Abstract
Approximately 15 million dry tons of food waste is produced annually in the United States (USA), and 92% of this waste is disposed of in landfills where it decomposes to produce greenhouse gases and water pollution. Hydrothermal liquefaction (HTL) is an attractive technology capable of converting a broad range of organic compounds, especially those with substantial water content, into energy products. The HTL process produces a bio-oil precursor that can be further upgraded to transportation fuels and an aqueous phase containing water-soluble organic impurities. Converting small oxygenated compounds that partition into the water phase into larger, hydrophobic compounds can reduce aqueous phase remediation costs and improve energy yields. HTL was investigated at 300 °C and a reaction time of 1 h for conversion of an institutional food waste to bio-oil, using either homogeneous Na2CO3 or heterogeneous CeZrOx to promote in situ conversion of water-soluble organic compounds into less oxygenated, oil-soluble products. Results with food waste indicate that CeZrOx improves both bio-oil higher heating value (HHV) and energy recovery when compared both to non-catalytic and Na2CO3-catalyzed HTL. The aqueous phase obtained using CeZrOx as an HTL catalyst contained approximately half the total organic carbon compared to that obtained using Na2CO3—suggesting reduced water treatment costs using the heterogeneous catalyst. Experiments with model compounds indicated that the primary mechanism of action was condensation of aldehydes, a reaction which simultaneously increases molecular weight and oxygen-to-carbon ratio—consistent with the improvements in bio-oil yield and HHV observed with institutional food waste. The catalyst was stable under hydrothermal conditions (≥16 h at 300 °C) and could be reused at least three times for conversion of model aldehydes to water insoluble products. Energy and economic analysis suggested favorable performance for the heterogeneous catalyst compared either to non-catalytic HTL or Na2CO3-catalyzed HTL, especially once catalyst lifetime differences were considered. The results of this study establish the potential of heterogeneous catalysts to improve HTL economics and energetics.
1. Introduction
A variety of sustainable energy solutions are being developed to displace the use of petroleum-derived fuels that contribute to increasing greenhouse gas levels in the atmosphere. Specifically, the growing demand for transportation fuels has driven alternative energy research for conversion of biomass into fuels [1]. The Energy Independence and Security Act 2007 Renewable Fuel Standards (RFS) program targets the production of 36 billion gallons of renewable fuel by 2022 [2]. Feed costs are a major challenge to economical production of biomass, the Department of Energy (DOE) National Renewable Energy Laboratory reported that the feed constitutes 71.5% of the cost of producing renewable biodiesel from biomass and municipal solid waste [3]. Food waste is an inexpensive, energy dense alternative to lignocellulosic biomass, with the potential to be converted into drop-in transportation fuels with thermochemical properties comparable to petroleum-derived fuels [4]. Repurposing food residues also helps divert material from landfills and reduce life-cycle greenhouse gas emissions caused by the biodegradation of organic waste. According to a recent DOE study, more than 15 million dry tons of food waste is generated annually in the United States (USA), 92% of which is discarded in landfills [5]. Repurposing food waste for biofuel production would reduce the environmental impact from landfills and reduce global reliance on crude oil.
Thermochemical and biochemical technologies can be used to process complex food wastes, mixtures that consist primarily of carbohydrates, proteins, and oils, but also minor components including minerals and salts [6]. Anaerobic digestion converts organic wastes into methane-rich biogas; however, digestion is a slow process, requiring large reactor volumes and yielding a product that must undergo significant upgrading for many applications [7,8]. When compared to digestion, gasification more rapidly converts organic wastes into a methane-rich syngas. Fast pyrolysis is the rapid thermal conversion of organic wastes or biomass to energy-rich oils [8]. However, both gasification and pyrolysis require dry feeds and the energy required to dry food waste detracts from the processes [8,9]. Thermochemical processing via hydrothermal liquefaction (HTL) is an attractive process for food wastes, which is capable of converting a broad range of wet organic solids at moderate temperatures and high pressures without the need for a costly biomass drying step [10]. HTL reactions are carried out at elevated temperatures (250–380 °C) and pressures (7–30 MPa) in a hydrothermal water reaction medium for relatively short residence times (10–60 min) to form a carbon rich bio-oil phase along with an aqueous byproduct phase [11,12]. HTL has been demonstrated for many organic-rich feeds and at a pilot plant scale of 2000 dry metric tons of waste per day [10].
A major issue in commercializing HTL is that considerable amounts of organic byproducts preferentially partition into the aqueous phase, rather than in the bio-oil phase. Molecules with high oxygen to carbon ratios (e.g., short-chain alcohols, acids, and esters) are particularly likely to exist in the aqueous phase due to their high water solubilities. Loss of organic compounds to the aqueous phase limits the HTL energy yield and necessitates downstream treatment of the water phase before it can be discharged. In their analysis HTL, Zhu et al. [10] found that economic performance of HTL is most sensitive to loss of carbon to the aqueous phase. HTL process conditions that reduce the production of water-soluble organic compounds can potentially improve energy yield, improve carbon yield, reduce waste treatment costs, and improve process economics.
Homogeneous alkali salts, such as Na2CO3, have been reported to improve HTL carbon yield, and that improvement is attributed to suppressing coke formation [10,13,14,15,16,17]. The limitation with homogeneous catalysts is the costly steps necessary to recover and reuse the catalyst after reaction. In comparison with either non-catalytic HTL or HTL catalyzed homogeneously, reusable heterogeneous catalysts have the potential to improve process economics and energy efficiency. Here, we investigate CeZrOx as a heterogeneous catalyst during HTL for in situ conversion of small, hydrophilic molecules that would otherwise partition into the aqueous phase, into larger, more hydrophobic molecules that instead partition into the bio-oil phase. CeZrOx was selected as the heterogeneous catalyst because of the stability of the parent oxides [13,18], and because it is known to catalyze condensation and coupling reactions [19,20,21]. In addition, we tested catalyst stability under the harsh reaction conditions required for HTL and performed catalytic activity tests on model organic compounds to investigate the upgrading mechanism. Finally, an energy analysis was performed to compare the benefits of the heterogeneous catalyst to previous work using homogeneous catalysts. This study provides a basis for understanding the use of heterogeneous catalysts for converting food wastes into liquid fuels under HTL conditions.
2. Results
2.1. Hydrothermal Liquefaction (HTL) or Food Waste
The feedstock used for HTL reactions was a mixture representative of institutional food waste and included seven commonly disposed food items. Selection of a traditional food waste mixture was important due to the varying effects on HTL yields that are influenced by protein, carbohydrate and fat content [22]. The list of solid ingredients used as the feedstock is included in Table 1, which also includes nutrient data calculated using values for each individual food item found in the United States Department of Agriculture Food Composition Database [23]. Table 2 shows that the food waste mixture contained 73% moisture, was highly oxygenated, and had a higher heating value (HHV) of 6.5 MJ/kg.

Table 1.
List of solid ingredients in the food waste feedstock and corresponding composition and higher heating value (HHV).

Table 2.
Food waste feedstock properties and properties of the hydrothermal liquefaction (HTL) water and oil products using different catalysts. Elemental analysis of HTL oil calculated on a dry basis. Reactions carried out at 300 °C under batch conditions for one hour.
The institutional food waste mixture was upgraded under 3 different conditions: (1) thermally, in the absence of any catalyst; (2) in the presence of Na2CO3 as a homogeneous catalyst; and, (3) in the presence of CeZrOx as a heterogeneous catalyst. CeZrOx was selected for its known activity for promoting the desired reactions as well as the known liquid-phase hydrothermal stability of metal oxides [24]. Dumesic and coworkers [19,20,21] have reported both esterification [21] and ketonization [20] reactions that are catalyzed by CeZrOx under vapor phase conditions. In addition, CeO2 is known for its redox activity that can assist in upgrading a variety of water soluble oxygenated species [18].
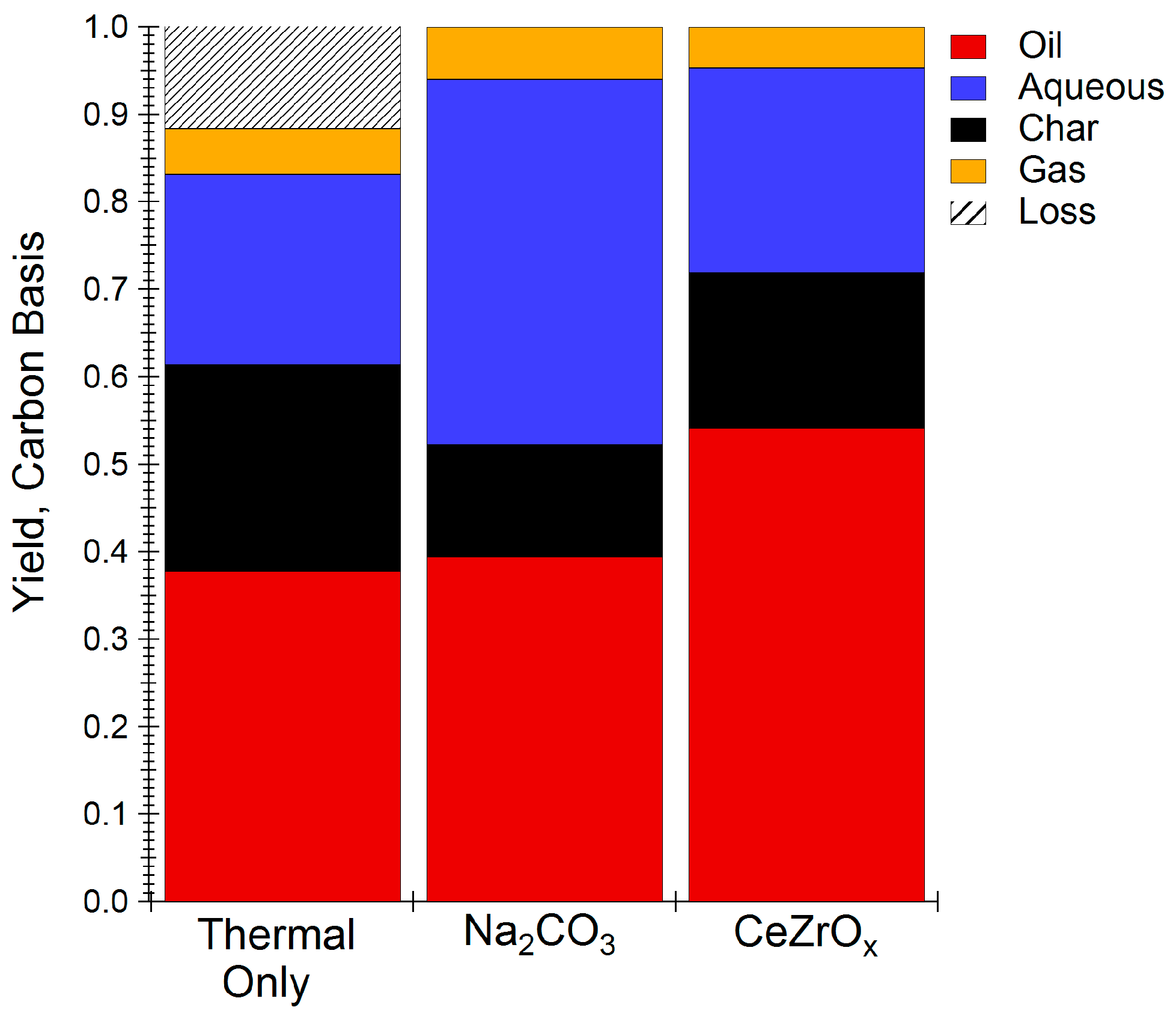
Figure 1 compares the carbon distribution of the major food waste HTL products, as oil, aqueous phase carbon, char, and gas. Non-catalytic HTL yielded 38.8% of the carbon in the oil phase, with the aqueous and solid char phases containing 21.7% and 23.6% of the carbon, respectively. The addition of Na2CO3 as a catalyst reduced coke formation by 10% relative to the thermal HTL reaction, as shown in Figure 1, consistent with previous work on food waste HTL [10]. On the other hand, use of CeZrOx, resulted in the greatest amount of carbon recovered in the oil phase and char, while simultaneously rejecting the least amount of carbon to the gas and aqueous phases. All of these results establish the benefits of using CeZrOx as an HTL catalyst for food waste upgrading.
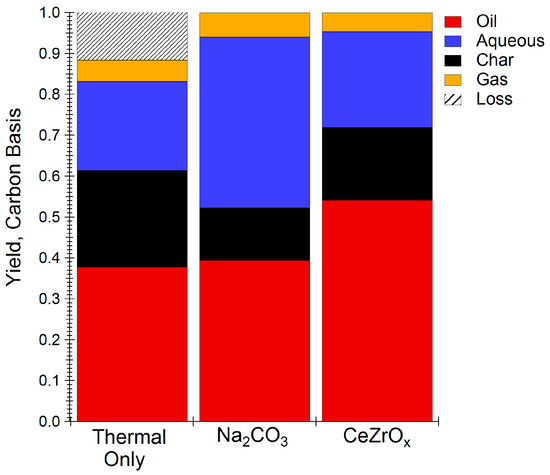
Figure 1.
HTL yields using different HTL catalysts. Reactions carried out at 300 °C for one hour. Oil, gas, and char yields are calculated on a dry basis. Plots are based on total carbon yield of HTL products.
Table 2 compares the properties of bio-oil obtained without catalyst, with Na2CO3, and with CeZrOx. The energy recovery obtained using CeZrOx was 38.8% energy recovery, which is greater than that obtained either under thermal conditions (27.6%) or with homogeneous catalyst (21.3%), and it is comparable to yields reported for HTL of algae, a feed with much greater energy density than food waste [25]. Although the HHV of oil from HTL reactions is slightly less using CeZrOx compared to uncatalyzed HTL reactions, the energy recovery improves due to the increased oil yield. In addition, the total organic carbon (TOC) of the water byproduct obtained from CeZrOx HTL was approximately 50% that obtained under Na2CO3 HTL conditions, indicating that the CeZrOx is more effective at reducing the loss of organic compounds to the water phase. The HHV of bio-oil obtained from CeZrOx-catalyzed HTL was 25% greater than that obtained when Na2CO3 was used as the catalyst, which is consistent with both the increased carbon content and the decreased moisture content of the CeZrOx oil product.
2.2. Hydrothermal Stability of CeZrOx Catalyst
The data in Section 2.1 indicate that CeZrOx may improve bio-oil yield and HHV when compared to Na2CO3 catalysis, while also reducing the organic content of the aqueous phase. However, activity is only one criterion for a commercial catalyst. In addition to activity, the catalyst must be stable at industrial timescales, a difficult challenge given that many catalyst materials degrade rapidly under HTL process conditions [24]. To be considered hydrothermally stable, a metal oxide catalyst must: (1) retain its crystal structure after hot liquid water (HLW) treatment without any lattice rearrangement; (2) maintain the oxidation state of active metals; and, (3) retain the active metals incorporated at the surface. Batch hydrothermal stability tests were performed to investigate the crystal phase, metal oxidation state, and the leaching stability of CeZrOx under HLW conditions (>16 h and 300 °C). Relative to reaction conditions (1 h), longer treatment times were used for stability tests (16 h), to provide data under more extreme conditions than were used to acquire the data in Table 1 and Table 2.
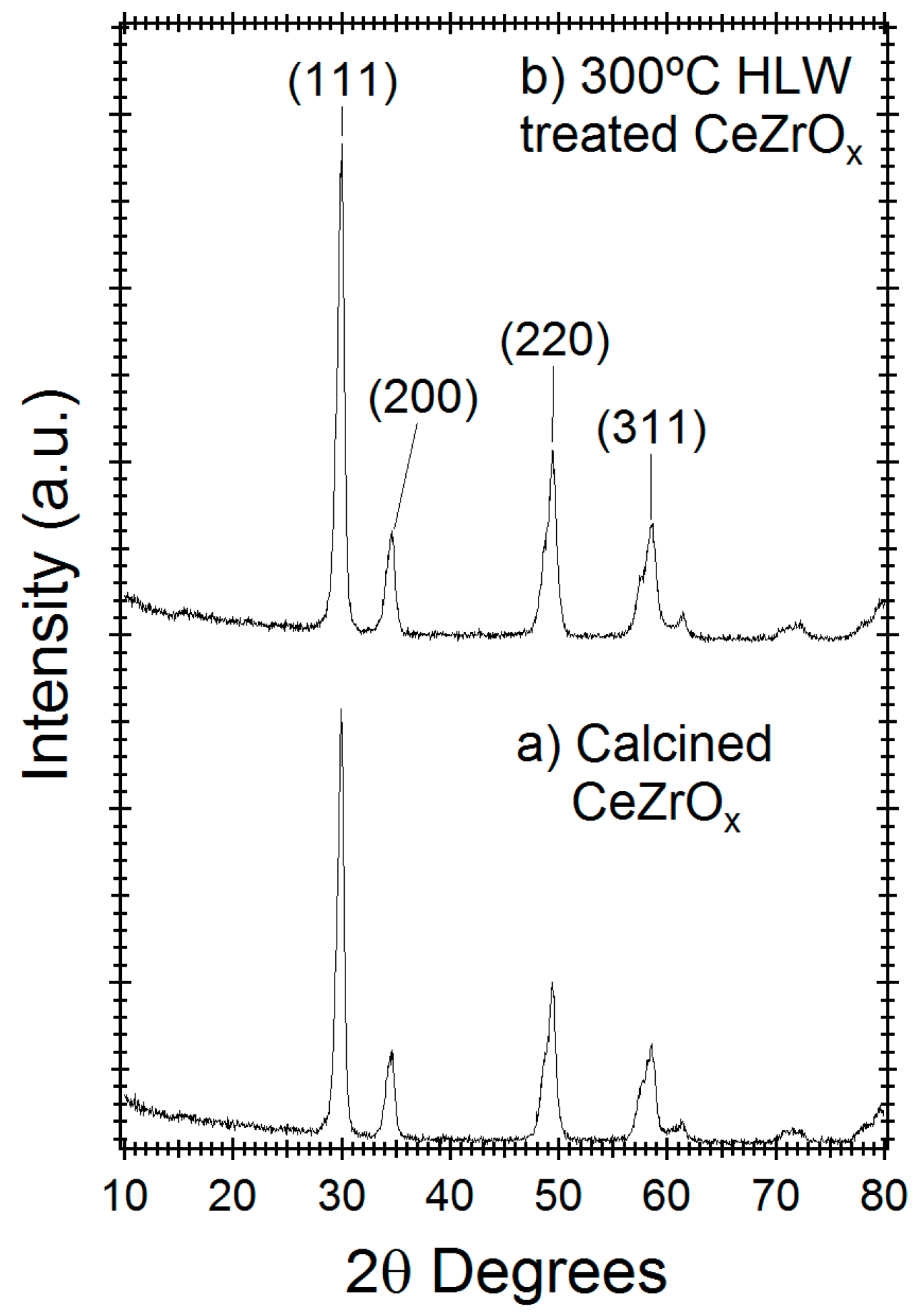
X-ray diffraction was used to study the crystal phase stability of CeZrOx. Figure 2 compares the diffractogram of untreated CeZrOx and HLW treated CeZrOx after 165 h at 300 °C. Based on the diffraction peaks located at 30.2, 34 and 50 2θ degrees, the calcined CeZrOx crystal is in either the cubic or tetragonal phase [26]. No new diffraction peaks appeared with hydrothermal processing, indicating that the crystal lattice was stable under HTL conditions and that no new crystalline phases formed during treatment. Moreover, the peak intensities of calcined and HLW treated CeZrOx are within 10% of the original material, indicating minimal amorphization during treatment.
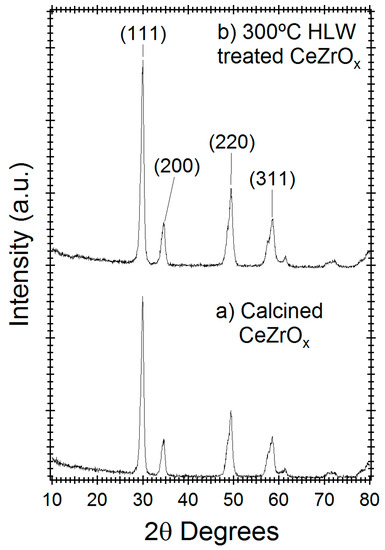
Figure 2.
X-ray powder diffractogram of (a) Calcined CeZrOx and (b) Calcined CeZrOx treated in HLW for 165 h at 300 °C.
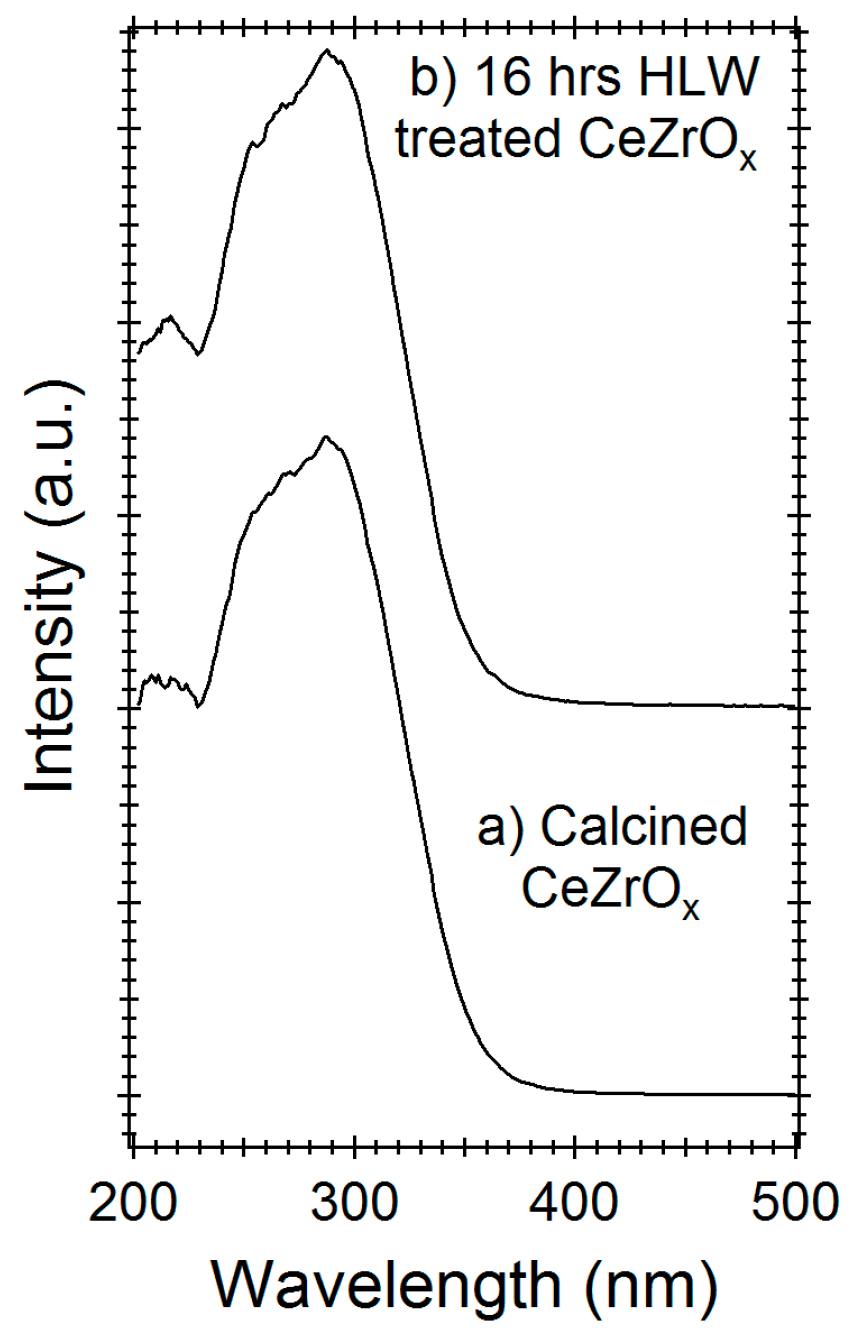
Diffuse reflectance UV-Vis-spectroscopy (DR-UV) can be used to differentiate cerium or zirconium oxides and their oxidation states [27]. Figure 3a shows the DR-UV spectra of calcined CeZrOx and 16-h HLW-treated CeZrOx, respectively. Both spectra have broad DR-UV bands centered at 295 nm, with a shoulder at 230–270 nm. Preferential leaching, oxidation, or the reduction of either Ce or Zr would cause this central band to shift, as shown by the work of Damyana et al. [27]. Treatment with HLW does not shift the location or relative intensity of this central band (Figure 3), indicating that the elemental composition and oxidation state of CeZrOx were both unchanged by HLW treatment.
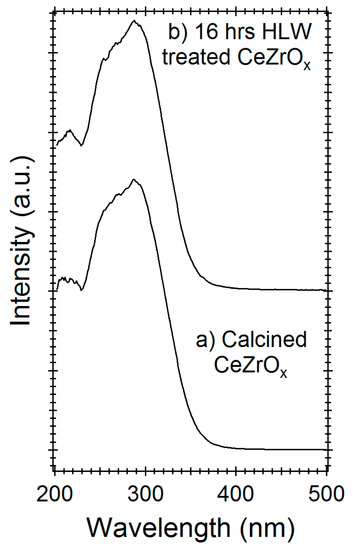
Figure 3.
Diffuse reflectance UV-Vis-spectroscopy (DR-UV) spectra of (a) untreated Cerium zirconium oxide (CeZrOx), and (b) CeZrOx treated in hot liquid water (HLW) for 16 h at 300 °C.
DR-UV is not sensitive to trace metal leaching (<1%) [28]. However, trace leaching can become problematic on extended use. Accordingly, the aqueous phase recovered after hydrothermal treatment of CeZrOx (again at 300 °C and for 16 h) was analyzed using inductively coupled plasma (ICP) with optical emission spectroscopy (OES). ICP-OES revealed that 0.25% of the cerium present in the catalyst leached into the aqueous phase during treatment. The minimal leaching into water that was observed with ICP-OES again supports the stability of the catalyst in hot, liquid water.
2.3. Model Chemistries for HTL Reaction
Relative to the homogeneous catalyst (Na2CO3), CeZrOx improved HTL oil yields, increased the energy recovery of the product, and reduced the TOC content in the water phase. Tests under extreme conditions (300 °C and 16 h) indicated that the catalyst retained crystallinity and underwent only minor leaching under reaction conditions. All of these findings warranted further understanding of CeZrOx for food waste upgrading. At this point, we sought to confirm that the CeZrOx catalyst acted by coupling of small oxygenated molecules into larger molecules with reduced oxygen content. Unfortunately, determining the catalytic role of CeZrOx with a molecularly complex mixture, such as food waste, is a difficult analytical challenge. As a result, data from food waste upgrading did not reveal the mechanism of CeZrOx, or indeed confirm if it acts catalytically at all. For this reason, a series of tests with simple model compounds was performed to confirm the catalytic role of CeZrOx.
CeZrOx catalyst activity was evaluated for reaction of small oxygenated molecules that are characteristic of food waste. An alcohol (isobutanol), carboxylic acid (propionic acid), aldehyde (pentanal) and ketone (pentanone) were selected for model HTL reactions based on their relative hydrophilicity and abundance in food waste. Moreover, we hypothesized that these reactants might undergo aldol condensation, esterification, and ketonization reactions to a desired product with increased molecular weight and decreased oxygen/carbon ratio [29]. Model HTL reactions were performed under batch conditions described in the Methods and Materials section.
Table 3 is a qualitative overview of the results obtained from the model compound HTL reactions, using CeZrOx as a catalyst. Pentanal was the most active of all model oxygenate reactants. All other compounds, including alcohols, carboxylic acid, and ketones, yielded only trace products (<0.3 wt % yield) in the presence of CeZrOx. Reactions with alcohol and/or carboxylic acid had no observable reaction products at concentrations greater than our detection limit of 0.05 wt % yield. Reactions with ketones formed only a trace amount of products at concentrations less than 0.3 wt %. Table 3 also lists mixtures consisting of aldehydes and a second compound as “slightly reactive”; in these cases, the observed reactivity was attributed primarily to the aldehyde. The lack of reactivity of acids contrasts with literature reports that show CeZrOx is active for ketonization and esterification [1,2,5]. The difference between the current results and those in the literature can be attributed to the high concentration of water present during the HTL reaction as water has been shown to greatly reduce CeZrOx activity for ketonization and esterification [19]. Apparently, the activity of CeZrOx towards aldols is less sensitive to water than its ketonization and esterification activity.

Table 3.
Single and mixed reactant model HTL activity using isobutanol, propionic acid, pentanone, pentanal, and equimolar mixtures of each pair. Reactions performed at 300 °C under batch conditions for one hour.
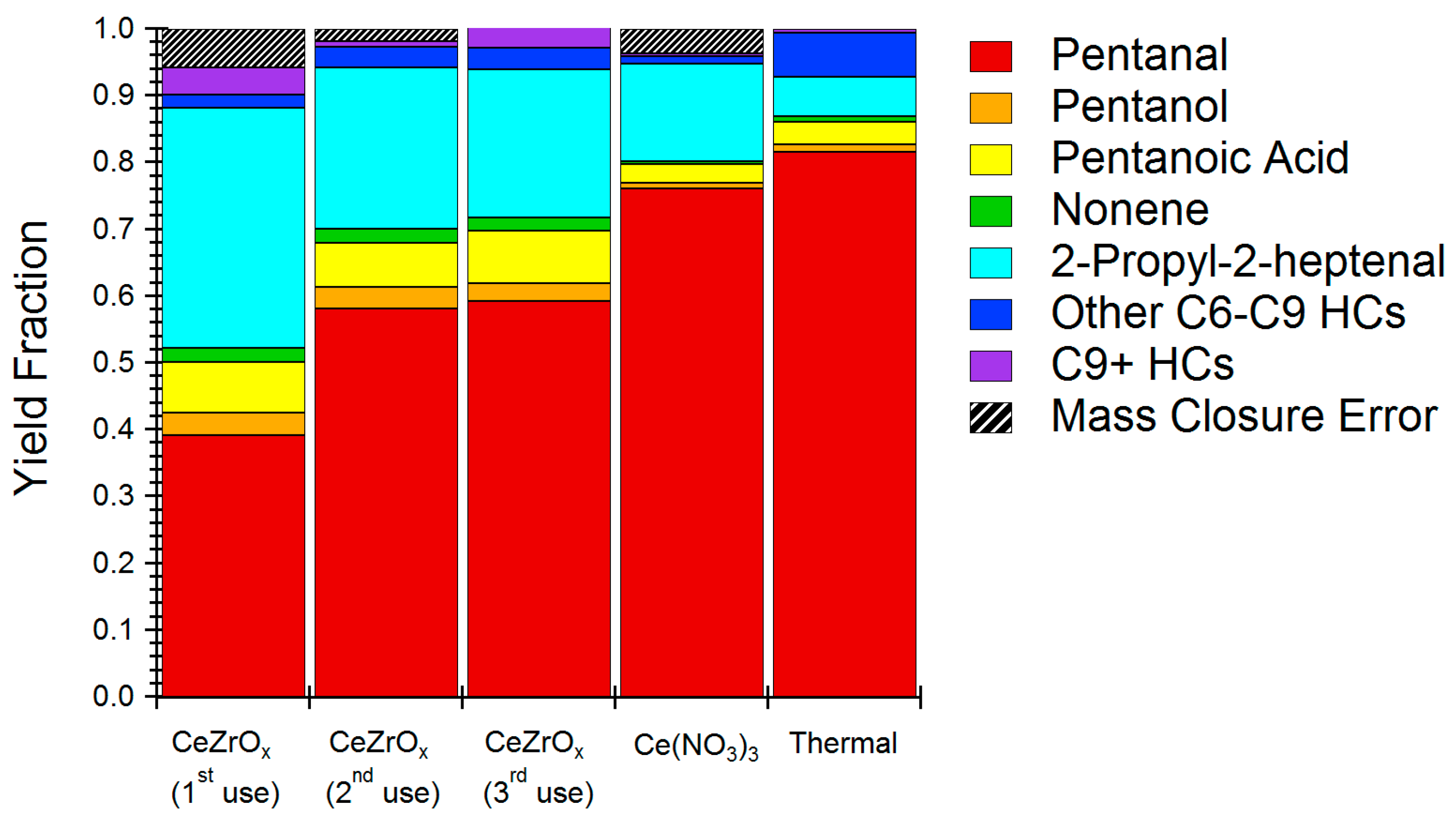
The pentanal reaction activity of CeZrOx was studied in more detail by quantifying yields of all the major reaction products (>0.3% of total). Specifically, we sought to determine which reactions were being catalyzed and if they would produce products with reduced water solubility compared to the reactants. Figure 4 shows the product distribution obtained for HTL reaction of pentanal in the presence of CeZrOx. First, pentanal conversion was measured at approximately 60%. In comparison, pentanal conversion under uncatalyzed, thermal conditions was about 20%. The main product of catalytic reaction was 2-propyl-2-heptenal; this product constituted 74% of the yield. Other products include pentanol, pentanoic acid, nonene, and octene. Similar products were formed under thermal conditions, albeit with a selectivity to 2-propyl-2-heptanal of only about 50%.
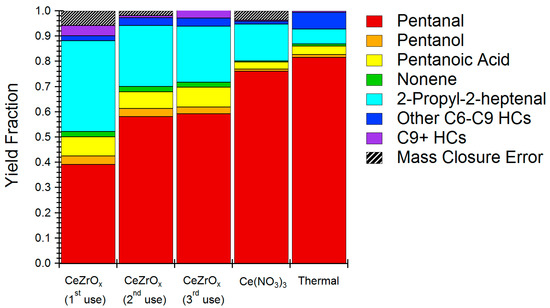
Figure 4.
Product distribution of model pentanal condensation reaction using CeZrOx catalyst, Ce(NO3)3 and no catalyst (thermal only). HCs in legend refers to other hydrocarbons
Production of 2-propyl-2-heptenal can be attributed to aldol condensation of pentanal. Aldol condensation with CeZrOx occurs via base catalyzed formation of an enolate [30]. The enolate couples with another pentanal, and, after dehydration, can form the 2-propyl heptenal product we observed. Aldol condensation is typically base catalyzed, and we therefore surmise that the primary effect of CeZrOx was to act as a Brønsted base. As desired, the product of aldehyde condensation is much less water soluble than the reactant, having both greater molecular weight and a reduced oxygen content. Given the abundance of aldehydes both in food waste and HTL bio-oil, the results of the model experiments strongly suggest that CeZrOx catalyzed aldol condensation reactions during the treatment of institutional food waste, thereby improving carbon recovery and reducing loss of carbon to the aqueous phase when compared to the homogeneous catalyst.
Aside from 2-propyl-2-heptenal, CeZrOx produces 3-nonene, an interesting product given its complete de-oxygenation. Formation of 3-nonene is likely attributable to decarbonylation chemistry of 2-propyl-2-heptanal. Interestingly, the product distribution was not especially dependent on the type of catalyst used, indicating that CeZrOx acts primarily to increase rates, rather than to alter selectivity. Aside from 2-propyl-2-heptanal, the remaining products were a mixture of alcohol and alkenes, which were likely the products of oxidation, reduction, or condensation reactions, and a pentanoic acid, which was likely produced by oxidation of the aldehyde by cerium oxide [31].
An effective heterogeneous catalyst must be reusable. Accordingly, we evaluated the reusability of CeZrOx for pentanal upgrading without calcination or regeneration steps in between runs. Figure 4 summarizes the results, showing that pentanal conversion decreased from 60% on first use to about 45% on second and third uses. Selectivity for 2-propyl-2-heptanal remained stable. Reusability tests confirmed that CeZrOx can be reused with modest loss of activity and without changing the distribution of products. Based on post-reaction analysis of the catalyst, coke formation was likely a key deactivation mechanism and could be addressed by combusting char and coke produced during HTL reactions to regenerate the catalyst.
Catalyst stability tests indicated minor cerium leaching under HTL conditions; nonetheless, even modest leaching might contribute to homogeneous catalysis, rather than the desired heterogeneous effect. Figure 4 includes the product distribution of pentanal upgrading under HTL conditions using 0.25 wt % Ce(NO3)3 as a homogeneous catalyst to simulate the effect of leached cerium. The Ce(NO3)3 concentration was selected based on the amount of cerium leaching quantified during the hydrothermal stability studies of CeZrOx. While 2-propyl-2-heptenal remained the major product when using Ce3+ as a catalyst, the overall pentanal conversion reduced from 60% for heterogeneous catalyst to 24% for the homogeneous reaction. The results of catalyst leaching tests again suggest that CeZrOx acts primarily as a heterogeneous catalyst during HTL chemistry.
3. Discussion
Catalytic upgrading of food waste using CeZrOx as a heterogeneous catalysts yielded a bio-oil with increased carbon content, decreased oxygen content, and increased HHV when compared to the bio-oil produced using a homogeneous catalyst. Its activity was attributed to catalysis of aldol condensation reactions, which have the dual benefit of increasing carbon yield and decreasing the organic content of the aqueous byproduct. Moreover, CeZrOx exhibited minimal loss of activity on repeated usage. To estimate the potential benefits of the reusable catalyst on HTL, we compared the current results to those presented in the literature.
Table 4 compares the energy recovery, oil yield, and oil HHV improvement from the presented food waste catalytic HTL runs with similar studies reported in the literature, using energy recovery as the primary metric of comparison. Inter-comparison of HTL results must take into account the effects of feed, reaction temperature, and catalyst loading on performance; hence, Table 4 provides data on the experimental conditions relevant to energy recovery analysis. Energy recovery can depend strongly on the feedstock. To account for feedstock dependence, Table 4 provides data on food waste (the current study), vegetable oil, sawdust, and several algae types. Compared to other feeds, vegetable oil has a high energy recovery and mass yield due to the relative ease of converting straight chain lipids into bio-oil compared to carbohydrate-rich steams, such as food waste or biomass [32]. In contrast, lignocellulosic feedstocks, such as sawdust (Table 4), are more recalcitrant than simple oils and therefore result in lower energy recovery [33]. Algae is a popular candidate as an energy crop for fuel production due to its high growth rate and high energy density, which can be attributed to high lipid content, a component similar to vegetable oil [34]. However, HTL of algae results in lower energy recovery than reported for vegetable oil, likely due to the combined effects of lower operating temperature and more dilute feedstock (6 wt % feed) [31]. Catalytic food waste HTL results in a similar energy recovery compared to microalgae, which is surprising given that microalgae has high lipid content, whereas the food waste mixture used here is primarily composed carbohydrates. The close agreement in the energy recovery for the two feeds may be attributable to the greater reaction temperature used for food waste HTL (250 vs. 300 °C) and the relative effectiveness of the catalyst (zeolites vs. CeZrOx).

Table 4.
Comparison of energy recoveries, oil yield improvements and oil HHV improvements with the use of a heterogeneous catalyst.
Table 4 compares HTL performance based on oil yield improvement and oil HHV to differentiate the effect of feedstock properties from catalytic effects. Oil yield improvement is defined as the ratio of HTL bio-oil yields obtained with the use of a catalyst to that obtained without the use of a catalyst. Similarly, oil HHV improvement is defined as the ratio of HTL bio-oil HHV obtained with the use of a catalyst to that obtained without the use of a catalyst. Table 4 shows that CeZrOx catalyst improved oil yield by 59% relative to the yield obtained from non-catalytic HTL of food waste and that the HHV of the thermal and CeZrOx oils were within 10% of one another. Meanwhile, Na2CO3 catalysis had no effect on oil yield and decreased the HHV of the oil product by 68% when compared to the oil obtained from non-catalytic HTL. The net result is that CeZrOx improves HTL energy yield from 27.6% for the non-catalytic performance to 38.8%, while Na2CO3 actually reduces the energy yield.
Next, we compared the results that were obtained for food waste HTL to results reported for other feeds. The relative oil yield and HHV improvements obtained using Na2CO3 and CeZrOx on food waste are similar to those reported in the study by Nazari et al. [33], which used sawdust as a HTL feedstock. The similar performance may be consistent with the fact that carbohydrates dominate the composition of both biomass and food waste, despite the fact that the carbohydrates present in biomass (especially cellulose) are generally more stable than those present in food waste (starch). In contrast, the lipid-rich feeds (vegetable oil, algae) do not benefit as greatly from catalyst addition as the carbohydrate-rich feeds (sawdust and food waste), suggesting that catalysts are not as necessary for efficient energy recovery from the lipid-rich feeds as they are for carbohydrate-rich feeds.
Beyond the single use analysis shown in Table 4, a re-usable heterogeneous catalyst, such as CeZrOx, has considerable lifetime benefits compared with thermal processes or with processes utilizing homogeneous catalysts. Table 5 provides estimates of the total oil heating value derived from catalytically produced HTL bio-oil obtained between a single use and up to 165 reuses. For single use, the energy yield of CeZrOx is approximately twice that of Na2CO3, 0.24 MJ per gram of catalyst (MJ/g) compared to 0.103 MJ/g. Next, the lifetime of homogeneous catalysts was taken as the equivalent of two uses, as consistent with results reported by Jena et al. [35] that indicate approximately 50% loss of homogeneous catalyst per use. The reusability of CeZrOx was estimated to be either 3, 10, 100 or 165 uses. Three uses for CeZrOx is estimated as a lower limit based on the relative activity maintained in coupling reactions from the model HTL chemistry. The upper range of reusability for CeZrOx is based on the hydrothermal stability study (i.e., 165 h). The actual lifetime may in fact be greater than that indicated in stability tests because the stability study indicated negligible crystallinity loss after 165 h, at which point the study was terminated. On the other hand, food waste HTL conditions may be more aggressive than hydrothermal conditions, due to the presence of acidic byproducts and heteroatoms, especially sulfur. Therefore, a range between 3 and 165 h of catalyst reuse is used to estimate catalyst lifetime, which takes into account all available data. Using these estimates of catalyst lifetime, the total lifetime energy yield of CeZrOx after either 3 or 165 uses was estimated at 0.73 MJ/g or 39.9 MJ/g, respectively, as compared to 0.21 MJ/g for Na2CO3—a 4 to 200-fold improvement. For comparison, Table 5 provides similar analysis for both homogeneously and heterogeneously catalyzed HTL of different algae types. While the data are scattered by differences in reaction conditions and algae feed characteristics, the lifetime energy recovery obtained using solid Ca3(PO4)2 (0.5 MJ/g) is roughly equal to the best results obtained using homogeneous catalysts (i.e., 0.8 MJ/g under optimized conditions using acetic acid) and much better than the typical result, which is in the range of 0.05 MJ/g. This inter-comparison further establishes the benefits of the reusability of heterogeneous catalysts.

Table 5.
Lifetime energy yields for heterogeneous HTL reactions using either a homogeneous or a heterogeneous catalyst. CeZrOx reuse range based on hydrothermal stability study.
In addition to energy efficiency and yield, cost considerations must also be weighed in the overall analysis. Although bulk pricing data for CeZrOx are not readily available, a simple calculation can be performed. Based on pricing for kg quantities, CeZrOx is approximately 30-times more expensive per gram than Na2CO3, both at purities of 99%. Factoring in the 59% increase in energy yield and considering cost on the basis of energy yield, CeZrOx is more economical than Na2CO3 when CeZrOx is reused at least 25 times and Na2CO3 is reused twice. While more detailed analysis will require obtaining the bulk pricing data for the two catalysts, the preliminary economic analysis is promising. In summary, therefore, the present study suggests that heterogeneous catalysts such as CeZrOx have potential for energy efficient and economical promotion of HTL conversion of food waste to energy.
4. Materials and Methods
4.1. Materials
All of the HTL experiments used a common food waste feedstock made up of common food items listed in Table 1. Nutrient data were calculated using USDA data for individual food items found on the Nutrient Data Laboratory website and also listed in Table 1 [23]. Food was mixed together with deionized (DI) water to create a slurry with 15 wt % solids, which was stored under refrigeration between experiments. A 15 wt % reactant slurry was selected based on prior HTL studies with similar solid loadings, and balances process intensity, feed handling, water use, and heat transfer considerations [40].
Cerium zirconium oxide (CeZrOx) nanopowder (99% purity) was purchased from Sigma Aldrich (St. Louis, MO, USA) (P/N: 634174) and anhydrous Na2CO3 was purchased from Alpha Aesar (P/N: 11552) for use as catalysts. CeZrOx was calcined at 550 °C in a furnace for at least 1 h prior to use. All of the reactants for model HTL studies and products used for gas chromatography (GC) analysis were purchased from Sigma Aldrich, with a minimum purity of at least 99%.
4.2. Food Waste HTL Reactions
Food waste HTL experiments were performed in a 300 mL stainless-steel bench-top reactor purchased from Parr Instruments (Model 4561) rated for use up to 20.6 MPa and 350 °C. The reactor was heated using an external heating jacket and was equipped with a magnetic stirring drive. Reactor temperature was maintained to within 5 °C of the desired set point using a proportional-integral-derivative (PID) controller. For each experiment, the reactor was loaded with 200 g of slurry and 5 wt % of either CeZrOx or Na2CO3 catalyst. The reactor was sealed and heated for approximately 50 min to 300 °C without any initial pressurization. The reactor temperature was maintained at 300 °C for one hour before cooling to room temperature using a water bath. In the majority of experiments on institutional food waste, the reactor headspace was neither purged nor was it pressurized with inert gas as the goal of institutional food waste tests was to simulate realistic reactor operation. In a handful of runs, the effect of N2 pressurization on HTL yields from institutional food waste was tested. The results of these tests indicated that N2 pressurization had no effect on HTL yields within the limits of experimental uncertainty. Thermal HTL reactions using institutional food waste were performed in duplicate with measured yields agreeing to within ±5%. Catalytic HTL reactions were performed several times using different analytic procedures to ensure data reproducibility. Loss in carbon balance closure in Figure 1 for the uncatalyzed HTL reaction is attributed to the losses during product extraction or charring on the reactor walls, impeller, and other surfaces.
4.3. Food Waste HTL Product Analysis
Once cooled, the reactor pressure was recorded and the vessel was depressurized. The gas yield was calculated based on the final gas pressure and the ideal gas law. The molar composition of the gas was assumed to be 80% CO2, 10% CO and 10% H2; these values were based on literature sources that showed HTL gas is typically composed of 70–90% CO2, 5–15% H2 5–15%, and 5–14% CO [34,41]. Methane, ethylene, and ethane never accounted for more than 1–2% of HTL gases, and therefore the concentrations of these gases were assumed to be negligible [34,41]. Methane, ethylene, and ethane never accounted for more than 1–2% of HTL gases, and therefore the concentrations of these gases were considered negligible.
The liquid and solid HTL products were removed from the reactor into a vacuum filtration funnel fitted with 1.2 μm filter paper. Water and dissolved organics passed through the filter and were set aside for TOC analysis. Oil and solids remaining on the filter paper were washed with acetone to dissolve and collect the oil. The reactor walls and impeller were also washed with acetone to collect any residual material left in the reactor. Acetone was removed from the oil fraction using a rotary evaporator heated to 50 °C. Solids that were left on the filter papers were dried in an oven at 105 °C for 24 h before being ashed in a furnace at 650 °C. All food waste HTL runs had at least a 90% mass balance closure (gravimetric analysis).
Oil and water samples produced during catalytic HTL were analyzed with gas chromatography equipped with a mass spectrometer detector. Water samples were directly injected after filtration, while oil samples were diluted to 4 wt % in acetone, filtered, and then injected. Higher heating values for the oil were obtained with a semimicro calorimeter (25720, Parr, Moline, IL, USA) using O2. Benzoic acid was used to calibrate the instrument prior to analysis. The CHON content of the oil phase was performed by an outside laboratory (Midwest Microlabs) and obtained using an elemental analyzer. The total organic carbon (TOC) of aqueous HTL samples was outsourced (Flowers Chemical Laboratories) and obtained using a TOC analyzer.
4.4. Model Food Waste HTL Reactions
HTL reactions with model compounds were performed at the same reaction conditions as used for actual food waste. A 300 mL Parr reactor was initially loaded with 15 g of organic model compound, 85 g of water and 5 g of CeZrOx catalyst. For the mixed model HTL activity runs, a 50 mol % ratio for the two reactants was used as a basis. The reactor was then sealed and purged with nitrogen before loading 7.6 MPa of N2. Initial N2 pressurization was selected to provide the most careful control of the composition of the reaction mixture, as the goal of model compounds was the unambiguous identification of specific reaction pathways. The reactor was heated to 300 °C and mixed with an impeller set at approximately 700 revolutions per minute. The reaction proceeded at 300 °C and 20 MPa for one hour before quenching and separating out the aqueous, oil, and solid catalyst phases.
Gas chromatograph with mass spectrometer detection (GC-MS) was used for product identification, based on matches with the National Institute of Standards and Technology mass spectra data base. Product quantification was performed using GC with flame ionization detection (FID). Pure pentanal, 1-octene, 1-nonene and pentanol were used as calibration standards. Trans-2-decenal was used as a calibration standard for 2-propyl-2-heptenal quantification, as 2-propyl-2-heptenal was not available commercially. Model HTL reactions without activity, labeled “No Product” in Table 3, had no detectable product peaks in the GC-MS (<0.05% yield). All of the products considered “trace” in Table 3 constituted less than 0.3% of total yield, as estimated from FID peak areas. Reactant conversion reproducibility for tests performed under thermal conditions without a catalyst without a catalyst, with Ce(NO3)4, or with CeZrOx was ±5 wt %. Likewise, product yields were reproducible to within ±2 wt %.
4.5. Hydrothermal Stability of CeZrOx
A total of 1.0 g of CeZrOx and 100 mL of water were loaded into a 300 mL stainless-steel batch reactor and initially pressurized with N2 to 7.6 MPa. The reactor was then heated to 300 °C, which pressurized the vessel to 20.6 MPa. The stability study was performed for 16 or 165 h at 300 °C before quenching the reactor and extracting the catalyst for post-characterization. A small fraction of the water was removed from the reactor and kept for analysis followed by centrifugation and filtration steps to remove the CeZrOx nanoparticles.
CeZrOx samples were characterized before and after hydrothermal treatment using a variety of techniques. X-ray diffraction was performed using a Rigaku automatic instrument with the Bragg-Bretano theta-theta configuration. XRD patterns were obtained with a Cu Kα at 27.5 kV and 5 mA. Diffuse reflectance UV-Vis-spectroscopy (DR-UV) analysis was performed on powder CeZrOx using a ThermoScientific Evolution 300 UV-Vis spectrophotometer equipped with a Praying Mantis diffuse reflection cell. BaSO4 was used as a white reflectance standard. Samples were analyzed over the range from 200 to 1100 nm and plotted using the Kubelka and Munk diffuse reflectance model. Cerium content contained in the leachate obtained from hydrothermal treatment of CeZrOx was measured using a ICP-MS (inductively coupled plasma with mass spectrometer detector, NexION 350X, PerkinElmer, Hopkinton, MA, USA). The instrument was calibrated with an ICP standard and the liquid sample was diluted 1/50 in water before analysis for cerium content.
5. Conclusions
The conversion of food waste to energy has potential for diverting waste from landfills, a disposal method which contributes to both pollution and greenhouse gas emissions. HTL has shown promise for waste-to-energy conversion, especially for waste streams with high water content. A major challenge for HTL is simultaneously recovering a high quality bio-oil, maximizing energy recovery, and minimizing loss to the aqueous phase. This work establishes CeZrOx as a heterogeneous catalyst for HTL that yields a bio-oil with improved HHV, increases energy recovery relative to non-catalytic and Na2CO3-catalyzed HTL, and reduces the carbon loss to the aqueous phase relative to thermal conditions. Stability tests indicated that the CeZrOx crystal structure, elemental composition, and oxidation state were stable during exposure to HTL conditions (water at 300 °C for ≥16 h), with approximately 0.2% leaching of Ce being measured at the same conditions. Model compound reactions indicated that condensation of aldehydes was the main mechanism of catalytic action, consistent with increased bio-oil HHV and decreased aqueous phase carbon loss observed for the CeZrOx catalyzed HTL of institutional food waste. The catalyst could be reused up to three times with minimal loss of activity, which was the maximum number tested. Energy analysis indicated that reuse of the heterogeneous catalyst improves lifetime energy recovery by a factor of 200 when compared to single use homogeneous catalyst (39.9 MJ/g for CeZrOx compared to 0.21 MJ/g for Na2CO3). Economically, the heterogeneous catalyst is more cost effective than Na2CO3 provided it can be reused at least 25 times. This work suggests that CeZrOx, and possibly other water-stable oxides, have potential for base-catalyzed upgrading of food waste under HTL conditions.
Acknowledgments
This work was funded by a Department of Energy SBIR (Grant Number DE-SC0015784). Marianna Bailey, Jeremy Hemingway and Nicholas Carabillo supported some of the experiments performed on the model food waste mixture.
Author Contributions
Alex D. Paulsen, Ted J. Amundsen and Paul E. Yelvington designed and performed food waste HTL experiments as well as water and oil phase characterization; Alex R. Maag performed the CeZrOx stability experiments and model HTL reactions; Alex R. Maag, Geoffrey A. Tompsett and Michael T. Timko analyzed the data and wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
References
- U.S. Energy Information Administration. International Energy Outlook 2016 Chapter 8: Transportation Sector Energy Consumption; U.S. Energy Information Administration: Washington, DC, USA, 2016.
- Environmental Protection Agency. U.S. Energy Independence and Security Act; Environmental Protection Agency: Washington, DC, USA, 2007; p. 882.
- Davis, R.; Tao, L.; Tan, E.C.D.; Biddy, M.J.; Beckham, G.T.; Scarlata, C.; Jacobson, J.; Cafferty, K.; Ross, J.; Lukas, J.; et al. Process Design and Economics for the Conversion of Lignocellulosic Biomass to Hydrocarbons: Dilute-Acid and Enzymatic Deconstruction of Biomass to Sugars and Biological Conversion of Sugars to Hydrocarbons; National Renewable Energy Laboratory: Golden, CO, USA, 2013.
- Pavlovič, I.; Knez, Ž.; Škerget, M. Hydrothermal reactions of agricultural and food processing wastes in sub- and supercritical water: A review of fundamentals, mechanisms, and state of research. J. Agric. Food Chem. 2013, 61, 8003–8025. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Energy. Biofuels and Bioproducts from Wet and Gaseous Waste Streams: Challenges and Opportunities; U.S. Department of Energy: Washington, DC, USA, 2017.
- Déniel, M.; Haarlemmer, G.; Roubaud, A.; Weiss-Hortala, E.; Fages, J. Energy valorisation of food processing residues and model compounds by hydrothermal liquefaction. Renew. Sustain. Energy Rev. 2016, 54, 1632–1652. [Google Scholar] [CrossRef]
- Appels, L.; Lauwers, J.; Degrve, J.; Helsen, L.; Lievens, B.; Willems, K.; Van Impe, J.; Dewil, R. Anaerobic digestion in global bio-energy production: Potential and research challenges. Renew. Sustain. Energy Rev. 2011, 15, 4295–4301. [Google Scholar] [CrossRef]
- Huber, G.W.; Iborra, S.; Corma, A. Synthesis of transportation fuels from biomass: Chemistry, catalysts, and engineering. Chem. Rev. 2006, 106, 4044–4098. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, J.; Saidina Amin, N. A review on operating parameters for optimum liquid oil yield in biomass pyrolysis. Renew. Sustain. Energy Rev. 2012, 16, 5101–5109. [Google Scholar] [CrossRef]
- Zhu, Y.; Biddy, M.J.; Jones, S.B.; Elliott, D.C.; Schmidt, A.J. Techno-economic analysis of liquid fuel production from woody biomass via hydrothermal liquefaction (HTL) and upgrading. Appl. Energy 2014, 129, 384–394. [Google Scholar] [CrossRef]
- Yan, W.H.; Duan, P.G.; Wang, F.; Xu, Y.P. Composition of the bio-oil from the hydrothermal liquefaction of duckweed and the influence of the extraction solvents. Fuel 2016, 185, 229–235. [Google Scholar] [CrossRef]
- Posmanik, R.; Cantero, D.A.; Malkani, A.; Sills, D.L.; Tester, J.W. Biomass conversion to bio-oil using sub-critical water: Study of model compounds for food processing waste. J. Supercrit. Fluids 2017, 119, 26–35. [Google Scholar] [CrossRef]
- Elliott, D.C.; Hart, T.R.; Neuenschwander, G.G. Chemical processing in high-pressure aqueous environments. 8. Improved catalysts for hydrothermal gasification. Ind. Eng. Chem. Res. 2006, 45, 3776–3781. [Google Scholar] [CrossRef]
- Zhu, Y.; Albrecht, K.O.; Elliott, D.C.; Hallen, R.T.; Jones, S.B. Development of hydrothermal liquefaction and upgrading technologies for lipid-extracted algae conversion to liquid fuels. Algal Res. 2013, 2, 455–464. [Google Scholar] [CrossRef]
- Zhang, Y. Hydrothermal Liquefaction to Convert Biomass into Crude Oil; Blackwell Publishing: Hoboken, NJ, USA, 2010; ISBN 9780813802527. [Google Scholar]
- Ross, A.B.; Biller, P.; Kubacki, M.L.; Li, H.; Lea-Langton, A.; Jones, J.M. Hydrothermal processing of microalgae using alkali and organic acids. Fuel 2010, 89, 2234–2243. [Google Scholar] [CrossRef]
- Yang, Y.F.; Feng, C.P.; Inamori, Y.; Maekawa, T. Analysis of energy conversion characteristics in liquefaction of algae. Resour. Conserv. Recycl. 2004, 43, 21–33. [Google Scholar] [CrossRef]
- Beckers, J.; Rothenberg, G. Sustainable selective oxidations using ceria-based materials. Green Chem. 2010, 12, 939. [Google Scholar] [CrossRef]
- Gärtner, C.A.; Serrano-Ruiz, J.C.; Braden, D.J.; Dumesic, J.A. Catalytic upgrading of bio-oils by ketonization. ChemSusChem 2009, 2, 1121–1124. [Google Scholar] [CrossRef] [PubMed]
- Gaertner, C.A.; Serrano-Ruiz, J.C.; Braden, D.J.; Dumesic, J.A. Catalytic coupling of carboxylic acids by ketonization as a processing step in biomass conversion. J. Catal. 2009, 266, 71–78. [Google Scholar] [CrossRef]
- Gaertner, C.A.; Serrano-Ruiz, J.C.; Braden, D.J.; Dumesic, J.A. Ketonization reactions of carboxylic acids and esters over ceria-zirconia as biomass-upgrading processes. Ind. Eng. Chem. Res. 2010, 49, 6027–6033. [Google Scholar] [CrossRef]
- Zastrow, D.J.; Jennings, P.A. Hydrothermal liquefaction of food waste and model food. In Proceedings of the 2013 AIChE Annual Meeting Online Proceedings, San Francisco, CA, USA, 3–8 November 2013; pp. 1–9. [Google Scholar]
- Welcome to the USDA Food Composition Database. Available online: https://ndb.nal.usda.gov/ndb/ (accessed on 14 September 2017).
- Xiong, H.; Pham, H.N.; Datye, A.K. Hydrothermally stable heterogeneous catalysts for conversion of biorenewables. Green Chem. 2014, 16, 4627–4643. [Google Scholar] [CrossRef]
- Gollakota, A.R.K.; Kishore, N.; Gu, S. A review on hydrothermal liquefaction of biomass. Renew. Sustain. Energy Rev. 2018, 81, 1378–1392. [Google Scholar] [CrossRef]
- Sánchez Escribano, V.; Fernández López, E.; Panizza, M.; Resini, C.; Gallardo Amores, J.M.; Busca, G. Characterization of cubic ceria-zirconia powders by X-ray diffraction and vibrational and electronic spectroscopy. Solid State Sci. 2003, 5, 1369–1376. [Google Scholar] [CrossRef]
- Damyanova, S.; Pawelec, B.; Arishtirova, K.; Huerta, M.V.M.; Fierro, J.L.G. Study of the surface and redox properties of ceria-zirconia oxides. Appl. Catal. A Gen. 2008, 337, 86–96. [Google Scholar] [CrossRef]
- Besson, M.; Gallezot, P. Deactivation of metal catalysts in liquid phase organic reactions. Catal. Today 2003, 81, 547–559. [Google Scholar] [CrossRef]
- Kumar, R.; Enjamuri, N.; Shah, S.; Al-Fatesh, A.S.; Bravo-Suárez, J.J.; Chowdhury, B. Ketonization of oxygenated hydrocarbons on metal oxide based catalysts. Catal. Today 2017, 302, 16–49. [Google Scholar] [CrossRef]
- Postole, G.; Chowdhury, B.; Karmakar, B.; Pinki, K.; Banerji, J.; Auroux, A. Knoevenagel condensation reaction over acid-base bifunctional nanocrystalline CexZr1−xO2 solid solutions. J. Catal. 2010, 269, 110–121. [Google Scholar] [CrossRef]
- Saber, M.; Golzary, A.; Hosseinpour, M.; Takahashi, F.; Yoshikawa, K. Catalytic hydrothermal liquefaction of microalgae using nanocatalyst. Appl. Energy 2016, 183, 566–576. [Google Scholar] [CrossRef]
- Robin, T.; Jones, J.M.; Ross, A.B. Catalytic hydrothermal processing of lipids using metal doped zeolites. Biomass Bioenergy 2017, 98, 26–36. [Google Scholar] [CrossRef]
- Nazari, L.; Yuan, Z.; Souzanchi, S.; Ray, M.B.; Xu, C. Hydrothermal liquefaction of woody biomass in hot-compressed water: Catalyst screening and comprehensive characterization of bio-crude oils. Fuel 2015, 162, 74–83. [Google Scholar] [CrossRef]
- Duan, P.; Savage, P.E. Hydrothermal liquefaction of a microalga with heterogeneous catalysts. Ind. Eng. Chem. Res. 2011, 50, 52–61. [Google Scholar] [CrossRef]
- Jena, U.; Das, K.C.; Kastner, J.R. Comparison of the effects of Na2CO3, Ca3(PO4)2, and NiO catalysts on the thermochemical liquefaction of microalga Spirulina platensis. Appl. Energy 2012, 98, 368–375. [Google Scholar] [CrossRef]
- Chen, D.; Ma, Q.; Wei, L.; Li, N.; Shen, Q.; Tian, W. Catalytic hydroliquefaction of rice straw for bio-oil production using Ni/CeO2 catalysts. J. Anal. Appl. Pyrolysis 2018, 130, 169–180. [Google Scholar] [CrossRef]
- Shakya, R.; Whelen, J.; Adhikari, S.; Mahadevan, R.; Neupane, S. Effect of temperature and Na2CO3 catalyst on hydrothermal liquefaction of algae. Algal Res. 2015, 12, 80–90. [Google Scholar] [CrossRef]
- Shuping, Z.; Yulong, W.; Mingde, Y.; Kaleem, I.; Chun, L.; Tong, J. Production and characterization of bio-oil from hydrothermal liquefaction of microalgae Dunaliella tertiolecta cake. Energy 2010, 35, 5406–5411. [Google Scholar] [CrossRef]
- Yang, W.; Li, X.; Liu, S.; Feng, L. Direct hydrothermal liquefaction of undried macroalgae Enteromorpha prolifera using acid catalysts. Energy Convers. Manag. 2014, 87, 938–945. [Google Scholar] [CrossRef]
- Elliott, D.C.; Biller, P.; Ross, A.B.; Schmidt, A.J.; Jones, S.B. Hydrothermal liquefaction of biomass: Developments from batch to continuous process. Bioresour. Technol. 2015, 178, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Von Keitz, M.; Valentas, K. Thermal effects on hydrothermal biomass liquefaction. Appl. Biochem. Biotechnol. 2008, 147, 143–150. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).