Eye Movements in Mild Traumatic Brain Injury: Ocular Biomarkers
Abstract
Introduction
Methods of Literature Search
Ocular Biomarkers in mTBI
Saccades
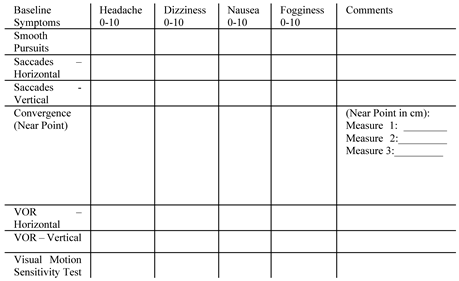
Clinical Measurement
Saccades and mTBI
Reflexive Saccade Tasks
Complex Saccade Tasks
Smooth Pursuit
Clinical Measurement
Smooth Pursuit and mTBI
Smooth Pursuit in ‘Postconcussion Syndrome’
Vestibulo-ocular reflex (VOR)
Clinical Measurement
VOR and mTBI
Vergence
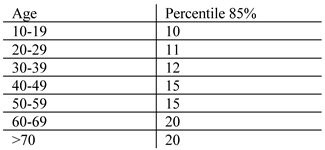
Clinical Measurement
Vergence and mTBI
Pupillary Light Reflex
Clinical Measurement
Pupillary Light Reflex and mTBI
Accommodation
Clinical Measurement
Accommodation and mTBI
Limitations
Summary
Conclusion
Ethics and Conflict of Interest
Acknowledgements
References
- Abraham, N. G., K. Srinivasan, and J. Thomas. 2015. Normative data for near point of convergence, accommodation, and phoria. Oman journal of ophthalmology 8, 1: 14–18. [Google Scholar] [PubMed]
- Ahn, B. Y., K. D. Choi, J. S. Kim, K. P. Park, J. H. Bae, and T. H. Lee. 2007. Impaired ipsilateral smooth pursuit and gaze-evoked nystagmus in paramedian pontine lesion. Neurology 68, 17: 1436. [Google Scholar] [CrossRef] [PubMed]
- Akhand, O., L. J. Balcer, and S. L. Galetta. 2019. Assessment of vision in concussion. Curr Opin Neurol 32, 1: 68–74. [Google Scholar] [CrossRef] [PubMed]
- Alabi, E. B., and T. L. Simpson. 2020. Pupil response to noxious corneal stimulation. PLoS One 15, 1: e0227771. [Google Scholar] [CrossRef]
- Alberta, P. 2014. Vestibular/Ocular-Motor Screening (VOMS) for Concussion. Retrieved April 15 from https://www.physiotherapyalberta.ca/files/vomstool.pdf.
- Alhilali, L. M., K. Yaeger, M. Collins, and S. Fakhran. 2014. Detection of central white matter injury underlying vestibulopathy after mild traumatic brain injury. Radiology 272, 1: 224–232. [Google Scholar] [CrossRef]
- Alkathiry, A. A., A. P. Kontos, J. M. Furman, S. L. Whitney, E. R. Anson, and P. J. Sparto. 2019. Vestibulo-Ocular Reflex Function in Adolescents With Sport-Related Concussion: Preliminary Results. Sports Health 11, 6: 479–485. [Google Scholar] [CrossRef]
- Alshehri, M. M., P. J. Sparto, J. M. Furman, S. Fedor, A. Mucha, L. C. Henry, and S. L. Whitney. 2016. The usefulness of the video head impulse test in children and adults postconcussion. J Vestib Res 26, 5-6: 439–446. [Google Scholar] [CrossRef] [PubMed]
- Antoniades, C., U. Ettinger, B. Gaymard, I. Gilchrist, A. Kristjánsson, C. Kennard, R. John Leigh, I. Noorani, P. Pouget, N. Smyrnis, A. Tarnowski, D. S. Zee, and R. H. S. Carpenter. 2013. An internationally standardised antisaccade protocol. Vision Research 84: 1–5. [Google Scholar] [CrossRef]
- Astafiev, S. V., G. L. Shulman, N. V. Metcalf, J. Rengachary, C. L. MacDonald, D. L. Harrington, J. Maruta, J. S. Shimony, J. Ghajar, M. Diwakar, M.-X. Huang, R. R. Lee, and M. Corbetta. 2015. Abnormal White Matter Blood-Oxygen-Level-Dependent Signals in Chronic Mild Traumatic Brain Injury. Journal of Neurotrauma 32, 16: 1254–1271. [Google Scholar] [CrossRef]
- Aston-Jones, G., and J. D. Cohen. 2005. An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu. Rev. Neurosci. 28: 403–450. [Google Scholar] [CrossRef]
- Ayton, L. N., L. A. Abel, T. R. Fricke, and N. A. McBrien. 2008. Does the Developmental Eye Movement (DEM) Test Actually Measure Eye Movements? Validation of a Clinical Saccade Test. Invest Ophthalmol Vis Sci 49, 13: 18101810. [Google Scholar]
- Babicz, M. A., S. P. Woods, P. Cirino, C. Presley, Z. Colton, and K. Podell. 2020. Vestibular/Ocular Motor Screening is Independently Associated With Concussion Symptom Severity in Youths. Clin J Sport Med. [Google Scholar] [CrossRef]
- Bahrami, N., D. Sharma, S. Rosenthal, E. M. Davenport, J. E. Urban, B. Wagner, Y. Jung, C. G. Vaughan, G. A. Gioia, J. D. Stitzel, C. T. Whitlow, and J. A. Maldjian. 2016. Subconcussive Head Impact Exposure and White Matter Tract Changes over a Single Season of Youth Football. Radiology 281, 3: 919–926. [Google Scholar] [CrossRef]
- Baker, J. F., B. M. Devitt, J. Green, and C. McCarthy. 2013. Concussion among under 20 rugby union players in Ireland: incidence, attitudes and knowledge. Ir J Med Sci 182, 1: 121–125. [Google Scholar] [CrossRef]
- Balaban, C., M. E. Hoffer, M. Szczupak, H. Snapp, J. Crawford, S. Murphy, K. Marshall, C. Pelusso, S. Knowles, and A. Kiderman. 2016. Oculomotor, Vestibular, and Reaction Time Tests in Mild Traumatic Brain Injury. PLoS One 11, 9: e0162168–e0162168. [Google Scholar] [CrossRef]
- Barnes, G. R. 2008. Cognitive processes involved in smooth pursuit eye movements. Brain and Cognition 68, 3: 309–326. [Google Scholar] [CrossRef] [PubMed]
- Barnes, G. R., and P. T. Asselman. 1991. The mechanism of prediction in human smooth pursuit eye movements. The Journal of physiology 439: 439–461. [Google Scholar] [CrossRef]
- Beatty, J. 1982. Task-evoked pupillary responses, processing load, and the structure of processing resources. Psychological bulletin 91, 2: 276. [Google Scholar] [CrossRef] [PubMed]
- Bell, S. L., F. Barker, H. Heselton, E. MacKenzie, D. Dewhurst, and A. Sanderson. 2015. A study of the relationship between the video head impulse test and air calorics. Eur Arch Otorhinolaryngol 272, 5: 1287–1294. [Google Scholar] [CrossRef]
- Belliveau, A. P., A. N. Somani, and R. H. Dossani. 2022. Pupillary Light Reflex. In StatPearls. StatPearls Publishing LLC. [Google Scholar]
- Berman, J. M., and J. M. Fredrickson. 1978. Vertigo after head injury—A five year follow-up. The Journal of otolaryngology 7, 3: 237–245. http://europepmc.org/abstract/MED/151151.
- Bertrand, A. L., J. B. Garcia, E. B. Viera, A. M. Santos, and R. H. Bertrand. 2013. Pupillometry: the influence of gender and anxiety on the pain response. Pain Physician 16, 3: E257–266. [Google Scholar]
- Bin Zahid, A., M. E. Hubbard, J. Lockyer, O. Podolak, V. M. Dammavalam, M. Grady, M. Nance, M. Scheiman, U. Samadani, and C. L. Master. 2018. Eye Tracking as a Biomarker for Concussion in Children. Clin J Sport Med. [Google Scholar] [CrossRef]
- Binda, P., M. Pereverzeva, and S. O. Murray. 2013. Attention to Bright Surfaces Enhances the Pupillary Light Reflex. The Journal of Neuroscience 33, 5: 2199. [Google Scholar] [CrossRef]
- Brahm, K. D., H. M. Wilgenburg, J. Kirby, S. Ingalla, C. Y. Chang, and G. L. Goodrich. 2009. Visual impairment and dysfunction in combat-injured servicemembers with traumatic brain injury [Article]. Optometry and Vision Science 86, 7: 817–825. [Google Scholar] [CrossRef] [PubMed]
- Cameron, I. G. M., J. M. Riddle, and M. D’Esposito. 2015. Dissociable Roles of Dorsolateral Prefrontal Cortex and Frontal Eye Fields During Saccadic Eye Movements [Original Research]. Frontiers in human neuroscience 9. [Google Scholar] [CrossRef]
- Capó-Aponte, J., T. Urosevich, D. Walsh, L. Temme, and A. Tarbett. 2013. Pupillary light reflex as an objective biomarker for early identification of blast-induced mTBI. J Spine S 4, 2: 1–4. [Google Scholar]
- Capó-Aponte, J. E., K. L. Jorgensen-Wagers, J. A. Sosa, D. V. Walsh, G. L. Goodrich, L. A. Temme, and D. W. Riggs. 2017. Visual Dysfunctions at Different Stages after Blast and Non-blast Mild Traumatic Brain Injury. Optometry and Vision Science 94, 1. https://journals.lww.com/optvissci/Fulltext/2017/01000/Visual_Dysfunctions_at_Different_Stages_after.4.aspx. [Google Scholar] [CrossRef]
- Capó-Aponte, J. E., T. G. Urosevich, L. A. Temme, A. K. Tarbett, and N. K. Sanghera. 2012. Visual Dysfunctions and Symptoms During the Subacute Stage of Blast-Induced Mild Traumatic Brain Injury. Military medicine 177, 7: 804–813. [Google Scholar] [CrossRef] [PubMed]
- Carroll, L. J., J. D. Cassidy, L. Holm, J. Kraus, and V. G. Coronado. 2004. Methodological issues and research recommendations for mild traumatic brain injury: the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J Rehabil Med, 113–125. [Google Scholar] [CrossRef]
- Champagne, A. A., E. Peponoulas, I. Terem, A. Ross, M. Tayebi, Y. Chen, N. S. Coverdale, P. M. F. Nielsen, A. Wang, V. Shim, S. J. Holdsworth, and D. J. Cook. 2019. Novel strain analysis informs about injury susceptibility of the corpus callosum to repeated impacts. Brain Communications 1, 1. [Google Scholar] [CrossRef]
- Büttner-Ennever, J. A. 2006. The extraocular motor nuclei: organization and functional neuroanatomy. Prog Brain Res 151: 95–125. [Google Scholar]
- Chan, R. V. P., and J. D. Trobe. 2002. Spasm of Accommodation Associated with Closed Head Trauma. Journal of Neuro-Ophthalmology 22, 1. https://journals.lww.com/jneuroophthalmology/Fulltext/2002/03000/Spasm_of_Accommodation_Associated_with_Closed_Head.5.aspx. [Google Scholar] [CrossRef]
- Cifu, D. X., J. R. Wares, K. W. Hoke, P. A. Wetzel, G. Gitchel, and W. Carne. 2015. Differential eye movements in mild traumatic brain injury versus normal controls. J Head Trauma Rehabil 30, 1: 21–28. [Google Scholar] [CrossRef]
- Ciuffreda, K. J., N. Kapoor, D. Rutner, I. B. Suchoff, M. E. Han, and S. Craig. 2007. Occurrence of oculomotor dysfunctions in acquired brain injury: A retrospective analysis. Optometry Journal of the American Optometric Association 78, 4: 155–161. [Google Scholar] [CrossRef] [PubMed]
- Contreras, R., J. Ghajar, S. Bahar, and M. Suh. 2011. Effect of cognitive load on eye-target synchronization during smooth pursuit eye movement. Brain Res 1398: 55–63. [Google Scholar] [CrossRef]
- Convergence Insufficiency Treatment Trial Study, G. 2008. The convergence insufficiency treatment trial: design, methods, and baseline data. Ophthalmic epidemiology 15, 1: 24–36. [Google Scholar] [CrossRef]
- Cortez, M. M., N. A. Rea, L. A. Hunter, K. B. Digre, and K. Brennan. 2017. Altered pupillary light response scales with disease severity in migrainous photophobia. Cephalalgia 37, 8: 801–811. [Google Scholar] [CrossRef] [PubMed]
- Clark, J. J. 1999. Spatial attention and latencies of saccadic eye movements. Vision Research 39, 3: 585–602. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Crampton, A., E. Teel, M. Chevignard, and I. Gagnon. 2021. Vestibular-ocular reflex dysfunction following mild traumatic brain injury: A narrative review. Neurochirurgie. [Google Scholar] [CrossRef]
- Cochrane, G. D., J. B. Christy, A. Almutairi, C. Busettini, M. W. Swanson, and K. K. Weise. 2019. Visuo-oculomotor Function and Reaction Times in Athletes with and without Concussion. Optometry and vision science: official publication of the American Academy of Optometry 96, 4: 256–265. [Google Scholar] [CrossRef] [PubMed]
- Cochrane, G. D., J. B. Christy, and R. W. Motl. 2021. Comprehensive Clinical Assessment of Vestibular Function in Multiple Sclerosis. J Neurol Phys Ther 45, 3: 228–234. [Google Scholar] [CrossRef]
- Cohen, M., Z. Groswasser, R. Barchadski, and A. Appel. 1989. Convergence insufficiency in braininjured patients. Brain Injury 3, 2: 187–191. [Google Scholar] [CrossRef]
- Danna-Dos-Santos, A., S. Mohapatra, M. Santos, and A. M. Degani. 2018. Long-term effects of mild traumatic brain injuries to oculomotor tracking performances and reaction times to simple environmental stimuli. Scientific Reports 8, 1: 4583. [Google Scholar] [CrossRef]
- Das, V. E. 2011. Cells in the supraoculomotor area in monkeys with strabismus show activity related to the strabismus angle. Annals of the New York Academy of Sciences 1233: 85–90. [Google Scholar] [CrossRef]
- Dias, E. C., and M. A. Segraves. 1999. Muscimolinduced inactivation of monkey frontal eye field: effects on visually and memory-guided saccades. J Neurophysiol 81, 5: 2191–2214. [Google Scholar] [CrossRef] [PubMed]
- Colebatch, J. G., and J. C. Rothwell. 2004. Motor unit excitability changes mediating vestibulocollic reflexes in the sternocleidomastoid muscle. Clin Neurophysiol 115, 11: 2567–2573. [Google Scholar] [CrossRef]
- DiCesare, C. A., A. W. Kiefer, P. Nalepka, and G. D. Myer. 2017. Quantification and analysis of saccadic and smooth pursuit eye movements and fixations to detect oculomotor deficits. Behav Res Methods 49, 1: 258–266. [Google Scholar] [CrossRef] [PubMed]
- Diwakar, M., D. L. Harrington, J. Maruta, J. Ghajar, F. ElGabalawy, L. Muzzatti, M. Corbetta, M. X. Huang, and R. R. Lee. 2015. Filling in the gaps: Anticipatory control of eye movements in chronic mild traumatic brain injury. Neuroimage Clin 8: 210–223. [Google Scholar] [CrossRef] [PubMed]
- Duane, A. 1922. Studies in monocular and binocular accommodation, with their clinical application. Transactions of the American Ophthalmological Society 20: 132. [Google Scholar] [CrossRef]
- Edinger, L. 1885. Über den Verlauf der centralen Hirnnervenbahnen mit Demonstrationen von Präparaten. Arch Psychiat Nervenkr 16: 858–859. [Google Scholar]
- Einhäuser, W., J. Stout, C. Koch, and O. Carter. 2008. Pupil dilation reflects perceptual selection and predicts subsequent stability in perceptual rivalry. Proceedings of the National Academy of Sciences 105, 5: 1704. [Google Scholar] [CrossRef]
- Ellis, M. J., D. Cordingley, S. Vis, K. Reimer, J. Leiter, and K. Russell. 2015. Vestibulo-ocular dysfunction in pediatric sports-related concussion. J Neurosurg Pediatr 16, 3: 248–255. [Google Scholar] [CrossRef]
- Evans, K., Tamara Espinoza, Brian Geary, Kristopher Hendershot, Nicole Kosoris, Michelle LaPlaca, and et al. 2016. Combining Visual Target Tracking and Time Estimation to Assess Vestibular and Oculomotor Function after Concussion. Medicine & Science in Sports & Exercise 48: 629–630. [Google Scholar]
- Fife, T. D., R. J. Tusa, J. M. Furman, D. S. Zee, E. Frohman, R. W. Baloh, T. Hain, J. Goebel, J. Demer, and L. Eviatar. 2000. Assessment: Vestibular testing techniques in adults and children. Neurology 55, 10: 1431. [Google Scholar]
- Galetta, K. M., J. Barrett, M. Allen, F. Madda, D. Delicata, A. T. Tennant, C. C. Branas, M. G. Maguire, L. V. Messner, S. Devick, S. L. Galetta, and L. J. Balcer. 2011. The King-Devick test as a determinant of head trauma and concussion in boxers and MMA fighters. Neurology 76, 17: 1456–1462. [Google Scholar] [CrossRef] [PubMed]
- Gall, R. W. B., H. Bedell, and P. Pease. 1995. Vergence facility: establishment of clinical norms. American Academy of Optometry(Conference Abstract). https://www.aaopt.org/detail/knowledge-basearticle/vergence-facility-establishment-clinicalnorms.
- Gallaway, M., M. Scheiman, and G. L. Mitchell. 2017. Vision Therapy for Post-Concussion Vision Disorders. Optometry and Vision Science 94, 1. https://journals.lww.com/optvissci/Fulltext/2017/01000/Vision_Therapy_for_Post_Concussion_Vision.11.aspx. [Google Scholar] [CrossRef] [PubMed]
- Gamlin, P. D. 1999. Subcortical neural circuits for ocular accommodation and vergence in primates. Ophthalmic and Physiological Optics 19, 2: 81–89. [Google Scholar] [CrossRef] [PubMed]
- Gamlin, P. D. 2002. Neural mechanisms for the control of vergence eye movements. Ann N Y Acad Sci 956: 264–272. [Google Scholar] [CrossRef]
- Gannon, R. P., G. N. Willson, M. E. Roberts, and H. J. Pearse. 1978. Auditory and Vestibular Damage in Head Injuries at Work. Archives of Otolaryngology 104, 7: 404–408. [Google Scholar] [CrossRef]
- Gattu, R., F. W. Akin, A. T. Cacace, C. D. Hall, O. D. Murnane, and E. M. Haacke. 2016. Vestibular, balance, microvascular and white matter neuroimaging characteristics of blast injuries and mild traumatic brain injury: Four case reports. Brain Inj 30, 12: 1501–1514. [Google Scholar] [CrossRef]
- Gilmartin, B. 1998. Autonomic correlates of near-vision in emmetropia and myopia. Myopia and nearwork, 117–146. [Google Scholar]
- Green, W., K. J. Ciuffreda, P. Thiagarajan, D. SzymanoWicz, D. P. Ludlam, and N. Kapoor. 2010. Accommodation in mild traumatic brain injury. Journal of rehabilitation research and development 47, 3: 183–200. [Google Scholar] [CrossRef]
- Green, W., K. J. Ciuffreda, P. Thiagarajan, D. Szymanowicz, D. P. Ludlam, and N. Kapoor. 2010. Static and dynamic aspects of accommodation in mild traumatic brain injury: A review. Optometry Journal of the American Optometric Association 81, 3: 129–136. [Google Scholar] [CrossRef]
- Group, B. D. W., A. J. Atkinson, Jr., W. A. Colburn, V. G. DeGruttola, D. L. DeMets, G. J. Downing, D. F. Hoth, J. A. Oates, C. C. Peck, and R. T. Schooley. 2001. Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clinical pharmacology & therapeutics 69, 3: 89–95. [Google Scholar]
- Group, C. I. T. T. S. Procedures Manual. Retrieved July 4, 2021 from http://publicfiles.jaeb.org/pedig/manuals/CITS_Procedures_Manual_v_5_0_03_04_14.pdf.
- Group, C. I. T. T. S. 2008. The convergence insufficiency treatment trial: design, methods, and baseline data. Ophthalmic epidemiology 15, 1: 24–36. [Google Scholar]
- Halmagyi, G. M., I. S. Curthoys, P. D. Cremer, C. J. Henderson, M. J. Todd, M. J. Staples, and D. M. D'Cruz. 1990. The human horizontal vestibulo-ocular reflex in response to highacceleration stimulation before and after unilateral vestibular neurectomy. Exp Brain Res 81, 3: 479–490. [Google Scholar] [CrossRef] [PubMed]
- Hassan, L. I., S. M. Ibrahim, M. Abdu, and A. MohamedSharif. 2018. Prevalence of convergence insufficiency among secondary school students in Khartoum, Sudan. Oman journal of ophthalmology 11, 2: 129–133. [Google Scholar] [CrossRef]
- Heitger, M. H., T. J. Anderson, and R. D. Jones. 2002. Saccade sequences as markers for cerebral dysfunction following mild closed head injury. Prog Brain Res 140: 433–448. [Google Scholar]
- Heitger, M. H., T. J. Anderson, R. D. Jones, J. C. DalrympleAlford, C. M. Frampton, and M. W. Ardagh. 2004. Eye movement and visuomotor arm movement deficits following mild closed head injury. Brain 127, Pt 3: 575–590. [Google Scholar] [CrossRef]
- Heitger, M. H., R. D. Jones, and T. J. Anderson. 2008. A new approach to predicting postconcussion syndrome after mild traumatic brain injury based upon eye movement function. Conf Proc IEEE Eng Med Biol Soc 2008: 3570–3573. [Google Scholar]
- Heitger, M. H., R. D. Jones, J. C. Dalrymple-Alford, C. M. Frampton, M. W. Ardagh, and T. J. Anderson. 2006. Motor deficits and recovery during the first year following mild closed head injury. Brain Inj 20, 8: 807–824. [Google Scholar] [CrossRef]
- Heitger, M. H., R. D. Jones, J. C. Dalrymple-Alford, C. M. Frampton, M. W. Ardagh, and T. J. Anderson. 2007. Mild head injury--a close relationship between motor function at 1 week post-injury and overall recovery at 3 and 6 months. J Neurol Sci 253, 1-2: 34–47. [Google Scholar] [CrossRef] [PubMed]
- Heitger, M. H., R. D. Jones, A. D. Macleod, D. L. Snell, C. M. Frampton, and T. J. Anderson. 2009. Impaired eye movements in post-concussion syndrome indicate suboptimal brain function beyond the influence of depression, malingering or intellectual ability. Brain 132, 10: 2850–2870. [Google Scholar] [CrossRef]
- Hoffer, M. E., C. Balaban, M. Szczupak, J. Buskirk, H. Snapp, J. Crawford, S. Wise, S. Murphy, K. Marshall, C. Pelusso, S. Knowles, and A. Kiderman. 2017. The use of oculomotor, vestibular, and reaction time tests to assess mild traumatic brain injury (mTBI) over time. Laryngoscope Investigative Otolaryngology 2, 4: 157–165. [Google Scholar] [CrossRef]
- Hon, K. L., A. K. C. Leung, and A. R. Torres. 2019. Concussion: A Global Perspective. Seminars in Pediatric Neurology 30: 117–127. [Google Scholar] [CrossRef]
- Honce, J. M., E. Nyberg, I. Jones, and L. Nagae. 2016. Neuroimaging of Concussion. Phys Med Rehabil Clin N Am 27, 2: 411–428. [Google Scholar] [CrossRef] [PubMed]
- Hughes, F. E., M. P. Treacy, E. S. Duignan, and P. B. Mullaney. 2017. Persistent pseudomyopia following a whiplash injury in a previously emmetropic woman. American journal of ophthalmology case reports 8: 28–30. [Google Scholar] [CrossRef] [PubMed]
- Johnson, B., M. Hallett, and S. Slobounov. 2015. Follow-up evaluation of oculomotor performance with fMRI in the subacute phase of concussion. Neurology 85, 13: 1163–1166. [Google Scholar] [CrossRef]
- Johnson, B., K. Zhang, M. Hallett, and S. Slobounov. 2015. Functional neuroimaging of acute oculomotor deficits in concussed athletes. Brain Imaging Behav 9, 3: 564–573. [Google Scholar] [CrossRef] [PubMed]
- Hunfalvay, M., C.-M. Roberts, N. Murray, A. Tyagi, H. Kelly, and T. Bolte. 2019. Horizontal and vertical self-paced saccades as a diagnostic marker of traumatic brain injury. Concussion (London, England) 4, 1: CNC60–CNC60. [Google Scholar] [CrossRef]
- Kaifie, A., M. Reugels, T. Kraus, and M. Kursawe. 2021. The pupillary light reflex (PLR) as a marker for the ability to work or drive–a feasibility study. Journal of Occupational Medicine and Toxicology 16, 1: 39. [Google Scholar] [CrossRef]
- Ikeda, N., T. Ikeda, and T. Kohno. 2016. Traumatic myopia secondary to ciliary spasm after blunt eye trauma and reconsideration of its pathogenesis. Graefes Arch Clin Exp Ophthalmol 254, 7: 1411–1417. [Google Scholar] [CrossRef]
- Ikeda, N., T. Ikeda, M. Nagata, and O. Mimura. 2002. Pathogenesis of transient high myopia after blunt eye trauma. Ophthalmology 109, 3: 501–507. [Google Scholar] [CrossRef]
- Jacob, R. J., S. S. Jennylee, W. Kylene, A. A. Andrea, T. L. Matthew, K. F. Robert, E. O. Mark, J. S. Nicholas, and P. B. Steven. 2019. Pupillary changes after clinically asymptomatic highacceleration head impacts in high school football athletes. Journal of Neurosurgery JNS, 1–6. [Google Scholar]
- Jang, I., I. Y. Chun, J. R. Brosch, S. Bari, Y. Zou, B. R. Cummiskey, T. A. Lee, R. J. Lycke, V. N. Poole, T. E. Shenk, D. O. Svaldi, G. G. Tamer, Jr., U. Dydak, L. J. Leverenz, E. A. Nauman, and T. M. Talavage. 2019. Every hit matters: White matter diffusivity changes in high school football athletes are correlated with repetitive head acceleration event exposure. Neuroimage. Clinical 24: 101930–101930. [Google Scholar] [CrossRef]
- Kardon, R., N. Miller, N. Newman, V. Biousse, and J. Kerrison. 2005. Walsh and Hoyt's Clinical Neuro-ophthalmology. Anatomy and physiology of the autonomic nervous system 6: 649–712. [Google Scholar]
- Kelly, K. M., A. Kiderman, S. Akhavan, M. R. Quigley, E. D. Snell, E. Happ, A. S. Synowiec, E. R. Miller, M. A. Bauer, L. P. Oakes, Y. Eydelman, C. W. Gallagher, T. Dinehart, J. H. Schroeder, and R. C. Ashmore. 2019. Oculomotor, Vestibular, and Reaction Time Effects of Sports-Related Concussion: VideoOculography in Assessing Sports-Related Concussion. J Head Trauma Rehabil 34, 3: 176–188. [Google Scholar] [CrossRef]
- Kelly, M. 2017. Technical Report of the Use of a Novel Eye Tracking System to Measure Impairment Associated with Mild Traumatic Brain Injury. Cureus 9, 5: e1251. [Google Scholar] [CrossRef] [PubMed]
- Kerr, A. G. 1980. The effects of blast on the ear. The Journal of Laryngology & Otology 94, 1: 107110. [Google Scholar]
- Kim, S. I., Y. J. Cha, and S. E. Park. 2008. A case report on the change of the refractive power after a blunt trauma. Korean Journal of Ophthalmology 22, 1: 53–57. [Google Scholar] [CrossRef] [PubMed]
- Knell, G., T. Caze, and S. O. Burkhart. 2021. Evaluation of the vestibular and ocular motor screening (VOMS) as a prognostic tool for protracted recovery following paediatric sports-related concussion. BMJ Open Sport & Exercise Medicine 7, 1: e000970. [Google Scholar] [CrossRef]
- Kongsgaard, U. E., and G. Høiseth. 2019. Dynamic assessment of the pupillary reflex in patients on high-dose opioids. Scandinavian Journal of Pain 19, 3: 465–471. [Google Scholar] [CrossRef]
- Koretz, J. F., A. M. Bertasso, M. W. Neider, B. A. TrueGabelt, and P. L. Kaufman. 1987. Slitlamp studies of the rhesus monkey eye: II. Changes in crystalline lens shape, thickness and position during accommodation and aging. Exp Eye Res 45, 2: 317–326. [Google Scholar] [CrossRef]
- Kowal, L. 1992. Ophthalmic manifestations of head injury. Australian and New Zealand Journal of Ophthalmology 20, 1: 35–40. [Google Scholar] [CrossRef]
- Laeng, B., and U. Sulutvedt. 2014. The eye pupil adjusts to imaginary light. Psychological science 25, 1: 188–197. [Google Scholar] [CrossRef]
- Landry, A. P., W. K. C. Ting, Z. Zador, A. Sadeghian, and M. D. Cusimano. 2019. Using artificial neural networks to identify patients with concussion and postconcussion syndrome based on antisaccades. Journal of Neurosurgery JNS 131, 4: 1235–1242. [Google Scholar] [CrossRef] [PubMed]
- Lee, A. G., and S. L. Galetta. 2016. Subconcussive Head Trauma and Near Point of Convergence. JAMA Ophthalmol 134, 7: 770–771. [Google Scholar] [CrossRef] [PubMed]
- Leube, A., K. Rifai, and K. Rifai. 2017. Sampling rate influences saccade detection in mobile eye tracking of a reading task. Journal of Eye Movement Research 10, 3. [Google Scholar] [CrossRef]
- Loewenfeld, I. E. 1958. Mechanisms of reflex dilatation of the pupil. Documenta Ophthalmologica 12, 1: 185–448. [Google Scholar]
- London, R., B. Wick, and D. Kirschen. 2003. Posttraumatic pseudomyopia. Optometry (St. Louis, Mo.) 74, 2: 111–120. [Google Scholar] [PubMed]
- Lynch, J. 1987. Frontal eye field lesions in monkeys disrupt visual pursuit. Experimental Brain Research 68, 2: 437–441. [Google Scholar] [CrossRef]
- Lynch, J. C., J. E. Hoover, and P. L. Strick. 1994. Input to the primate frontal eye field from the substantia nigra, superior colliculus, and dentate nucleus demonstrated by transneuronal transport. Exp Brain Res 100, 1: 181–186. [Google Scholar] [CrossRef]
- Maas, A. I. R., D. K. Menon, P. D. Adelson, N. Andelic, M. J. Bell, A. Belli, P. Bragge, A. Brazinova, A. Büki, R. M. Chesnut, G. Citerio, M. Coburn, D. J. Cooper, A. T. Crowder, E. Czeiter, M. Czosnyka, R. Diaz-Arrastia, J. P. Dreier, A. C. Duhaime, A. Ercole, and et al. 2017. Traumatic brain injury: Integrated appraoches to improve prevention, clinical care, and research. The Lancet Neurology 16: 9871048. [Google Scholar] [CrossRef]
- Magliulo, G., S. Gagliardi, M. C. Appiani, G. Iannella, and M. Re. 2014. Vestibular Neurolabyrinthitis: A Follow-Up Study With Cervical and Ocular Vestibular Evoked Myogenic Potentials and the Video Head Impulse Test. Annals of Otology, Rhinology & Laryngology 123, 3: 162–173. [Google Scholar]
- Magone, M. T., E. Kwon, and S. Y. Shin. 2014. Chronic visual dysfunction after blast-induced mild traumatic brain injury. Journal of rehabilitation research and development 51, 1: 71–80. [Google Scholar] [CrossRef]
- Maller, J. J., B. Lithgow, C. Gurvich, S. Haghgooie, O. R. Pouya, P. B. Fitzgerald, and J. Kulkarni. 2014. Separating mental disorders using vestibular field potentials. Archives of Neuroscience 2, 2: e19257. [Google Scholar] [CrossRef]
- Maruta, J., K. J. Heaton, A. L. Maule, and J. Ghajar. 2014. Predictive visual tracking: specificity in mild traumatic brain injury and sleep deprivation. Mil Med 179, 6: 619–625. [Google Scholar] [CrossRef] [PubMed]
- Maruta, J., E. M. Palacios, R. D. Zimmerman, J. Ghajar, and P. Mukherjee. 2016. Chronic PostConcussion Neurocognitive Deficits. I. Relationship with White Matter Integrity [Original Research]. Frontiers in human neuroscience 10, 35. [Google Scholar] [CrossRef] [PubMed]
- Maruta, J., L. A. Spielman, B. B. Yarusi, Y. Wang, J. M. Silver, and J. Ghajar. 2016. Chronic PostConcussion Neurocognitive Deficits. II. Relationship with Persistent Symptoms. Frontiers in human neuroscience 10: 45–45. [Google Scholar]
- Maruta, J., M. Suh, S. N. Niogi, P. Mukherjee, and J. Ghajar. 2010. Visual tracking synchronization as a metric for concussion screening. J Head Trauma Rehabil 25, 4: 293305. [Google Scholar] [CrossRef]
- Master, C. L., S. R. Master, D. J. Wiebe, E. P. Storey, J. E. Lockyer, O. E. Podolak, and M. F. Grady. 2018. Vision and vestibular system dysfunction predicts prolonged concussion recovery in children. Clinical Journal of Sport Medicine 28, 2: 139–145. [Google Scholar] [CrossRef]
- Master, C. L., M. Scheiman, M. Gallaway, A. Goodman, R. L. Robinson, S. R. Master, and M. F. Grady. 2015. Vision Diagnoses Are Common After Concussion in Adolescents. Clinical Pediatrics 55, 3: 260–267. [Google Scholar] [CrossRef]
- Mathôt, S., E. Dalmaijer, J. Grainger, and S. Van der Stigchel. 2014. The pupillary light response reflects exogenous attention and inhibition of return. Journal of Vision 14, 14: 7–7. [Google Scholar] [CrossRef]
- Mathôt, S., L. van der Linden, J. Grainger, and F. Vitu. 2013. The pupillary light response reveals the focus of covert visual attention. PLoS One 8, 10: e78168–e78168. [Google Scholar] [CrossRef]
- Mathôt, S., L. van der Linden, J. Grainger, and F. Vitu. 2015. The pupillary light response reflects eyemovement preparation. Journal of Experimental Psychology: Human Perception and Performance 41, 1: 28. [Google Scholar]
- Mathôt, S., and S. Van der Stigchel. 2015. New Light on the Mind’s Eye: The Pupillary Light Response as Active Vision. Current Directions in Psychological Science 24, 5: 374–378. [Google Scholar] [CrossRef] [PubMed]
- Matuseviciene, G., J. Johansson, M. Möller, A. K. Godbolt, T. Pansell, and C. N. Deboussard. 2018. Longitudinal changes in oculomotor function in young adults with mild traumatic brain injury in Sweden: an exploratory prospective observational study. BMJ Open 8, 2: e018734. [Google Scholar] [CrossRef] [PubMed]
- May, P. J., S. Warren, M. O. Bohlen, M. Barnerssoi, and A. K. E. Horn. 2016. A central mesencephalic reticular formation projection to the Edinger–Westphal nuclei. Brain Structure and Function 221, 8: 4073–4089. [Google Scholar] [CrossRef]
- Meehan, A., A. Lewandowski, L. K. Weaver, D. Hebert, and K. Deru. 2019. Prospective study of anxiety, post-traumatic stress and depression on postural control, gait, otolith and visuospatial function in military service members with persistent post-concussive symptoms. Undersea Hyperb Med 46, 3: 271–287. [Google Scholar]
- Meehan, W. P., 3rd, R. C. Mannix, M. J. O'Brien, and M. W. Collins. 2013. The prevalence of undiagnosed concussions in athletes. Clin J Sport Med 23, 5: 339–342. [Google Scholar] [CrossRef] [PubMed]
- Merezhinskaya, N., R. K. Mallia, D. Park, D. W. Bryden, K. Mathur, and F. M. Barker, II. 2019. Visual Deficits and Dysfunctions Associated with Traumatic Brain Injury: A Systematic Review and Meta-analysis. Optometry and Vision Science 96, 8. https://journals.lww.com/optvissci/Fulltext/2019/08000/Visual_Deficits_and_Dysfunctions_Associated_with.2.aspx. [Google Scholar] [CrossRef]
- Mezzalira, R., R. S. M. Bittar, M. M. do Carmo BiléckiStipsky, C. Brugnera, and S. S. Grasel. 2017. Sensitivity of caloric test and video head impulse as screening test for chronic vestibular complaints. Clinics (Sao Paulo, Brazil) 72, 8: 469–473. [Google Scholar] [CrossRef]
- Moschovakis, A. K. 1996. The superior colliculus and eye movement control. Curr Opin Neurobiol 6, 6: 811–816. [Google Scholar] [CrossRef]
- Mossman, B., S. Mossman, G. Purdie, and E. Schneider. 2015. Age dependent normal horizontal VOR gain of head impulse test as measured with video-oculography [Article]. Journal of Otolaryngology Head and Neck Surgery 44(July): Article 29. [Google Scholar] [CrossRef]
- Mucha, A., M. W. Collins, R. J. Elbin, J. M. Furman, C. Troutman-Enseki, R. M. DeWolf, G. Marchetti, and A. P. Kontos. 2014. A Brief Vestibular/Ocular Motor Screening (VOMS) assessment to evaluate concussions: preliminary findings. The American journal of sports medicine 42, 10: 2479–2486. [Google Scholar] [CrossRef]
- Murray, N. G., V. N. Ambati, M. M. Contreras, A. P. Salvatore, and R. J. Reed-Jones. 2014. Assessment of oculomotor control and balance post-concussion: a preliminary study for a novel approach to concussion management. Brain Inj 28, 4: 496–503. [Google Scholar] [CrossRef] [PubMed]
- Naber, M., G. A. Alvarez, and K. Nakayama. 2013. Tracking the allocation of attention using human pupillary oscillations. Frontiers in psychology 4: 919–919. [Google Scholar] [CrossRef] [PubMed]
- Naber, M., S. Frässle, and W. Einhäuser. 2011. Perceptual Rivalry: Reflexes Reveal the Gradual Nature of Visual Awareness. PLoS One 6, 6: e20910. [Google Scholar] [CrossRef] [PubMed]
- Nij Bijvank, J. A., A. Petzold, L. J. Balk, H. S. Tan, B. M. J. Uitdehaag, M. Theodorou, and L. J. van Rijn. 2018. A standardized protocol for quantification of saccadic eye movements: DEMoNS. PLoS One 13, 7: e0200695. [Google Scholar] [CrossRef]
- Nobre, A. C., D. R. Gitelman, E. C. Dias, and M. M. Mesulam. 2000. Covert Visual Spatial Orienting and Saccades: Overlapping Neural Systems. Neuroimage 11, 3: 210–216. [Google Scholar] [CrossRef]
- Ohtsuka, K., and T. Enoki. 1998. Transcranial magnetic stimulation over the posterior cerebellum during smooth pursuit eye movements in man. Brain 121, 3: 429–435. [Google Scholar] [CrossRef]
- Ostadimoghaddam, H., H. Hashemi, P. Nabovati, A. Yekta, and M. Khabazkhoob. 2017. The distribution of near point of convergence and its association with age, gender and refractive error: a population-based study. Clinical and Experimental Optometry 100, 3: 255–259. [Google Scholar] [CrossRef]
- Park, J. C., A. L. Moura, A. S. Raza, D. W. Rhee, R. H. Kardon, and D. C. Hood. 2011. Toward a Clinical Protocol for Assessing Rod, Cone, and Melanopsin Contributions to the Human Pupil Response. Invest Ophthalmol Vis Sci 52, 9: 6624–6635. [Google Scholar] [CrossRef]
- Murray, N. G., B. Szekely, A. Islas, B. Munkasy, R. Gore, M. Berryhill, and R. J. Reed-Jones. 2020. Smooth Pursuit and Saccades after SportRelated Concussion. J Neurotrauma 37, 2: 340–346. [Google Scholar] [CrossRef]
- Pearce, K. L., A. Sufrinko, B. C. Lau, L. Henry, M. W. Collins, and A. P. Kontos. 2015. Near Point of Convergence After a Sport-Related Concussion: Measurement Reliability and Relationship to Neurocognitive Impairment and Symptoms. The American journal of sports medicine 43, 12: 3055–3061. [Google Scholar] [CrossRef]
- Peiffer, A. J., J. MacDonald, D. Duerson, G. Mitchell, A. T. E. Hartwick, and C. E. McDaniel. 2020. The Influence of Binocular Vision Symptoms on Computerized Neurocognitive Testing of Adolescents With Concussion. Clinical Pediatrics 59, 11: 961–969. [Google Scholar] [CrossRef]
- Perez, N., and J. Rama-Lopez. 2003. Head-impulse and caloric tests in patients with dizziness. Otol Neurotol 24, 6: 913–917. [Google Scholar] [CrossRef] [PubMed]
- Phillipou, A., J. Douglas, D. Krieser, L. Ayton, and L. Abel. 2014. Changes in saccadic eye movement and memory function after mild closed head injury in children. Developmental Medicine & Child Neurology 56, 4: 337–345. [Google Scholar]
- Piquado, T., D. Isaacowitz, and A. Wingfield. 2010. Pupillometry as a measure of cognitive effort in younger and older adults. Psychophysiology 47, 3: 560–569. [Google Scholar] [CrossRef] [PubMed]
- Podolak, O. E., N. Joshi, K. Ciuffreda, F. Mohamed, S. Sharma, R. Kessler, C. McDonald, K. B. Arbogast, M. Grady, and C. L. Master. 2019. The utility of pupillary light reflex as an objective biomarker of acute concussion in the adolescent athlete. Orthopaedic journal of sports medicine 7, 3_suppl: 2325967119S2325900155. [Google Scholar] [CrossRef]
- Porcar, E., and A. Martinez-Palomera. 1997. Prevalence of general binocular dysfunctions in a population of university students. Optometry and vision science: official publication of the American Academy of Optometry 74, 2: 111–113. [Google Scholar] [CrossRef]
- Raghuram, A., S. A. Cotter, S. Gowrisankaran, J. Kanji, D. R. Howell, W. P. Meehan, and A. S. Shah. 2019. Postconcussion: Receded Near Point of Convergence is not Diagnostic of Convergence Insufficiency. American Journal of Ophthalmology 206: 235–244. [Google Scholar] [CrossRef]
- Rambold, H., A. Churchland, Y. Selig, L. Jasmin, and S. G. Lisberger. 2002. Partial ablations of the flocculus and ventral paraflocculus in monkeys cause linked deficits in smooth pursuit eye movements and adaptive modification of the VOR. J Neurophysiol 87, 2: 912–924. [Google Scholar] [CrossRef]
- Richard, B., A. Johnson, and D. Ellemberg. 2009. Persistent abnormalities in the control of eye movements following a sport-related concussion. Journal of Vision 9, 8: 423–423. [Google Scholar] [CrossRef]
- Rodriguez, A. I., J. Chiao, and G. Spencer. 2022. Vestibular-Evoked Myogenic Potential Outcomes Associated with Pediatric SportsRelated Concussion. Laryngoscope 132, 2: 436–442. [Google Scholar] [CrossRef]
- Rouse, M. W., E. Borsting, L. Hyman, M. Hussein, S. A. Cotter, M. Flynn, M. Scheiman, M. Gallaway, and P. N. De Land. 1999. Frequency of convergence insufficiency among fifth and sixth graders. The Convergence Insufficiency and Reading Study (CIRS) group. Optometry and vision science: official publication of the American Academy of Optometry 76, 9: 643–649. [Google Scholar] [CrossRef] [PubMed]
- Ruskell, G. L. 2003. Access of autonomic nerves through the optic canal, and their orbital distribution in man. Anat Rec A Discov Mol Cell Evol Biol 275, 1: 973–978. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-González, M. C., J. M. Sánchez-González, C. DeHita-Cantalejo, M. Vega-Holm, J. J. JiménezRejano, and E. Gutiérrez-Sánchez. 2020. The Effect of Age on Binocular Vision Normative Values. J Pediatr Ophthalmol Strabismus 57, 6: 363–371. [Google Scholar] [CrossRef]
- Schall, J. D., and D. P. Hanes. 1993. Neural basis of saccade target selection in frontal eye field during visual search. Nature 366, 6454: 467–469. [Google Scholar] [CrossRef]
- Skopelja, E. N., C. Lepage, S. Bouix, O. Pasternak, A. P. Lin, M. E. Shenton, and I. K. Koerte. 2018. Sex differences in white matter alterations following repetitive subconcussive head impacts in collegiate ice hockey players. Neuroimage Clin 17: 642–649. [Google Scholar]
- Schall, J. D., A. Morel, D. J. King, and J. Bullier. 1995. Topography of visual cortex connections with frontal eye field in macaque: convergence and segregation of processing streams. The Journal of Neuroscience 15, 6: 4464. [Google Scholar] [CrossRef] [PubMed]
- Schneider, K. J., W. H. Meeuwisse, L. PalaciosDerflingher, and C. A. Emery. 2018. Changes in measures of cervical spine function, vestibulo-ocular reflex, dynamic balance, and divided attention following sport-related concussion in elite youth ice hockey players. Journal of Orthopaedic & Sports Physical Therapy 48, 12: 974–981. [Google Scholar]
- Sedaghat, M. R., H. Momeni-Moghaddam, S. A. Naroo, M. Etezad-Razavi, and M. Moshirfar. 2019. Induced Myopia Secondary to Blunt Trauma. Case Reports in Ophthalmological Medicine 2019: 1632828. [Google Scholar] [CrossRef]
- Shook, B., M. Schlag-Rey, and J. Schlag. 1990. Primate supplementary eye field: I. Comparative aspects of mesencephalic and pontine connections. Journal of Comparative Neurology 301, 4: 618–642. [Google Scholar] [CrossRef]
- Slobounov, S. M., A. Walter, H. C. Breiter, D. C. Zhu, X. Bai, T. Bream, P. Seidenberg, X. Mao, B. Johnson, and T. M. Talavage. 2017. The effect of repetitive subconcussive collisions on brain integrity in collegiate football players over a single football season: A multi-modal neuroimaging study. NeuroImage: Clinical 14: 708–718. [Google Scholar] [CrossRef]
- Sollmann, N., P. S. Echlin, V. Schultz, P. V. Viher, A. E. Lyall, Y. Tripodis, D. Kaufmann, E. Hartl, P. Kinzel, L. A. Forwell, A. M. Johnson, S. Somisetty, and J.M. Das. 2019. Neuroanatomy, Vestibulo-ocular reflex. StatPearls Publishing: Retrieved April 15 from https://www.ncbi.nlm.nih.gov/books/NBK545297/. [Google Scholar]
- Stanton, G. B., M. E. Goldberg, and C. J. Bruce. 1988. Frontal eye field efferents in the macaque monkey: II. Topography of terminal fields in midbrain and pons. J Comp Neurol 271, 4: 493–506. [Google Scholar] [CrossRef]
- Steele, C. A., A. B. Tullo, I. B. Marsh, and J. K. Storey. 1987. Traumatic myopia; an ultrasonographic and clinical study. Br J Ophthalmol 71, 4: 301–303. [Google Scholar] [CrossRef]
- Stubbs, J. L., S. L. Corrow, B. R. Kiang, J. C. Corrow, H. L. Pearce, A. Y. Cheng, J. J. S. Barton, and W. J. Panenka. 2019. Working memory load improves diagnostic performance of smooth pursuit eye movement in mild traumatic brain injury patients with protracted recovery. Scientific Reports 9, 1: 291. [Google Scholar] [CrossRef] [PubMed]
- Sufrinko, A. M., A. Mucha, T. Covassin, G. Marchetti, R. Elbin, M. W. Collins, and A. P. Kontos. 2017. Sex differences in vestibular/ocular and neurocognitive outcomes following sport-related concussion. Clinical journal of sport medicine: official journal of the Canadian Academy of Sport Medicine 27, 2: 133. [Google Scholar] [CrossRef]
- Suh, M., S. Basu, R. Kolster, R. Sarkar, B. McCandliss, and J. Ghajar. 2006. Increased oculomotor deficits during target blanking as an indicator of mild traumatic brain injury. Neuroscience Letters 410, 3: 203–207. [Google Scholar] [CrossRef] [PubMed]
- Suhr, C., M. Shust, R. Prasad, D. Wilcox, and C. Chronister. 2015. Recognizing TBI-related Vision Disorders. https://www.reviewofoptometry.com/article/recognizing-tbirelated-vision-disorders.
- Suzuki, Y., M. Kase, H. Kato, and K. Fukushima. 1997. Stability of ocular counterrolling and Listing's plane during static roll-tilts. Invest Ophthalmol Vis Sci 38, 10: 2103–2111. [Google Scholar] [PubMed]
- Sye, G., S. J. Sullivan, and P. McCrory. 2006. High school rugby players’ understanding of concussion and return to play guidelines. British Journal of Sports Medicine 40, 12: 1003–1005. [Google Scholar] [CrossRef]
- Szymanowicz, D., K. J. Ciuffreda, P. Thiagarajan, D. P. Ludlam, W. Green, and N. Kapoor. 2012. Vergence in mild traumatic brain injury: a pilot study [Report]. Journal of Rehabilitation Research & Development 49: 1083. [Google Scholar]
- Thiagarajan, P., and K. J. Ciuffreda. 2015. Pupillary responses to light in chronic non-blast-induced mTBI. Brain Inj 29, 12: 1420–1425. [Google Scholar] [CrossRef]
- Thiagarajan, P., K. J. Ciuffreda, and D. P. Ludlam. 2011. Vergence dysfunction in mild traumatic brain injury (mTBI): a review. Ophthalmic and Physiological Optics 31, 5: 456–468. [Google Scholar] [CrossRef]
- Ting, W. K.-C., T. A. Schweizer, J. Topolovec-Vranic, and M. D. Cusimano. 2016. Antisaccadic Eye Movements Are Correlated with Corpus Callosum White Matter Mean Diffusivity, Stroop Performance, and Symptom Burden in Mild Traumatic Brain Injury and Concussion. Frontiers in neurology 6: 271–271. [Google Scholar] [CrossRef] [PubMed]
- Taghdiri, F., J. Chung, S. Irwin, N. Multani, A. Tarazi, A. Ebraheem, M. Khodadadi, R. Goswami, R. Wennberg, D. Mikulis, R. Green, K. Davis, C. Tator, M. Eizenman, and M. C. Tartaglia. 2018. Decreased Number of Self-Paced Saccades in Post-Concussion Syndrome Associated with Higher Symptom Burden and Reduced White Matter Integrity. J Neurotrauma 35, 5: 719–729. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M. 2005. Involvement of the Central Thalamus in the Control of Smooth Pursuit Eye Movements. The Journal of Neuroscience 25, 25: 5866. [Google Scholar] [CrossRef]
- Tanaka, M., and S. G. Lisberger. 2002. Role of Arcuate Frontal Cortex of Monkeys in Smooth Pursuit Eye Movements. II. Relation to Vector Averaging Pursuit. Journal of Neurophysiology 87, 6: 2700–2714. [Google Scholar] [CrossRef]
- Terao, M., and S. y. Nishida. 2020. Direction of Apparent Motion During Smooth Pursuit Is Determined Using a Mixture of Retinal and Objective Proximities. i-Perception 11, 3: 2041669520937320. [Google Scholar] [CrossRef] [PubMed]
- Truong, J., N. Joshi, and K. J. Ciuffreda. 2018. Influence of refractive error on pupillary dynamics in the normal and mild traumatic brain injury (mTBI) populations. Journal of optometry 11, 2: 93–102. [Google Scholar] [CrossRef] [PubMed]
- Truong, J. Q., and K. J. Ciuffreda. 2016a. Comparison of pupillary dynamics to light in the mild traumatic brain injury (mTBI) and normal populations. Brain Inj 30, 11: 1378–1389. [Google Scholar] [CrossRef]
- Truong, J. Q., and K. J. Ciuffreda. 2016b. Quantifying pupillary asymmetry through objective binocular pupillometry in the normal and mild traumatic brain injury (mTBI) populations. Brain Injury 30, 11: 1372–1377. [Google Scholar] [CrossRef]
- Tuohimaa, P. 1978. Vestibular disturbances after acute mild head injury. Acta Otolaryngol Suppl 359: 3–67. [Google Scholar]
- Tyler, C. W., L. T. Likova, K. N. Mineff, and S. C. Nicholas. 2015. Deficits in the Activation of Human Oculomotor Nuclei in Chronic Traumatic Brain Injury. Frontiers in neurology 6: 173–173. [Google Scholar] [CrossRef]
- van Esch, B. F., G. E. Nobel-Hoff, P. P. van Benthem, H. J. van der Zaag-Loonen, and T. D. Bruintjes. 2016. Determining vestibular hypofunction: start with the video-head impulse test. Eur Arch Otorhinolaryngol 273, 11: 3733–3739. [Google Scholar] [CrossRef] [PubMed]
- Weber, K. P., S. M. Rosengren, R. Michels, V. Sturm, D. Straumann, and K. Landau. 2012. Single motor unit activity in human extraocular muscles during the vestibulo-ocular reflex. The Journal of physiology 590, 13: 3091–3101. [Google Scholar] [CrossRef]
- Welgampola, M. S., and J. P. Carey. 2010. Waiting for the evidence: VEMP testing and the ability to differentiate utricular versus saccular function. Otolaryngol Head Neck Surg 143, 2: 281–283. [Google Scholar] [CrossRef]
- Westphal, C. 1887. Ueber einen Fall von chronischer progressiver Lähmung der Augenmuskeln (Ophthalmoplegia externa) nebst Beschreibung von Ganglienzellengruppen im Bereiche des Oculomotoriuskerns. Archiv für Psychiatrie und Nervenkrankheiten 18, 3: 846–871. [Google Scholar] [CrossRef]
- Wetzel, P. A., A. S. Lindblad, H. Raizada, N. James, C. Mulatya, M. A. Kannan, Z. Villamar, G. T. Gitchel, and L. K. Weaver. 2018. Eye Tracking Results in Postconcussive Syndrome Versus Normative Participants. Invest Ophthalmol Vis Sci 59, 10: 4011–4019. [Google Scholar] [CrossRef] [PubMed]
- Zaleski, A., J. Bogle, A. Starling, D. A. Zapala, L. Davis, M. Wester, and M. Cevette. 2015. Vestibular evoked myogenic potentials in patients with vestibular migraine. Otol Neurotol 36, 2: 295–302. [Google Scholar] [CrossRef]
- Zonner, S. W., K. Ejima, C. C. Fulgar, C. N. Charleston, M. E. Huibregtse, Z. W. Bevilacqua, and K. Kawata. 2019. Oculomotor Response to Cumulative Subconcussive Head Impacts in US High School Football Players: A Pilot Longitudinal Study. JAMA Ophthalmol 137, 3: 265–270. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
Copyright © 2022. This article is licensed under a Creative Commons Attribution 4.0 International License.
Share and Cite
McDonald, M.A.; Holdsworth, S.J.; Danesh-Meyer, H.V. Eye Movements in Mild Traumatic Brain Injury: Ocular Biomarkers. J. Eye Mov. Res. 2022, 15, 1-31. https://doi.org/10.16910/jemr.15.2.4
McDonald MA, Holdsworth SJ, Danesh-Meyer HV. Eye Movements in Mild Traumatic Brain Injury: Ocular Biomarkers. Journal of Eye Movement Research. 2022; 15(2):1-31. https://doi.org/10.16910/jemr.15.2.4
Chicago/Turabian StyleMcDonald, Matthew A., Samantha J. Holdsworth, and Helen V. Danesh-Meyer. 2022. "Eye Movements in Mild Traumatic Brain Injury: Ocular Biomarkers" Journal of Eye Movement Research 15, no. 2: 1-31. https://doi.org/10.16910/jemr.15.2.4
APA StyleMcDonald, M. A., Holdsworth, S. J., & Danesh-Meyer, H. V. (2022). Eye Movements in Mild Traumatic Brain Injury: Ocular Biomarkers. Journal of Eye Movement Research, 15(2), 1-31. https://doi.org/10.16910/jemr.15.2.4



