Experimental Study of Reverse Underground Coal Gasification
Abstract
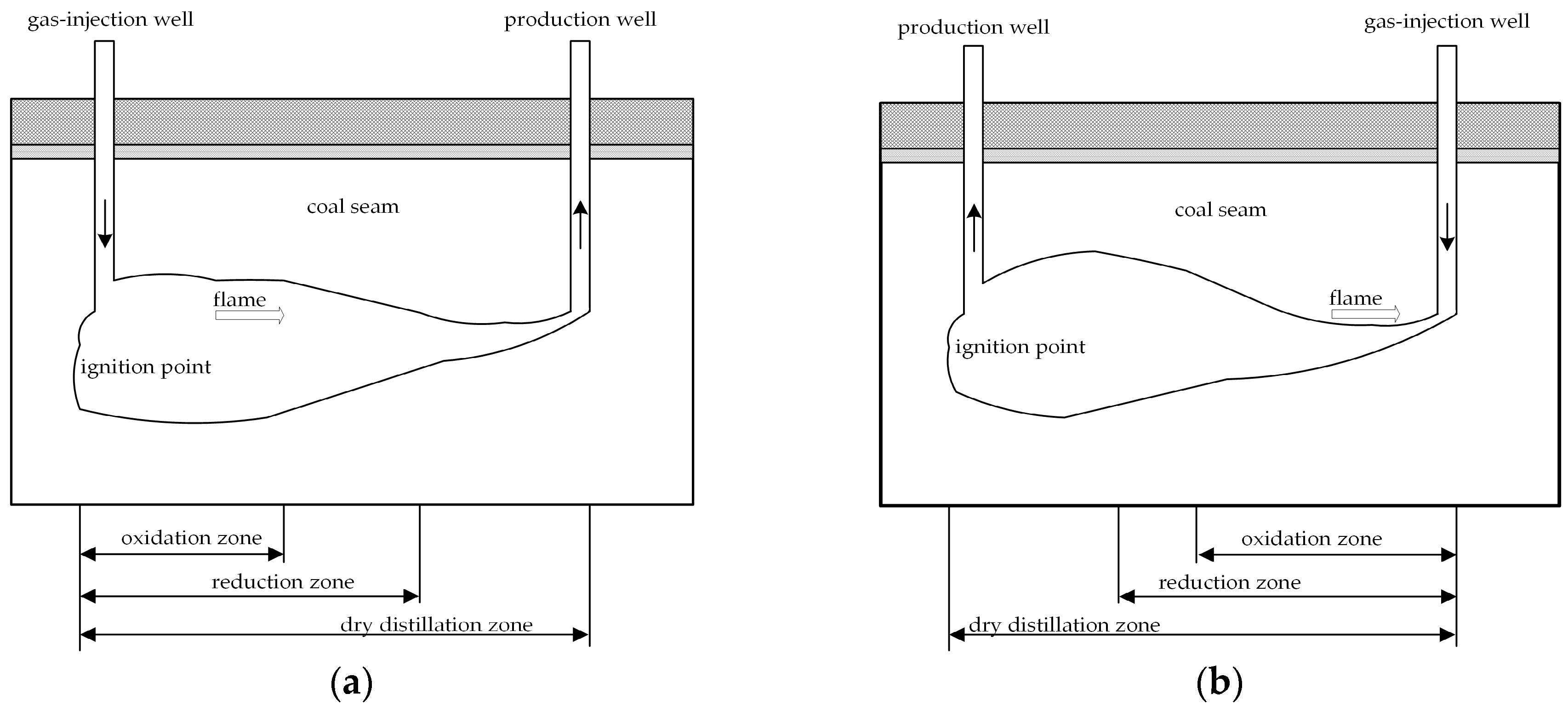
:1. Introduction
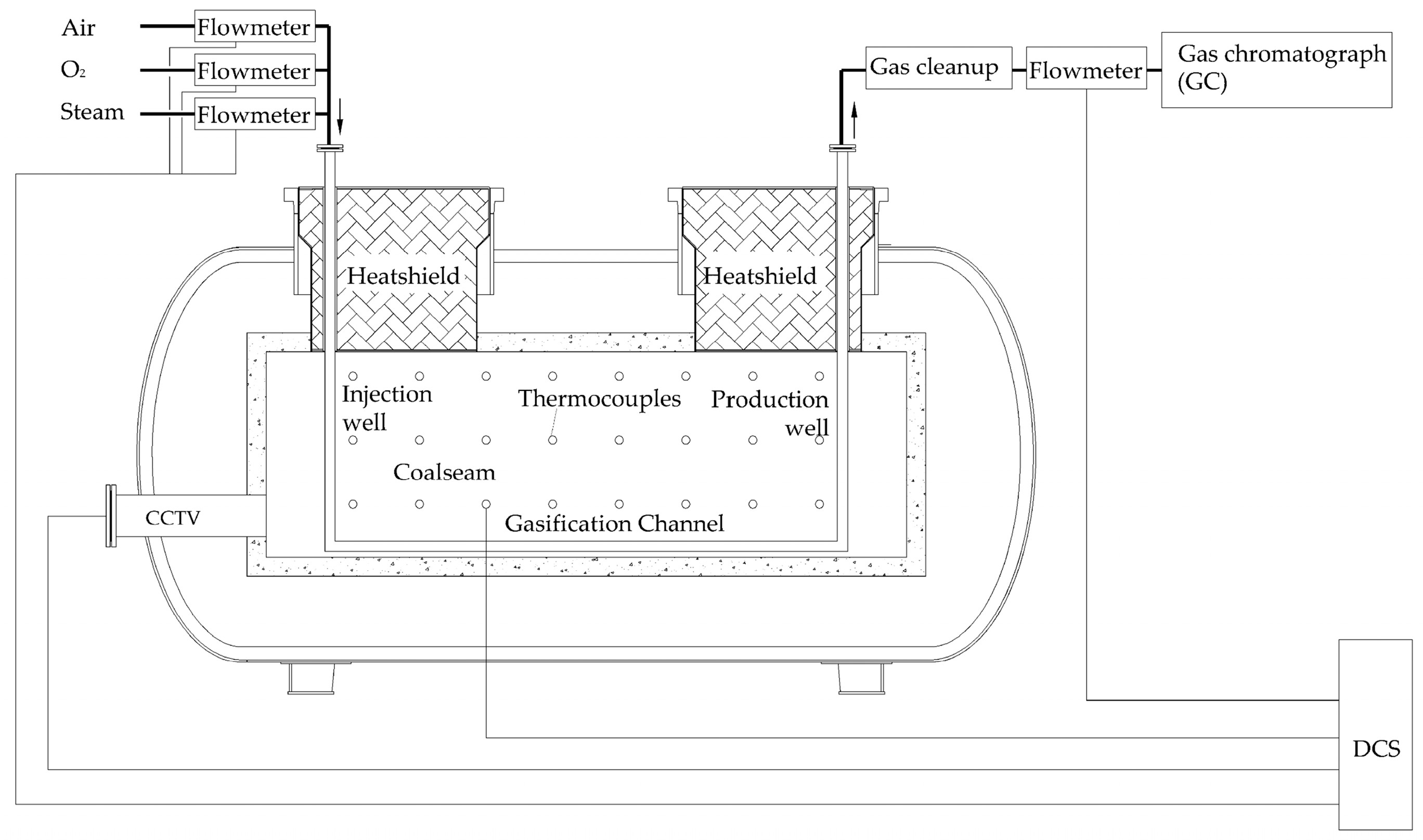
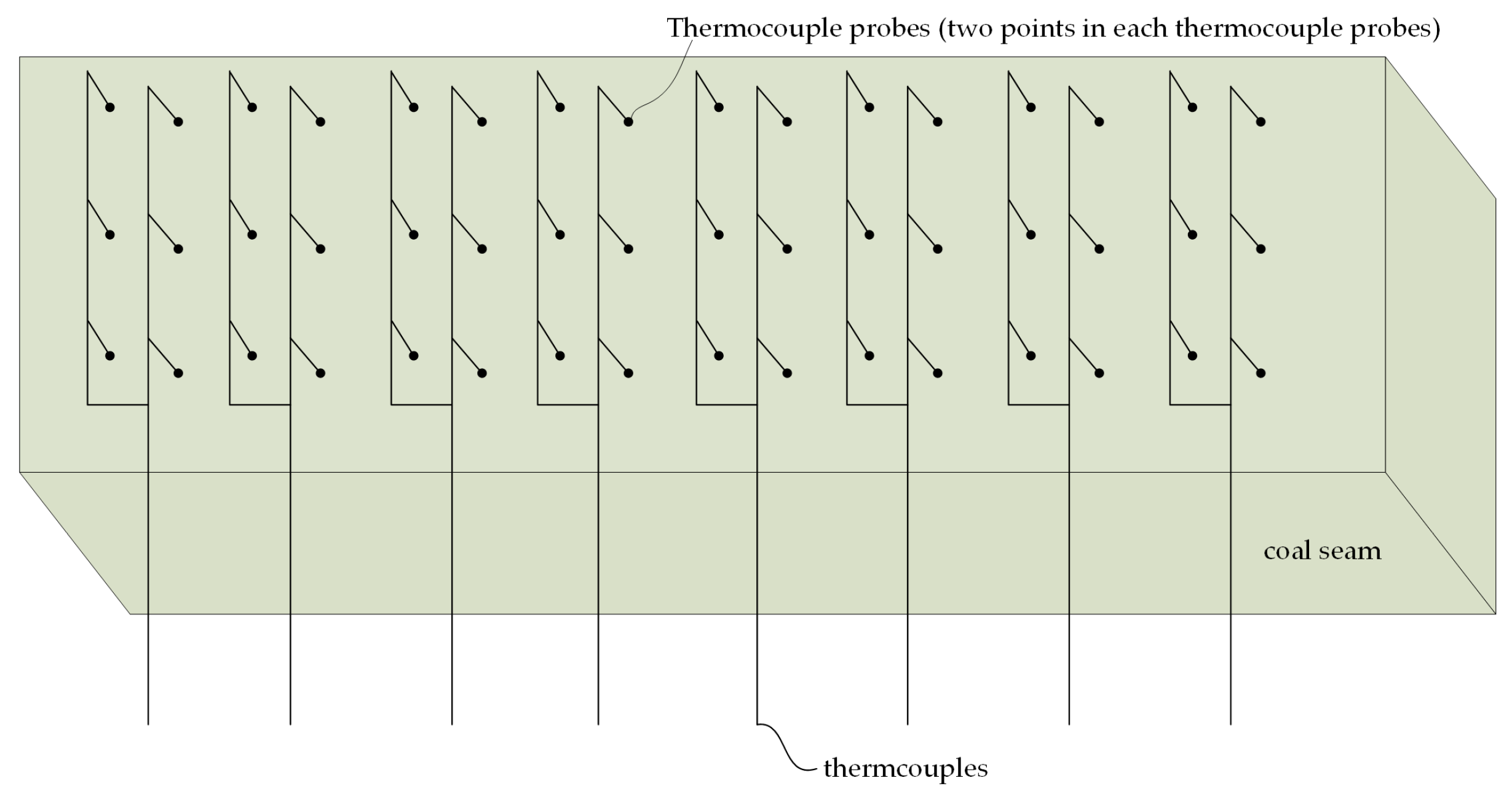
2. Experimental
2.1. Methodology and Material
2.2. Experimental Reactor
2.3. Experimental Procedure
3. Experimental Results
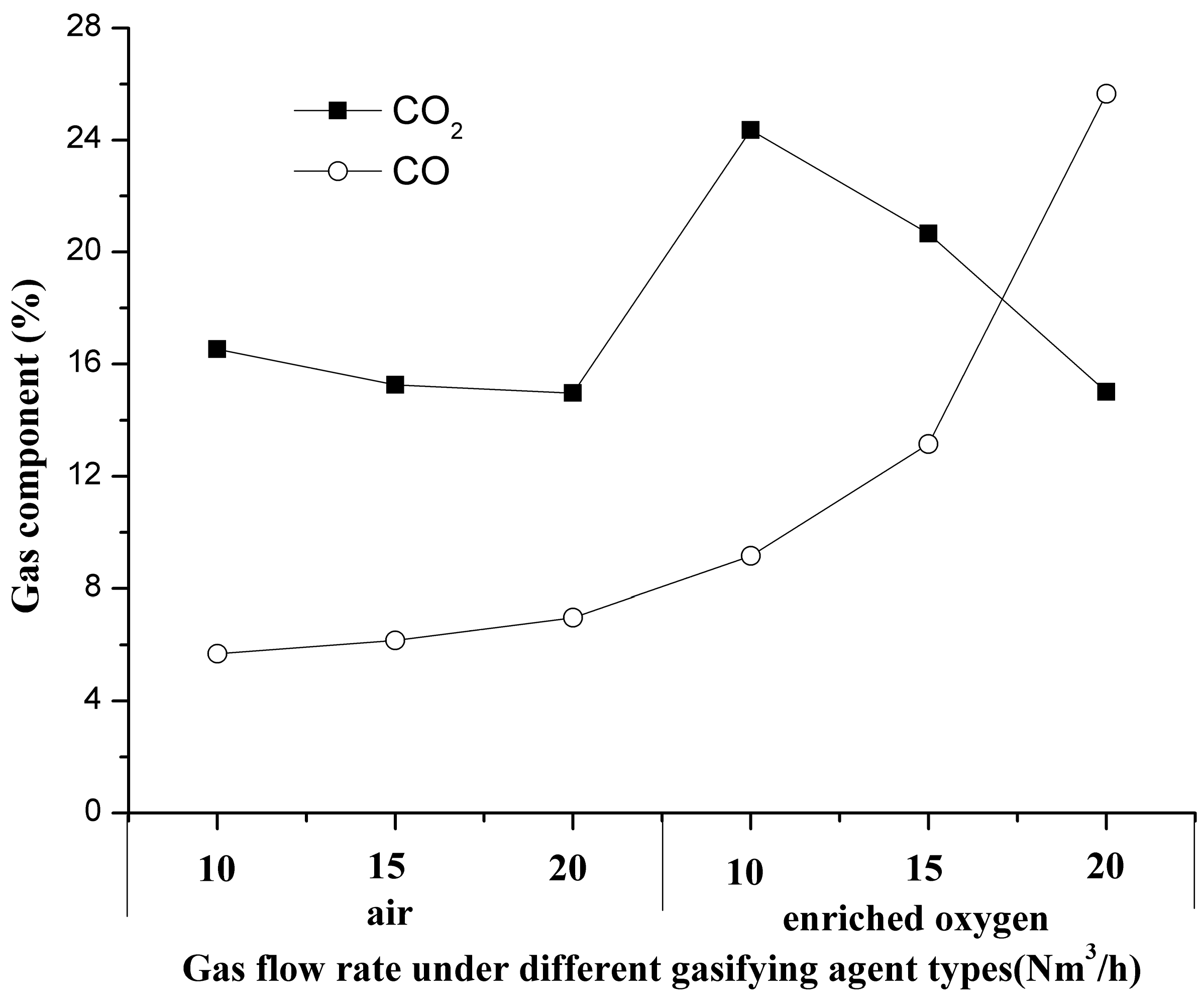
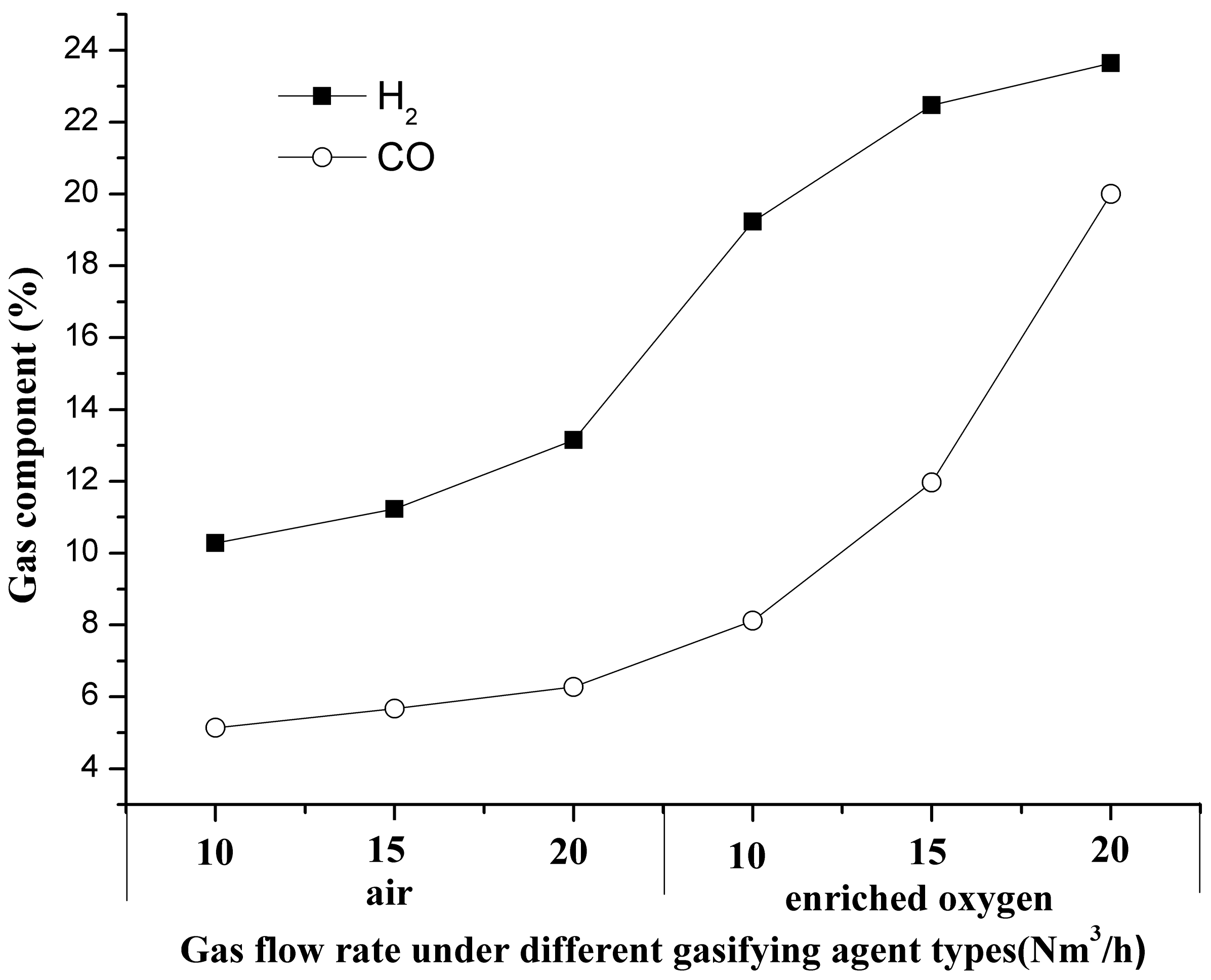
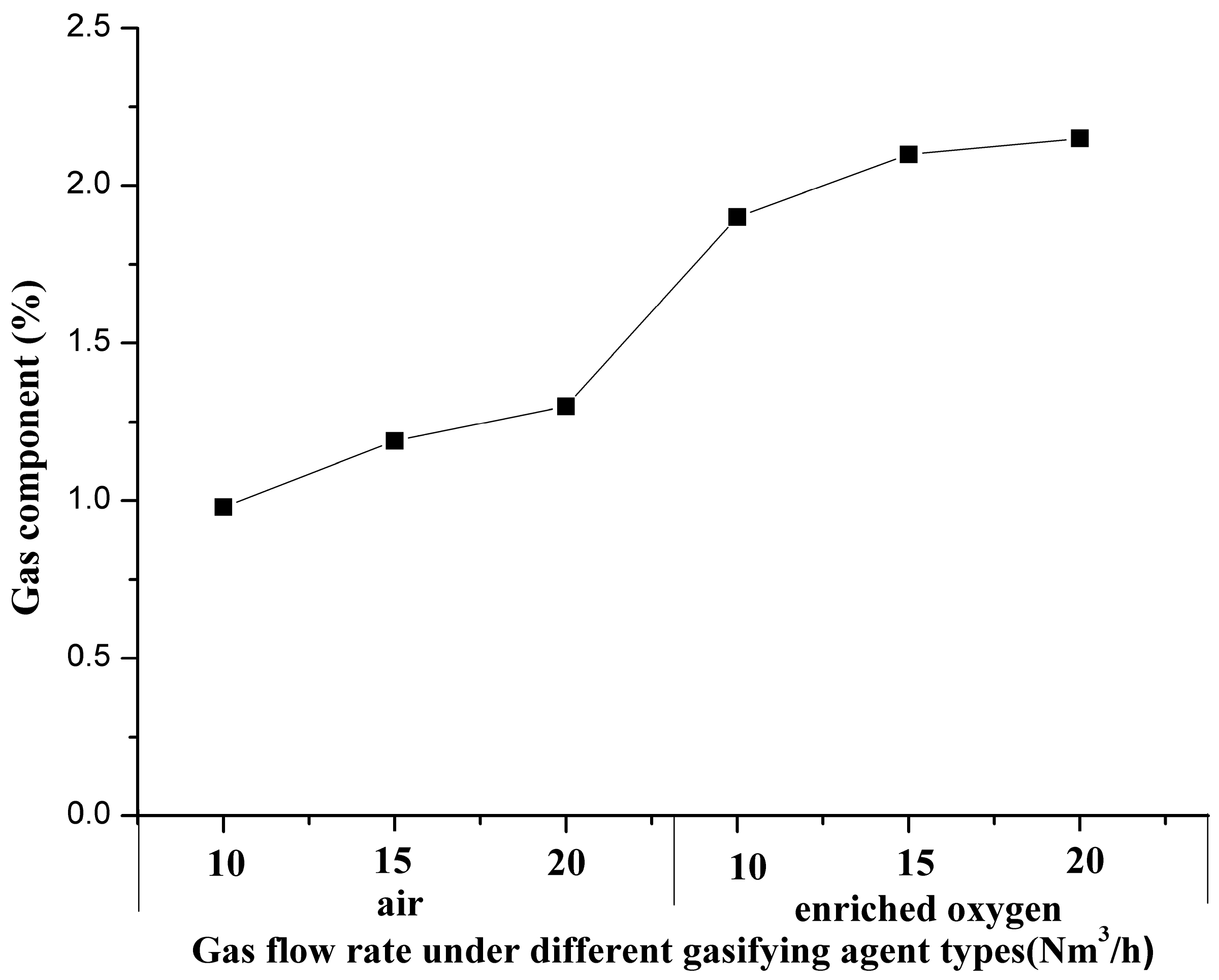
3.1. Characteristics of Gas Components
3.2. Temperature Field Transformation
3.3. Growth Rate of the Flame Front
3.4. Characteristics of Gas Components in the Three Zones
3.4.1. Characteristics of Gas Components in the Oxidation Zone
3.4.2. Characteristics of Gas Components in the Reduction Zone
3.4.3. Characteristics of Gas Components in the Dry Distillation Zone
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Glossary
| CCTV | closed-circuit industrial television |
| DCS | distributed control system |
| UCG | underground coal gasification |
| CRIP | controlled retraction injection point |
References
- Stańczyk, K.; Smoliński, A.; Kapusta, K.; Wiatowski, M.; Świadrowski, J.; Kotyrba, A.; et al. Dynamic experimental simulation of hydrogen oriented underground gasification of lignite. Fuel 2010, 89, 3307–3314. [Google Scholar] [CrossRef]
- Prabu, V.; Jayanti, S. Underground coal-air gasification based solid oxide fuel cell system. Appl. Energy 2012, 94, 406–414. [Google Scholar] [CrossRef]
- Prabu, V. Integration of in-situ CO2-oxy coal gasification with advanced power generating systems performing in a chemical looping approach of clean combustion. Appl. Energy 2015, 140, 1–13. [Google Scholar] [CrossRef]
- Olateju, B.; Kumar, A. Techno-economic assessment of hydrogen production from underground coal gasification (UCG) in Western Canada with carbon capture and sequestration (CCS) for upgrading bitumen from oil sands. Appl. Energy 2013, 111, 428–440. [Google Scholar] [CrossRef]
- Imran, M.; Kumar, D.; Kumar, N.; Qayyum, A.; Saeed, A.; Bhatti, M.S. Environmental concerns of underground coal gasification. Renew. Sustain. Energy Rev. 2014, 31, 600–610. [Google Scholar] [CrossRef]
- Kacur, J.; Durdán, M.; Laciak, M.; Flegner, P. Impact analysis of the oxidant in the process of underground coal gasification. Measurement 2014, 51, 147–155. [Google Scholar] [CrossRef]
- Doerell, P.E. All future energy will have to be “clean”. Appl. Energy 1999, 64, 79–88. [Google Scholar] [CrossRef]
- Friedmann, S.J.; Upadhye, R.; Kong, F.M. Prospects for underground coal gasification in carbon-constrained world. Energy Procedia 2009, 1, 4551–4557. [Google Scholar] [CrossRef]
- Liu, S.Q.; Wang, Y.Y.; Zhao, K.; Yang, N. Enhanced-hydrogen gas production through underground gasification of lignite. Min. Sci. Technol. 2009, 19, 389–394. [Google Scholar] [CrossRef]
- Perkins, G.; Sahajwalla, V. Modelling of heat and mass transport phenomena and chemical reaction in underground coal gasification. Chem. Eng. Res. Des. 2007, 85, 329–343. [Google Scholar] [CrossRef]
- Yang, L.H.; Zhang, X.; Liu, S.Q.; Yu, L.; Zhang, W.L. Field test of large-scale hydrogen manufacturing from underground coal gasification (UCG). Int. J. Hydrogen Energy 2008, 33, 1275–1285. [Google Scholar] [CrossRef]
- Yang, L.H. Study of the model experiment of blinding-hole UCG. Fuel Process. Technol. 2003, 82, 11–25. [Google Scholar] [CrossRef]
- Prabu, V.; Jayanti, S. Simulation of cavity formation in underground coal gasification using bore hole combustion experiments. Energy 2011, 36, 5854–5864. [Google Scholar] [CrossRef]
- Kapadia, P.R.; Wang, J.; Kallos, M.S.; Gates, I.D. Practical process design for in situ gasification of bitumen. Appl. Energy 2013, 107, 281–296. [Google Scholar] [CrossRef]
- Yang, L.H.; Liang, J.; Yu, L. Clean coal technology—Study on the pilot project experiment of underground coal gasification. Energy 2003, 28, 1445–1450. [Google Scholar] [CrossRef]
- Dmitry, N.; Ovid, A.; Klimenko, A. Flame propagation in a gasification channel. Energy 2010, 35, 1264–1273. [Google Scholar] [Green Version]
- Blinderman, M.; Saulov, D.; Klimenko, A. Forward and reverse combustion linking in underground coal gasification. Energy 2008, 33, 446–454. [Google Scholar] [CrossRef]
- Bhutto, A.; Bazmi, A.; Zahedi, G. Underground coal gasification: From fundamentals to applications. Prog. Energy Combust. Sci. 2013, 39, 189–214. [Google Scholar] [CrossRef]
- Blinderman, M.; Klimenko, A. Theory of reverse combustion linking. Combust. Flame 2007, 150, 232–245. [Google Scholar] [CrossRef] [Green Version]
- Cui, Y.; Liang, J.; Wang, Z.; Zhang, X.; Fan, C.; Liang, D.; Wang, X. Forward and reverse combustion gasification of coal with production. Appl. Energy 2014, 131, 9–19. [Google Scholar] [CrossRef]
- Britten, J.A.; Krantz, W.B.; Gunn, R.D. Modeling studies of reverse combustion linking at high pressures. In Proceedings of the 8th Underground Coal Conversion Symposium; Sandia National Laboratories Report Sand 82-2355. Sandia National Laboratories: Albuquerque, NM, USA, 1982; pp. 515–523. [Google Scholar]
- Liu, S.Q.; Liang, J.; Yu, L. Study on auxiliary gasification technology of CO2 control in the process of underground coal gasification. Coal Convers. 1999, 22, 50–53. (In Chinese) [Google Scholar]
- Gregg, D.; Hill, R.W.; Olness, D. An Overview of the Soviet Effort in Underground Gasification of Coal; Report No. UCR-50024; Lawrence Livermore Laboratories: Livermore, CA, USA, 1976. [Google Scholar]
- Hommertm, P.; Beard, S. Description of reverse combustion linking and forward gasification during Underground Coal Gasification. In Proceedings of the 3rd Underground Coal Conversion Symposium, Fallen Leaf Lake, CA, USA, 6–9 June 1977; pp. 267–279. [Google Scholar]
- Glaser, R.; Gunn, R.; Krantz, W.; Breidung, P.; Gudenau, H. Unexpected aspects of reverse combustion: Effects of pressure, volatility and chemical reactivity. In Proceedings of the 9th Underground Coal Conversion Symposium, Bloomingdale, IL, USA, 7–10 August 1983; pp. 219–226. [Google Scholar]
- Su, R.; Engleman, V. Reverse combustion linking phenomena in bituminous coal at high pressure. In Proceedings of the 5th Underground Coal Conversion Symposium, Alexandria, VA, USA, 18–21 June 1979; pp. 323–330. [Google Scholar]
- Skafa, P. Underground Coal Gasification; Gosgortechizdat: Moscow, Russia, 1960. (In Russian) [Google Scholar]
- Cupps, C.; Land, C.; Marchant, L. Field experiment of in-situ oil recovery from a Utah tar sand by reverse combustion. AIChE Symp. Ser. 1976, 155, 61–67. [Google Scholar]
- Gunn, R.; Krantz, W. Reverse combustion instabilities in tar sands and coal. Soc. Pet. Eng. J. 1980, 20, 267–277. [Google Scholar] [CrossRef]
- Evgeny, S.; Arvind, V. Underground coal gasification: A brief review of current status. Ind. Eng. Chem. Res. 2009, 48, 7865–7875. [Google Scholar]
- Camp, D.W. Underground coal gasification research and development in the United States. Undergr. Coal Gasif. Combust. 2018, 59–127. [Google Scholar] [CrossRef]
- Liang, J.; Yu, L. Study of two-stage underground coal gasification in counter directions. J. China Coal Soc. 1996, 21, 68–72. (In Chinese) [Google Scholar]
- Yang, L.H.; Liang, J. Model test two-stage underground coal gasification in counter directions with gently inclined coal seam. J. Chongqing Univ. 2003, 26, 47–50. (In Chinese) [Google Scholar]
- Jiang, L.; Chen, Z.; Ali, S.M.F. Modelling of reverse combustion linking in underground coal gasification. Fuel 2017, 207, 302–311. [Google Scholar] [CrossRef]
- Su, F.; Hamanaka, A.; Itakura, K.; Zhang, W.; Deguchi, G.; Sato, K.; Kodama, J. Monitoring and evaluation of simulated underground coal gasification in an ex-situ experimental artificial coal seam system. Appl. Energy 2018, 223, 82–92. [Google Scholar] [CrossRef]
- Laciak, M.; Kostúr, K.; Durdán, M.; Kačur, J.; Flegner, P. The analysis of the underground coal gasification in experimental equipment. Energy 2016, 114, 332–343. [Google Scholar] [CrossRef]
- Liang, J. Underground coal gasification stability and control technology. J. China Univ. Min. Technol. 2002, 31, 358–361. (In Chinese) [Google Scholar]




| Proximate Analysis a | Ultimate Analysis | |||||||
|---|---|---|---|---|---|---|---|---|
| M | A | V | FC | C | H | O | N | S |
| 11.87 | 22.89 | 32.88 | 32.36 | 44.93 | 4.35 | 14.41 | 1.01 | 0.5 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Chen, F.; Wang, Y.; Liu, G.; Yao, H.; Liu, S. Experimental Study of Reverse Underground Coal Gasification. Energies 2018, 11, 2949. https://doi.org/10.3390/en11112949
Liu H, Chen F, Wang Y, Liu G, Yao H, Liu S. Experimental Study of Reverse Underground Coal Gasification. Energies. 2018; 11(11):2949. https://doi.org/10.3390/en11112949
Chicago/Turabian StyleLiu, Hongtao, Feng Chen, Yuanyuan Wang, Gang Liu, Hong Yao, and Shuqin Liu. 2018. "Experimental Study of Reverse Underground Coal Gasification" Energies 11, no. 11: 2949. https://doi.org/10.3390/en11112949
APA StyleLiu, H., Chen, F., Wang, Y., Liu, G., Yao, H., & Liu, S. (2018). Experimental Study of Reverse Underground Coal Gasification. Energies, 11(11), 2949. https://doi.org/10.3390/en11112949




