Phase Equilibria of the CH4-CO2 Binary and the CH4-CO2-H2O Ternary Mixtures in the Presence of a CO2-Rich Liquid Phase
Abstract
1. Introduction
2. Experiments
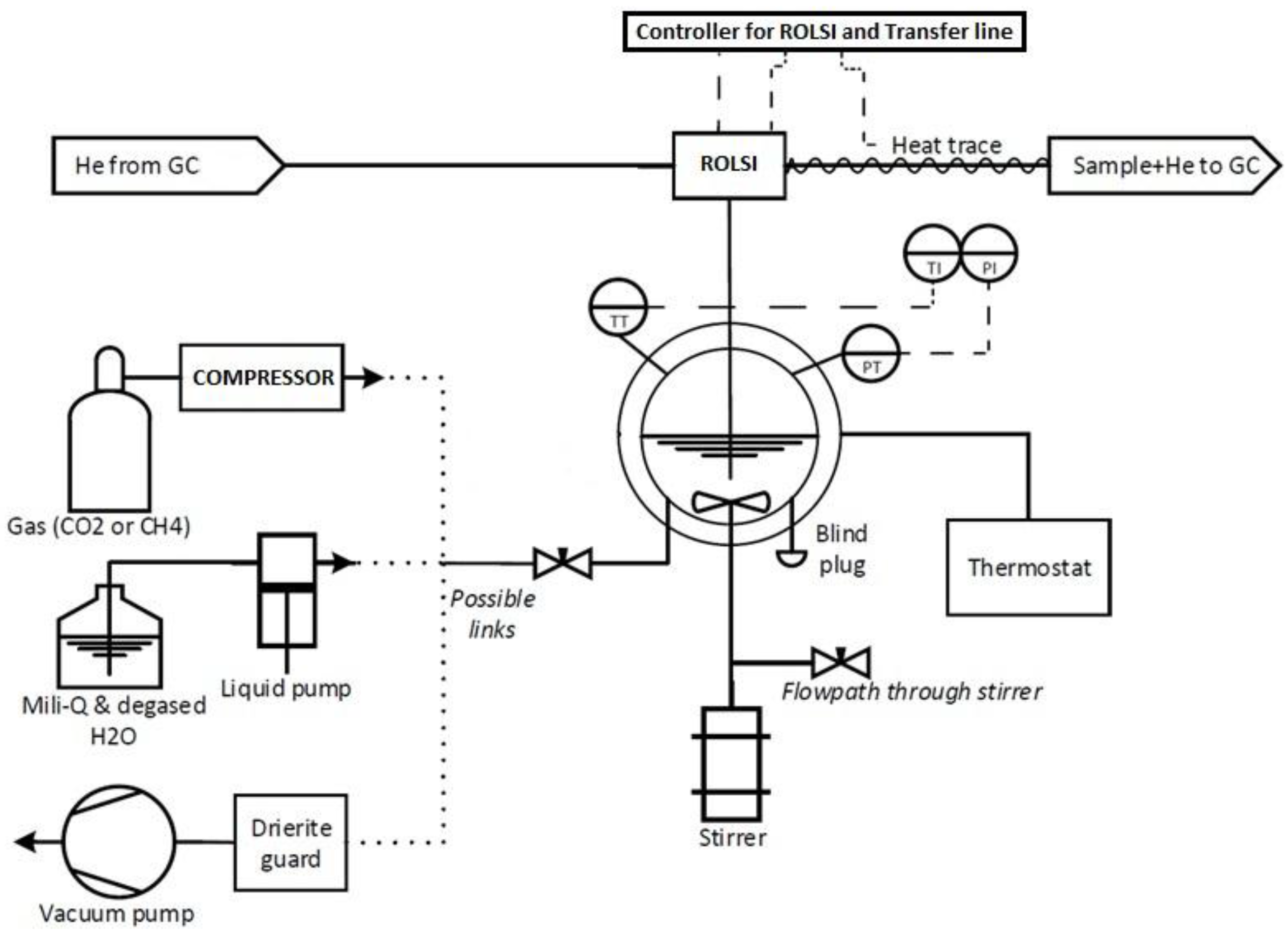
2.1. Experimental Apparatus
2.2. Materials
2.3. Experimental Procedure
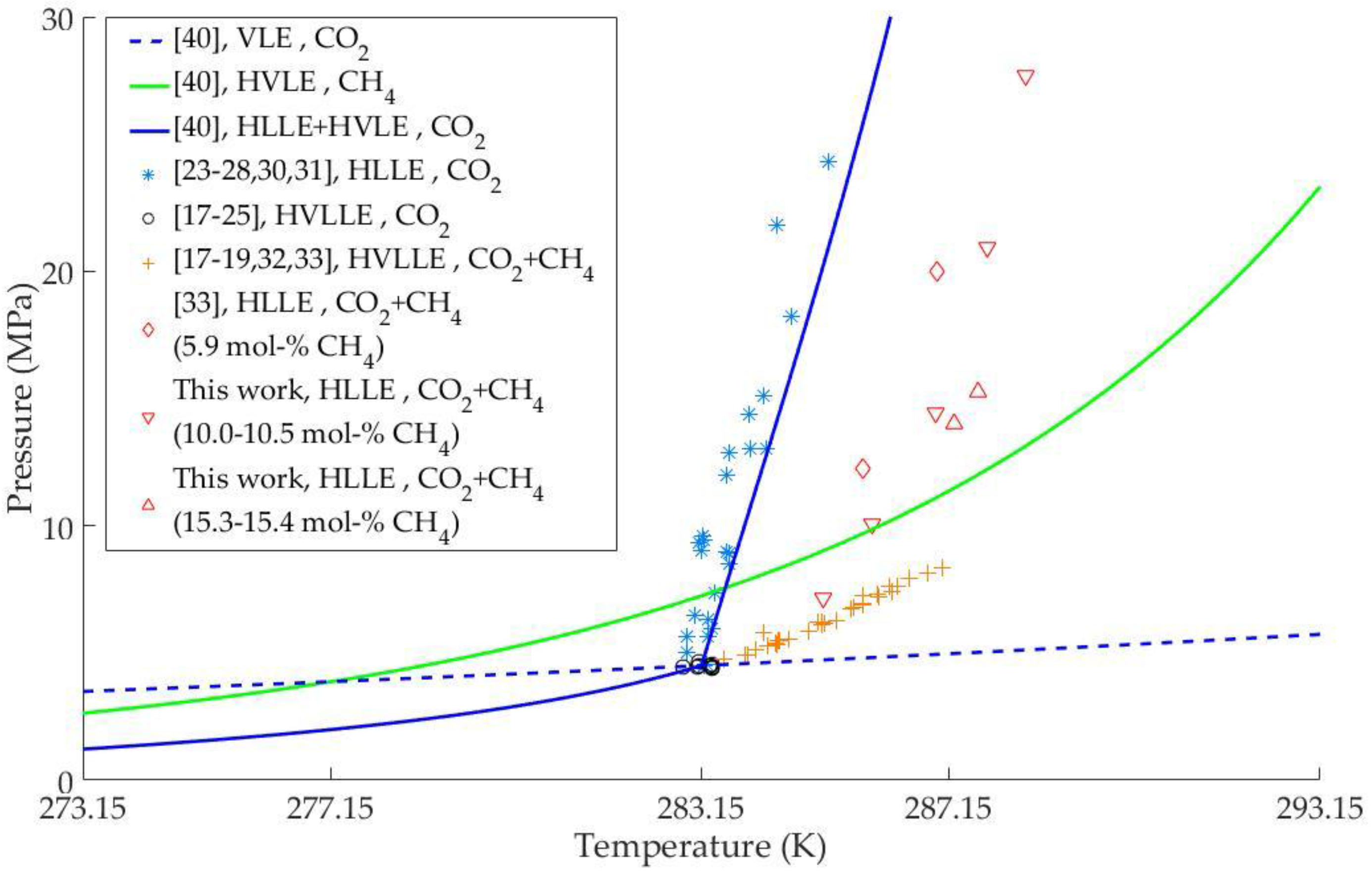
3. Results and Discussion
3.1. CH4-CO2 Binary System
3.2. CH4-CO2-H2O Ternary System
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| Symbol | Unit | |
| CO2-rich liquid phase composition of molecule i | xi | mole fraction |
| CO2-rich liquid phase composition of molecule i without H2O | x*i | mole fraction |
| Vapour phase composition of molecule i | yi | mole fraction |
| Global composition of molecule i in the system | zi | mole fraction |
| Global composition of molecule i in the system without H2O | z*i | mole fraction |
References
- Lewicki, J.L.; Birkholzer, J.; Tsang, C.F. Natural and industrial analogues for leakage of CO2 from storage reservoirs: Identification of features, events, and processes and lessons learned. Environ. Geol. 2007, 52, 457–467. [Google Scholar] [CrossRef]
- Boiron, M.C.; Cathelineau, M.; Ruggieri, G.; Jeanningros, A.; Gianelli, G.; Banks, D.A. Active contact metamorphism and CO2–CH4 fluid production in the Larderello geothermal field (Italy) at depths between 2.3 and 4 km. Chem. Geol. 2007, 237, 303–328. [Google Scholar] [CrossRef]
- Sakai, H.; Gamo, T.; Kim, E.S.; Tsutsumi, M.; Tanaka, T.; Ishibashi, J.; Wakita, H.; Yamano, M.; Oomori, T. Venting of carbon dioxide-rich fluid and hydrate formation in mid-Okinawa trough backarc basin. Science 1990, 248, 1093–1097. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, F.; Kuypers, M.M.; Tsunogai, U.; Ishibashi, J.I.; Nakamura, K.I.; Treude, T.; Ohkubo, S.; Nakaseama, M.; Gena, K.; Chiba, H.; et al. Microbial community in a sediment-hosted CO2 lake of the southern Okinawa Trough hydrothermal system. Proc. Natl. Acad. Sci. USA 2006, 103, 14164–14169. [Google Scholar] [CrossRef] [PubMed]
- Konno, U.; Tsunogai, U.; Nakagawa, F.; Nakaseama, M.; Ishibashi, J.I.; Nunoura, T.; Nakamura, K.I. Liquid CO2 venting on the seafloor: Yonaguni Knoll IV hydrothermal system, Okinawa Trough. Geophys. Res. lett. 2006, 33. [Google Scholar] [CrossRef]
- Lupton, J.; Butterfield, D.; Lilley, M.; Evans, L.; Nakamura, K.I.; Chadwick, W.; Resing, J.; Emblez, R.; Olson, E.; Proskurowski, G.; et al. Submarine venting of liquid carbon dioxide on a Mariana Arc volcano. Geochem. Geophys. 2006, 7. [Google Scholar] [CrossRef]
- Ebinuma, T. Method for Dumping and Disposing of Ccarbon Dioxide Gas and Apparatus Therefor. U.S. Patent 5,261,490, 16 November 1993. [Google Scholar]
- Nakano, S.; Yamamoto, K.; Ohgaki, K. Natural gas exploitation by carbon dioxide from gas hydrate fields—High-pressure phase equilibrium for an ethane hydrate system. Proc. Inst. Mech. Eng. A J. Power Energy 1998, 212, 159–163. [Google Scholar] [CrossRef]
- Ota, M.; Morohashi, K.; Abe, Y.; Watanabe, M.; Smith, R.L., Jr.; Inomata, H. Replacement of CH4 in the hydrate by use of liquid CO2. Energy Convers. Manag. 2005, 46, 1680–1691. [Google Scholar] [CrossRef]
- Schicks, J.M.; Spangenberg, E.; Giese, R.; Steinhauer, B.; Klump, J.; Luzi, M. New approaches for the production of hydrocarbons from hydrate bearing sediments. Energies 2011, 4, 151–172. [Google Scholar] [CrossRef]
- Deusner, C.; Bigalke, N.; Kossel, E.; Haeckel, M. Methane production from gas hydrate deposits through injection of supercritical CO2. Energies 2012, 5, 2112–2140. [Google Scholar] [CrossRef]
- Boswell, R.; Schoderbek, D.; Collett, T.S.; Ohtsuki, S.; White, M.; Anderson, B.J. The Ignik Sikumi Field Experiment, Alaska North Slope: Design, Operations, and Implications for CO2-CH4 Exchange in Gas Hydrate Reservoirs. Energy Fuels 2016, 31, 140–153. [Google Scholar] [CrossRef]
- Kaminishi, G.I.; Arai, Y.; Saito, S.; Maeda, S. Vapor-liquid equilibria for binary and ternary systems containing carbon dioxide. J. Chem. Eng. Jpn. 1968, 1, 109–116. [Google Scholar] [CrossRef]
- Arai, Y.; Kaminishi, G.I.; Saito, S. The experimental determination of the PVTX relations for the carbon dioxide-nitrogen and the carbon dioxide-methane systems. J. Chem. Eng. Jpn. 1971, 4, 113–122. [Google Scholar] [CrossRef]
- Xu, N.; Dong, J.; Wang, Y.; Shi, J. High pressure vapor liquid equilibria at 293 K for systems containing nitrogen, methane and carbon dioxide. Fluid Phase Equilib. 1992, 81, 175–186. [Google Scholar] [CrossRef]
- Bian, B.; Wang, Y.; Shi, J.; Zhao, E.; Lu, B.C.Y. Simultaneous determination of vapor-liquid equilibrium and molar volumes for coexisting phases up to the critical temperature with a static method. Fluid Phase Equilib. 1993, 90, 177–187. [Google Scholar] [CrossRef]
- Seo, Y.T.; Kang, S.P.; Lee, H.; Lee, C.S.; Sung, W.M. Hydrate phase equilibria for gas mixtures containing carbon dioxide: A proof-of-concept to carbon dioxide recovery from multicomponent gas stream. Korean J. Chem. Eng. 2000, 17, 659–667. [Google Scholar] [CrossRef]
- Seo, Y.T.; Lee, H. Multiple-phase hydrate equilibria of the ternary carbon dioxide, methane, and water mixtures. J. Phys. Chem. B 2001, 105, 10084–10090. [Google Scholar] [CrossRef]
- Bi, Y.; Yang, T.; Guo, K. Determination of the upper-quadruple-phase equilibrium region for carbon dioxide and methane mixed gas hydrates. J. Pet. Sci. Eng. 2013, 101, 62–67. [Google Scholar] [CrossRef]
- Unruh, C.H.; Katz, D.L. Gas hydrates of carbon dioxide-methane mixtures. J. Petrol. Technol. 1949, 1, 83–86. [Google Scholar] [CrossRef]
- Robinson, D.B.; Metha, B.R. Hydrates in the propane carbon dioxide-water system. J. Can. Petrol. Technol. 1971, 10. [Google Scholar] [CrossRef]
- Yoon, J.H.; Lee, H. Clathrate phase equilibria for the water-phenol-carbon dioxide system. AIChE J. 1997, 43, 1884–1893. [Google Scholar] [CrossRef]
- Fan, S.S.; Guo, T.M. Hydrate formation of CO2-rich binary and quaternary gas mixtures in aqueous sodium chloride solutions. J. Chem. Eng. Data 1999, 44, 829–832. [Google Scholar] [CrossRef]
- Mooijer-Van Den Heuvel, M.M.; Witteman, R.; Peters, C.J. Phase behaviour of gas hydrates of carbon dioxide in the presence of tetrahydropyran, cyclobutanone, cyclohexane and methylcyclohexane. Fluid Phase Equilib. 2001, 182, 97–110. [Google Scholar] [CrossRef]
- Ruffine, L.; Trusler, J.P.M. Phase behaviour of mixed-gas hydrate systems containing carbon dioxide. J. Chem. Thermodyn. 2010, 42, 605–611. [Google Scholar] [CrossRef]
- Takenouchi, S.; Kennedy, G.C. The binary system H2O-CO2 at high temperatures and pressures. Am. J. Sci. 1964, 262, 1055–1074. [Google Scholar] [CrossRef]
- Ng, H.J.; Robinson, D.B. Hydrate formation in systems containing methane, ethane, propane, carbon dioxide or hydrogen sulfide in the presence of methanol. Fluid Phase Equilib. 1985, 21, 145–155. [Google Scholar] [CrossRef]
- Ohgaki, K.; Makihara, Y.; Takano, K. Formation of CO2 hydrate in pure and sea waters. J. Chem. Eng. Jpn. 1993, 26, 558–564. [Google Scholar] [CrossRef]
- Nakano, S.; Moritoki, M.; Ohgaki, K. High-pressure phase equilibrium and Raman microprobe spectroscopic studies on the CO2 hydrate system. J. Chem. Eng. Data 1998, 43, 807–810. [Google Scholar] [CrossRef]
- Chapoy, A.; Burgass, R.; Tohidi, B.; Austell, J.M.; Eickhoff, C. Effect of common impurities on the phase behavior of carbon-dioxide-rich systems: Minimizing the risk of hydrate formation and two-phase flow. SPE J. 2011, 16, 921–930. [Google Scholar] [CrossRef]
- Alsiyabi, I.; Chapoy, A.; Tohidi, B. Effect of common impurities on the hydrate stability of carbon dioxide systems. In Proceedings of the 8th International Conference on Gas Hydrates (ICGH8-2014), Beijing, China, 28 July–1 August 2014. [Google Scholar]
- Al Ghafri, S.Z.; Forte, E.; Maitland, G.C.; Rodriguez-Henríquez, J.J.; Trusler, J.M. Experimental and modeling study of the phase behavior of (methane + CO2 + water) mixtures. J. Phys. Chem. B 2014, 118, 14461–14478. [Google Scholar] [CrossRef] [PubMed]
- Chapoy, A.; Burgass, R.; Tohidi, B.; Alsiyabi, I. Hydrate and phase behavior modeling in CO2-Rich pipelines. J. Chem. Eng. Data 2014, 60, 447–453. [Google Scholar] [CrossRef]
- Vitu, S.; Jaubert, J.N.; Pauly, J.; Daridon, J.L.; Barth, D. Bubble and dew points of carbon dioxide + a five-component synthetic mixture: Experimental data and modeling with the PPR78 model. J. Chem. Eng. Data 2008, 52, 1851–1855. [Google Scholar] [CrossRef]
- Duan, Z.; Hu, J. A new cubic equation of state and its applications to the modeling of vapor-liquid equilibria and volumetric properties of natural fluids. Geochim. Cosmochim. Acta 2004, 68, 2997–3009. [Google Scholar] [CrossRef]
- Van Konynenburg, P.H.; Scott, R.L. Critical lines and phase equilibria in binary van der Waals mixtures. Philos. Trans. R. Soc. A 1980, 298, 495–540. [Google Scholar] [CrossRef]
- Poling, B.E.; Prausnitz, J.M.; O’Connell, J.P. The Properties of Gases and Liquids; McGraw-Hill: New York, NY, USA, 2001; Volume 5. [Google Scholar]
- Kaminishi, G.; Toriumi, T. Gas-liquid equilibrium under high pressures VI. Vapor-liquid phase equilibrium in the CO2-H2, CO2-N2, and CO2-O2 systems. Kogyo Kagaku Zasshi 1966, 69, 175–178. [Google Scholar] [CrossRef]
- Xu, N.; Yao, J.; Wang, Y.; Shi, J.; Lu, B.C.Y. Vapor-liquid equilibria of five binary systems containing R-22. Fluid Phase Equilib. 1991, 69, 261–270. [Google Scholar] [CrossRef]
- Kossel, E.; Bigalke, N.K.; Pinero, E.; Haeckel, M. The SUGAR Toolbox: A Library of Numerical Algorithms and Data for Modelling of Gas Hydrate Systems and Marine Environments; PANGAEA: Bremen, Germany, 2013. [Google Scholar]
- Kastanidis, P.; Romanos, G.E.; Stubos, A.K.; Economou, I.G.; Tsimpanogiannis, I.N. Two- and three-phase equilibrium experimental measurements for the ternary CH4 + CO2 + H2O mixture. Fluid Phase Equilib. 2017, 451, 96–105. [Google Scholar] [CrossRef]
- Ruffine, L.; Donval, J.P.; Charlou, J.L.; Cremière, A.; Zehnder, B.H. Experimental study of gas hydrate formation and destabilisation using a novel high-pressure apparatus. Mar. Pet. Geol. 2010, 27, 1157–1165. [Google Scholar] [CrossRef]
- Guilbot, P.; Valtz, A.; Legendre, H.; Richon, D. Rapid on-line sampler-injector: A reliable tool for HT-HP sampling and on-line GC analysis. Analusis 2000, 28, 426–431. [Google Scholar] [CrossRef]
- Tohidi, B.; Burgass, R.W.; Danesh, A.; Østergaard, K.K.; Todd, A.C. Improving the accuracy of gas hydrate dissociation point measurements. Ann. N. Y. Acad. Sci. 2000, 912, 924–931. [Google Scholar] [CrossRef]
- NIST Chemistry WebBook, SRD 69. Available online: Webbook.nist.gov/chemistry/fluid (accessed on 10 September 2017).
- Span, R.; Wagner, W. A new equation of state for carbon dioxide covering the fluid region from the triple-point temperature to 1100 K at pressures up to 800 MPa. J. Phys. Chem. Ref. Data 1996, 25, 1509–1596. [Google Scholar] [CrossRef]
- Sloan, E.D., Jr.; Koh, C. Clathrate Hydrates of Natural Gases; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]

| Isotherm Studied (K) | Pressure Range (MPa) | Number of Data Points (xCH4, yCH4) | Reference |
|---|---|---|---|
| 273.15 | 5.20–8.08 | (3, 4) | [13] |
| 283.15 | 6.12–8.08 | (3, 3) | |
| 273.15 | 4.15–8.41 | (3, 9) | [14] |
| 288.15 | 5.38–8.04 | (5, 7) | |
| 288.50 | 5.12–8.15 | (10, 10) | [15] |
| 293.40 | 5.73–7.98 | (13, 13) | |
| 301.00 | 6.86–7.70 | (6, 6) + Critical point | [16] |
| 274.15 | 3.64–8.33 | (9, 0) | This work |
| 277.15 | 3.94–8.20 | (11, 0) | |
| 283.15 | 4.60–8.08 | (4, 0) | |
| 288.15 | 5.17–7.63 | (4, 0) | |
| 290.15 | 5.44–7.82 | (6, 0) |
| System | Phases | T (K) | CH4 Composition | Data | Reference |
|---|---|---|---|---|---|
| p (MPa) | |||||
| CO2-H2O | H-LH2O-LCO2-V Point in p-T space | 283.19 (±0.46) | - | 9 | [17,18,19,20,21,22,23,24,25] |
| 4.49 (±0.20) | |||||
| H-LH2O-LCO2 Line in p-T space | 282.92–294.00 | - | 61 | [23,24,25,26,27,28,29,30,31] | |
| 4.5–494 | |||||
| CH4-CO2-H2O | H-LH2O-LCO2-V Line in p-T space | 283.86–285.56 | 0.0517–0.1750 | 3 | [17] |
| 4.930–6.720 | (yCH4, vapor phase) | ||||
| 283.86–285.76 | 0.0596–0.2026 | 4 | [18] | ||
| 4.930–7.251 | (yCH4, vapor phase) | ||||
| 283.51–287.04 | 0.05–0.22 | 18 | [19] | ||
| 4.74–8.37 | (z*CH4, gas load) | ||||
| 283.90–286.19 | - | 5 | [32] | ||
| 4.925–7.62 | |||||
| 284.15 | 0.059 | 1 | [33] | ||
| 5.81 | (z*CH4, gas load) | ||||
| H-LH2O-LCO2 Surface in p-T space | 285.75–286.95 | 0.059 | 2 | [33] | |
| 12.25–19.97 | (z*CH4, gas load) | ||||
| 285.11–288.39 | 0.100–0.154 | 7 | This work | ||
| 7.17–27.71 | (z*CH4, gas load) |
| RD % | T (K) | p (MPa) | xCH4 | Reference |
|---|---|---|---|---|
| −5.4 (xCH4) | 288.15 | 7.63 | 0.1097 | This work |
| 7.65 | 0.1160 | [14] | ||
| −2.1 (xCH4) | 283.15 | 8.08 | 0.1732 | This work |
| 8.08 | 0.177 | [13] | ||
| 1.7 (p) | 274.15 | 3.64 | 0 | This work |
| 3.58 | 0 | [45,46] | ||
| 1.8 (p) | 277.15 | 3.94 | 0 | This work |
| 3.87 | 0 | [45,46] | ||
| 2.2 (p) | 283.15 | 4.60 | 0 | This work |
| 4.50 | 0 | [45,46] | ||
| 1.6 (p) | 288.15 | 5.17 | 0 | This work |
| 5.09 | 0 | [45,46] | ||
| 1.9 (p) | 290.15 | 5.44 | 0 | This work |
| 5.34 | 0 | [45,46] |
| T (K) | p (MPa) | xCH4 | T (K) | p (MPa) | xCH4 |
|---|---|---|---|---|---|
| 274.15 | 3.64 | 0 | 277.15 | 8.02 | 0.2005 |
| 274.15 | 4.11 | 0.0166 | 277.15 | 8.13 | 0.2107 |
| 274.15 | 4.99 | 0.0518 | 277.15 | 8.20 | 0.2185 |
| 274.15 | 5.90 | 0.0929 | 283.15 | 4.60 | 0 |
| 274.15 | 6.60 | 0.1284 | 283.15 | 5.37 | 0.0293 |
| 274.15 | 7.06 | 0.1545 | 283.15 | 7.24 | 0.1145 |
| 274.15 | 7.56 | 0.186 | 283.15 | 8.08 | 0.1732 |
| 274.15 | 8.04 | 0.2206 | 288.15 | 5.17 | 0 |
| 274.15 | 8.33 | 0.2577 | 288.15 | 6.62 | 0.0566 |
| 277.15 | 3.94 | 0 | 288.15 | 6.80 | 0.0654 |
| 277.15 | 4.69 | 0.0266 | 288.15 | 7.63 | 0.1097 |
| 277.15 | 5.26 | 0.0495 | 290.15 | 5.44 | 0 |
| 277.15 | 5.97 | 0.0817 | 290.15 | 6.09 | 0.0243 |
| 277.15 | 6.51 | 0.1083 | 290.15 | 6.69 | 0.0496 |
| 277.15 | 7.04 | 0.1358 | 290.15 | 7.27 | 0.0772 |
| 277.15 | 7.51 | 0.1643 | 290.15 | 7.59 | 0.0951 |
| 277.15 | 7.71 | 0.1786 | 290.15 | 7.82 | 0.1107 |
| Title | Mix. 1 | Mix. 2 | Mix. 3 | Mix. 4 | Mix. 5 | Mix. 6 | Mix. 7 | |
|---|---|---|---|---|---|---|---|---|
| Composition Mole fraction | z*CH4 | 0.105 | 0.105 | 0.105 | 0.100 | 0.100 | 0.153 | 0.154 |
| zCH4 | 0.031 | 0.043 | 0.038 | 0.029 | 0.052 | 0.103 | 0.090 | |
| zCO2 | 0.266 | 0.369 | 0.328 | 0.263 | 0.467 | 0.572 | 0.492 | |
| zH2O | 0.703 | 0.588 | 0.634 | 0.708 | 0.481 | 0.325 | 0.418 | |
| x*CH4 | 0.102 | 0.110 | 0.111 | 0.106 | 0.106 | 0.154 | 0.155 | |
| without gas hydrate | (0.088) | (0.108) | (0.108) | (0.105) | (0.102) | (0.154) | (0.157) | |
| x*CH4 | 0.095 | 0.102 | 0.106 | 0.092 | 0.089 | 0.152 | 0.149 | |
| with gas hydrate | (0.060) | (0.072) | (0.063) | (0.097) | (0.073) | (0.138) | (0.133) | |
| Gas hydrate dissociation | T (K) | 285.11 | 285.90 | 286.93 | 287.77 | 288.39 | 287.24 | 287.61 |
| p (MPa) | 7.17 | 10.07 | 14.45 | 20.94 | 27.71 | 13.99 | 15.25 | |
| (6.33) | (7.52) | (11.73) | (16.54) | (20.87) | (10.72) | (12.08) | ||
| Title | Mix. 1 | Mix. 2 | Mix. 3 | Mix. 4 | Mix. 5 | Mix. 6 | Mix. 7 |
|---|---|---|---|---|---|---|---|
| Stirrer rotation speed (rpm) | 1000 | 1000 | 1000 | 100 | 100 | 700 | 700 |
| Time of incipient formation (min) | 18 | 14 | 33 | 18 | 10 | 10 | 56 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Legoix, L.N.; Ruffine, L.; Donval, J.-P.; Haeckel, M. Phase Equilibria of the CH4-CO2 Binary and the CH4-CO2-H2O Ternary Mixtures in the Presence of a CO2-Rich Liquid Phase. Energies 2017, 10, 2034. https://doi.org/10.3390/en10122034
Legoix LN, Ruffine L, Donval J-P, Haeckel M. Phase Equilibria of the CH4-CO2 Binary and the CH4-CO2-H2O Ternary Mixtures in the Presence of a CO2-Rich Liquid Phase. Energies. 2017; 10(12):2034. https://doi.org/10.3390/en10122034
Chicago/Turabian StyleLegoix, Ludovic Nicolas, Livio Ruffine, Jean-Pierre Donval, and Matthias Haeckel. 2017. "Phase Equilibria of the CH4-CO2 Binary and the CH4-CO2-H2O Ternary Mixtures in the Presence of a CO2-Rich Liquid Phase" Energies 10, no. 12: 2034. https://doi.org/10.3390/en10122034
APA StyleLegoix, L. N., Ruffine, L., Donval, J.-P., & Haeckel, M. (2017). Phase Equilibria of the CH4-CO2 Binary and the CH4-CO2-H2O Ternary Mixtures in the Presence of a CO2-Rich Liquid Phase. Energies, 10(12), 2034. https://doi.org/10.3390/en10122034





