Improved Microbial Electrolysis Cell Hydrogen Production by Hybridization with a TiO2 Nanotube Array Photoanode
Abstract
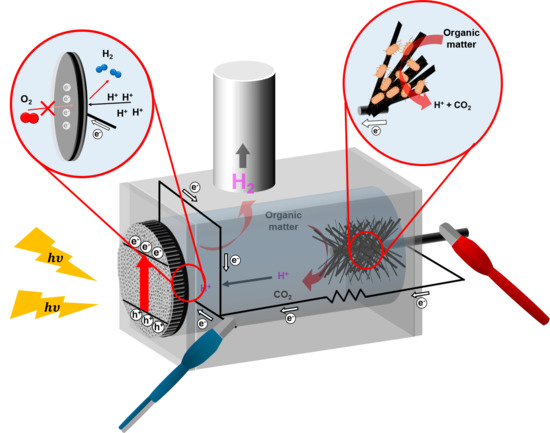
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Synthesis of TNT Array Photoanodes
2.3. MFC Operation for Bioanode Preparation
2.4. Hybrid MEC Fabrication and Operation
2.5. Hybrid MEC Performance Evaluation
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Matthews, H.D.; Caldeira, K. Stabilizing climate requires near-zero emissions. Geophys. Res. Lett. 2008, 35. [Google Scholar] [CrossRef] [Green Version]
- Grimes, C.; Varghese, O.; Ranjan, S. Light, Water, Hydrogen: The Solar Generation of Hydrogen by Water Photoelectrolysis; Springer: New York, NY, USA, 2008; ISBN 978-0-387-33198-0. [Google Scholar]
- Satyapal, S. Hydrogen and Fuel Cells Overview, DLA Worldwide Energy Conference, National Harbor, MD. 2017. Available online: https://www.energy.gov/eere/fuelcells/downloads/hydrogen-and-fuel-cells-overview (accessed on 14 August 2018).
- Logan, B.E. Microbial Fuel Cells; John Wiley & Sons, Inc.: New York, NY, USA, 2008; pp. 6–7. ISBN 9780470239483. [Google Scholar]
- Shizas, I.; Bagley, D.M. Experimental determination of energy content of unknown organics in municipal wastewater streams. J. Energy Eng. 2004, 130, 45–53. [Google Scholar] [CrossRef]
- Liu, H.; Grot, S.; Logan, B.E. Electrochemically assisted microbial production of hydrogen from acetate. Environ. Sci. Technol. 2005, 39, 4317–4320. [Google Scholar] [CrossRef] [PubMed]
- Call, D.; Logan, B.E. Hydrogen production in a single chamber microbial electrolysis cell lacking a membrane. Environ. Sci. Technol. 2008, 42, 3401–3406. [Google Scholar] [CrossRef] [PubMed]
- Rozendal, R.A.; Hamelers, H.V.M.; Euverink, G.J.W.; Metz, S.J.; Buisman, C.J.N. Principle and perspectives of hydrogen production through biocatalyzed electrolysis. Int. J. Hydrogen Energy 2006, 31, 1632–1640. [Google Scholar] [CrossRef]
- Rabaey, K.; Boon, N.; Siciliano, S.D.; Verhaege, M.; Verstraete, W. Biofuel cells select for microbial consortia that self-mediate electron transfer. Appl. Environ. Microbiol. 2004, 70, 5373–5382. [Google Scholar] [CrossRef] [PubMed]
- Bond, D.R.; Lovley, D.R. Electricity production by Geobacter sulfurreducens attached to electrodes. Appl. Environ. Microbiol. 2003, 69, 1548–1555. [Google Scholar] [CrossRef] [PubMed]
- Gorby, Y.A.; Yanina, S.; McLean, J.S.; Rosso, K.M.; Moyles, D.; Dohnalkova, A.; Beveridge, T.J.; Chang, I.S.; Kim, B.H.; Kim, K.S. Electrically conductive bacterial nanowires produced by Shewanella oneidensis strain MR-1 and other microorganisms. Proc. Natl. Acad. Sci. USA 2006, 103, 11358–11363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reguera, G.; McCarthy, K.D.; Mehta, T.; Nicoll, J.S.; Tuominen, M.T.; Lovley, D.R. Extracellular electron transfer via microbial nanowires. Nature 2005, 435, 1098–1101. [Google Scholar] [CrossRef] [PubMed]
- Min, B.; Logan, B.E. Continuous electricity generation from domestic wastewater and organic substrates in a flat plate microbial fuel cell. Environ. Sci. Technol. 2004, 38, 5809–5814. [Google Scholar] [CrossRef] [PubMed]
- Logan, B.E.; Regan, J.M. Electricity-producing bacterial communities in microbial fuel cells. Trends Microbiol. 2006, 14, 512–518. [Google Scholar] [CrossRef] [PubMed]
- Strik, D.P.; Timmers, R.A.; Helder, M.; Steinbusch, K.J.J.; Hamelers, H.V.M.; Buisman, C.J.N. Microbial solar cells: Applying photosynthetic and electrochemically active organisms. Trends Biotechnol. 2011, 29, 41–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qi, X.; Ren, Y.; Liang, P.; Wang, X. New insights in photosynthetic microbial fuel cell using anoxygenic phototrophic bacteria. Bioresour. Technol. 2018, 258, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Qian, F.; Wang, G.; Li, Y. Solar-driven microbial photoelectrochemical cells with a nanowire photocathode. Nano Lett. 2010, 10, 4686–4691. [Google Scholar] [CrossRef] [PubMed]
- Ansari, S.A.; Khan, M.M.; Ansari, M.O.; Cho, M.H. Improved electrode performance in microbial fuel cells and the enhanced visible light-induced photoelectrochemical behaviour of PtOx@ M-TiO2 nanocomposites. Ceram. Int. 2015, 41, 9131–9139. [Google Scholar] [CrossRef]
- Wang, S.; Yang, X.; Zhu, Y.; Su, Y.; Li, C. Solar-assisted dual chamber microbial fuel cell with a CuInS2 photocathode. RSC Adv. 2014, 4, 23790–23796. [Google Scholar] [CrossRef]
- Kim, H.; Lee, K.; Razzaq, A.; Lee, S.H.; Grimes, C.A.; In, S. Photocoupled bioanode: A new approach for improved microbial fuel cell performance. Energy Technol. 2018, 6, 257–262. [Google Scholar] [CrossRef]
- Cheng, S.; Logan, B.E. Sustainable and efficient biohydrogen production via electrohydrogenesis. Proc. Natl. Acad. Sci. USA 2007, 104, 18871–18873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tice, R.C.; Kim, Y. Methanogenesis control by electrolytic oxygen production in microbial electrolysis cells. Int. J. Hydrogen Energy 2014, 39, 3079–3086. [Google Scholar] [CrossRef]
- Logan, B.E. Scaling up microbial fuel cells and other bioelectrochemical systems. Appl. Microbiol. Biotechnol. 2010, 85, 1665–1671. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Liang, P.; Liu, P.; Wang, D.; Miao, B.; Huang, X. A novel microbial fuel cell sensor with biocathode sensing element. Biosens. Bioelectron. 2017, 94, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Qiu, R.; Zhang, B.G.; Li, J.X.; Lv, Q.; Wang, S.; Gu, Q. Enhanced vanadium (V) reduction and bioelectricity generation in microbial fuel cells with biocathode. J. Power Sources 2017, 359, 379–383. [Google Scholar] [CrossRef]
- Rago, L.; Cristiani, P.; Villa, F.; Zecchin, S.; Colombo, A.; Cavalca, L.; Schievano, A. Influences of dissolved oxygen concentration on biocathodic microbial communities in microbial fuel cells. Bioelectrochemistry 2017, 116, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Jeremiasse, A.W.; Hamelers, H.V.; Buisman, C.J. Microbial electrolysis cell with a microbial biocathode. Bioelectrochemistry 2010, 78, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Jafary, T.; Daud, W.R.W.; Ghasemi, M.; Kim, B.H.; Md Jahim, J.; Ismail, M.; Lim, S.S. Biocathode in microbial electrolysis cell; present status and future prospects. Renew. Sustain. Energy Rev. 2015, 47, 23–33. [Google Scholar] [CrossRef]
- Jafary, T.; Wan Daud, W.R.; Ghasemi, M.; Abu Bakar, M.H.; Sedighi, M.; Kim, B.H.; Carmona-Martínez, A.A.; Jahim, J.M.; Ismail, M. Clean hydrogen production in a full biological microbial electrolysis cell. Int. J. Hydrogen Energy 2018. [Google Scholar] [CrossRef]
- Huang, L.; Jiang, L.; Wang, Q.; Quan, X.; Yang, J.; Chen, L. Cobalt recovery with simultaneous methane and acetate production in biocathode microbial electrolysis cells. Chem. Eng. J. 2014, 253, 281–290. [Google Scholar] [CrossRef]
- Chen, Y.; Shen, J.; Huang, L.; Pan, Y.; Quan, X. Enhanced Cd(II) removal with simultaneous hydrogen production in biocathode microbial electrolysis cells in the presence of acetate or NaHCO3. Int. J. Hydrogen Energy 2016, 41, 13368–13379. [Google Scholar] [CrossRef]
- Shen, R.; Liu, Z.; He, Y.; Zhang, Y.; Lu, J.; Zhu, Z.; Si, B.; Zhang, C.; Xing, X.-H. Microbial electrolysis cell to treat hydrothermal liquefied wastewater from cornstalk and recover hydrogen: Degradation of organic compounds and characterization of microbial community. Int. J. Hydrogen Energy 2016, 41, 4132–4142. [Google Scholar] [CrossRef]
- Escapa, A.; Mateos, R.; Martínez, E.J.; Blanes, J. Microbial electrolysis cells: An emerging technology for wastewater treatment and energy recovery. From laboratory to pilot plant and beyond. Renew. Sustain. Energy Rev. 2016, 55, 942–956. [Google Scholar] [CrossRef]
- Cusick, R.D.; Bryan, B.; Parker, D.S.; Merrill, M.D.; Mehanna, M.; Kiely, P.D.; Liu, G.; Logan, B.E.J.A.M. Biotechnology, Performance of a pilot-scale continuous flow microbial electrolysis cell fed winery wastewater. Appl. Microbiol. Biotechnol. 2011, 89, 2053–2063. [Google Scholar] [CrossRef] [PubMed]
- Heidrich, E.S.; Dolfing, J.; Scott, K.; Edwards, S.R.; Jones, C.; Curtis, T.P.J.A.M. Biotechnology, Production of hydrogen from domestic wastewater in a pilot-scale microbial electrolysis cell. Appl. Microbiol. Biotechnol. 2013, 97, 6979–6989. [Google Scholar] [CrossRef] [PubMed]
- Heidrich, E.S.; Edwards, S.R.; Dolfing, J.; Cotterill, S.E.; Curtis, T.P. Performance of a pilot scale microbial electrolysis cell fed on domestic wastewater at ambient temperatures for a 12 month period. Bioresour. Technol. 2014, 173, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.K.; Harnisch, F.; Wirth, S.; Wahlandt, H.; Dockhorn, T.; Dichtl, N.; Schröder, U. Evaluating the effects of scaling up on the performance of bioelectrochemical systems using a technical scale microbial electrolysis cell. Bioresour. Technol. 2014, 163, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Cotterill, S.E.; Dolfing, J.; Jones, C.; Curtis, T.P.; Heidrich, E.S. Low Temperature Domestic Wastewater Treatment in a Microbial Electrolysis Cell with 1 m2 Anodes: Towards System Scale-Up. Fuel Cells 2017, 17, 584–592. [Google Scholar] [CrossRef]
- Cotterill, S.E.; Dolfing, J.; Curtis, T.P.; Heidrich, E.S. Community Assembly in Wastewater-Fed Pilot-Scale Microbial Electrolysis Cells. Front. Energy Res. 2018, 6. [Google Scholar] [CrossRef]
- Mehanna, M.; Kiely, P.D.; Call, D.F.; Logan, B.E. Microbial Electrodialysis Cell for Simultaneous Water Desalination and Hydrogen Gas Production. Environ. Sci. Technol. 2010, 44, 9578–9583. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Logan, B.E. Hydrogen production from inexhaustible supplies of fresh and salt water using microbial reverse-electrodialysis electrolysis cells. Proc. Natl. Acad. Sci. USA 2011, 108, 16176–16181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Jin, X.; Zhao, N.; Angelidaki, I.; Zhang, Y. Novel bio-electro-Fenton technology for azo dye wastewater treatment using microbial reverse-electrodialysis electrolysis cell. Bioresour. Technol. 2017, 228, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Cusick, R.D.; Logan, B.E. Phosphate recovery as struvite within a single chamber microbial electrolysis cell. Bioresour. Technol. 2012, 107, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Zamora, P.; Georgieva, T.; Ter Heijne, A.; Sleutels, T.H.J.A.; Jeremiasse, A.W.; Saakes, M.; Buisman, C.J.N.; Kuntke, P. Ammonia recovery from urine in a scaled-up Microbial Electrolysis Cell. J. Power Sources 2017, 356, 491–499. [Google Scholar] [CrossRef]
- Almatouq, A.; Babatunde, A.O. Concurrent hydrogen production and phosphorus recovery in dual chamber microbial electrolysis cell. Bioresour. Technol. 2017, 237, 193–203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.; Liu, G.; Zhang, R.; Qin, B.; Luo, Y. Development of the Microbial Electrolysis Desalination and Chemical-Production Cell for Desalination as Well as Acid and Alkali Productions. Environ. Sci. Technol. 2012, 46, 2467–2472. [Google Scholar] [CrossRef] [PubMed]
- Ye, B.; Luo, H.; Lu, Y.; Liu, G.; Zhang, R.; Li, X. Improved performance of the microbial electrolysis desalination and chemical-production cell with enlarged anode and high applied voltages. Bioresour. Technol. 2017, 244, 913–919. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Li, H.; Lu, Y.; Liu, G.; Zhang, R. Treatment of reverse osmosis concentrate using microbial electrolysis desalination and chemical production cell. Desalination 2017, 408, 52–59. [Google Scholar] [CrossRef]
- Ye, B.; Lu, Y.; Luo, H.; Liu, G.; Zhang, R. Tetramethyl ammonium hydroxide production using the microbial electrolysis desalination and chemical-production cell with long anode. Bioresour. Technol. 2018, 251, 403–406. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Logan, B.E. Simultaneous removal of organic matter and salt ions from saline wastewater in bioelectrochemical systems. Desalination 2013, 308, 115–121. [Google Scholar] [CrossRef]
- Carmona-Martínez, A.A.; Trably, E.; Milferstedt, K.; Lacroix, R.; Etcheverry, L.; Bernet, N. Long-term continuous production of H2 in a microbial electrolysis cell (MEC) treating saline wastewater. Water Res. 2015, 81, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Shehab, N.A.; Ortiz-Medina, J.F.; Katuri, K.P.; Hari, A.R.; Amy, G.; Logan, B.E.; Saikaly, P.E. Enrichment of extremophilic exoelectrogens in microbial electrolysis cells using Red Sea brine pools as inocula. Bioresour. Technol. 2017, 239, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Alonso, M.D.; Fresno, F.; Suárez, S.; Coronado, J.M.J.E.; Science, E. Development of alternative photocatalysts to TiO2: Challenges and opportunities. Energy Environ. Sci. 2009, 2, 1231–1257. [Google Scholar] [CrossRef]
- Henderson, M.A. A surface science perspective on TiO2 photocatalysis. Surf. Sci. Rep. 2011, 66, 185–297. [Google Scholar] [CrossRef]
- Sclafani, A.; Herrmann, J.M. Comparison of the Photoelectronic and Photocatalytic Activities of Various Anatase and Rutile Forms of Titania in Pure Liquid Organic Phases and in Aqueous Solutions. J. Phys. Chem. 1996, 100, 13655–13661. [Google Scholar] [CrossRef]
- Angelome, P.C.; Andrini, L.; Calvo, M.E.; Requejo, F.G.; Bilmes, S.A.; Soler-Illia, G.J. Mesoporous anatase TiO2 films: Use of Ti K XANES for the quantification of the nanocrystalline character and substrate effects in the photocatalysis behavior. J. Phys. Chem. C 2007, 111, 10886–10893. [Google Scholar] [CrossRef]
- Colbeau-Justin, C.; Kunst, M.; Huguenin, D. Structural influence on charge-carrier lifetimes in TiO2 powders studied by microwave absorption. J. Mater. Sci. 2003, 38, 2429–2437. [Google Scholar] [CrossRef]
- Yamada, Y.; Kanemitsu, Y. Determination of electron and hole lifetimes of rutile and anatase TiO2 single crystals. Appl. Phys. Lett. 2012, 101, 133907. [Google Scholar] [CrossRef] [Green Version]
- Martini, I.; Hodak, J.H.; Hartland, G.V. Effect of Structure on Electron Transfer Reactions between Anthracene Dyes and TiO2 Nanoparticles. J. Phys. Chem. B 1998, 102, 9508–9517. [Google Scholar] [CrossRef]
- Roy, P.; Berger, S.; Schmuki, P. TiO2 Nanotubes: Synthesis and Applications. Angew. Chem. Int. Ed. 2011, 50, 2904–2939. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Lee, K.-S.; Sorcar, S.; Razzaq, A.; Grimes, C.A.; In, S.-I. Wastewater treatment and electricity generation from a sunlight-powered single chamber microbial fuel cell. J. Photochem. Photobiol. A Chem. 2018, 358, 432–440. [Google Scholar] [CrossRef]
- Melody, B.; Kinard, T.; Lessner, P.J.E. The Non-Thickness-Limited Growth of Anodic Oxide Films on Valve Metals. Electrochem. Solid-State Lett. 1998, 1, 126–129. [Google Scholar] [CrossRef]
- Bard, A.; Parsons, R.; Jordan, J.J.N.Y. Standard Potentials in Aqueous Solution; CRC Press (Taylor & Francis): New York, NY, USA, 1985; ISBN 9780824772918. [Google Scholar]
- Prakasam, H.E.; Shankar, K.; Paulose, M.; Varghese, O.K.; Grimes, C.A. A New Benchmark for TiO2 Nanotube Array Growth by Anodization. J. Phys. Chem. C 2007, 111, 7235–7241. [Google Scholar] [CrossRef]
- Regonini, D.; Bowen, C.R.; Jaroenworaluck, A.; Stevens, R. A review of growth mechanism, structure and crystallinity of anodized TiO2 nanotubes. Mater. Sci. Eng. R Rep. 2013, 74, 377–406. [Google Scholar] [CrossRef] [Green Version]
- Varghese, O.K.; Gong, D.; Paulose, M.; Grimes, C.A.; Dickey, E.C. Crystallization and high-temperature structural stability of titanium oxide nanotube arrays. J. Mater. Res. 2003, 18, 156–165. [Google Scholar] [CrossRef]
- Liu, H.; Logan, B.E. Electricity generation using an air-cathode single chamber microbial fuel cell in the presence and absence of a proton exchange membrane. Environ. Sci. Technol. 2004, 38, 4040–4046. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Liu, H.; Logan, B.E. Increased performance of single-chamber microbial fuel cells using an improved cathode structure. Electrochem. Commun. 2006, 8, 489–494. [Google Scholar] [CrossRef]
- Cheng, S.; Logan, B.E. Ammonia treatment of carbon cloth anodes to enhance power generation of microbial fuel cells. Electrochem. Commun. 2007, 9, 492–496. [Google Scholar] [CrossRef]
- Lovley, D.R.; Phillips, E.J.P. Novel mode of microbial energy metabolism: Organic carbon oxidation coupled to dissimilatory reduction of iron or manganese. Appl. Environ. Microbiol. 1988, 54, 1472–1480. [Google Scholar] [PubMed]
- Gong, D.; Grimes, C.A.; Varghese, O.K.; Hu, W.; Singh, R.S.; Chen, Z.; Dickey, E.C. Titanium oxide nanotube arrays prepared by anodic oxidation. J. Mater. Res. 2001, 16, 3331–3334. [Google Scholar] [CrossRef]
- Shankar, K.; Basham, J.I.; Allam, N.K.; Varghese, O.K.; Mor, G.K.; Feng, X.; Paulose, M.; Seabold, J.A.; Choi, K.-S.; Grimes, C.A. Recent advances in the use of TiO2 nanotube and nanowire arrays for oxidative photoelectrochemistry. J. Phys. Chem. C 2009, 113, 6327–6359. [Google Scholar] [CrossRef]
- Varghese, O.K.; Paulose, M.; Grimes, C.A. Long vertically aligned titania nanotubes on transparent conducting oxide for highly efficient solar cells. Nat. Nanotechnol. 2009, 4, 592–597. [Google Scholar] [CrossRef] [PubMed]
- Sorcar, S.; Razzaq, A.; Tian, H.; Grimes, C.A.; In, S.-I. Facile electrochemical synthesis of anatase nano-architectured titanium dioxide films with reversible superhydrophilic behavior. J. Ind. Eng. Chem. 2017, 46, 203–211. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, W.; Cui, X.; Yao, W.; Duan, T. One-step hydrothermal synthesis of iron and nitrogen co-doped TiO2 nanotubes with enhanced visible-light photocatalytic activity. CrystEngComm 2015, 17, 8368–8376. [Google Scholar] [CrossRef]
- Ieropoulos, I.; Winfield, J.; Greenman, J. Effects of flow-rate, inoculum and time on the internal resistance of microbial fuel cells. Bioresour. Technol. 2010, 101, 3520–3525. [Google Scholar] [CrossRef] [PubMed]
- Watson, V.J.; Logan, B.E. Analysis of polarization methods for elimination of power overshoot in microbial fuel cells. Electrochem. Commun. 2011, 13, 54–56. [Google Scholar] [CrossRef]
- Kim, B.; An, J.; Chang, I.S. Elimination of power overshoot at bioanode through assistance current in microbial fuel cells. ChemSusChem 2017, 10, 612–617. [Google Scholar] [CrossRef] [PubMed]






© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, K.N.; Lee, S.H.; Kim, H.; Park, Y.H.; In, S.-I. Improved Microbial Electrolysis Cell Hydrogen Production by Hybridization with a TiO2 Nanotube Array Photoanode. Energies 2018, 11, 3184. https://doi.org/10.3390/en11113184
Kim KN, Lee SH, Kim H, Park YH, In S-I. Improved Microbial Electrolysis Cell Hydrogen Production by Hybridization with a TiO2 Nanotube Array Photoanode. Energies. 2018; 11(11):3184. https://doi.org/10.3390/en11113184
Chicago/Turabian StyleKim, Ki Nam, Sung Hyun Lee, Hwapyong Kim, Young Ho Park, and Su-Il In. 2018. "Improved Microbial Electrolysis Cell Hydrogen Production by Hybridization with a TiO2 Nanotube Array Photoanode" Energies 11, no. 11: 3184. https://doi.org/10.3390/en11113184
APA StyleKim, K. N., Lee, S. H., Kim, H., Park, Y. H., & In, S.-I. (2018). Improved Microbial Electrolysis Cell Hydrogen Production by Hybridization with a TiO2 Nanotube Array Photoanode. Energies, 11(11), 3184. https://doi.org/10.3390/en11113184