Integrating Life Cycle Inventory and Process Design Techniques for the Early Estimate of Energy and Material Consumption Data
Abstract
:1. Introduction
2. Materials and Methods
2.1. Life Cycle Assessment
2.2. Process Scale-Up and Definition of Input/Output Data
3. Results
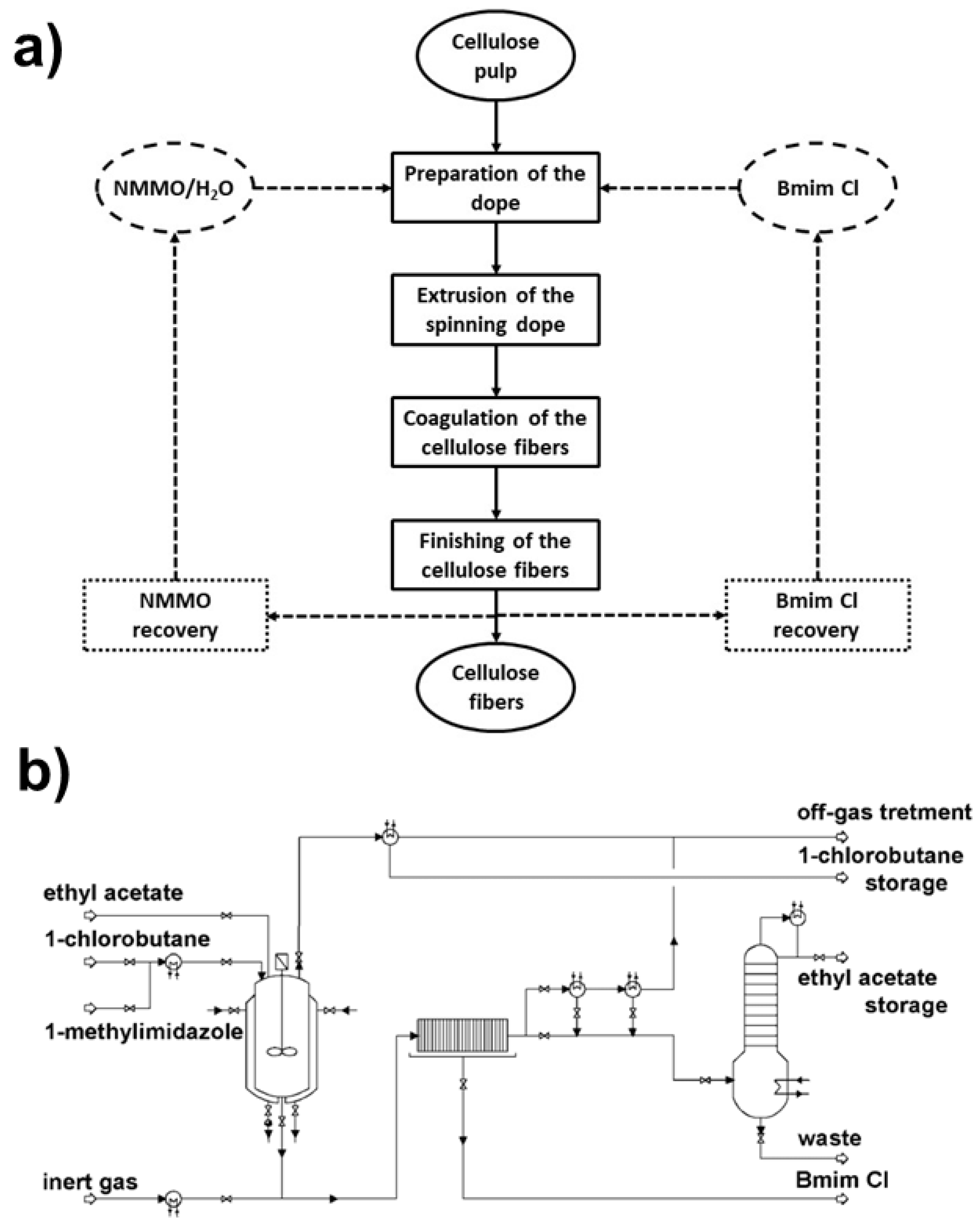
3.1. Ionic-Liquid Case Study
Modeling and Scale-Up of the Processes
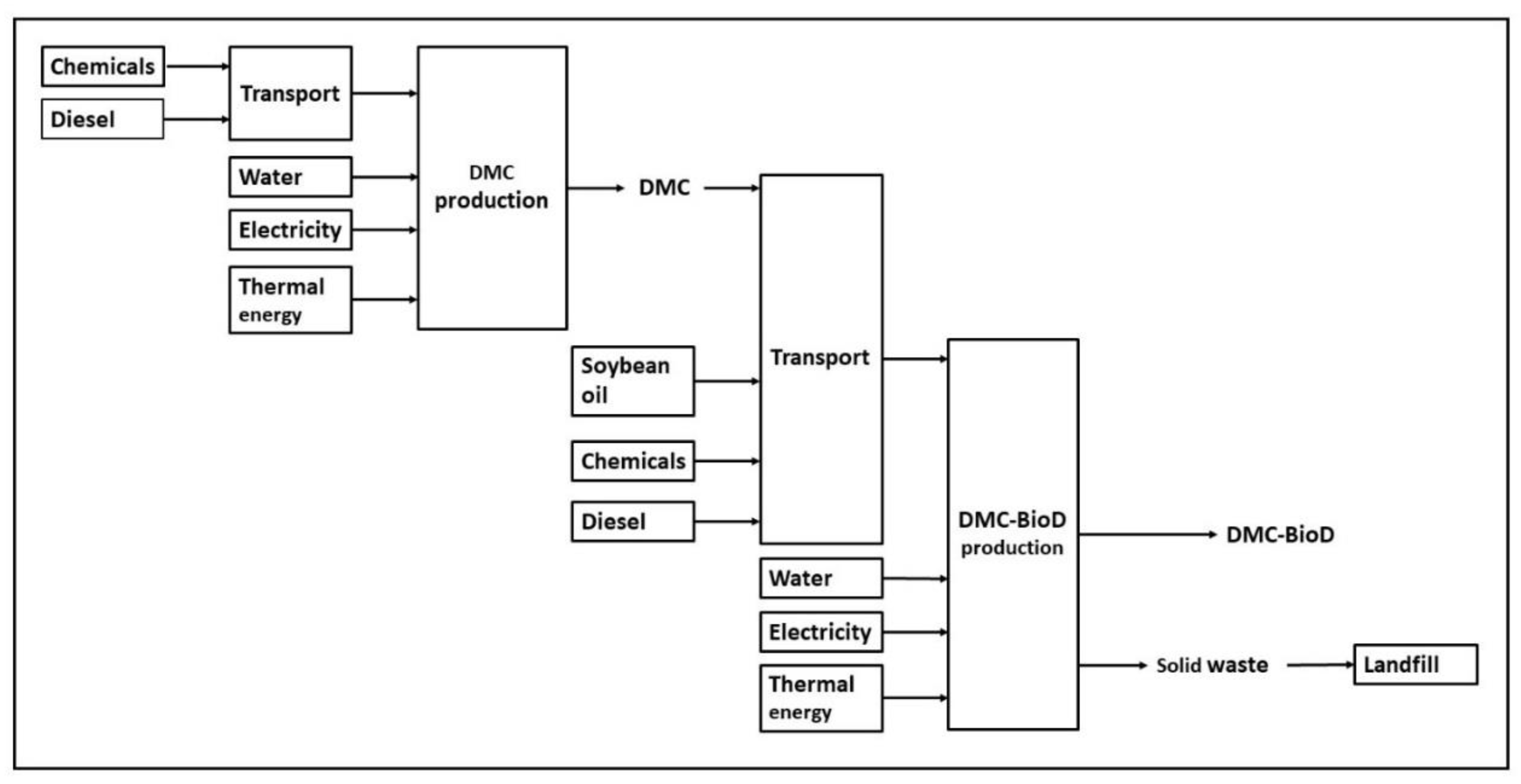
3.2. DMC-BioD Case Study
Modeling and Scale-Up of the Processes
3.3. PHA Extraction Case Study
Modeling and Scale-Up of the Processes
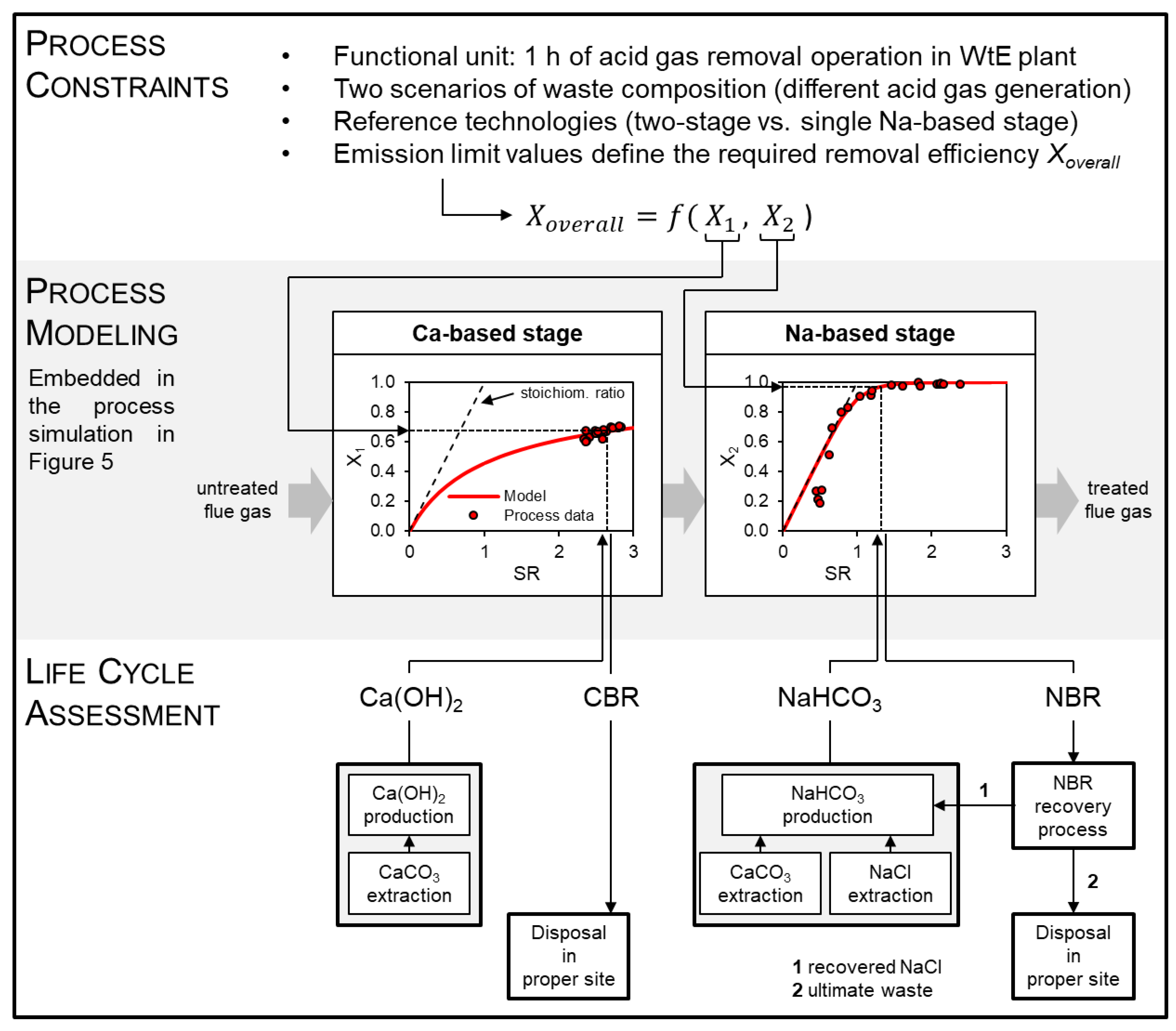
3.4. Use of Alternative Sorbents for Acid Gas Removal in Waste-to-Energy Plants
3.4.1. Modeling and Scale-Up of the Processes
- Ca-based stage. Calcium hydroxide is the less reactive of the two sorbents and it is only partially converted in the residence times typical of dry sorbent injection systems [78]. The solid residues of the reaction of Ca(OH)2 with acid gases, known as Ca-based residues (CBR), are to date non-recyclable and, thus, are to be sent to proper disposal sites [79]. Therefore, the Ca-based stage can be equipped with a solids recirculation system, which helps maximizing sorbent conversion.
- Na-based stage. Sodium bicarbonate presents higher affinity towards acid gases, but the sorbent as commercially supplied requires comminution in a grinding mill before injection to promote its reactivity [80]. In contrast with CBR, Na-based residues (NBR) can be recycled off-site: dedicated plants regenerate fresh bicarbonate from the residue, with ~85 wt % efficiency [81].
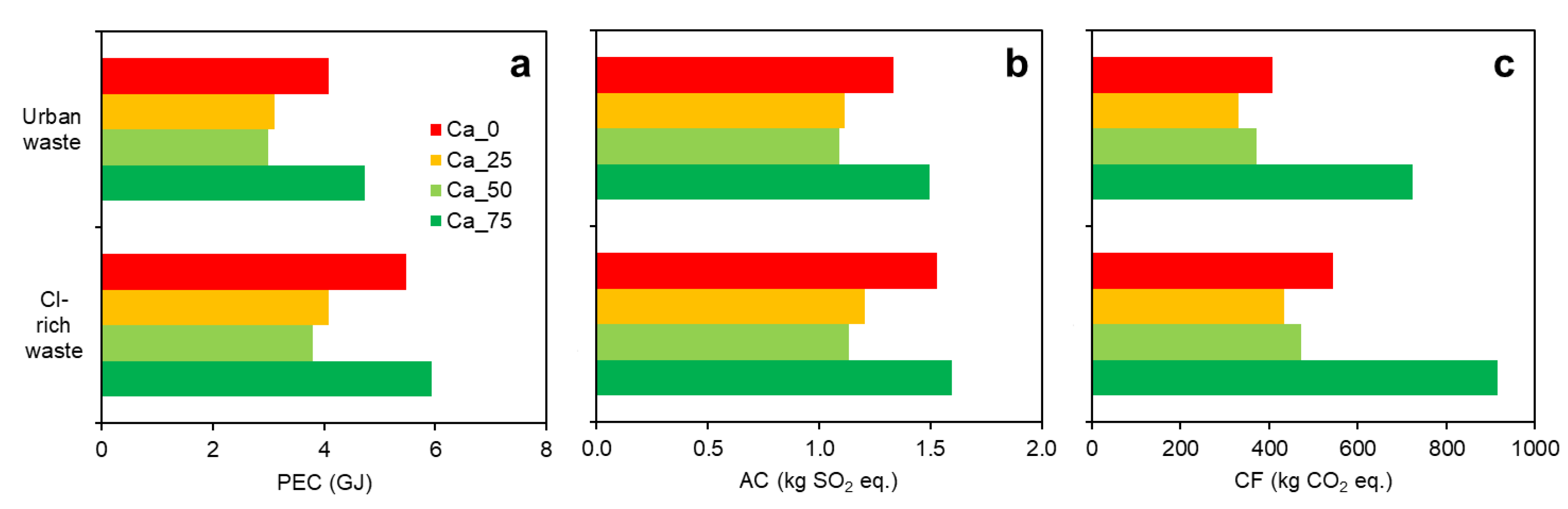
3.4.2. LCIA Results and Discussion
4. Conclusions
Author Contributions
Conflicts of Interest
Abbreviations
| Acronym | Term |
| AC | Acidification |
| BmimCl | 1-butyl-3-methylimidazolium chloride |
| CBR | Ca-based residues |
| CF | Carbon Footprint |
| CPS | Chemical Process Simulation |
| DCE | 1,2-dichloroethane |
| DMC | dimethyl carbonate |
| DMC-BioD | dimethyl carbonate-biodiesel |
| ELCD | European reference Life Cycle Database |
| EtOH | ethanol |
| FU | Functional Unit |
| I/O | Input/Output |
| ISO | International Organization for Standardization |
| LCA | Life Cycle Assessment |
| LCI | Life Cycle Inventory |
| LCIA | Life Cycle Impact Assessment |
| MeOH | methanol |
| NBR | Na-based residues |
| NMM | N-methylmorpholine |
| NMMO/H2O | N-methyl-morpholine-N-oxide monohydrated |
| PD | Preliminary design |
| PEC | Primary Energy Consumption |
| PFD | Process Flow Diagram |
| PHB | poly-hydroxybutyrate |
| PS | Preliminary sizing |
| R&D | Research and Development |
| SR | stoichiometric ratio |
| WtE | waste-to-energy plant |
References
- Ruiz-Mercado, G.J.; Smith, R.L.; Gonzalez, M.A. Sustainability indicators for chemical processes: I. Taxonomy. Ind. Eng. Chem. Res. 2012, 51, 2309–2328. [Google Scholar] [CrossRef]
- Tufvesson, L.M.; Tufvesson, P.; Woodley, J.M.; Borjesson, P. Life cycle assessment in green chemistry: Overview of key parameters and methodological concerns. Int. J. Life Cycle Assess. 2013, 18, 431–444. [Google Scholar] [CrossRef]
- Broeren, M.L.M.; Zijp, M.C.; Waaijers-van der Loop, S.L.; Heugens, E.H.W.; Posthuma, L.; Worrell, E.; Shen, L. Environmental assessment of bio-based chemicals in early-stage development: A review of methods and indicators. Biofuels Bioprod. Biorefin. 2017, 11, 701–718. [Google Scholar] [CrossRef]
- European Commission. Communication from the Commission to the Council and the European Parliament—Integrated Product Policy, Building on the Environmental Life-Cycle Thinking; COM (2003) 302 Final; European Commission: Brussels, Belgium, 2003. [Google Scholar]
- Environmental Management—Life Cycle Assessment—Principles and Framework; ISO 14040; ISO: Geneva, Switzerland, 2006.
- Hellweg, S.; Canals, L.M.I. Emerging approaches, challenges and opportunities in life cycle assessment. Science 2014, 344, 1109–1113. [Google Scholar] [CrossRef] [PubMed]
- UNEP/SETAC. Why Take a Life Cycle Approach? United Nations Publications: New York, NY, USA, 2004. [Google Scholar]
- Arvidsson, R.; Svanström, M. A Framework for Energy Use Indicators and Their Reporting in Life Cycle Assessment. Integr. Environ. Assess. Manag. 2016, 12, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Huijbregts, M.A.J.; Hellweg, S.; Frischknecht, R.; Hendriks, H.W.M.; Hungerbühler, K.; Hendriks, A.J. Cumulative energy demand as predictor for the environmental burden of commodity production. Environ. Sci. Technol. 2010, 44, 2189–2196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huijbregts, M.A.J.; Rombouts, L.J.A.; Hellweg, S.; Frischknecht, R.; Hendriks, A.J.; van de Meent, D.; Ragas, A.M.J.; Reijnders, L.; Struijs, J. Is cumulative fossil energy demand a useful indicator for the environmental performance of products? Environ. Sci. Technol. 2006, 40, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Sinnott, R.K. Coulson & Richardson’s Chemical Engineering, 3rd ed.; Butterworth-Heinemann: Oxford, UK, 1999; Volume 6. [Google Scholar]
- Bisio, A.; Kabel, R.L. Scale-Up of Chemical Processes: Conversion from Laboratory Scale Tests to Successful Commercial Size Design; Wiley: New York, NY, USA, 1985. [Google Scholar]
- Zlokarnik, M. Scale-Up in Chemical Engineering; Wiley: Weinheim, Germany, 2002. [Google Scholar]
- Righi, S.; Morfino, A.; Galletti, P.; Samorì, C.; Tugnoli, A.; Stramigioli, C. Comparative cradle-to-gate life cycle assessments of cellulose dissolution with 1-butyl-3-methylimidazolium chloride and N-methyl-morpholine-N-oxide. Green Chem. 2011, 13, 367–375. [Google Scholar] [CrossRef]
- Righi, S.; Bandini, V.; Fabbri, D.; Cordella, M.; Stramigioli, C.; Tugnoli, A. Modeling of an alternative process technology for biofuel production and assessment of its environmental impacts. J. Clean. Prod. 2016, 122, 42–51. [Google Scholar] [CrossRef]
- Guthrie, K.M. Data and techniques for preliminary capital cost estimating. Chem. Eng. 1969, 76, 114–142. [Google Scholar]
- Remer, D.S.; Chai, L.H. Estimate costs of scaled-up process plants. Chem. Eng. 1990, 97, 138–175. [Google Scholar]
- Caduff, M.; Huijbregts, M.A.J.; Koehler, A.; Althaus, H.-J.; Hellweg, S. Scaling Relationships in Life Cycle Assessment. J. Ind. Ecol. 2014, 18, 393–406. [Google Scholar] [CrossRef]
- Wernet, G.; Papadokonstantakis, S.; Hellweg, S.; Hungerbuhler, K. Bridging data gaps in environmental assessments: Modeling impacts of fine and basic chemical production. Green Chem. 2009, 11, 1826–1831. [Google Scholar] [CrossRef]
- Calvo-Serrano, R.; Gonzalez-Miquel, M.; Papadokonstantakis, S.; Guillen-Gosalbez, G. Predicting the cradle-to-gate environmental impact of chemicals from molecular descriptors and thermodynamic properties via mixed-integer programming. Comput. Chem. Eng. 2018, 108, 179–193. [Google Scholar] [CrossRef]
- Piccinno, F.; Hischier, R.; Seeger, S.; Som, C. From laboratory to industrial scale: A scale-up framework for chemical processes in life cycle assessment studies. J. Clean. Prod. 2016, 135, 1085–1097. [Google Scholar] [CrossRef]
- Caduff, M.; Huijbregts, M.A.J.; Althaus, H.-J.; Hendriks, A.J. Power-Law Relationships for Estimating Mass, Fuel Consumption and Costs of Energy Conversion Equipments. Environ. Sci. Technol. 2011, 45, 751–754. [Google Scholar] [CrossRef] [PubMed]
- Shibasaki, M.; Fischer, M.; Barthel, L. Effects on life cycle assessment d Scale up of processes. In Advances in Life Cycle Engineering for Sustainable Manufacturing Businesses; Takata, S., Umeda, Y., Eds.; Springer: London, UK, 2007; pp. 377–381. [Google Scholar]
- Zhou, Y.; Lee, C.K.; Sharratt, P. Bridging the Gap from Pilot Plant Experimental Records to Life Cycle Inventory. Ind. Eng. Chem. Res. 2017, 56, 10393–10412. [Google Scholar] [CrossRef]
- Smith, R.L.; Ruiz-Mercado, G.J.; Meyer, D.E.; Gonzalez, M.A.; Abraham, J.P.; Barrett, W.M.; Randall, P.M. Coupling Computer-Aided Process Simulation and Estimations of Emissions and Land Use for Rapid Life Cycle Inventory Modeling. ACS Sustain. Chem. Eng. 2017, 5, 3786–3794. [Google Scholar] [CrossRef]
- Zaimes, G.G.; Beck, A.W.; Janupala, R.R.; Resasco, D.E.; Crossley, S.P.; Lobban, L.L.; Khanna, V. Multistage torrefaction and in situ catalytic upgrading to hydrocarbon biofuels: Analysis of life cycle energy use and greenhouse gas emissions. Energy Environ. Sci. 2017, 10, 1034–1050. [Google Scholar] [CrossRef]
- Winjobi, O.; Shonnard, D.R.; Zhou, W. Production of Hydrocarbon Fuel Using Two-Step Torrefaction and Fast Pyrolysis of Pine. Part 1: Techno-economic Analysis. ACS Sustain. Chem. Eng. 2017, 5, 4529–4540. [Google Scholar] [CrossRef]
- Lari, G.M.; Pastore, G.; Haus, M.; Ding, Y.; Papadokonstantakis, S.; Mondelli, C.; Perez-Ramirez, J. Environmental and economical perspectives of a glycerol biorefinery. Energy Environ. Sci. 2018. [Google Scholar] [CrossRef]
- Schakel, W.; Hung, C.R.; Tokheim, L.-A.; Stromman, A.-H.; Worrell, E.; Ramirez, A. Impact of fuel selection on the environmental performance of post-combustion calcium looping applied to a cement plant. Appl. Energy 2018, 210, 75–87. [Google Scholar] [CrossRef]
- Environmental Management—Life Cycle Assessment—Requirements and Guidelines; ISO 14044; ISO: Geneva, Switzerland, 2006.
- Sugiyama, H.; Hirao, M.; Fischer, U.; Hungerbühler, K. Activity modeling for integrating environmental, health and safety (EHS) consideration as a new element in industrial chemical process design. J. Chem. Eng. Jpn. 2008, 41, 884–897. [Google Scholar] [CrossRef]
- Sugiyama, H.; Fischer, U.; Hungerbühler, K.; Hirao, M. Decision framework for chemical process design including different stages of environmental, health, and safety assessment. AIChE J. 2008, 54, 1037–1053. [Google Scholar] [CrossRef]
- Piccinno, F.; Hischier, R.; Seeger, S.; Som, C. Predicting the environmental impact of a future nanocellulose production at industrial scale: Application of the life cycle assessment scale-up framework. J. Clean. Prod. 2018, 174, 283–295. [Google Scholar] [CrossRef]
- Ayres, R.U. Life cycle analysis: A critique. Resour. Conserv. Recycl. 1995, 14, 199–223. [Google Scholar] [CrossRef]
- Tugnoli, A.; Santarelli, F.; Cozzani, V. An approach to quantitative sustainability assessment in the early stages of process design. Environ. Sci. Technol. 2008, 42, 4555–4562. [Google Scholar] [CrossRef] [PubMed]
- Alexander, B.; Barton, G.; Petrie, J.; Romagnoli, J. Process synthesis and optimization tools for environmental design: Methodology and structure. Comput. Chem. Eng. 2000, 24, 1195–1200. [Google Scholar] [CrossRef]
- Brunet, R.; Cortés, D.; Guillén-Gosalbez, G.; Jiménez, L.; Boer, D. Minimization of the LCA impact of thermodynamic cycles using a combined simulation-optimization approach. Appl. Therm. Eng. 2012, 48, 367–377. [Google Scholar] [CrossRef]
- Aspen Plus® 10.2-1. Aspen Technology Inc.: Burlington, MA, USA, 2000.
- McCorsley, C.C.; Asheville, N.C. Process for Shaped Cellulose Article Prepared from a Solution Containing Cellulose Dissolved in a Tertiary Amine N-Oxide Solvent. U.S. Patent 4,246,221, 20 January 1981. [Google Scholar]
- Kosan, B.; Michels, C.; Meister, F. Dissolution and forming of cellulose with ionic liquids. Cellulose 2008, 15, 59–66. [Google Scholar] [CrossRef]
- Park, S.; Kazlauskas, R.J. Improved Preparation and Use of Room-Temperature Ionic Liquids in Lipase-Catalyzed Enantio- and Regioselective Acylations. J. Org. Chem. 2001, 66, 8395–8401. [Google Scholar] [CrossRef] [PubMed]
- Hydrocarbon Processing; The Leonard Process Company: North Hollywood, CA, USA, 1979; Volume 58, p. 194f.
- Forkner, M.W.; Robson, J.H.; Snellings, W.M. Kirk-Othmer Encyclopedia of Chemical Technology, 4th ed.; Wiley: New York, NY, USA, 1994; Volume 12, p. 695. [Google Scholar]
- Mattioda, G.; Blanc, A. Ullmann’s Encyclopedia of Industrial Chemistry, 5th ed.; Verlag Chemie: New York, NY, USA, 1989; Volume A12, p. 491. [Google Scholar]
- Howe, B.K.; Hardy, F.R.F.; Clarke, D.A. Vapour Phase Oxidation of Hydroxy Compounds. GB Patent 1,272,592, 3 May 1972. [Google Scholar]
- Ebel, K.; Koehle, H.; Gamer, A.; Jäckh, A. Ullmann’s Encyclopedia of Industrial Chemistry, 5th ed.; Verlag Chemie: New York, NY, USA, 1989; Volume A13, p. 661. [Google Scholar]
- Von Däniken, A.; Chudacoff, M. Vergleichende ökologische Bewertung von Anstrichstoffen in Baubereich. Band 2: Daten; Buwal Schriftenreihe Unwelt Nr. 232: Bern, Switzerland, 1995. [Google Scholar]
- Scholten, H.; Rindtorff, K. Process for Producing Aqueous N-Methylmorpholine-N-oxide Solutions. U.S. Patent 4,748,241, 31 May 1988. [Google Scholar]
- Simon, J.; Becker, R.; Lebkücher, R.; Neuhauser, H. N-Alkylation of Amines. U.S. Patent 5,917,039, 29 June 1999. [Google Scholar]
- Fabbri, D.; Bevoni, V.; Notari, M.; Rivetti, F. Properties of a Potential Biofuel Obtained from Soybean Oil by Transmethylation with Dimethyl Carbonate. Fuel 2007, 86, 690–697. [Google Scholar] [CrossRef]
- Ecoinvent Centre. Ecoinvent Data and Reports v2.0 Final Reports Ecoinvent 2000; Swiss Centre for Life Cycle Inventories: Dübendorf, Switerland, 2007. [Google Scholar]
- GaBi Professional Database. Available online: http://www.gabisoftware.com/support/gabi/ (accessed on 20 August 2015).
- Notari, M.; Rivetti, F. Use of a Mixture of Esters of Fatty Acids as Fuel or Solvent. Patent WO 2004/052874, 24 June 2004. [Google Scholar]
- Aspen HYSYS 7.1. User’s Guide; Aspen Technology Inc.: Burlington, MA, USA, 2009.
- Rivetti, F.; Romano, U. Procedure for the Production of Alkyl Carbonates. Patent EP534545, 19 September 1992. [Google Scholar]
- Righi, S.; Baioli, F.; Samorì, C.; Galletti, P.; Tagliavini, E.; Stramigioli, C.; Tugnoli, A.; Fantke, P. A life cycle assessment of poly-hydroxybutyrate extraction from microbial biomass using dimethyl carbonate. J. Clean. Prod. 2017, 168, 692–707. [Google Scholar] [CrossRef]
- Samorì, C.; Basaglia, M.; Casella, S.; Favaro, L.; Galletti, P.; Giorgini, L.; Marchi, D.; Mazzocchetti, L.; Torri, C.; Tagliavini, E. Dimethyl carbonate and switchable anionic surfactants: Two effective tools for the extraction of polyhydroxyalkanoates from microbial biomass. Green Chem. 2015, 17, 1047–1056. [Google Scholar] [CrossRef]
- Senior, P.J.; Wright, L.F.; Alderson, B. Extraction Process. U.S. Patent 4,324,907, 13 April 1982. [Google Scholar]
- Perry, R.H.; Green, D.W.; Maloney, J.O. Perry’s Chemical Engineers’ Handbook, 6th ed.; McGraw-Hill: New-York, NY, USA, 1984. [Google Scholar]
- Harding, K.G.; Dennis, J.S.; von Blottnitz, H.; Harrison, S.T.L. Environmental analysis of plastic production processes: Comparing petroleum-based polypropylene and polyethylene with biologically-based poly-b-hydroxybutyric acid using life cycle analysis. J. Biotechnol. 2007, 130, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Morfino, A. Collection, Production and Analysis of the Inventory Data of the Life Cycle of the Ionic Liquid Bmim-BF4 (In Italian). Bachelor’s Thesis, University of Bologna, Bologna, Italy, 2009; p. 91. [Google Scholar]
- Baker, C.G.J.; McKenzie, K.A. Energy consumption of industrial spray dryers. Dry. Technol. 2005, 23, 365–386. [Google Scholar] [CrossRef]
- EEA. EMEP/EEA Air Pollutant Emission Inventory Guidebook 2013; Technical Guidance to Prepare National Emission Inventories; EEA Technical Report 12; Publications Office of the European Union: Luxembourg, 2013. [Google Scholar]
- Kujawski, W. Application of pervaporation and vapor permeation in environmental protection. Pol. J. Environ. Stud. 2000, 9, 13–26. [Google Scholar]
- Neel, J. Introduction to Pervaporation. Pervaporation Membrane Separation Processes; Elsevier: Amsterdam, The Netherlands, 1991. [Google Scholar]
- Chi, C.; Li, Y.; Sun, R.; Ma, X.; Duan, L.; Wang, Z. HCl removal performance of Mg-stabilized carbide slag from carbonation/calcination cycles for CO2 capture. RSC Adv. 2016, 6, 104303–104310. [Google Scholar] [CrossRef]
- Damgaard, A.; Riber, C.; Fruergaard, T.; Hulgaard, T.; Christensen, T.H. Life cycle assessment of the historical development of air pollution control and energy recovery in waste incineration. Waste Manag. 2010, 30, 1244–1250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hunt, G.; Heiszwolf, J.J.; Sewell, M. Enhanced Hydrated Lime—A Simple Solution for Acid Gas Compliance. IEEE Trans. Ind. Appl. 2017, 54, 796–807. [Google Scholar] [CrossRef]
- Dal Pozzo, A.; Guglielmi, D.; Antonioni, G.; Tugnoli, A. Sustainability analysis of dry treatment technologies for acid gas removal in waste-to-energy plants. J. Clean. Prod. 2017, 162, 1061–1074. [Google Scholar] [CrossRef]
- Dal Pozzo, A.; Guglielmi, D.; Antonioni, G.; Tugnoli, A. Two-stage vs. single stage acid gas treatment systems: A performance assessment based on economic and environmental indices. In Proceedings of the 9th International Conference on Environmental Engineering and Management (ICEEM09), Bologna, Italy, 6–9 September 2017. [Google Scholar]
- Foo, R.; Berger, R.; Heiszwolf, J.J. Reaction Kinetic Modeling of DSI for MATS Compliance. In Proceedings of the Power Plant Pollutant Control and Carbon Management “MEGA” Symposium, Baltimore, MD, USA, 16–19 August 2016. [Google Scholar]
- Marocco, L.; Mora, A. CFD modeling of the Dry-Sorbent-Injection process for flue gas desulfurization using hydrated lime. Sep. Purif. Technol. 2013, 108, 205–214. [Google Scholar] [CrossRef]
- Gutiérrez Ortiz, F.J.; Ollero, P. A realistic approach to modeling an in-duct desulfurization process based on an experimental pilot plant study. Chem. Eng. J. 2008, 141, 141–150. [Google Scholar] [CrossRef]
- Dal Pozzo, A.; Antonioni, G.; Guglielmi, D.; Stramigioli, C.; Cozzani, V. Comparison of alternative flue gas dry treatment technologies in waste-to-energy processes. Waste Manag. 2016, 51, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-D.; Jeon, S.-M.; Hasolli, N.; Lee, K.-S.; Lee, J.-R.; Han, J.-W.; Kim, H.-T.; Park, Y.-O. HCl removal characteristics of calcium hydroxide at the dry-type sorbent reaction accelerator using municipal waste incinerator flue gas at a real site. Korean J. Chem. Eng. 2017, 34, 747–756. [Google Scholar] [CrossRef]
- Brna, T.G. Cleaning of Flue Gases from Waste Combustors. Combust. Sci. Technol. 1990, 74, 83–98. [Google Scholar] [CrossRef]
- Antonioni, G.; Dal Pozzo, A.; Guglielmi, D.; Tugnoli, A.; Cozzani, V. Enhanced modelling of heterogeneous gas-solid reactions in acid gas removal dry processes. Chem. Eng. Sci. 2016, 148, 140–154. [Google Scholar] [CrossRef]
- Dal Pozzo, A.; Moricone, R.; Antonioni, G.; Tugnoli, A.; Cozzani, V. Hydrogen Chloride Removal from Flue Gas by Low-Temperature Reaction with Calcium Hydroxide. Energy Fuels 2018, 32, 747–756. [Google Scholar] [CrossRef]
- Margallo, M.; Taddei, M.B.M.; Hernandez-Pellon, A.; Aldaco, R.; Irabien, A. Environmental sustainability assessment of the management of municipal solid waste incineration residues: A review of the current situation. Clean. Technol. Environ. Policy 2015, 17, 1333–1353. [Google Scholar] [CrossRef]
- Walawska, B.; Szymanek, A.; Pajdak, A.; Nowak, M. Flue gas desulfurization by mechanically and thermally activated sodium bicarbonate. Pol. J. Chem. Technol. 2014, 16, 56–62. [Google Scholar] [CrossRef]
- Brivio, S. The SOLVAL platform: A sodium sludges valorization, an industrial reality for the environment (in Italian). L’Ambiente 2005, 3, 48–49. [Google Scholar]
- ELCD. European Reference Life Cycle Database. Available online: eplca.jrc.ec.europa.eu/ELCD3/index.xhtml (accessed on 2 February 2018).
- Swedish Life Cycle Center. CPM LCA Database. Available online: cpmdatabase.cpm.chalmers.se (accessed on 2 February 2018).
- Ninane, L.; Adam, J.F.; Humblot, C. Method for Producing an Aqueous Industrial Sodium Chloride Solution. U.S. Patent 5478447, 26 December 1995. [Google Scholar]
- Molerus, O. Overview: Pneumatic transport of solids. Powder Technol. 1996, 88, 309–321. [Google Scholar] [CrossRef]
- US EPA. Air Pollution Control Cost Manual, 6th ed.; United States Environmental Protection Agency Office of Air Quality Planning and Standards: Research Triangle Park, NC, USA, 2002. Available online: www3.epa.gov/ttncatc1/dir1/c_allchs.pdf (accessed on 2 February 2018).
- European Commission. Reference Document on BAT in Common Waste Water and Waste Gas Treatment/Management Systems in the Chemical Sector; Technical Report; Publications Office of the European Union: Luxembourg, 2016; Available online: https://ec.europa.eu/jrc/en/publication/eur-scientific-and-technical-research-reports/best-available-techniques-bat-reference-document-common-waste-water-and-waste-gas (accessed on 2 February 2018).
- Institute of Environmental Sciences (CML). CML-IA Characterization Factors; Universiteit Leiden: Leiden, The Netherlands, 2016; Available online: www.universiteitleiden.nl/en/research/research-output/science/cml-ia-characterisation-factors (accessed on 2 February 2018).




| Process Life Cycle Stage | Process Chemistry | Conceptual Design | Detailed Design | Plant Operation |
|---|---|---|---|---|
| Design activities | Selection of the chemical route and operative conditions | Process definition | Equipment and layout design, utilities design | Plant management and optimization |
| Information available | Stoichiometry, yields, temperature, pressure | Unit operations, energy and material balances | Equipment type and size, piping and instrumentation, operating procedure | Field data on energy and material balances |
| LCI modeling: Material input/output | Main raw materials and products | Raw materials, products and wastes | Raw materials, products, wastes, fugitive emissions | Raw materials, products, wastes, fugitive emissions |
| LCI modeling: Energy input/output | None | Process related energy demand | Plant energy demand (including losses) | Plant energy demand (including losses) |
| Data quality for LCI | Measured (in laboratory conditions) | Estimated data (process specific) | Estimated data (plant specific) | Measured data (plant specific) |
| Case Study | Problem with Data Availability | Modeling Based Directly on Available Data | Modeling Assisted by Preliminary Process Design | |||||
|---|---|---|---|---|---|---|---|---|
| Practical Problem | Unit Processes Affected | Available Data | I/O Contribution to LCI | Modeling Approach | I/O Contribution to LCI | |||
| Material I/O | Energy I/O | Material I/O | Energy I/O | |||||
| Case study 1 NMMO/H2O process | Industrial level application but no access to plant data | Cellulose dissolution by NMMO/H2O | Literature data on dissolution process Limited data on solvent recovery | Main input/output No data on solvent losses | No data available | PD of the process PS of the main equipment | Estimated input/output | Estimated thermal (including losses) and electric energy |
| NMMO synthesis | Limited literature data on chemical synthesis process, no data on product separation | Main input/output | No data available | PD of the process PS of the main equipment | Estimated input/output | Estimated thermal (including losses) and electric energy | ||
| Case study 1 Ionic liquid | Lab scale application only | Cellulose dissolution by BmimCl | No process data (laboratory scale solubility test) | No data available | No data available | PD of the process and PS of the equipment based on the NMMO/ H2O case | Estimated input/output | Estimated thermal (including losses) and electric energy |
| BmimCl systhesis | Laboratory synthesis protocol | Input/output at laboratory scale (not optimized) | Data for laboratory scale equipment | PD of the process PS of the main equipment | Estimated input/output | Estimated thermal (including losses) and electric energy | ||
| Case study 2 DMC-BioD | Lab scale application only + Industrial level application but no access to plant data | DMC-BioD production | Laboratory synthesis protocol | Input/output at laboratory scale (not optimized) | Data for laboratory scale equipment | PD of the process PS of the main equipment | Estimated input/output | Estimated thermal (including losses) and electric energy |
| DMC production | Limited literature data on production process | Main input/output | No data available | PD of the process PS of the main equipment | Estimated input/output | Estimated thermal (including losses) and electric energy | ||
| Case study 3 PHA extraction by DMC | Lab scale application only | Extraction process | Laboratory synthesis protocol | Input/output at laboratory scale (not optimized) | Data for laboratory scale equipment | PD of the process PS of the main equipment | Estimated input/output | Estimated thermal (incl. losses) and electric energy |
| Case study 3 PHA extraction by 1,2-DCE | Industrial level application but no access to plant data | Extraction process | Limited literature data on process | Main input/output | No data available | PD of the process PS of the main equipment | Estimated input/output | Estimated thermal (including losses) and electric energy |
| Case study 4 Sorbents for acid gas removal | Industrial level application but limited access to plant data | Flue gas treatment system at the WtE plant | Available plant data refer to specific modes of operation of the process (not optimized) | Main input/output data (no optimization or correlation to waste type) | Data available for specific operative condition | Non-linear model of the reaction, PD of the process, PS of the main equipment | Estimated input/output for different modes of operation | Estimated energy consumption for different modes of operation |
| Process | IO | Flow | Unit | Value |
|---|---|---|---|---|
| Glyoxal production | Input | Thermal energy | MJ | 1.53 |
| Electric energy | MJ | 0.43 | ||
| Ethylene glycol | kg | 2.03 | ||
| Output | Glyoxal | kg | 1.16 | |
| Methylamine production | Input | Thermal energy | MJ | 1.86 |
| Electric energy | MJ | 0.02 | ||
| Ammonia | kg | 0.14 | ||
| Methanol | kg | 0.27 | ||
| Output | Methylamine | kg | 0.25 | |
| 1-methylimidazole production | Input | Thermal energy | MJ | 0.74 |
| Electric energy | MJ | 0.48 | ||
| Ammonia | kg | 0.14 | ||
| Formaldehyde | kg | 0.65 | ||
| Glyoxal | kg | 1.16 | ||
| Methylamine | kg | 0.25 | ||
| Output | 1-methylimidazole | kg | 0.49 | |
| 1-chlorobutane production | Input | Thermal energy | MJ | 0.27 |
| Electric energy | MJ | 0.08 | ||
| Butanol | kg | 0.50 | ||
| Hydrochloric acid | kg | 0.25 | ||
| Output | 1-chlorobutane | kg | 0.61 | |
| Ehylacetate production | Input | Thermal energy | MJ | 0.19 |
| Electric energy | MJ | 0.50 | ||
| Ethanol | kg | 0.06 | ||
| Output | Ehylacetate | kg | 0.04 | |
| Bmim Cl production | Input | Thermal energy | MJ | 1.50 |
| Electric energy | MJ | 0.21 | ||
| 1-methylimidazole | kg | 0.49 | ||
| 1-chlorobutane | kg | 0.61 | ||
| Ehylacetate | kg | 0.04 | ||
| Output | Bmim Cl | kg | 1.00 |
| Process | I/O | Flow | Unit | Value |
|---|---|---|---|---|
| Methylamine production | Input | Thermal energy | MJ | 1.72 |
| Electric energy | MJ | 0.02 | ||
| Ammonia | kg | 0.13 | ||
| Methanol | kg | 0.25 | ||
| Output | Methylamine | kg | 0.23 | |
| NMM production | Input | Thermal energy | MJ | 4.24 |
| Electric energy | MJ | 0.02 | ||
| Methylamine | kg | 0.23 | ||
| Diethylene glycol | kg | 0.44 | ||
| Output | NMM | kg | 0.51 | |
| NMMO/H2O (59% sol) | Input | Thermal energy | MJ | 3.93 |
| Electric energy | MJ | 0.09 | ||
| Hydrogen peroxide | kg | 0.50 | ||
| NMM | kg | 0.51 | ||
| Output | NMMO/H2O (59%sol) | kg | 1.00 |
| Process | Input/Output | Flow | Unit | Value | Source | Note |
|---|---|---|---|---|---|---|
| DMC production | Input | CO | kg | 1.04 × 100 | [55] | from production plant |
| H2 | kg | 5.58 × 10−3 | [55] | from production plant | ||
| O2 | kg | 2.53 × 10−1 | [55] | from production plant | ||
| N2 | kg | 3.90 × 10−2 | [55] | from production plant | ||
| CH3OH | kg | 7.46 × 10−1 | [55] | from production plant | ||
| HCl | kg | 4.46 × 10−3 | [55] | from production plant | ||
| NaOH | kg | 9.00 × 10−4 | [55] | from production plant | ||
| H2O | kg | 7.62 × 10−3 | [55] | from production plant | ||
| Electricity | MJ | 9.50 × 10−1 | [54] | from electricity grid mix | ||
| Thermal energy | MJ | 1.30 × 101 | [54] | thermal energy from natural gas | ||
| Output | DMC | kg | 1.00 × 100 | [55] | to DMC-BioD production | |
| CO2 | kg | 5.00 × 10−3 | [54] | emissions to air | ||
| N2 | kg | 4.50 × 10−3 | [54] | emissions to air | ||
| O2 | kg | 4.50 × 10−2 | [54] | emissions to air | ||
| Wastewater | kg | 1.70 × 10−1 | [54] | emissions to sea water | ||
| DMC-BioD production | Input | Soybean oil | kg | 2.66 × 10−2 | [53] | from production plant |
| DMC | kg | 3.58 × 10−3 | [53] | from DMC production | ||
| NaCH3O | kg | 8.53 × 10−5 | [53] | from production plant | ||
| H3PO4 | kg | 2.25 × 10−4 | [53] | from production plant | ||
| CH3OH | kg | 1.97 × 10−4 | [53] | from production plant | ||
| H2O | kg | 3.38 × 10−5 | [54] | from production plant | ||
| Electricity | MJ | 1.38 × 10−3 | [54] | from electricity grid mix | ||
| Thermal energy | MJ | 4.41 × 10−2 | [54] | thermal energy from natural gas | ||
| Output | DMC-BioD | MJ | 1.00 × 100 | [53] | ||
| NaH2PO4 | kg | 1.20 × 10−5 | [54] | to landfill |
| Equipment | Data | Source |
|---|---|---|
| Centrifuges | Specific power | [59] |
| Volumetric capacity | [59] | |
| Operating time | [60] | |
| Batch reaction vessels | Specific power | [61] |
| Volume | [61] | |
| Air dryers | Energy consumption | [62] |
| Purge flow | [54] | |
| Heat loss | [54] | |
| Catalytic oxidizer | Emission factors | [63] |
| Pervaporation systems | General information | [64] |
| General information | [65] |
| Process | Flow | Dry | Slurry | U.M. | ||
|---|---|---|---|---|---|---|
| Laboratory Scale | Industrial Scale-Up | Laboratory Scale | Industrial Scale-Up | |||
| Centrifugation | Electricity | 1.7 × 101 | 2.6 × 10−1 | 1.7 × 101 | 2.6 × 10−1 | MJ |
| Drying | Electricity | 3.8 × 104 | 1.3 × 100 | NR | NR | MJ |
| Steam | NR | 1.1 × 101 | NR | NR | MJ | |
| Solubilization | Electricity | 3.6 × 102 | 7.6 × 10−2 | 3.6 × 102 | 1.1 × 10−1 | MJ |
| Steam | NR | 2.7 × 100 | NR | 5.5 × 100 | MJ | |
| DMC recovery | DMC | 0 | 99.0–99.8 | 0 | 92.4–93.3 | % |
| Process | I/O | Flow | Unit | Value | Note |
|---|---|---|---|---|---|
| Centrifuge 1 | Input | Pure microbial culture | kg | 1.5 × 102 | from cultivation phase |
| Electricity | MJ | 3.8 × 10−1 | from electricity grid mix | ||
| Output | Concentrated wet biomass | kg | 8.9 | to batch reactor | |
| Water | kg | 1.4 × 102 | reusable for a successive cultivation | ||
| Air dryer 1 | Input | Concentrated wet biomass | kg | 8.9 | from centrifuge 1 |
| Electricity | MJ | 1.9 | from electricity grid mix | ||
| Steam | MJ | 1.7 × 101 | steam from natural gas | ||
| Output | Dried biomass | kg | 1.5 | to batch reactor | |
| Water vapor | kg | 7.5 | emission to air | ||
| Batch reactor | Input | DMC new | kg | 5.1 × 10−2 | from production plant |
| Dried biomass | kg | 1.5 | from air dryer 1 | ||
| DMC recovered | kg | 3.2 × 101 | from condenser 1 and condenser 2 | ||
| Electricity | MJ | 1.1 × 10−1 | from electricity grid mix | ||
| Steam | MJ | 4.0 | from natural gas | ||
| Output | Biomass–DMC mixture | kg | 3.3 × 101 | to centrifuge 2 | |
| Centrifuge 2 | Input | Biomass–DMC mixture | kg | 3.3 × 101 | from batch reactor |
| Electricity | MJ | 7.9 × 10−2 | from electricity grid mix | ||
| Output | PHB–DMC solution | kg | 3.2 × 101 | to air dryer 3 | |
| Residual biomass–DMC mixture | kg | 9.5 × 10−1 | to air dryer 2 | ||
| Air dryer 2 | Input | Residual biomass–DMC mixture | kg | 9.5 × 10−1 | from centrifuge 2 |
| Electricity | MJ | 2.6 × 10−2 | from electricity grid mix | ||
| Steam | MJ | 2.3 × 10−1 | from natural gas | ||
| Output | Residual biomass | kg | 4.9 × 10−1 | to waste incineration | |
| DMC | kg | 4.6 × 10−1 | to condenser 1 | ||
| Condenser 1 | Input | DMC | kg | 4.6 × 10−1 | from air dryer 2 |
| Electricity | MJ | 1.1 × 10−2 | from electricity grid mix | ||
| Output | DMC recovered | kg | 4.6 × 10−1 | to batch reactor | |
| DMC purge | kg | 7.3 × 10−4 | to catalytic oxidizer | ||
| Air dryer 3 | Input | PHB–DMC solution | kg | 3.2 × 101 | from centrifuge 2 |
| Electricity | MJ | 1.8 | from electricity grid mix | ||
| Steam | MJ | 1.6 × 101 | steam from natural gas | ||
| Output | PHB | kg | 1.0 | raw material | |
| DMC | kg | 3.1 × 101 | to condenser 2 | ||
| Condenser 2 | Input | DMC | kg | 3.1 × 101 | from air dryer 3 |
| Electricity | MJ | 7.5 × 10−1 | from electricity grid mix | ||
| Output | DMC recovered | kg | 3.1 × 101 | to batch reactor | |
| DMC purge | kg | 5.0 × 10−2 | to catalytic oxidizer | ||
| Catalytic oxidizer | Input | DMC purge | kg | 5.1 × 10−2 | from condenser 1 and condenser 2 |
| Output | DMC emission | kg | 2.7 × 10−4 | emission to air | |
| CO2 | kg | 7.4 × 10−2 | emission to air | ||
| Water vapor | kg | 3.0 × 10−2 | emission to air | ||
| NOx | kg | 3.2 × 10−5 | emission to air |
| Equipment | Energy Consumption Per FU (kWh/h) | Source for Modeling | |||
|---|---|---|---|---|---|
| Acid gas treatment system | Ca_0 | Ca_25 | Ca_50 | Ca_75 | |
| Dilute-phase conveying (sorbent feeds) | 3.00 | 6.00 | 6.00 | 6.00 | [85] |
| Air-classifying mill (NaHCO3 feed) | 35.45 | 24.05 | 16.31 | 8.82 | [86] |
| Dense-phase conveying (residue streams) | 1.50 | 3.00 | 3.00 | 3.00 | [85] |
| Air pulse cleaning | 0.25 | 0.25 | 0.25 | 0.25 | [86] |
| NBR recycling plant | Ca_0 | Ca_25 | Ca_50 | Ca_75 | |
| Stirrer | 0.11 | 0.08 | 0.05 | 0.03 | [54] |
| Filter press | 4.54 | 3.05 | 2.09 | 1.16 | [87] |
| Pump | 0.15 | 0.10 | 0.07 | 0.04 | [54] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Righi, S.; Baioli, F.; Dal Pozzo, A.; Tugnoli, A. Integrating Life Cycle Inventory and Process Design Techniques for the Early Estimate of Energy and Material Consumption Data. Energies 2018, 11, 970. https://doi.org/10.3390/en11040970
Righi S, Baioli F, Dal Pozzo A, Tugnoli A. Integrating Life Cycle Inventory and Process Design Techniques for the Early Estimate of Energy and Material Consumption Data. Energies. 2018; 11(4):970. https://doi.org/10.3390/en11040970
Chicago/Turabian StyleRighi, Serena, Filippo Baioli, Alessandro Dal Pozzo, and Alessandro Tugnoli. 2018. "Integrating Life Cycle Inventory and Process Design Techniques for the Early Estimate of Energy and Material Consumption Data" Energies 11, no. 4: 970. https://doi.org/10.3390/en11040970
APA StyleRighi, S., Baioli, F., Dal Pozzo, A., & Tugnoli, A. (2018). Integrating Life Cycle Inventory and Process Design Techniques for the Early Estimate of Energy and Material Consumption Data. Energies, 11(4), 970. https://doi.org/10.3390/en11040970






