Gelation Behavior Study of a Resorcinol–Hexamethyleneteramine Crosslinked Polymer Gel for Water Shut-Off Treatment in Low Temperature and High Salinity Reservoirs
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Equipment
2.3. Procedures
2.3.1. Gel Preparation
2.3.2. Gelation Time
2.3.3. Determination of Gel Strength
3. Results and Discussion
3.1. Crosslinking Reaction Process between Polymer and Resorcinol-Hexamethylenetetramine
3.2. Gelling Property
3.3. Mass Fraction of Polymer Impact on the Crosslinking Reaction Process
3.4. Mass Fraction of Resorcinol Impact on Crosslinking Reaction Process
3.5. Mass Fraction of Hexamethylenetetramine Impact on Crosslinking Reaction Process
3.6. pH Impact on Crosslinking Reaction Process
3.7. Temperature Impact on Crosslinking Reaction Process
4. Conclusions
- (1)
- The polymer gel is majorly composed of anionic polymer, resorcinol, HMTA, and conditioner. The applicable concentration ranges for each component are 0.25~0.3% YG100 + 0.6~0.9% resorcinol + 0.2~0.4% HMTA + 0.08~0.27% conditioner (oxalic acid). The gelation time can be controlled within 13 to 40 h. The gel strength can be adjusted from 0.037 to 0.064 MPa at 50 °C.
- (2)
- The complex viscosity in relationship with frequency is drawn to reflect the reaction process of polymer and crosslinker. This process is further divided into three identical periods: induction period, fast crosslinking period, and stable period.
- (3)
- The induction period determines the mobility of the gel system, where it shows very low complex viscosity. After the induction period, the viscosity increases and mobility of the gel system decreases dramatically.
- (4)
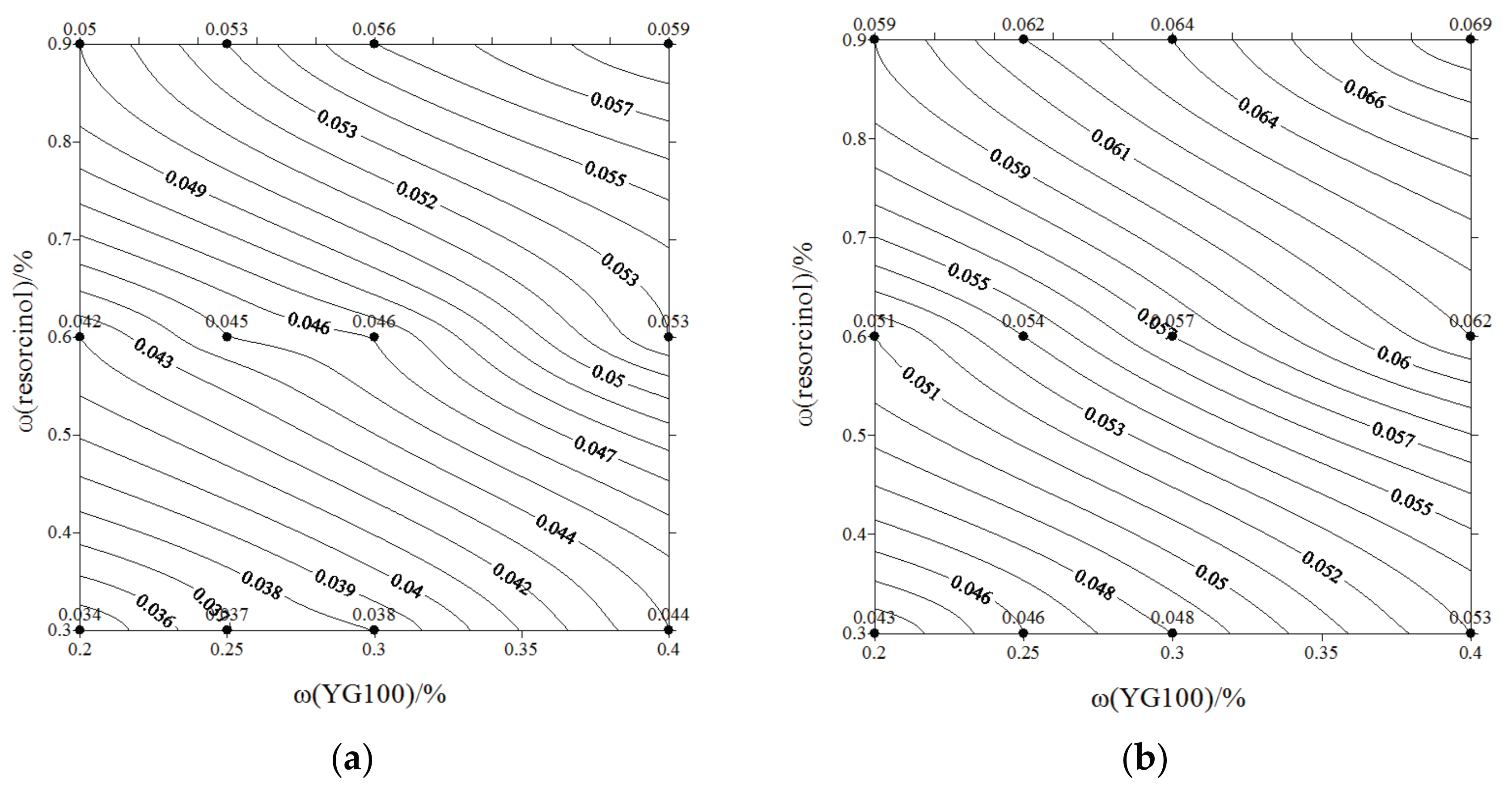
- The gel strength at different pH values are displayed in contour maps. With higher doses of components, lower pH, and higher temperature, the induction period becomes shorter, and the crosslinking process displays higher efficiency, resulting in a better gel stability and gel strength performance.
- (5)
- The gelation behavior was examined from the impact of various factors on the crosslinking reaction process. The relationship between each impact factors and the initial crosslinking time was described with mathematical equations.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hamouda, A.A.; Amiri, H.A.A. Factors affecting alkaline sodium silicate gelation for in-depth reservoir profile modification. Energies 2014, 7, 568–590. [Google Scholar] [CrossRef]
- Dai, C.; Zhao, G.; You, Q.; Zhao, M. A study on environment-friendly polymer gel for water shut-off treatments in low-temperature reservoirs. J. Appl. Polym. Sci. 2014, 131, 40154. [Google Scholar] [CrossRef]
- Chung, T.; Bae, W.; Nguyen, N.; Dang, C.; Lee, W.; Jung, B. A review of polymer conformance treatment: A successful guideline for water control in mature fields. Energy Sources Part A Recovery Utili. Environ. Eff. 2011, 34, 122–133. [Google Scholar] [CrossRef]
- Ju, B.; Qiu, X.; Dai, S.; Fan, T.; Wu, H.; Wang, X. A Study to Prevent Bottom Water From Coning in Heavy-Oil Reservoirs: Design and Simulation Approaches. J. Energy Resour. Technol. 2008, 130, 033102. [Google Scholar] [CrossRef]
- Vossoughi, S. Profile modification using in situ gelation technology—A review. J. Pet. Sci. Eng. 2000, 26, 199–209. [Google Scholar] [CrossRef]
- Liang, J.-T.; Sun, H.; Seright, R.S. Why Do Gels Reduce Water Permeability More Than Oil Permeability? SPE Reserv. Eng. 1995, 10, 282–286. [Google Scholar] [CrossRef]
- Seright, R.S. Disproportionate Permeability Reduction With Pore-Filling Gels. SPE J. 2009, 14, 5–13. [Google Scholar] [CrossRef]
- Liu, Y.; Bai, B.; Shuler, P. Application and Development of Chemical-Based Conformance Control Treatments in China Oilfields. In Proceedings of the SPE/DOE Symposium on Improved Oil Recovery, Tulsa, OK, USA, 22–26 April 2006. [Google Scholar]
- Kabir, A.H. Chemical Water & Gas Shutoff Technology—An Overview. In Proceedings of the SPE Asia Pacific Improved Oil Recovery Conference, Kuala Lumpur, Malaysia, 6–9 October 2001. [Google Scholar]
- Zhong, H.; Zhang, W.; Fu, J.; Lu, J.; Yin, H. The Performance of Polymer Flooding in Heterogeneous Type II Reservoirs—An Experimental and Field Investigation. Energies 2017, 10, 454. [Google Scholar] [CrossRef]
- Zhu, D.; Wei, L.; Wang, B.; Feng, Y. Aqueous hybrids of silica nanoparticles and hydrophobically associating hydrolyzed polyacrylamide used for EOR in high-temperature and high-salinity reservoirs. Energies 2014, 7, 3858–3871. [Google Scholar] [CrossRef]
- Sengupta, B.; Sharma, V.; Udayabhanu, G. A Study of the Effect of the Concentration of Constituents on the Characteristics of a Cross-Linked Polyacrylamide Gel. Pet. Sci. Technol. 2012, 30, 1865–1881. [Google Scholar] [CrossRef]
- Zhang, H.; Bai, B. Preformed-Particle-Gel Transport Through Open Fractures and Its Effect on Water Flow. SPE J. 2011, 16, 388–400. [Google Scholar]
- Al-Muntasheri, G.A. Conformance control with polymer gels: What it takes to be successful. Arab. J. Sci. Eng. 2012, 37, 1131–1141. [Google Scholar] [CrossRef]
- El-Karsani, K.S.M.; Al-Muntasheri, G.A.; Hussein, I.A. Polymer systems for water shutoff and profile modification: A review over the last decade. SPE J. 2014, 19, 135–149. [Google Scholar] [CrossRef]
- Akhlaghi Amiri, H.A.; Hamouda, A.A.; Roostaei, A. Sodium Silicate Behavior in Porous Media Applied for In-Depth Profile Modifications. Energies 2014, 7, 2004–2026. [Google Scholar] [CrossRef]
- Sydansk, R.D.; Southwell, G.P. More Than 12 Years of Experience with a Successful Conformance-Control Polymer Gel Technology. In Proceedings of the SPE/AAPG Western Regional Meeting, Long Beach, CA, USA, 19–22 June 2000. [Google Scholar]
- Lockhart, T.P. Chemical Properties of Chromium/Polyacrylamide Gels. SPE Adv. Technol. Ser. 1994, 2, 13–24. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, S.; Li, L.; Wu, R.; Liu, D.; Wu, J.; Wu, W. High-temperature-resistant polymer gel system with metal–organic mixed cross-linking agents. J. Appl. Polym. Sci. 2015, 132, 42261. [Google Scholar] [CrossRef]
- Goel, N.; Shah, S.N.; Yuan, W.L.; O’Rear, E.A. Suspension characteristics of borate-crosslinked gels: Rheology and atomic force microscopy measurements. J. Appl. Polym. Sci. 2001, 82, 2978–2990. [Google Scholar] [CrossRef]
- Bryant, S.L.; Bartosek, M.; Lockhart, T.P. Laboratory evaluation of phenol—Formaldehyde/polymer gelants for high-temperature applications. J. Pet. Sci. Eng. 1997, 17, 197–209. [Google Scholar] [CrossRef]
- Albonico, P.; Bartosek, M.; Malandrino, A.; Bryant, S.; Lockhart, T.P. Studies on Phenol-Formaldehyde Crosslinked Polymer Gels in Bulk and in Porous Media. In Proceedings of the SPE International Symposium on Oilfield Chemistry, San Antonio, TX, USA, 14–17 February 1995. [Google Scholar]
- ElKarsani, K.S.; Al-Muntasheri, G.A.; Sultan, A.S.; Hussein, I.A. Performance of PAM/PEI gel system for water shut-off in high temperature reservoirs: Laboratory study. J. Appl. Polym. Sci. 2015, 132, 41869. [Google Scholar] [CrossRef]
- Dovan, H.; Hutchins, R.; Sandiford, B. Delaying Gelation of Aqueous Polymers at Elevated Temperatures Using Novel Organic Crosslinkers. In Proceedings of the International Symposium on Oilfield Chemistry, Houston, TX, USA, 18–21 February 1997. [Google Scholar]
- Jia, H.; Zhao, J.-Z.; Jin, F.-Y.; Pu, W.-F.; Li, Y.-M.; Li, K.-X.; Li, J.-M. New insights into the gelation behavior of polyethyleneimine cross-linking partially hydrolyzed polyacrylamide gels. Ind. Eng. Chem. Res. 2012, 51, 12155–12166. [Google Scholar] [CrossRef]
- Zhuang, Y.; Pandey, S.; McCool, N.C.; Willhite, G. Permeability modification with sulfomethylated resorcinol-formaldehyde gel system. SPE Reserv. Eval. Eng. 2000, 3, 386–393. [Google Scholar] [CrossRef]
- Gommes, C.J.; Roberts, A.P. Structure development of resorcinol-formaldehyde gels: Microphase separation or colloid aggregation. Phys. Rev. E 2008, 77, 041409. [Google Scholar] [CrossRef] [PubMed]
- Yadav, U.S.; Mahto, V. Experimental studies, modeling and numerical simulation of gelation behavior of a partially hydrolyzed polyacrylamide-hexamine-pyrocatechol polymer gel system for profile modification jobs. Int. J. Adv. Pet. Eng. Technol. 2012, 1, 1–16. [Google Scholar]
- Liu, Y.; Dai, C.; Wang, K.; Zhao, M.; Zhao, G.; Yang, S.; Yan, Z.; You, Q. New insights into the hydroquinone (HQ)–hexamethylenetetramine (HMTA) gel system for water shut-off treatment in high temperature reservoirs. J. Ind. Eng. Chem. 2016, 35, 20–28. [Google Scholar] [CrossRef]
- He, H.; Wang, Y.; Zhang, J.; Xu, X.; Zhu, Y.; Bai, S. Comparison of Gelation Behavior and Morphology of Resorcinol–Hexamethylenetetramine–HPAM Gel in Bulk and Porous Media. Transp. Porous Media 2015, 109, 377–392. [Google Scholar] [CrossRef]
- Albonico, P.; Burrafato, G.; Di Lullo, A.; Lockhart, T. Effective Gelation-Delaying Additives for Cr+ 3/polymer Gels. In Proceedings of the SPE International Symposium on Oilfield Chemistry, New Orleans, LA, USA, 2–5 March 1993. [Google Scholar]
- Willhite, G.P.; Pancake, R.E. Controlling Water Production Using Gelled Polymer Systems. SPE Reserv. Eval. Eng. 2008, 11, 454–465. [Google Scholar] [CrossRef]
- Sydansk, R.D.; Argabright, P.A. Conformance Improvement in a Subterranean Hydrocarbon-Bearing Formation Using a Polymer Gel. Google Patents US4683949 A, 4 August 1987. [Google Scholar]
- He, H.; Wang, Y.; Zhang, J. Novel Gel with Controllable Strength for In-Depth Conformance Control: Bulk Gelation Performance and Propagation Properties in Porous Media. J. Dispers. Sci. Technol. 2015, 36, 626–633. [Google Scholar] [CrossRef]
- Haas, P.A.; Pitt, W.W., Jr.; Robinson, S.M.; Ryon, A.D. Preparation of metal oxide gel spheres with hexamethylenetetramine as an ammonia donor. Ind. Eng. Chem. Prod. Res. Dev. 1983, 22, 461–466. [Google Scholar] [CrossRef]












| pH | ω(YG100) % | ω(resorcinol) % | ω(HMTA) % | Gel Strength MPa |
|---|---|---|---|---|
| 6 | 0.2 | 0.3 | 0.2 | 0.034 |
| 0.25 | 0.3 | 0.2 | 0.037 | |
| 0.3 | 0.3 | 0.2 | 0.038 | |
| 0.4 | 0.3 | 0.2 | 0.044 | |
| 0.2 | 0.6 | 0.4 | 0.042 | |
| 0.25 | 0.6 | 0.4 | 0.045 | |
| 0.3 | 0.6 | 0.4 | 0.046 | |
| 0.4 | 0.6 | 0.4 | 0.053 | |
| 0.2 | 0.9 | 0.6 | 0.05 | |
| 0.25 | 0.9 | 0.6 | 0.053 | |
| 0.3 | 0.9 | 0.6 | 0.056 | |
| 0.4 | 0.9 | 0.6 | 0.059 | |
| 5 | 0.2 | 0.3 | 0.2 | 0.043 |
| 0.25 | 0.3 | 0.2 | 0.046 | |
| 0.3 | 0.3 | 0.2 | 0.048 | |
| 0.4 | 0.3 | 0.2 | 0.053 | |
| 0.2 | 0.6 | 0.4 | 0.051 | |
| 0.25 | 0.6 | 0.4 | 0.054 | |
| 0.3 | 0.6 | 0.4 | 0.057 | |
| 0.4 | 0.6 | 0.4 | 0.062 | |
| 0.2 | 0.9 | 0.6 | 0.059 | |
| 0.25 | 0.9 | 0.6 | 0.062 | |
| 0.3 | 0.9 | 0.6 | 0.064 | |
| 0.4 | 0.9 | 0.6 | 0.069 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Fang, Y.; Chen, A.; You, Q.; Dai, C.; Cheng, R.; Liu, Y. Gelation Behavior Study of a Resorcinol–Hexamethyleneteramine Crosslinked Polymer Gel for Water Shut-Off Treatment in Low Temperature and High Salinity Reservoirs. Energies 2017, 10, 913. https://doi.org/10.3390/en10070913
Sun Y, Fang Y, Chen A, You Q, Dai C, Cheng R, Liu Y. Gelation Behavior Study of a Resorcinol–Hexamethyleneteramine Crosslinked Polymer Gel for Water Shut-Off Treatment in Low Temperature and High Salinity Reservoirs. Energies. 2017; 10(7):913. https://doi.org/10.3390/en10070913
Chicago/Turabian StyleSun, Yongpeng, Yanchao Fang, Ang Chen, Qing You, Caili Dai, Rui Cheng, and Yifei Liu. 2017. "Gelation Behavior Study of a Resorcinol–Hexamethyleneteramine Crosslinked Polymer Gel for Water Shut-Off Treatment in Low Temperature and High Salinity Reservoirs" Energies 10, no. 7: 913. https://doi.org/10.3390/en10070913
APA StyleSun, Y., Fang, Y., Chen, A., You, Q., Dai, C., Cheng, R., & Liu, Y. (2017). Gelation Behavior Study of a Resorcinol–Hexamethyleneteramine Crosslinked Polymer Gel for Water Shut-Off Treatment in Low Temperature and High Salinity Reservoirs. Energies, 10(7), 913. https://doi.org/10.3390/en10070913






