Capacity Decay Mechanism of the LCO + NMC532/Graphite Cells Combined with Post-Mortem Technique
Abstract
:1. Introduction
2. Studies of Full Cells
2.1. Long-Term Cycling Studies of Full Cells at 1 C Rate
2.2. Detailed Studies of Thermodynamic Characteristics of Full Cells at a Low Rate
2.3. Analysis of Kinetic Characteristics of Full Cells
3. Studies of Half Cells
3.1. Harvest of Half Cells
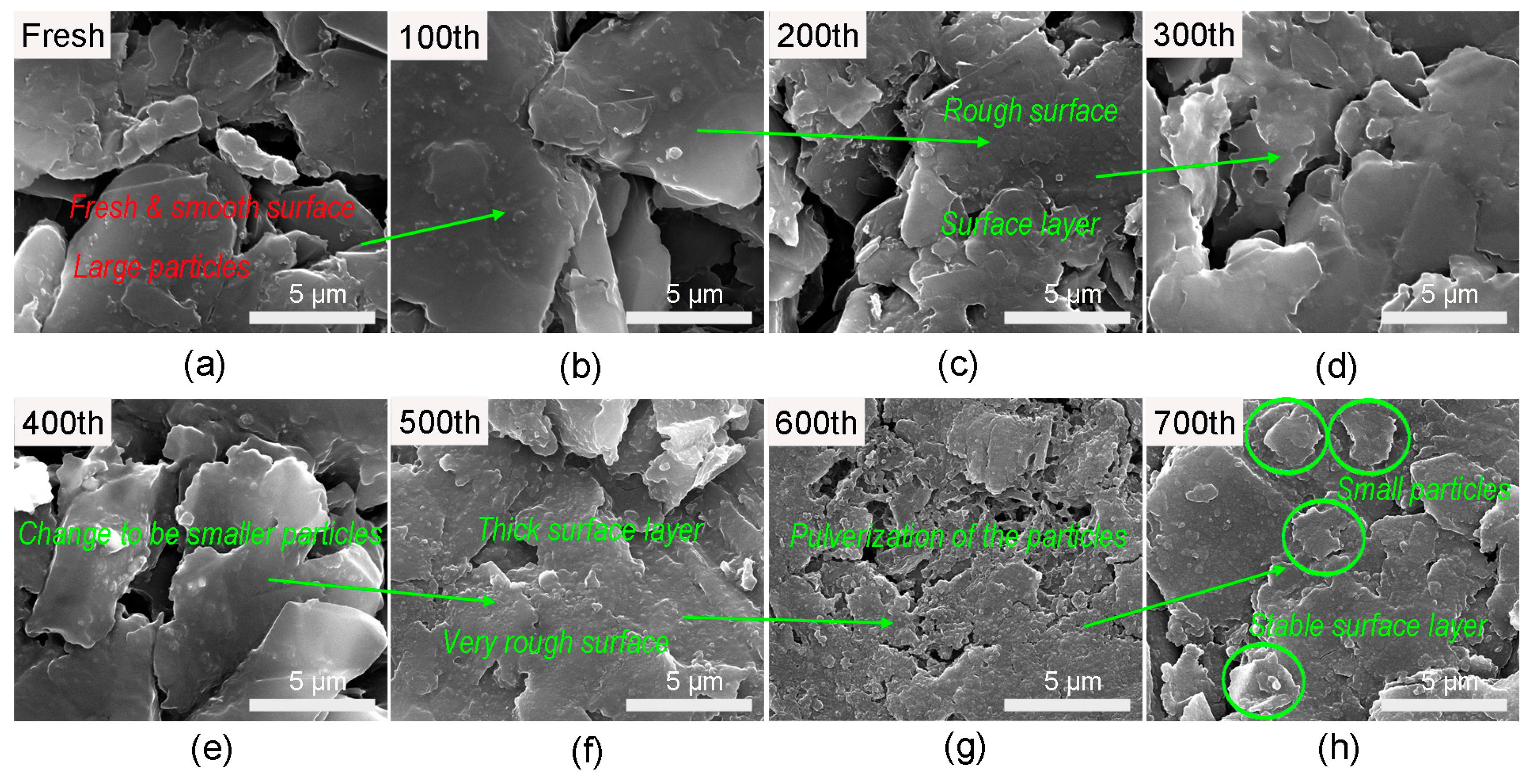
3.2. Morphology and Surface Chemistry Studies of the Harvested Electrodes
4. Materials and Methods
4.1. Electrochemical Performance Tests of the Full Cells
4.2. Morphology and Surface Chemistry Characterization of the Harvested Electrodes
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tarascon, J.-M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Whittingham, M.S. Lithium batteries and cathode materials. Chem. Rev. 2004, 104, 4271–4302. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.; Meng, Y.S.; Bréger, J.; Gray, C.P.; Ceder, G. Electrodes with high power and high capacity for rechargeable lithium batteries. Science 2006, 311, 977–980. [Google Scholar] [CrossRef] [PubMed]
- Armand, M.; Tarascon, J.-M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Scrosati, B.; Hassoun, J.; Sun, Y.-K. Lithium-ion batteries. A look into the future. Energy Environ. Sci. 2011, 4, 3287–3295. [Google Scholar] [CrossRef]
- Thackeray, M.M.; Wolverton, C.; Isaacs, E.D. Electrical energy storage for transportation-approaching the limits of, and going beyond, lithium-ion batteries. Energy Environ. Sci. 2012, 5, 7854–7863. [Google Scholar] [CrossRef]
- Mizushima, K.; Jones, P.C.; Wiseman, P.J.; Goodenough, J.B. LixCoO2 (0 < x ≤ −1): A new cathode material for batteries of high energy density. Mater. Res. Bull. 1980, 15, 783–789. [Google Scholar]
- Antolini, E. LiCoO2: Formation, structure, lithium and oxygen nonstoichiometry, electrochemical behaviour and transport properties. Solid State Ion. 2004, 170, 159–171. [Google Scholar] [CrossRef]
- Liu, Z.; Yu, A.; Lee, J.Y. Synthesis and characterization of LiNi1-x-yCoxMnyO2 as the cathode materials of secondary lithium batteries. J. Power Sources 1999, 81–82, 416–419. [Google Scholar] [CrossRef]
- Hwang, B.J.; Tsai, Y.W.; Chen, C.H.; Santhanam, R. Influence of Mn content on the morphology and electrochemical performance of LiNi1-x-yCoxMnyO2 cathode materials. J. Mater. Chem. 2003, 13, 1962–1968. [Google Scholar] [CrossRef]
- Sun, Y.-K.; Myung, S.-T.; Park, B.-C.; Prakash, J.; Belharouak, I.; Amine, K. High-energy cathode material for long-life and safe lithium batteries. Nat. Mater. 2009, 8, 320–324. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Zheng, J.; Cui, S.; Song, X.; Su, Y.; Deng, W.; Wu, Z.; Wang, X.; Wang, W.; Rao, M.; Lin, Y.; Wang, C.; Amine, K.; Pan, F. Kinetics tuning of Li-ion diffusion in layered Li(NixMnyCoz)O2. J. Am. Chem. Soc. 2015, 137, 8364–8367. [Google Scholar] [CrossRef] [PubMed]
- Noh, M.; Cho, J. Optimized synthetic conditions of LiNi0.5Co0.2Mn0.3O2 Cathode materials for high rate lithium batteries via co-precipitation method. J. Electrochem. Soc. 2013, 160, A105–A111. [Google Scholar] [CrossRef]
- Su, Y.; Cui, S.; Zhuo, Z.; Yang, W.; Wang, X.; Pan, F. Enhancing the high-voltage cycling performance of LiNi0.5Mn0.3Co0.2O2 by retarding its interfacial reaction with an electrolyte by atomic-layer-deposited Al2O3. ACS Appl. Mater. Interfaces 2015, 7, 25105–25112. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Su, C.-C.; Peebles, C.; Feng, Z.; Connell, J.G.; Liao, C.; Wang, Y.; Shkrob, I.A.; Zhang, Z. Mechanistic insight in the function of phosphite additives for protection of LiNi0.5Co0.2Mn0.3O2 cathode in high voltage Li-ion cells. ACS Appl. Mater. Interfaces 2016, 8, 11450–11458. [Google Scholar] [CrossRef] [PubMed]
- Flandrois, S.; Simon, B. Carbon materials for lithium-ion rechargeable batteries. Carbon 1999, 37, 165–180. [Google Scholar] [CrossRef]
- An, S.J.; Li, J.; Daniel, C.; Mohanty, D.; Nagpure, S.; Wood, D.L., III. The state of understanding of the lithium-ion-battery graphite solid electrolyte interphase (SEI) and its relationship to formation cycling. Carbon 2016, 105, 52–76. [Google Scholar] [CrossRef]
- Wong, D.; Shrestha, B.; Wetz, D.A.; Heinzel, J.M. Impact of high rate discharge on the aging of lithium nickel cobalt aluminum oxide batteries. J. Power Sources 2015, 280, 363–372. [Google Scholar] [CrossRef]
- Dubarry, M.; Liaw, B.Y. Identify capacity fading mechanism in a commercial LiFePO4 cell. J. Power Sources 2009, 194, 541–549. [Google Scholar] [CrossRef]
- Sarre, G.; Blanchard, P.; Broussely, M. Aging of lithium-ion batteries. J. Power Sources 2004, 127, 65–71. [Google Scholar] [CrossRef]
- Zhang, S.S. The effect of the charging protocol on the cycle life of a Li-ion battery. J. Power Sources 2006, 161, 1385–1391. [Google Scholar] [CrossRef]
- Guan, T.; Zuo, P.; Sun, S.; Du, C.; Zhang, L.; Cui, Y.; Yang, L.; Gao, Y.; Yin, G.; Wang, F. Degradation mechanism of LiCoO2/mesocarbon microbeads battery based on accelerated aging tests. J. Power Sources 2014, 268, 816–823. [Google Scholar] [CrossRef]
- Kobayashi, H.; Shikano, M.; Koike, S.; Sakaebe, H.; Tatsumi, K. Investigation of positive electrodes after cycle testing of high-power Li-ion battery cells I. An approach to the power fading mechanism using XANES. J. Power Sources 2007, 174, 380–386. [Google Scholar] [CrossRef]
- Abraham, D.P.; Liu, J.; Chen, C.H.; Hyung, Y.E.; Stoll, M.; Elsen, N.; MacLaren, S.; Twesten, R.; Haasch, R.; Sammannb, E.; Petrov, I.; Amine, K.; Henriksen, G. Diagnosis of power fade mechanisms in high-power lithium-ion cells. J. Power Sources 2003, 119–121, 511–516. [Google Scholar] [CrossRef]
- Zheng, H.; Sun, Q.; Liu, G.; Song, X.; Battaglia, V.S. Correlation between dissolution behavior and electrochemical cycling performance for LiNi1/3Co1/3Mn1/3O2-based cells. J. Power Sources 2012, 207, 134–140. [Google Scholar] [CrossRef]
- Lee, Y.-J.; Choi, H.-Y.; Ha, C.-W.; Yu, J.-H.; Hwang, M.-J.; Doh, C.-H.; Choi, J.-H. Cycle life modeling and the capacity fading mechanisms in a graphite/LiNi0.6Co0.2Mn0.2O2 cell. J. Appl. Electrochem. 2015, 45, 419–426. [Google Scholar] [CrossRef]
- Jung, S.-K.; Gwon, H.; Hong, J.; Park, K.-Y.; Seo, D.-H.; Kim, H.; Hyun, J.; Yang, W.; Kang, K. Understanding the degradation mechanisms of LiNi0.5Co0.2Mn0.3O2 cathode material in lithium ion batteries. Adv. Energy Mater. 2013, 4, 1300787. [Google Scholar] [CrossRef]
- Gilbert, J.A.; Bareño, J.; Spila, T.; Trask, S.E.; Miller, D.J.; Polzin, B.J.; Jansen, A.N.; Abraham, D.P. Cycling behavior of NCM523/graphite lithium-ion cells in the 3–4.4 V range: Diagnostic studies of full cells and harvested electrodes. J. Electrochem. Soc. 2017, 164, A6054–A6065. [Google Scholar] [CrossRef]
- Markervich, E.; Salitra, G.; Levi, M.D.; Aurbach, D. Capacity fading of lithiated graphite electrodes studied by a combination of electroanalytical methods; Raman spectroscopy and SEM. J. Power Sources 2005, 146, 146–150. [Google Scholar] [CrossRef]
- Dubarry, M.; Svoboda, V.; Hwu, R.; Liaw, B.Y. Incremental capacity analysis and close-to-equilibrium OCV measurements to quantify capacity fade in commercial rechargeable lithium batteries. Electrochem. Solid ST 2006, 9, A454–A457. [Google Scholar] [CrossRef]
- Reimers, J.N.; Dahn, J.R. Electrochemical and in situ X-ray diffraction studies of lithium intercalation in LixCoO2. J. Electrochem. Soc. 1992, 139, 2091–2097. [Google Scholar] [CrossRef]
- Huang, B.; Jang, Y.-I.; Chiang, Y.-M.; Sadoway, D.R. Electrochemical evaluation of LiCoO2 synthesized by decomposition and intercalation of hydroxides for lithium-ion battery applications. J. Appl. Electrochem. 1998, 28, 1365–1369. [Google Scholar] [CrossRef]
- Pentyala, N.; Guduru, R.K.; Mohanty, P.S. Binder free porous ultrafine/nano structured LiCoO2 cathode from plasma deposited cobalt. Electrochim. Acta 2011, 56, 9851–9859. [Google Scholar] [CrossRef]
- Dixit, M.; Kosa, M.; Lavi, O.S.; Markovsky, B.; Aurbach, D.; Major, D.T. Thermodynamic and kinetic studies of LiNi0.5Co0.2Mn0.3O2 as a positive electrode material for Li-ion batteries using first principles. Phys. Chem. Chem. Phys. 2016, 18, 6799–6812. [Google Scholar] [CrossRef] [PubMed]
- Dahn, J.R. Phase diagram of LixC6. Phys. Rev. B 1991, 44, 9170–9177. [Google Scholar] [CrossRef]
- Ohzuku, T.; Iwakoshi, Y.; Sawai, K. Formation of lithium-graphite intercalation compounds in nonaqueous electrolytes and their application as a negative electrode for a lithium ion (shuttlecock) cell. J. Electrochem. Soc. 1993, 140, 2490–2498. [Google Scholar] [CrossRef]
- Vetter, J.; Novák, P.; Wagner, M.R.; Veit, C.; Möller, K.-C.; Besenhard, J.O.; Winter, M.; Wohlfahrt-Mehrens, M.; Vogler, C.; Hammouche, A. Ageing mechanisms in lithium-ion batteries. J. Power Sources 2005, 147, 269–281. [Google Scholar] [CrossRef]
- Barré, A.; Deguilhem, B.; Grolleau, S.; Gérard, M.; Suard, F.; Riu, D. A review on lithium-ion battery ageing mechanisms and estimations for automotive applications. J. Power Sources 2013, 241, 680–689. [Google Scholar] [CrossRef]
- Dubarry, M.; Truchot, C.; Liaw, B.Y.; Gering, K.; Sazhin, S.; Jamison, D.; Michelbacher, C. Evaluation of commercial lithium-ion cells based on composite positive electrode for plug-in hybrid electric vehicle applications. Part II. Degradation mechanism under 2 C cycle aging. J. Power Sources 2011, 196, 10336–10343. [Google Scholar] [CrossRef]
- Zhang, Q.; White, R.E. Calendar life study of Li-ion pouch cells. J. Power Sources 2007, 173, 990–997. [Google Scholar] [CrossRef]
- Buchberger, I.; Seidlmayer, S.; Pokharel, A.; Pianaa, M.; Hattendorff, J.; Kudejova, P.; Gilles, R.; Gasteiger, H.A. Aging analysis of graphite/ LiNi1/3Mn1/3Co1/3O2 cells using XRD; PGAA and AC impedance. J. Electrochem. Soc. 2015, 162, A2737–A2746. [Google Scholar] [CrossRef]








| Peak | d/e | i | c/f | b/g | a/h | j |
|---|---|---|---|---|---|---|
| Redox couple | C2②*A③ | - | C2①*A② C1③*A② | C1③*A① | C2①*A① | - |
| Redox couple | - | C2②*A② | - | - | - | C1②*A① |
| Cycle Number (n) | 10% SOC CH | 40% SOC CH | 80% SOC CH | ||||||
|---|---|---|---|---|---|---|---|---|---|
| f1 (Hz) | f2 (Hz) | R2 (mΩ) | f1 (Hz) | f2 (Hz) | R2 (mΩ) | f1 (Hz) | f2 (Hz) | R2 (mΩ) | |
| 0 | 6.6 | 0.18 | 9.1 | NA | NA | NA | NA | NA | NA |
| 100 | 20.1 | 0.09 | 24.1 | 28.0 | 0.34 | 9.0 | NA | NA | NA |
| 200 | 28.0 | 0.05 | 37.1 | 28.0 | 0.18 | 12.5 | 38.5 | 0.34 | 8.4 |
| 300 | 28.0 | 0.05 | 49.3 | 28.0 | 0.18 | 14.5 | 38.5 | 0.34 | 9.5 |
| 400 | 28.0 | 0.05 | 59.4 | 28.0 | 0.18 | 17.3 | 38.5 | 0.34 | 11.2 |
| 500 | 38.5 | 0.02 | 85.4 | 28.0 | 0.09 | 22.1 | 38.5 | 0.18 | 16.3 |
| 600 | 38.5 | 0.02 | 94.4 | 28.0 | 0.09 | 31.1 | 38.5 | 0.18 | 18.7 |
| 700 | 32.6 | 0.01 | 170.0 | 28.0 | 0.05 | 39.6 | 38.5 | 0.18 | 21.0 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Jiang, J.; Zhang, W. Capacity Decay Mechanism of the LCO + NMC532/Graphite Cells Combined with Post-Mortem Technique. Energies 2017, 10, 1147. https://doi.org/10.3390/en10081147
Zhang L, Jiang J, Zhang W. Capacity Decay Mechanism of the LCO + NMC532/Graphite Cells Combined with Post-Mortem Technique. Energies. 2017; 10(8):1147. https://doi.org/10.3390/en10081147
Chicago/Turabian StyleZhang, Linjing, Jiuchun Jiang, and Weige Zhang. 2017. "Capacity Decay Mechanism of the LCO + NMC532/Graphite Cells Combined with Post-Mortem Technique" Energies 10, no. 8: 1147. https://doi.org/10.3390/en10081147
APA StyleZhang, L., Jiang, J., & Zhang, W. (2017). Capacity Decay Mechanism of the LCO + NMC532/Graphite Cells Combined with Post-Mortem Technique. Energies, 10(8), 1147. https://doi.org/10.3390/en10081147




