Assessment of Energy Performance and Emission Control Using Alternative Fuels in Cement Industry through a Process Model
Abstract
:1. Introduction
2. Principles of Cement Manufacturing Process
3. Clinker Chemistry
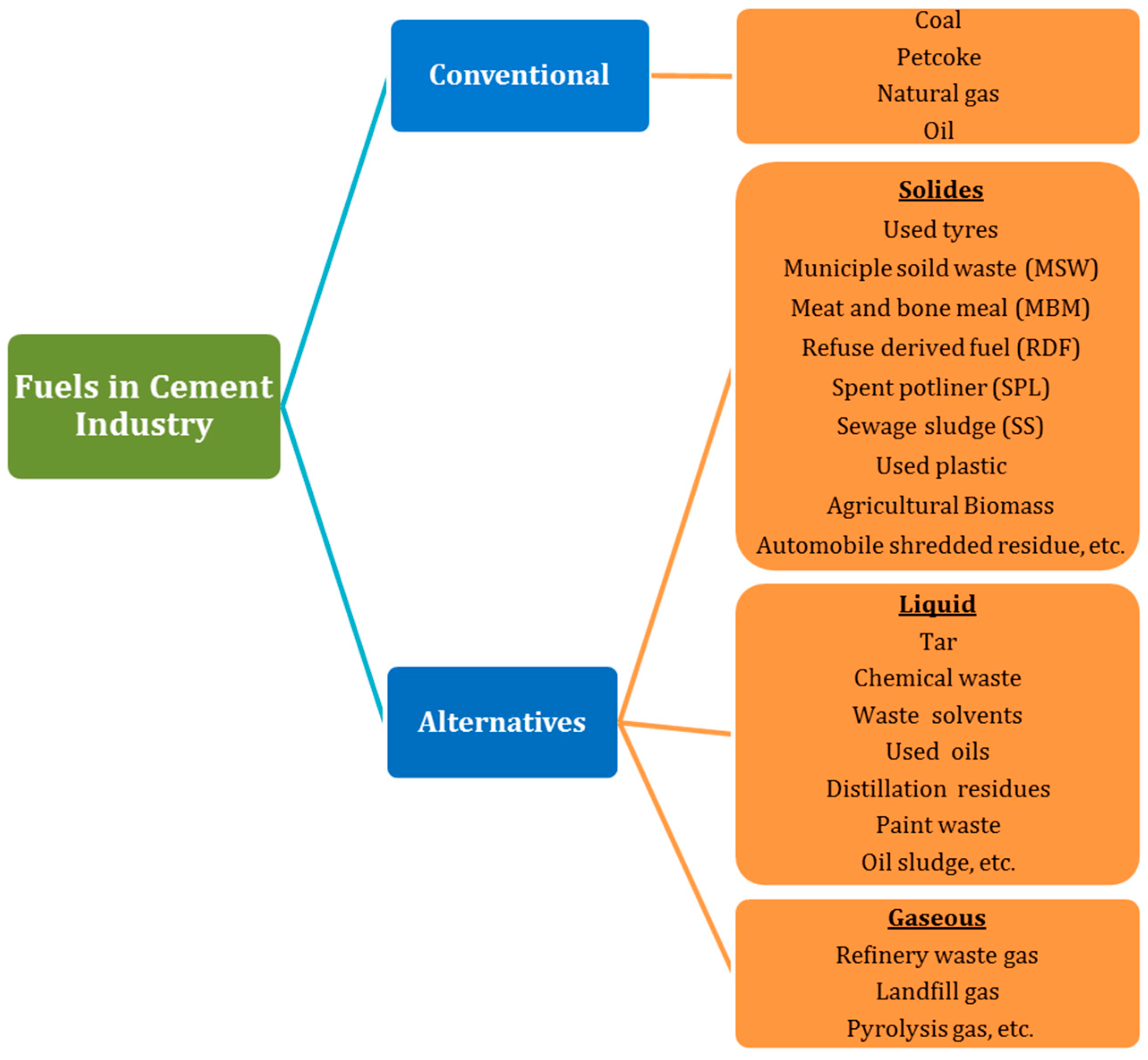
4. Alternative Fuels
5. Process Model
- In the burning zone, the coal and other fuel combustion has taken place in two different reactors. The combustion process consists of the fuel decomposition section and decomposed products combustion section with Gibbs free energy minimization technique. These have been simulated by using two reactor modules of Aspen Plus, namely RYIELD and RGIBBS.
- NOX generation from the kiln was due to the combustion of the fuel only.
- CO2 was produced through calcination process and combustion of fuels.
- Only CaCO3 and MgCO3 of raw feed were decomposed within the calciner.
- Ash took part in the clinker formation reaction and ash yields were determined from the ash analysis of coal and alternative fuel.
- All the clinker formation reactions in kiln occurred in three separate reactors to facilitate pyoprocessing in different set of temperature.
- Any air leakages in the calciner system have not been considered throughout the model.
- Heat of combustion,
- Heat of formation and,
- Heat capacity.
- Mass flow rates of all incoming streams.
- Temperature and pressure of all incoming material streams.
- Heating values and chemical composition of the coal and alternative fuels.
- Composition of raw meal in terms of mass or mole fraction.
- Particle size distribution of all fuel and raw feed.
- Dimension and efficiency of cyclone string.
- Heat flux data for the cooler section.
6. Results and Discussion
6.1. Emission Control
6.2. Quality Control
6.3. Energy Performance
7. Conclusions
Author Contributions
Conflicts of Interest
References
- Van Oss, H.G.; Padovani, A.C. Cement manufacture and the environment, Part I: Chemistry & Technology. J. Ind. Ecol. 2002, 6, 89–105. [Google Scholar]
- Van Oss, H.G.; Padovani, A.C. Cement manufacture and the environment, Part II: Environmental challenges and opportunities. J. Ind. Ecol. 2003, 7, 93–126. [Google Scholar]
- Rodrigues, F.A.; Joekes, I. Cement industry: Sustainability, challenges and perspectives. Environ. Chem. Lett. 2011, 9, 151–166. [Google Scholar] [CrossRef]
- Hills, T.; Leeson, D.; Florin, N.; Fennell, P. Carbon capture in the cement industry: Technologies, progress, and retrofitting. Environ. Sci. Technol. 2016, 50, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Carrasco-Maldonado, F.; Spörl, R.; Fleiger, K.; Hoenig, V.; Maier, J.; Scheffknecht, G. Oxy-fuel combustion technology for cement production—State of the art research and technology development. Int. J. Greenh. Gas Control 2016, 45, 189–199. [Google Scholar] [CrossRef]
- Castellani, B.; Gambelli, A.M.; Morini, E.; Nastasi, B.; Presciutti, A.; Filipponi, M.; Nicolini, A.; Rossi, F. Experimental investigation on CO2 methanation process for solar energy storage compared to CO2-based methanol synthesis. Energies 2017, 10, 855. [Google Scholar] [CrossRef]
- Hoffart, M. Tyre Derived Fuel. In Proceedings of the WasteMINZ Conference, Auckland, New Zealand, 13–16 October 2010. [Google Scholar]
- Kaantee, U.; Zevenhoven, R.; Backman, R.; Hupa, M. Cement manufacturing using alternative fuels and the advantages of process modelling. Fuel Process. Technol. 2004, 85, 293–301. [Google Scholar] [CrossRef]
- Lechtenberg, D. Spent cell linings from the aluminium smelting process as an alternative fuel and raw material for cement production. Carbon 2009, 15, 30. [Google Scholar]
- Mikulcic, H.; Klemes, J.J.; Vujanovic, M.; Urbaniec, K.; Duic, N. Reducing greenhouse gasses emissions by fostering the deployment of alternative raw materials and energy sources in the cleaner cement manufacturing process. J. Clean. Prod. 2016, 136, 119–132. [Google Scholar] [CrossRef]
- Horsley, C.; Emmert, M.H.; Sakulich, A. Influence of alternative fuels on trace element content of ordinary Portland cement. Fuel 2016, 184, 481–489. [Google Scholar] [CrossRef]
- Richards, G.; Agranovski, I.E. Dioxin-like pcb emissions from cement kilns during the use of alternative fuels. J. Hazard. Mater. 2017, 323, 698–709. [Google Scholar] [CrossRef] [PubMed]
- Georgiopoulou, M.; Lyberatos, G. Life cycle assessment of the use of alternative fuels in cement kilns: A case study. J. Environ. Manag. 2017, in press. [Google Scholar] [CrossRef] [PubMed]
- Ariyaratne, W.K.H.; Malagalage, A.; Melaaen, M.C.; Tokheim, L. CFD modelling of meat and bone meal combustion in a cement rotary kiln—Investigation of fuel particle size and fuel feeding position impacts. Chem. Eng. Sci. 2015, 123, 596–608. [Google Scholar] [CrossRef]
- Mastorakos, E.; Massias, A.; Tsakiroglou, C.D. CFD predictions for cement kilns including flame modelling, heat transfer and clinker chemistry. Appl. Math. Model. 1999, 23, 55–76. [Google Scholar] [CrossRef]
- Mujumdar, K.S.; Ranade, V.V. Simulation of rotary cement kilns using a one-dimensional model. Chem. Eng. Res. Des. 2006, 84, 165–177. [Google Scholar] [CrossRef]
- Mujumdar, K.S.; Ganesh, K.V.; Kulkarni, S.B.; Ranade, V.V. Rotary cement kiln simulator (RoCKS): Integrated modelling of pre-heater, calciner, kiln and clinker cooler. Chem. Eng. Sci. 2007, 62, 2590–2607. [Google Scholar] [CrossRef]
- Spang, H.A. A dynamic model of a cement kiln. Automatica 1972, 8, 309–323. [Google Scholar] [CrossRef]
- Boateng, A.A.; Barr, P.V. A thermal model for the rotary kiln including heat transfer within the bed. Int. J. Heat Mass Transf. 1996, 39, 2131–2147. [Google Scholar] [CrossRef]
- Martins, M.A.; Oliveira, L.S.; Franca, A.S. Modeling and simulation of Limestone calcinations in rotary kilns. ZKG Int. 2002, 55, 76–87. [Google Scholar]
- Paul, M.; Mujumdar, K.S.; Ranade, V.V. Modeling of rotary cement kilns. In Proceedings of the International Symposium & 55th Annual Session of IIChE (Chemcon), Hyderabad, India, 19–22 December 2002. [Google Scholar]
- Giddings, D.; Pickering, S.J.; Simmons, K.; Eastwick, C.N. Combustion and aerodynamic behaviour of car tyre chips in a cement works precalciner. J. Inst. Energy 2002, 75, 91–99. [Google Scholar]
- Marin, O.; Charon, O.; Dugue, J.; Dukhan, S.; Zhou, W. Simulating the impact of oxygen enrichment in a cement rotary kiln using advanced computational methods. Combust. Sci. Technol. 2001, 164, 193–207. [Google Scholar] [CrossRef]
- Zhang, Y.; Cao, S.; Shao, S.; Chen, Y.; Liu, S.; Zhang, S. Aspen Plus-based simulation of a cement calciner and optimization analysis of air pollutants emission. Clean Technol. Environ. Policy 2011, 13, 459–468. [Google Scholar] [CrossRef]
- Rahman, A.; Rasul, M.G.; Khan, M.M.K.; Sharma, S. Cement calciner model development for optimizing the usage of alternative fuels. In Proceedings of the 12th International Conference on Sustainable Energy Technologies (SET 2013), Hong Kong, China, 26–29 August 2013; pp. 1784–1794. [Google Scholar]
- Rahman, A.; Rasul, M.G.; Khan, M.M.K.; Sharma, S. Aspen Plus based simulation for energy recovery from waste to utilize in cement plant preheater tower. Energy Procedia 2014, 61, 922–927. [Google Scholar] [CrossRef]
- Hokfors, B. Phase Chemistry in Process Models for Cement Clinker and Lime Production. Ph.D. Thesis, Thermal Energy Conversion Laboratory, Department of Applied Physics and Electronics, UMEA University, Umeå, Sweden, 2014. [Google Scholar]
- European Commission (EC). Reference Document on the Best Available Techniques in the Cement and Lime Manufacturing Industries; BAT Reference Document (BREF); European IPPC Bureau: Seville, Spain, 2001. [Google Scholar]
- Cembureau, European Cement Association. Best Available Techniques for the Cement Industry. 1999. Chapter 3. pp. 15–43. Available online: http://cembureau.be/ (accessed on 10 May 2013).
- Murray, A.; Price, L. Use of Alternative Fuels in Cement Manufacture: Analysis of Fuel Characteristics and Feasibility for Use in the Chinese Cement Sector; Ernest Orlando Lawrence Berkeley National Laboratory: Berkeley, CA, USA, 2008.
- CIF Fast Facts 2015, Australian Cement Industry Statistics 2015. Available online: http://www.cement.org.au/Portals/0/Documents/Fast%20Facts/CIF%20Fast%20Facts%202015%20(low%20res).pdf (accessed on 22 July 2016).
- Tokheim, L.A. The Impact of Staged Combustion on the Operation of a Precalciner Cement Kiln. Ph.D. Thesis, Telemark College, Porsgrunn, Norway, 1999. [Google Scholar]
- Hewlett, P.C. Lea’s Chemistry of Cement and Concrete, 4th ed.; Butterworth-Heinemann Ltd.: Oxford, UK, 2003. [Google Scholar]
- Aldieb, M.A.; Ibrahim, H.G. Variation of feed chemical composition and its effect on clinker formation- simulation process. In Proceedings of the World Congress on Engineering and Computer Science, San Francisco, CA, USA, 20–22 October 2010; Volume 2. [Google Scholar]
- Taylor, H.F.W. Cement Chemistry, 2nd ed.; Thomas Telford Publishing: London, UK, 2004. [Google Scholar]
- Rahman, A.; Rasul, M.G.; Khan, M.M.K.; Sharma, S. Recent development on the uses of alternative fuels in cement manufacturing process. Fuel 2015, 145, 84–99. [Google Scholar] [CrossRef]
- Karell, M.A.; Blumenthal, M.H. Air regulatory impacts of the use of tire-derived fuel. Environ. Prog. 2001, 20, 80–86. [Google Scholar] [CrossRef]
- Trezza, M.A.; Scian, A.N. Scrap tire ashes in Portland cement production. Mater. Res. 2009, 12, 489–494. [Google Scholar] [CrossRef]
- Garg, A.; Smith, R.; Hill, D.; Longhurst, P.J.; Pollard, S.J.T.; Simms, N.J. An integrated appraisal of energy recovery options in the United Kingdom using solid recovered fuel derived from municipal solid waste. Waste Manag. 2009, 29, 2289–2297. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, R. Recycling of municipal solid waste for cement production: Pilot-scale test for transforming incineration ash of solid waste into cement clinker. Res. Conserv. Recycl. 2001, 31, 137–147. [Google Scholar] [CrossRef]
- Fryda, L.; Panopoulos, K.; Vourliotis, P.; Kakaras, E.; Pavlidou, E. Meat and bone meal as secondary fuel in fluidized bed combustion. Proc. Combust. Inst. 2007, 31, 2829–2837. [Google Scholar] [CrossRef]
- Kim, J.; Mun, T.; Kim, J.; Kim, J. Air gasification of mixed plastic wastes using a two-stage gasifier for the production of producer gas with low tar and a high caloric value. Fuel 2011, 90, 2266–2272. [Google Scholar] [CrossRef]
- Xiao, R.; Jin, B.; Zhou, H.; Zhong, Z.; Zhang, M. Air gasification of polypropylene plastic waste in fluidized bed gasifier. Energy Convers. Manag. 2007, 48, 778–786. [Google Scholar] [CrossRef]
- Jenkins, B.M.; Baxter, L.L.; Miles, T.R., Jr.; Miles, T.R. Combustion properties of biomass. Fuel Process. Technol. 1998, 54, 17–46. [Google Scholar] [CrossRef]
- GERIAP. Company Toolkit for Energy Efficiency, Industry Sectors—Cement. 2005. Available online: http://www.energyefficiencyasia.org/docs/IndustrySectorsCement_draftMay05.pdf (accessed on 10 May 2014).






| Reaction | Reaction Equation | Standard Reaction Enthalpy [kJ/kg] |
|---|---|---|
| I. Formation of oxides and decomposing reactions | ||
| Evaporation of water | H2O(l) → H2O(g) | 2453 |
| Decomposition of kaolinite | Al2O3·2SiO2·2H2O → Al2O3 + 2SiO2 + 2H2O | 780 |
| Oxidation of carbon | C + O2 → CO2 | −33,913 |
| Dissociation of MgCO3 | MgCO3 → MgO + CO2 | 1395 |
| Dissociation of CaCO3 | CaCO3 → CaO + CO2 | 1780 |
| II. Formation of intermediates | ||
| Formation of CA | CaO + Al2O3 → CaO·Al2O3 | −100 |
| Formation of C2F | 2CaO + Fe2O3 → 2CaO·Fe2O3 | −114 |
| Formation of β-C2S | 2CaO + SiO2 → 2CaO·SiO2 | −732 |
| III. Sintering reactions | ||
| Formation of C4AF | CA + C2F + CaO → C4AF | 25 |
| Formation of C3A | CA + 2CaO → C3A | 25 |
| Formation of C3S | β-C2S + CaO → C3S | 59 |
| Temperature | Reaction | Stage of Process |
|---|---|---|
| 100 °C | Evaporation of free water | Preheater |
| 500 °C and above | Evolution of combined water from clay | Preheater |
| 900 °C and above | Crystallization of amorphous dehydration products of clay | Precalciner, Early kiln |
| 900 °C and above | Evolution of calcium carbonate to form carbon dioxide | Precalciner, Early kiln |
| 900 °C–1200 °C | Reaction between lime and clay | Early to mid-kiln |
| 1250 °C–1280 °C | Commencement of liquid formation | Mid to late Kiln |
| Above 1280 °C | Further formation of liquid and completion of formation of cement compounds | Mid to late kiln |
| Coal (Plant Data) | Tyre | MSW | MBM | Plastic Waste | Bagasse | |
|---|---|---|---|---|---|---|
| Proximate analysis on dry basis (wt %) | ||||||
| Moisture | 4.2 | 0.62 | 31.2 | 1.35 | 0.6 | 0 |
| Ash | 19.1 | 4.81 | 35.17 | 10.54 | 0.4 | 11.95 |
| Volatile matter | 36.6 | 67.06 | 64.83 | 80.74 | 94.77 | 85.61 |
| Fixed carbon | 53.0 | 28.13 | 0 | 8.72 | 4.83 | 2.44 |
| Elemental analysis on dry basis (wt %) | ||||||
| C | 69.43 | 84.39 | 34.88 | 55.7 | 77.02 | 48.64 |
| H | 3.83 | 7.13 | 4.65 | 8.03 | 12.14 | 5.87 |
| N | 1.5 | 0.24 | 1.02 | 7.15 | 0 | 0.16 |
| S | 0.36 | 0.01 | 0.15 | 0 | 1.09 | 0.03 |
| Cl | 0.2 | 1.24 | 1.02 | 0.05 | 0 | 0.04 |
| O | 5.58 | 2.18 | 23.11 | 18.53 | 4.92 | 42.82 |
| LHV (MJ/kg) | 27.4 | 37.8 | 15.4 | 30.705 | 41.5 | 18.99 |
| Ash analysis (wt %) | ||||||
| SiO2 | 46.09 | 14.1 | 15.1 | 5.97 | 61.35 | 46.61 |
| Al2O3 | 20.64 | 2.7 | 15.6 | 1.81 | 25.13 | 17.69 |
| Fe2O3 | 7.84 | 1.1 | 4.7 | 0.59 | 5.44 | 14.14 |
| CaO | 16.19 | 47.0 | 36.6 | 45.6 | 4.72 | 4.47 |
| MgO | 1.16 | 0.7 | 2 | 1.43 | 0.94 | 3.33 |
| SO3 | 2.45 | 1.2 | 1.7 | - | 0.03 | 2.08 |
| TiO2 | 1.3 | <0.01 | - | 1.1 | - | 2.63 |
| P2O5 | 2.45 | <0.01 | 1.5 | 37.3 | - | 2.72 |
| Na2O | 0.31 | <0.01 | 1.8 | 2.07 | 0.42 | 0.79 |
| K2O | 1.57 | <0.01 | 1.3 | 1.86 | 1.52 | 0.15 |
| Cl2 | - | - | 9.7 | 0.11 | - | - |
| ZnO | - | 33.1 | - | - | - | - |
| Name | Function |
|---|---|
| Mixed | Used to handle conventional components that reach vapor–liquid–solid phase equilibrium |
| CISOLID (Conventional, Inert, Solid) | Used to handle conventional components that appear in the solid phase but do not participate in phase equilibrium |
| NC (Nonconventional) | Used to handle nonconventional components |
| Input Data (From Plant) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coal analysis | Proximate analysis | Moisture | Fixed carbon | Volatile matter | Ash | Calorific Value | ||||||
| 1.35 | 57.3 | 24.15 | 18.55 | 27.4 MJ/kg | ||||||||
| Ultimate analysis | C | H | N | Cl | S | O (by diff.) | ||||||
| 69.13 | 3.79 | 1.51 | 0 | 0.36 | 6.66 | |||||||
| Ash analysis | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | SO3 | TiO2 | P2O5 | Na2O | K2O | ||
| 0.4609 | 0.2064 | 0.0784 | 0.1619 | 0.0116 | 0.0245 | 0.013 | 0.0245 | 0.0031 | 0.0157 | |||
| Raw meal (wt %) | SiO2 | Al2O3 | CaO | MgO | K2O | Na2O | Fe2O3 | TiO2 | SO3 | L.O.I. | CaCO3 | |
| 13.68 | 3.54 | 43.49 | 0.66 | 0.23 | 0.15 | 2.49 | - | 0.16 | 35.39 | 78.13 | ||
| Output Data | ||||||||||||
| Clinker composition (oxide form) | ||||||||||||
| CaO | SiO2 | Al2O3 | Fe2O3 | MgO | P2O5 | TiO2 | Na2O | K2O | SO3 | Free CaO | ||
| Plant data | 66.79 | 21.84 | 5.72 | 3.89 | 1.1 | -- | -- | 0.31 | 0.37 | 0.22 | 1.1 | |
| Simulation results | 66.7793 | 21.6564 | 5.7106 | 3.8798 | 1.0089 | 0.052 | 0.028 | 0.245 | 0.362 | 0.2227 | 0.9369 | |
| Clinker composition (Compound form) and ratios | ||||||||||||
| C4AF | C3A | C2S | C3S | LSF | AR | SR | ||||||
| Plant data | 11.84 | 8.58 | 15.95 | 61.26 | 96.003 | 1.47 | 2.273 | |||||
| Simulation results | 11.80692 | 8.55310 | 15.90564 | 61.23595 | 95.14625 | 1.47190 | 2.25813 | |||||
| Air emission data | ||||||||||||
| Pollutant | Unit | Source | Available data | Simulation results | ||||||||
| CO2 | kg/tonne clinker | Standard limit | 977 | 803.6919 | ||||||||
| NOX | gm/tonne clinker | Average plant data | 2200 | 2076.50498 | ||||||||
| SO2 | gm/tonne clinker | Average plant data | 170 | 175.893923 | ||||||||
| Kiln Process | Thermal Energy Consumption (MJ/kg Clinker) |
|---|---|
| Wet process with internals | 5.86–6.28 |
| Long dry process with internals | 4.60 |
| 1-stage cyclone pre-heater | 4.18 |
| 2-stage cyclone pre-heater | 3.77 |
| 4-stage cyclone pre-heater | 3.55 |
| 4-stage cyclone pre-heater plus calciner | 3.14 |
| 5-stage pre-heater plus calciner plus high efficiency cooler | 3.01 |
| 6-stage pre-heater plus calciner plus high efficiency cooler | <2.93 |
| Fuel Mix | Only Coal | Coal 82% & Tyre 18% | Coal 85% & MSW 15% | Coal 80% & MBM 20% | Coal 88% & Plastic Waste 12% | Coal 95% & Bagasse 5% | |
|---|---|---|---|---|---|---|---|
| Properties | |||||||
| Excess air in the kiln | 10% | 5% | 5% | 5% | 5% | 5% | |
| Excess air in the calciner | 20.025% | 13.58% | 6.866% | 15.277% | 10.7879% | 8.64% | |
| Kiln outlet temperature (°C) | 1680.665 | 1680.708 | 1680.441 | 1679.998 | 1680.722 | 1680.205 | |
| Raw feed (kg/h) [change %] | 137,856 | 138,365 [+0.369%] | 137,510 [−0.25%] | 138,530 [+0.489%] | 138,475 [+0.449%] | 138,320 [+0.337%] | |
| Energy requirement (MJ/kg clinker) [change %] | 3.145 | 3.028 [−3.72%] | 3.152 [+0.22%] | 2.944 [−6.39%] | 2.983 [−5.15%] | 2.982 [−5.18%] | |
| CO2 emission (kg/tonne clinker) [change %] | 803.692 | 781.275 [−2.79%] | 780.585 [−2.88%] | 768.543 [−4.37%] | 778.564 [−3.13%] | 786.632 [−2.12%] | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahman, A.; Rasul, M.G.; Khan, M.M.K.; Sharma, S.C. Assessment of Energy Performance and Emission Control Using Alternative Fuels in Cement Industry through a Process Model. Energies 2017, 10, 1996. https://doi.org/10.3390/en10121996
Rahman A, Rasul MG, Khan MMK, Sharma SC. Assessment of Energy Performance and Emission Control Using Alternative Fuels in Cement Industry through a Process Model. Energies. 2017; 10(12):1996. https://doi.org/10.3390/en10121996
Chicago/Turabian StyleRahman, Azad, Mohammad G. Rasul, M.M.K. Khan, and Subhash C. Sharma. 2017. "Assessment of Energy Performance and Emission Control Using Alternative Fuels in Cement Industry through a Process Model" Energies 10, no. 12: 1996. https://doi.org/10.3390/en10121996
APA StyleRahman, A., Rasul, M. G., Khan, M. M. K., & Sharma, S. C. (2017). Assessment of Energy Performance and Emission Control Using Alternative Fuels in Cement Industry through a Process Model. Energies, 10(12), 1996. https://doi.org/10.3390/en10121996





