3 | 0.067 |
| Lumbar (%BW) | 79.2 ± 8.9 | 132.8 ± 52.3 | 0.046 * |
| Variables | ||||||||
|---|---|---|---|---|---|---|---|---|
| r | p-Value | r | p-Value | r | p-Value | r | p-Value | |
| O–C2 angle | −0.152 | n.s. | 0.089 | n.s. | −0.149 | n.s. | 0.116 | n.s. |
| C2–C7 angle | −0.456 | n.s. | −0.203 | n.s. | −0.022 | n.s. | 0.247 | n.s. |
| C2–C7 SVA | −0.108 | n.s. | 0.305 | n.s. | 0.165 | n.s. | 0.451 | n.s. |
| COG–SVA | −0.121 | n.s. | 0.385 | n.s. | 0.280 | n.s. | 0.615 | 0.029 * |
| T1 slope | −0.589 | 0.034 * | 0.028 | n.s. | 0.245 | n.s. | 0.613 | 0.026 * |
| TK | −0.203 | n.s. | 0.090 | n.s. | 0.269 | n.s. | 0.176 | n.s. |
| LL | 0.007 | n.s. | −0.363 | n.s. | −0.416 | n.s. | −0.566 | 0.035 * |
| PT | −0.168 | n.s. | 0.289 | n.s. | 0.187 | n.s. | 0.119 | n.s. |
| SS | 0.304 | n.s. | 0.255 | n.s. | 0.092 | n.s. | −0.139 | n.s. |
| PI | −0.033 | n.s. | 0.426 | n.s. | 0.072 | n.s. | 0.028 | n.s. |
| PI−LL | −0.165 | n.s. | 0.455 | n.s. | 0.455 | n.s. | 0.332 | n.s. |
| SVA | −0.180 | n.s. | 0.317 | n.s. | 0.310 | n.s. | 0.612 | 0.020 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miura, T.; Hongo, M.; Kasukawa, Y.; Kijima, H.; Kudo, D.; Saito, K.; Kimura, R.; Iwami, T.; Miyakoshi, N. Relationship between Intervertebral Disc Compression Force and Sagittal Spinopelvic Lower Limb Alignment in Elderly Women in Standing Position with Patient-Specific Whole Body Musculoskeletal Model. Int. J. Environ. Res. Public Health 2022, 19, 16452. https://doi.org/10.3390/ijerph192416452
Miura T, Hongo M, Kasukawa Y, Kijima H, Kudo D, Saito K, Kimura R, Iwami T, Miyakoshi N. Relationship between Intervertebral Disc Compression Force and Sagittal Spinopelvic Lower Limb Alignment in Elderly Women in Standing Position with Patient-Specific Whole Body Musculoskeletal Model. International Journal of Environmental Research and Public Health. 2022; 19(24):16452. https://doi.org/10.3390/ijerph192416452
Chicago/Turabian StyleMiura, Takanori, Michio Hongo, Yuji Kasukawa, Hiroaki Kijima, Daisuke Kudo, Kimio Saito, Ryota Kimura, Takehiro Iwami, and Naohisa Miyakoshi. 2022. "Relationship between Intervertebral Disc Compression Force and Sagittal Spinopelvic Lower Limb Alignment in Elderly Women in Standing Position with Patient-Specific Whole Body Musculoskeletal Model" International Journal of Environmental Research and Public Health 19, no. 24: 16452. https://doi.org/10.3390/ijerph192416452





