Technical Assessment of Different Operating Conditions of an On-Board Autothermal Reformer for Fuel Cell Vehicles
Abstract
:1. Introduction
2. System Description
2.1. Autothermal Reactor Modeling and Optimization
- Autothermal reactor (ATR): It is the reforming reactor in which the fuel is converted into a gaseous mixture of H, CO, CO and HO. The ATR is fed by fuel, steam and oxygen, and it is maintained under adiabatic conditions, so that no heat transfer occurs from or to it.
- Water gas shift reactor (WGSR): the water gas shift reactor (low temperature water shift reactor WGSR) in which CO is reacted with HO towards H and CO.
- Heat recovery line: Since the thermal efficiency of the fuel processor unit depends strongly on reactants’ preheating temperatures, as reported in [26], a heat recovery line is defined by cooling the syngas stream temperature in two heat exchangers. In particular, the water and fuel required by the reforming reaction are pre-heated in the heat exchanger HEX by cooling the syngas stream and then heated in the heat exchanger HEX; the oxygen sent to the autothermal reactor is already heated up to 351 C because of the compression up to 10 bar required by the membrane separation process [20].
- Separation unit (SEP): the membrane separation unit where the pure oxygen is produced; here, the air is compressed up to 10 bar, and then, by passing through the membrane, the oxygen is separated from nitrogen with a 95% removal efficiency.
- Inter-refrigerated compression line (IRCL): the syngas compression line, equipped with three compressors and two heat exchangers, needed in order to increase the syngas pressure up to the hydrogen buffer pressure, i.e., 250 bar [20].
- the steam to carbon ratio S/C at the autothermal reactor, defined as the ratio between the mole flow rate of the steam fed to the reactor and the carbon mole flow rate of the feeding fuel, in the range of 0.2–3.6.
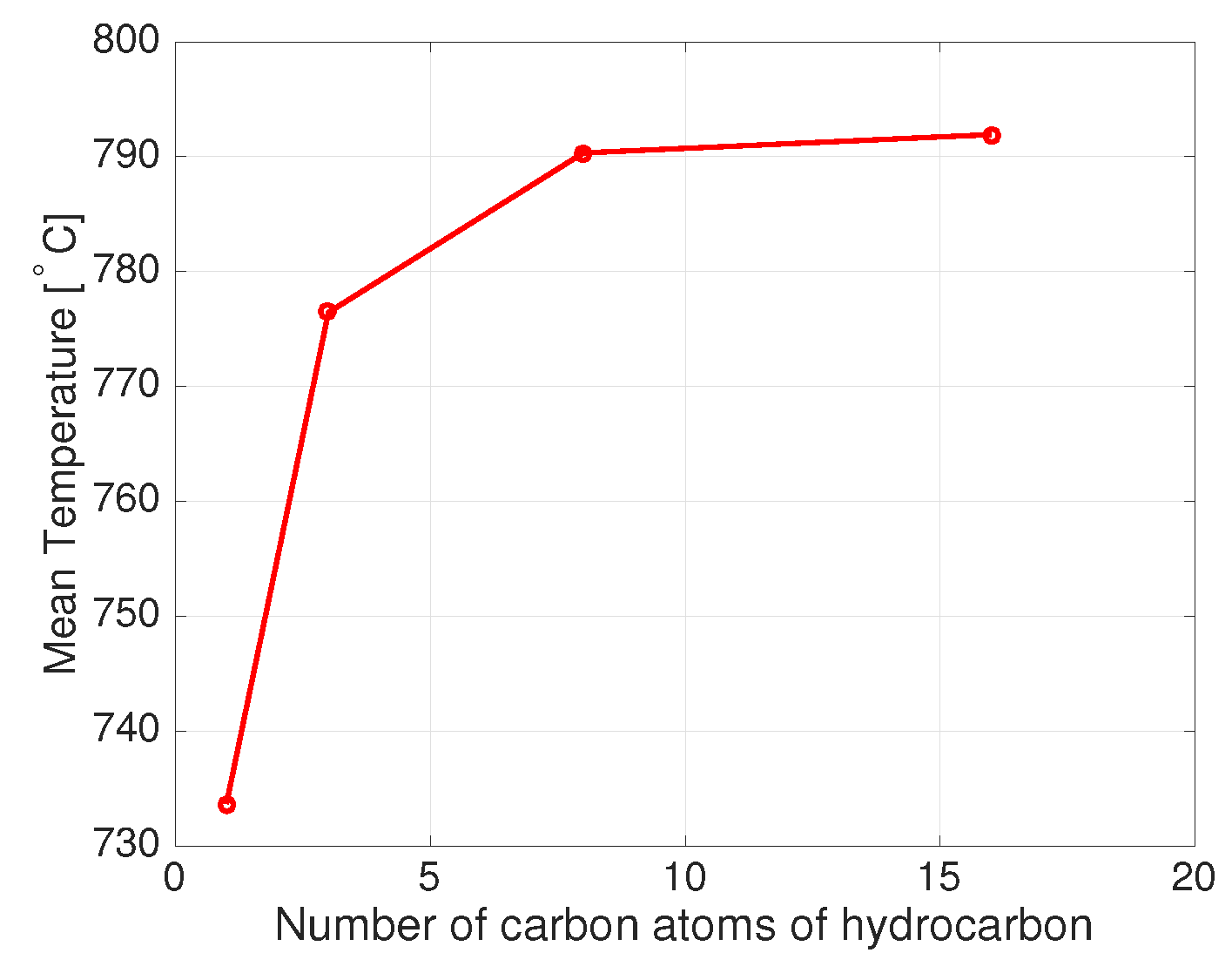
- the oxygen to carbon ratio O/C at the autothermal reactor, defined as the ratio between the mole flow rate of the oxygen fed to the reactor and the carbon mole flow rate of the feeding fuel, in the range of 0.02–0.26, 0.3–0.7, 1.3–1.7 and 2.5–3.5, for methane, propane, isooctane and n-hexadecane respectively.
- the pre-heat temperature of fuel and water feeding the autothermal reactor, recovering the heat internally to the process.
2.2. High Temperature Polymer Electrolyte Membrane Fuel Cell
3. Application to a Fuel Cell/Battery Vehicle
- -
- Artemis urban, urban driving cycle, for a total length of 50 km;
- -
- Extra-urban driving cycle, hereafter called Vail2NREL, for a total length of 140 km;
- -
- Federal Highway Driving Schedule (FHDS), highway driving cycle, for a total length of 250 km;
- -
- Worldwide harmonized Light vehicles Test Cycle (WLTC), combined driving cycle, for a total length of 180 km.
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Roskilly, A.P.; Palacin, R.; Yan, J. Novel technologies and strategies for clean transport systems. Appl. Energy 2015, 157, 563–566. [Google Scholar] [CrossRef]
- Chan, C.C. The state of the art of electric, hybrid, and fuel cell vehicles. Proc. IEEE 2007, 95, 704–718. [Google Scholar] [CrossRef]
- Lacandia, F.; Tribioli, L.; Onori, S.; Rizzoni, G. Adaptive energy management strategy calibration in PHEVs based on a sensitivity study. SAE Int. J. Altern. Powertrains 2013, 2, 443–455. [Google Scholar] [CrossRef]
- Ahmed, A.; Al-Amin, A.Q.; Ambrose, A.F.; Saidur, R. Hydrogen fuel and transport system: A sustainable and environmental future. Int. J. Hydrog. Energy 2016, 41, 1369–1380. [Google Scholar] [CrossRef]
- Purnima, P.; Jayanti, S. A high-efficiency, auto-thermal system for on-board hydrogen production for low temperature PEM fuel cells using dual reforming of ethanol. Int. J. Hydrog. Energy 2016, 41, 13800–13810. [Google Scholar] [CrossRef]
- Pettersson, L.J.; Westerholm, R. State of the art of multi-fuel reformers for fuel cell vehicles: Problem identification and research needs. Int. J. Hydrog. Energy 2001, 26, 243–264. [Google Scholar] [CrossRef]
- Brown, L.F. A comparative study of fuels for on-board hydrogen production for fuel-cell-powered automobiles. Int. J. Hydrog. Energy 2001, 26, 381–397. [Google Scholar] [CrossRef]
- Thomas, C.E.; James, B.D.; Lomax, F.D.; Kuhn, I.F. Fuel options for the fuel cell vehicle: Hydrogen, methanol or gasoline? Int. J. Hydrog. Energy 2000, 25, 551–567. [Google Scholar] [CrossRef]
- Larminie, J.; Lowry, J. Front Matter, in Electric Vehicle Technology Explained, Second Edition; John Wiley & Sons, Ltd.: Chichester, UK, 2012. [Google Scholar]
- Bowers, B.J.; Zhao, J.L.; Ruffo, M.; Khan, R.; Dattatraya, D.; Dushman, N.; Beziat, J.C.; Boudjemaa, F. Onboard fuel processor for PEM fuel cell vehicles. Int. J. Hydrog. Energy 2007, 32, 1437–1442. [Google Scholar] [CrossRef]
- Chrenko, D.; Gao, F.; Bluier, B.; Bouquain, D.; Miraoui, A. Methanol fuel processor and PEM fuel cell modeling for mobile application. Int. J. Hydrog. Energy 2010, 35, 6863–6871. [Google Scholar] [CrossRef]
- Xu, X.; Li, P.; Shen, Y. Small-scale reforming of diesel and jet fuels to make hydrogen and syngas for fuel cells: A review. Appl. Energy 2013, 108, 202–217. [Google Scholar] [CrossRef]
- Jaggi, V.; Jayanti, S. A conceptual model of a high-efficiency, stand-alone power unit based on a fuel cell stack with an integrated auto-thermal ethanol reformer. Appl. Energy 2013, 110, 295–303. [Google Scholar] [CrossRef]
- Cozzolino, R.; Chiappini, D.; Tribioli, L. A Numerical Model for CO Effect Evaluation in HT-PEMFCs: Part 1—Experimental Validation; American Institute of Physics: College Park, MD, USA, 2016; Volume 1738. [Google Scholar]
- Cozzolino, R.; Chiappini, D.; Tribioli, L. A Numerical Model for CO Effect Evaluation in HT-PEMFCs: Part 2—Application to Different Membranes; American Institute of Physics: College Park, MD, USA, 2016; Volume 1738. [Google Scholar]
- Ercolino, G.; Ashraf, M.A.; Specchia, V.; Specchia, S. Performance evaluation and comparison of fuel processors integrated with PEM fuel cell based on steam or autothermal reforming and on CO preferential oxidation or selective methanation. Appl. Energy 2015, 143, 138–153. [Google Scholar] [CrossRef]
- Martin, S.; Wörner, A. On-board reforming of biodiesel and bioethanol for high temperature PEM fuel cells: Comparison of autothermal reforming and steam reforming. J. Power Sources 2011, 196, 3163–3171. [Google Scholar] [CrossRef]
- Andreasen, S.J.; Ashworth, L.; Sahlin, S.; Jensen, H.C.B.; Kær, S.K. Test of hybrid power system for electrical vehicles using a lithium-ion battery pack and a reformed methanol fuel cell range extender. Int. J. Hydrog. Energy 2014, 39, 1856–1863. [Google Scholar] [CrossRef]
- Zhang, J.; Xie, Z.; Zhang, J.; Tang, Y.; Song, C.; Navessin, T.; Shi, Z.; Song, D.; Wang, H.; Wilkinson, D.P.; et al. High temperature PEM fuel cells. J. Power Sources 2006, 160, 872–891. [Google Scholar] [CrossRef]
- Tribioli, L.; Cozzolino, R.; Chiappini, D.; Iora, P. Energy management of a plug-in fuel cell/battery hybrid vehicle with on-board fuel processing. Appl. Energy 2016, 184, 140–154. [Google Scholar] [CrossRef]
- Ersoz, A.; Olgun, H.; Ozdogan, S. Reforming options for hydrogen production from fossil fuels for PEM fuel cells. J. Power Sources 2006, 154, 67–73. [Google Scholar] [CrossRef]
- Jin, Y.; Rui, Z.; Tian, Y.; Lin, Y.; Li, Y. Sequential simulation of dense oxygen permeation membrane reactor for hydrogen production from oxidative steam reforming of ethanol with ASPEN PLUS. Int. J. Hydrog. Energy 2010, 35, 6691–6698. [Google Scholar] [CrossRef]
- Yang, G.; Yu, H.; Peng, F.; Wang, H.; Yang, J.; Xie, D. Thermodynamic analysis of hydrogen generation via oxidative steam reforming of glycerol. Renew. Energy 2011, 36, 2120–2127. [Google Scholar] [CrossRef]
- Tzanetis, K.; Martavaltzi, C.; Lemonidou, A. Comparative exergy analysis of sorption enhanced and conventional methane steam reforming. Int. J. Hydrog. Energy 2012, 37, 16308–16320. [Google Scholar] [CrossRef]
- Gangadharan, P.; Kanchi, K.C.; Lou, H.H. Evaluation of the economic and environmental impact of combining dry reforming with steam reforming of methane. Chem. Eng. Res. Des. 2012, 90, 1956–1968. [Google Scholar] [CrossRef]
- Perna, A. Hydrogen from ethanol: Theoretical optimization of a PEMFC system integrated with a steam reforming processor. Int. J. Hydrog. Energy 2007, 32, 1811–1819. [Google Scholar] [CrossRef]
- Cozzolino, R.; Tribioli, L. On-Board Diesel Autothermal Reforming For PEM Fuel Cells: Simulation and Optimization; American Institute of Physics: College Park, MD, USA, 2015; Volume 1648. [Google Scholar]
- Minutillo, M. On-board fuel processor modeling for hydrogen-enriched gasoline fueled engine. Int. J. Hydrog. Energy 2005, 30, 1483–1490. [Google Scholar] [CrossRef]
- Tribioli, L.; Cozzolino, R.; Barbieri, M. Optimal Control of a Repowered Vehicle: Plug-in Fuel Cell against Plug-in Hybrid Electric Powertrain; American Institute of Physics: College Park, MD, USA, 2015; Volume 1648. [Google Scholar]
- Das, H.S.; Tan, C.W.; Yatim, A. Fuel cell hybrid electric vehicles: A review on power conditioning units and topologies. Renew. Sustain. Energy Rev. 2017, 76, 268–291. [Google Scholar] [CrossRef]
- Alternative Fuels Data Center. Available online: http://www.afdc.energy.gov/vehicles/natural_gas_filling_ tanks.html (accessed on 16 May 2017).










| ATR Power | 16 kW | 40 kW |
|---|---|---|
| Methane | 0.320 | 0.800 |
| Propane | 0.344 | 0.862 |
| Isooctane | 0.357 | 0.894 |
| N-hexadecane | 0.363 | 0.909 |
| T = 700 °C | T = 750 °C | |||||||
|---|---|---|---|---|---|---|---|---|
| Fuel Type | S/C | O/C | CO% | S/C | O/C | CO% | ||
| Methane | 1.2552 | 0.2450 | 84.81 | 0.74 | - | - | - | - |
| Propane | 1.3724 | 0.6300 | 82.70 | 0.82 | 1.4897 | 0.6600 | 84.43 | 0.61 |
| Isooctane | 1.2000 | 1.600 | 80.03 | 1.89 | 1.3724 | 1.700 | 77.44 | 0.91 |
| n-hexadecane | 1.2552 | 3.1750 | 81.22 | 1.51 | 1.4897 | 3.400 | 83.38 | 0.64 |
| T = 700 °C | T = 750 °C | |||||||
|---|---|---|---|---|---|---|---|---|
| Species | Methane | Propane | Isooctane | n-Hexadecane | Methane | Propane | Isooctane | n-Hexadecane |
| H | 0.735 | 0.698 | 0.677 | 0.675 | - | 0.702 | 0.683 | 0.680 |
| CO | 0.00842 | 0.00894 | 0.01946 | 0.01543 | - | 0.00684 | 0.00987 | 0.00722 |
| CO | 0.241 | 0.279 | 0.287 | 0.295 | - | 0.283 | 0.299 | 0.304 |
| CH | 0.00902 | 0.00753 | 0.00969 | 0.00801 | - | 0.00142 | 0.00170 | 0.00129 |
| N | 0.00656 | 0.00653 | 0.00685 | 0.00656 | - | 0.00674 | 0.00689 | 0.00700 |
| Operating Temperature | [°C] | 160 |
| Utilization Factor | - | 0.8 |
| Anode Pressure, | 1.3 | |
| Cathode Pressure, | 1.3 | |
| Fuel Stoichiometry, | - | 1.2 |
| Air Stoichiometry, | - | 2 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tribioli, L.; Cozzolino, R.; Chiappini, D. Technical Assessment of Different Operating Conditions of an On-Board Autothermal Reformer for Fuel Cell Vehicles. Energies 2017, 10, 839. https://doi.org/10.3390/en10070839
Tribioli L, Cozzolino R, Chiappini D. Technical Assessment of Different Operating Conditions of an On-Board Autothermal Reformer for Fuel Cell Vehicles. Energies. 2017; 10(7):839. https://doi.org/10.3390/en10070839
Chicago/Turabian StyleTribioli, Laura, Raffaello Cozzolino, and Daniele Chiappini. 2017. "Technical Assessment of Different Operating Conditions of an On-Board Autothermal Reformer for Fuel Cell Vehicles" Energies 10, no. 7: 839. https://doi.org/10.3390/en10070839
APA StyleTribioli, L., Cozzolino, R., & Chiappini, D. (2017). Technical Assessment of Different Operating Conditions of an On-Board Autothermal Reformer for Fuel Cell Vehicles. Energies, 10(7), 839. https://doi.org/10.3390/en10070839








