Gasification of Agroresidues for Syngas Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Feedstock
2.2. The Updraf Gasifier
2.3. TGA of Feedstock and Data Processing
2.4. Gasification Test Procedure
2.5. Sampling and Chemical Analyses
3. Results and Discussion
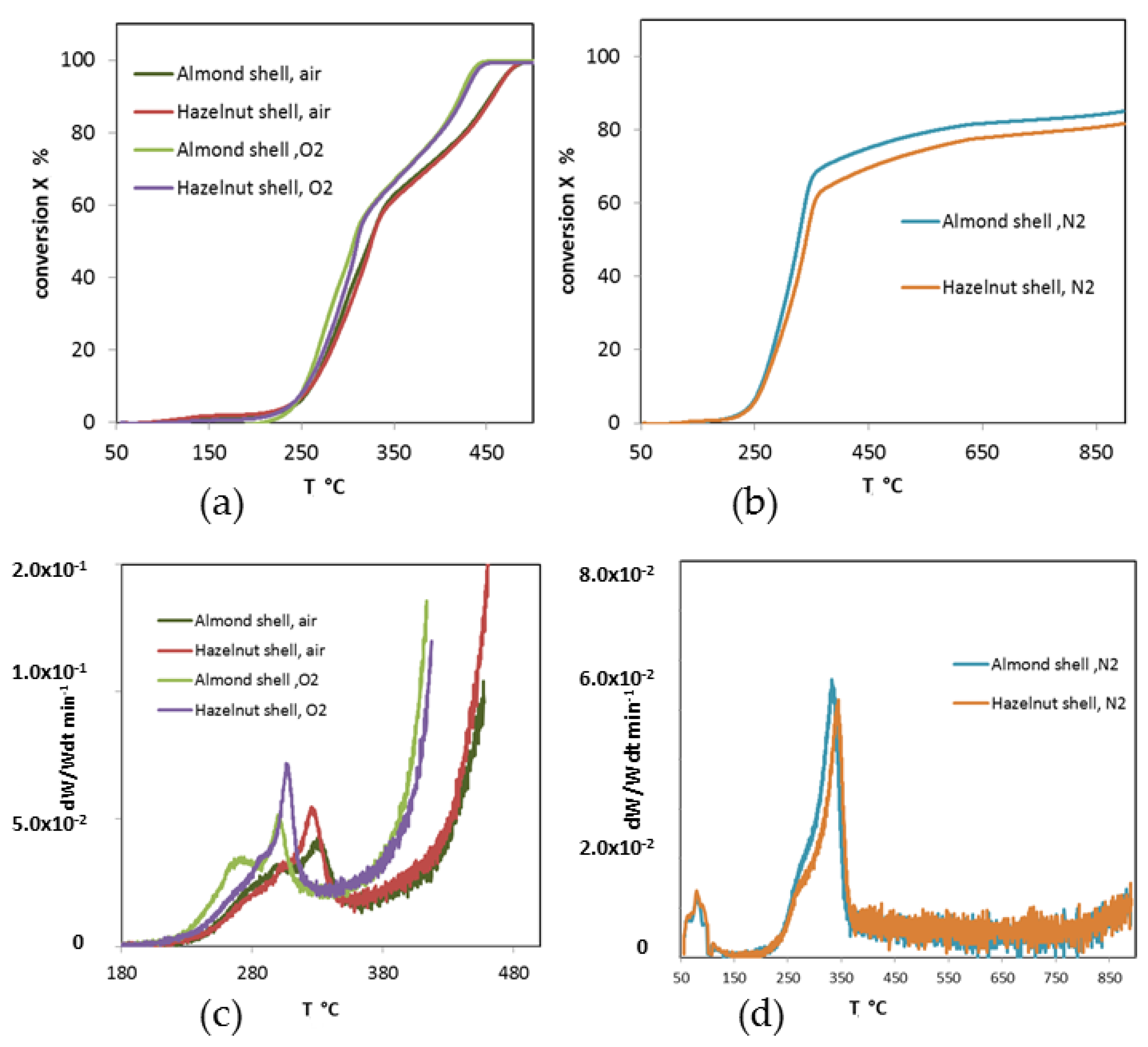
3.1. Thermogravimetric Analysis
3.2. Updraft Gasification
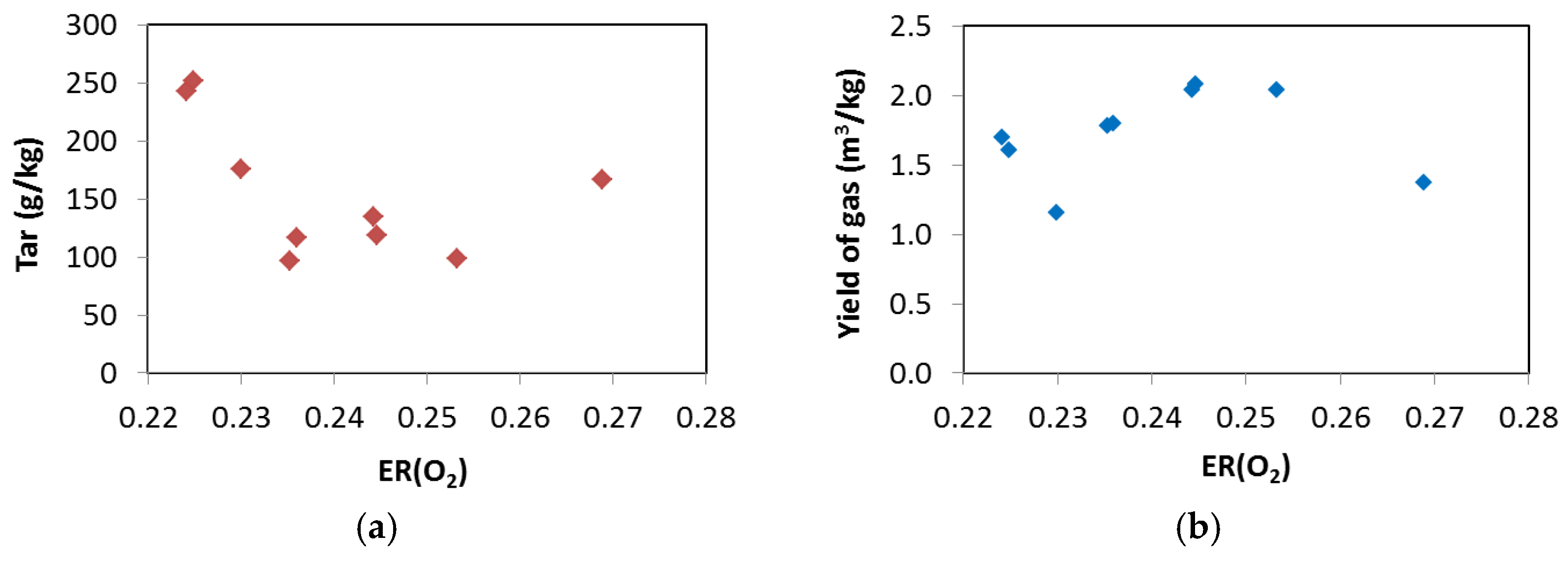
3.3. Process Yields
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schulz, H. Short history and present trends of Fischer–Tropsch synthesis. Appl. Catal. A 1999, 186, 3–12. [Google Scholar] [CrossRef]
- Ke, L.; Song, C.; Subramani, V. Hydrogen and Syngas Production and Purification Technologies; John Wiley & Sons: Hoboken, NJ, USA, 2009; Volume 104, pp. 113–119. ISBN 978-0-471-71975-5. [Google Scholar]
- IEA. Key World Energy Statistics. 2017. Available online: https://www.iea.org/publications/freepublications/publication/KeyWorld2017.pdf (accessed on 4 April 2018).
- Cerone, N.; Zimbardi, F.; Contuzzi, L.; Prestipino, M.; Carnevale, O.; Valerio, V. Air-steam and oxy-steam gasification of hydrolytic residues from biorefinery. Fuel Process. Technol. 2017, 167, 451–461. [Google Scholar] [CrossRef]
- Elliott, D.C.; Neuenschwander, G.G.; Hart, T.R.; Rotness, L.J.; Zacher, A.H.; Santosa, D.M.; Valkenburt, C.; Jones, S.B.; Tjokro Rahardjo, S.A. Catalytic Hydrothermal Gasification of Lignin-Rich Biorefinery Residues and Algae Final Report; Pacific Northwest National Laboratory (PNNL): Richland, WA, USA, 2009.
- Prins, M.J.; Ptasinski, K.J.; Janssen, F.J. From coal to biomass gasification: Comparison of thermodynamic efficiency. Energy 2007, 32, 1248–1259. [Google Scholar] [CrossRef]
- Brage, C.; Yu, Q.; Chen, G.; Sjöström, K. Tar evolution profiles obtained from gasification of biomass and coal. Biomass Bioenergy 2000, 18, 87–91. [Google Scholar] [CrossRef]
- Galvagno, A.; Prestipino, M.; Zafarana, G.; Chiodo, V. Analysis of an integrated agro-waste gasification and 120 kw SOFC CHP system: Modeling and experimental investigation. Energy Procedia 2016, 101, 528–535. [Google Scholar] [CrossRef]
- Dimitriou, I.; Goldingay, H.; Bridgwater, A.V. Techno-economic and uncertainty analysis of Biomass to Liquid (BTL) systems for transport fuel production. Renew. Sustain. Energy Rev. 2018, 88, 160–175. [Google Scholar] [CrossRef]
- Vera, D.; Jurado, F.; Torreglosa, J.P.; Ortega, M. Biomass Gasification for Power Generation Applications: A Modeling, Economic, and Experimental Study. In Advances in Renewable Energies and Power Technologie; Elsevier: New York, NY, USA, 2018; pp. 87–121. [Google Scholar]
- Loha, C.; Karmakar, M.K.; De, S.; Chatterjee, P.K. Gasifiers: Types, Operational Principles, and Commercial Forms. In Coal and Biomass Gasificatio; Springer: Singapore, 2018; pp. 63–91. [Google Scholar] [CrossRef]
- Ding, L.; Yoshikawa, K.; Fukuhara, M.; Kowata, Y.; Nakamura, S.; Xin, D.; Muhan, L. Development of an ultra-small biomass gasification and power generation system: Part 2. Gasification characteristics of carbonized pellets/briquettes in a pilot-scale updraft fixed bed gasifier. Fuel 2018, 220, 210–219. [Google Scholar] [CrossRef]
- Kurkela, E.; Simell, P.; Stǻhlberg, P.; Berna, G.; Barbagli, F.; Haavisto, I. Development of Novel Fixed-Bed Gasification for Biomass Residues and Agrobiofuels; VTT Research Notes; VTT Technical Research Centre of Finland: Espoo, Finland, 2000; ISBN 951-38-5757-3. [Google Scholar]
- James, R.A.M.; Yuan, W.; Boyette, M.D. The Effect of Biomass Physical Properties on Top-Lit Updraft Gasification of Woodchips. Energies 2016, 9, 283. [Google Scholar] [CrossRef]
- Rios, M.L.V.; González, A.M.; Lora, E.E.S.; Del Olmo, O.A.A. Reduction of tar generated during biomass gasification: A review. Biomass Bioenergy 2018, 108, 345–370. [Google Scholar] [CrossRef]
- Narváez, C.R.A.; Blanchard, R.; Dixon, R.; Ramírez, V.; Chulde, D. Low-cost syngas shifting for remote gasifiers: Combination of CO2 adsorption and catalyst addition in a novel and simplified packed structure. Energies 2018, 11, 311. [Google Scholar] [CrossRef]
- Daniell, J.; Köpke, M.; Simpson, S.D. Commercial biomass syngas fermentation. Energies 2012, 5, 5372–5417. [Google Scholar] [CrossRef]
- Molitor, B.; Richter, H.; Martin, M.E.; Jensen, R.O.; Juminaga, A.; Mihalcea, C.; Angenent, L.T. Carbon recovery by fermentation of CO-rich off gases–turning steel mills into biorefineries. Bioresour. Technol. 2016, 215, 386–396. [Google Scholar] [CrossRef] [PubMed]
- Cerone, N.; Zimbardi, F.; Villone, A.; Strjiugas, N.; Kiyikci, E.G. Gasification of wood and torrefied wood with air, oxygen, and steam in a fixed-bed pilot plant. Energy Fuels 2016, 30, 4034–4043. [Google Scholar] [CrossRef]
- Sánchez-Pérez, R.; Ortega, E.; Duval, H.; Martínez-Gómez, P.; Dicenta, F. Inheritance and relationships of important agronomic traits in almond. Euphytica 2007, 155, 381–391. [Google Scholar] [CrossRef]
- Cristofori, V.; Ferramondo, S.; Bertazza, G.; Bignami, C. Nut and kernel traits and chemical composition of hazelnut (Corylus avellana L.) cultivars. J. Sci. Food Agric. 2008, 88, 1091–1098. [Google Scholar] [CrossRef]
- Dogru, M.; Howarth, C.R.; Akay, G.; Keskinler, B.; Malik, A.A. Gasification of hazelnut shells in a downdraft gasifier. Energy 2002, 27, 415–427. [Google Scholar] [CrossRef]
- Rapagna, S.; Latif, A. Steam gasification of almond shells in a fluidised bed reactor: The influence of temperature and particle size on product yield and distribution. Biomass Bioenergy 1997, 12, 281–288. [Google Scholar] [CrossRef]
- Rapagna, S.; Jand, N.; Kiennemann, A.; Foscolo, P.U. Steam-gasification of biomass in a fluidised-bed of olivine particles. Biomass Bioenergy 2000, 19, 187–197. [Google Scholar] [CrossRef]
- Zimbardi, F. Evaluation of Reaction Order and Activation Energy of Char Combustion by Shift Technique. Combust. Sci. Technol. 2000, 156, 251–269. [Google Scholar] [CrossRef]
- Zhang, Y.; Ashizawa, M.; Kajitani, S.; Miura, K. Proposal of a semi-empirical kinetic model to reconcile with gasification reactivity profiles of biomass chars. Fuel 2008, 87, 475–481. [Google Scholar] [CrossRef]
- Feng, D.; Zhao, Y.; Zhang, Y.; Sun, S.; Gao, J. Steam Gasification of Sawdust Biochar Influenced by Chemical Speciation of Alkali and Alkaline Earth Metallic Species. Energies 2018, 11, 205. [Google Scholar] [CrossRef]
- Saddawi, A.; Jones, J.M.; Williams, A.; Wojtowicz, M.A. Kinetics of the thermal decomposition of biomass. Energy Fuels 2009, 24, 1274–1282. [Google Scholar] [CrossRef]
- Cheng, K.; Winter, W.T.; Stipanovic, A.J. A modulated-TGA approach to the kinetics of lignocellulosic biomass pyrolysis/combustion. Polym. Degrad. Stab. 2012, 97, 1606–1615. [Google Scholar] [CrossRef]
- Rabinovich, M.L.; Fedoryak, O.; Dobele, G.; Andersone, A.; Gawdzik, B.; Lindström, M.E.; Sevastyanova, O. Carbon adsorbents from industrial hydrolysis lignin: The USSR/Eastern European experience and its importance for modern biorefineries. Renew. Sustain. Energy Rev. 2016, 57, 1008–1024. [Google Scholar] [CrossRef]
- Demirbas, A. Effects of temperature and particle size on bio-char yield from pyrolysis of agricultural residues. J. Anal. Appl. Pyrolysis 2004, 72, 243–248. [Google Scholar] [CrossRef]
- Taba, L.E.; Irfan, M.F.; Daud, W.A.M.W.; Chakrabarti, M.H. The effect of temperature on various parameters in coal, biomass and CO-gasification: A review. Renew. Sustain. Energy Rev. 2012, 16, 5584–5596. [Google Scholar] [CrossRef]
- Fermoso, J.; Arias, B.; Plaza, M.G.; Pevida, C.; Rubiera, F.; Pis, J.J.; García-Peña, F.; Casero, P. High-pressure CO-gasification of coal with biomass and petroleum coke. Fuel Process. Technol. 2009, 90, 926–932. [Google Scholar] [CrossRef]
- Jayathilake, R.; Rudra, S. Numerical and Experimental Investigation of Equivalence Ratio (ER) and Feedstock Particle Size on Birchwood Gasification. Energies 2017, 10, 1232. [Google Scholar] [CrossRef]
- Saravanakumar, A.; Haridasan, T.M.; Reed, T.B.; Kasturi Bai, R. Experimental investigations of long stick wood gasification in a bottom lit updraft fixed bed gasifier. Fuel 2007, 86, 2846–2856. [Google Scholar] [CrossRef]
- Arena, U. Process and technological aspects of municipal solid waste gasification. A review. Waste Manag. 2012, 32, 625–639. [Google Scholar] [CrossRef] [PubMed]
- Tsai, W.T.; Liu, S.C.; Hsieh, C.H. Preparation and fuel properties of biochars from the pyrolysis of exhausted coffee residue. J. Anal. Appl. Pyrolysis 2012, 93, 63–67. [Google Scholar] [CrossRef]
- Cerone, N.; Zimbardi, F.; Contuzzi, L.; Alvino, E.; Carnevale, O.; Valerio, V. Updraft Gasification at Pilot Scale of Hydrolytic Lignin Residue. Energy Fuels 2014, 28, 3948–3956. [Google Scholar] [CrossRef]
- Czernik, S.; Bridgwater, A.V. Overview of applications of biomass fast pyrolysis oil. Energy Fuels 2004, 18, 590–598. [Google Scholar] [CrossRef]













| Almond Shells (M) | Standard Deviation | Hazelnut SHELLS (N) | Standard Deviation | Method | |
|---|---|---|---|---|---|
| Bulk density, kg/m3 | 417 | 299 | ASTM E873 | ||
| HHV MJ/kg | 19.5 | 0.1 | 19.4 | 0.1 | ISO 1928 |
| LHV a, MJ/kg | 18.1 | 0.1 | 17.8 | 0.1 | |
| Moisture b, % | 11.8 | 5 | |||
| Fix carbon, % | 18.2 | 0.2 | 20.9 | 0.4 | ASTM D 3172 |
| Volatile, % | 80.6 | 0.2 | 78 | 0.4 | ASTM D 3175 |
| Ash, % | 1.2 | 0.01 | 1.1 | 0.01 | ASTM D 1102 (600 °C) |
| Hexosans % | 31.2 | 0.8 | 22.2 | 0.7 | NREL/TP-510-42623 |
| Pentosans % | 28.0 | 0.8 | 12.2 | 0.1 | NREL/TP-510-42623 |
| Lignin (Klason)% | 30.2 | 0.3 | 40.9 | 0.5 | TAPPI 13M-54 |
| Lignin (Klason) ac. sol. % | 1.98 | 0.1 | 1.3 | 0.1 | TAPPI T250 |
| C % | 47.9 | 0.1 | 50.5 | 0.1 | UNI EN 15104 |
| H % | 6.3 | 0.2 | 6.64 | 0.05 | UNI EN 15104 |
| N % | 0.36 | 0.05 | 1.7 | 0.4 | UNI EN 15104 |
| O c % | 45.4 | 40.0 | |||
| Cl % | nd | 0.025 | 0.005 | UNI EN 15289 | |
| S (ppm) | 145 | 4 | 250 | 40 | UNI EN 15289 |
| Si (ppm) | 484 | 40 | 106 | 6 | CEN/TC 343 |
| Al (ppm) | 67.5 | 3.7 | 54 | 4 | CEN/TC 343 |
| Fe (ppm) | 1136 | 60 | 380 | 30 | CEN/TC 343 |
| Ca (ppm) | 941 | 26 | 3019 | 95 | CEN/TC 343 |
| K (ppm) | 3513 | 200 | 2560 | 120 | CEN/TC 343 |
| Mg (ppm) | 164 | 24 | 229 | 10 | CEN/TC 343 |
| Na (ppm) | nd | <53 | CEN/TC 343 | ||
| P (ppm) | nd | 162 | 10 | CEN/TC 343 | |
| Ni (ppm) | 63.3 | 0.7 | 46 | 5 | CEN/TC 345 |
| Cr (ppm) | nd | 78 | 9 | CEN/TC 345 | |
| Mn (ppm) | 13.4 | 0.6 | 23 | 2 | CEN/TC 345 |
| Zn (ppm) | 26.1 | 0.6 | 4.5 | 0.3 | CEN/TC 345 |
| Pb (ppm) | nd | <2.2 | CEN/TC 345 | ||
| Cu (ppm) | 9.4 | 0.1 | 7.4 | 0.3 | CEN/TC 345 |
| Mo (ppm) | 8.6 | 0.1 | nd | CEN/TC 345 | |
| Co (ppm) | nd | <2.19 | CEN/TC 345 | ||
| Cd (ppm) | nd | <2.19 | CEN/TC 345 | ||
| H/C, mol/mol | 1.58 | 1.58 | |||
| O/C, mol/mol | 0.71 | 0.59 | |||
| H2O for oxidation, kg/kg | 0.93 | 1.06 | |||
| O2 for combustion, kg/kg | 1.33 | 1.40 | |||
| Air for combustion, kg/kg | 5.75 | 6.08 |
| Reaction | Stoichiometry | Enthalpy |
|---|---|---|
| Combustion | C(grafite) + O2 ↔ CO2 | ∆H = −393.5 kJ/mol |
| Partial combustion | C(grafite) + ½ O2 ↔ CO | ∆H = −110.5 kJ/mol |
| Bouduard reaction | C(graphite) + CO2 ↔ 2CO | ∆H = 172.6 kJ/mol |
| Water gas reaction | C(grafite) + H2O ↔ CO + H2 | ∆H = 131.4 kJ/mol |
| Thermal cracking | CnHx ↔ nC + (x/2)H2 | for n = 1, ∆H = 74.9 kJ/mol |
| Steam reforming | CH4 + H2O ↔ CO2 + 3H2 | ∆H = 206 kJ/mol |
| Water Gas Shift | CO + H2O ↔ CO2 + H2 | ∆H = −41.2 kJ/mol |
| C-Methanation | C + 2H2 ↔ CH4 | ∆H = −78.84 kJ/mol |
| CO-Methanation | CO + 3 H2 ↔ CH4 + H2O | ∆H = −206 kJ/mol |
| CO2-Methanation | CO2 + 4 H2 ↔ CH4 + 2 H2O | ∆H = −165 kJ/mol |
| Reforming | CnHx + mH2O ↔ nCO +(m + x/2)H2 |
| Experiment Code a | Oxidant | Feeding Rate kg dry /h | Particle Residence Time, h | Air kg/h | O2 kg/h | ER (O2) kg/kg | Steam kg/h | ER (H2O) kg/kg | Average T in Bed, °C | Gas Residence Time b, s |
|---|---|---|---|---|---|---|---|---|---|---|
| MAS1(24) | Air | 12.4 | 2.81 | 16.7 | 0 | 0.24 | 0 | 0.00 | 767 | 6.2 |
| MAS2(24) | Air | 21.2 | 1.64 | 28.8 | 0 | 0.24 | 0 | 0.00 | 761 | 3.6 |
| MA1(22/24) | Air and steam | 22.4 | 1.55 | 28.9 | 0 | 0.22 | 5 | 0.24 | 701 | 3.1 |
| MA2(24/28) | Air and steam | 21.2 | 1.64 | 29.8 | 0 | 0.24 | 5.5 | 0.28 | 741 | 2.8 |
| MA3(22/19) | Air and steam | 22.4 | 1.55 | 29.0 | 0 | 0.22 | 4 | 0.19 | 715 | 3.2 |
| MA4(24/25) | Air and steam | 22.1 | 1.57 | 31.0 | 0 | 0.24 | 5.2 | 0.25 | 758 | 2.7 |
| MA5(25/30) | Air and steam | 21.6 | 1.60 | 31.5 | 0 | 0.25 | 6 | 0.30 | 739 | 2.7 |
| MO1(23/28) | Oxigen and steam | 21.2 | 1.64 | 0.0 | 6.5 | 0.23 | 5.5 | 0.28 | 748 | 6.5 |
| NA1(19/28) | Air and steam | 16.3 | 1.52 | 18.9 | 0 | 0.19 | 4.8 | 0.28 | 703 | 4.7 |
| NA2(24/22) | Air and steam | 20.4 | 1.22 | 29.3 | 0 | 0.24 | 4.8 | 0.22 | 760 | 3.3 |
| NA3(22/18) | Air and steam | 20.4 | 1.22 | 27.1 | 0 | 0.22 | 4 | 0.18 | 713 | 3.6 |
| NO1(28/23) | Oxigen and steam | 20.4 | 1.22 | 0.0 | 8 | 0.28 | 5 | 0.23 | 768 | 6.9 |
| NO2(28/28) | Oxigen and steam | 20.4 | 1.22 | 0.0 | 8 | 0.28 | 6 | 0.28 | 714 | 6.7 |
| MAO(27/23) | Enric. air and steam | 21.2 | 1.64 | 9.1 | 5.5 | 0.27 | 4.5 | 0.23 | 806 | 4.6 |
| Experiment Code | H2g/kg | COg/kg | CO2 g/kg | CnHm g/kg | Syngas a STP m3/kg | LHW MJ/m3 | Density kg/STPm3 | CGE % | Net CGE % | CLE % | Net CLE % | Plant Power b kW |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MAS1(24) | 15 | 635 | 451 | 40 | 1.78 | 5.76 | 0.98 | 57 | 57 | 11 | 11 | 35 |
| MAS2(24) | 18 | 615 | 360 | 34 | 1.79 | 5.62 | 1.11 | 56 | 56 | 13 | 13 | 59 |
| MA1(22/24) | 28 | 508 | 306 | 22 | 1.71 | 5.59 | 1.19 | 53 | 51 | 27 | 27 | 59 |
| MA2(24/28) | 39 | 548 | 213 | 29 | 2.09 | 5.56 | 1.05 | 64 | 62 | 13 | 13 | 68 |
| MA3(22/19) | 26 | 578 | 371 | 24 | 1.61 | 6.32 | 1.18 | 56 | 55 | 28 | 27 | 63 |
| MA4(24/25) | 36 | 630 | 643 | 34 | 2.03 | 6.10 | 1.11 | 69 | 66 | 15 | 14 | 76 |
| MA5(25/30) | 40 | 564 | 273 | 26 | 2.10 | 5.62 | 1.09 | 65 | 63 | 11 | 11 | 71 |
| MO1(23/28) | 35 | 580 | 479 | 37 | 1.15 | 10.4 | 1.10 | 66 | 64 | 20 | 19 | 70 |
| MAO(27/23) | 26 | 704 | 595 | 31 | 1.39 | 8.46 | 1.10 | 65 | 63 | 19 | 18 | 69 |
| NA1(19/28) | 25 | 564 | 403 | 55 | 1.77 | 6.43 | 1.14 | 64 | 61 | 19 | 18 | 52 |
| NA2(24/22) | 26 | 673 | 342 | 39 | 1.88 | 6.37 | 0.95 | 66 | 64 | 25 | 24 | 68 |
| NA3(22/18) | 29 | 699 | 265 | 24 | 2.01 | 5.90 | 1.09 | 66 | 64 | 23 | 22 | 67 |
| NO1(28/23) | 36 | 744 | 348 | 33 | 1.23 | 11.0 | 0.91 | 76 | 73 | 17 | 17 | 77 |
| NO2(28/28) | 40 | 691 | 259 | 23 | 1.22 | 10.6 | 1.10 | 73 | 70 | 19 | 18 | 74 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cerone, N.; Zimbardi, F. Gasification of Agroresidues for Syngas Production. Energies 2018, 11, 1280. https://doi.org/10.3390/en11051280
Cerone N, Zimbardi F. Gasification of Agroresidues for Syngas Production. Energies. 2018; 11(5):1280. https://doi.org/10.3390/en11051280
Chicago/Turabian StyleCerone, Nadia, and Francesco Zimbardi. 2018. "Gasification of Agroresidues for Syngas Production" Energies 11, no. 5: 1280. https://doi.org/10.3390/en11051280
APA StyleCerone, N., & Zimbardi, F. (2018). Gasification of Agroresidues for Syngas Production. Energies, 11(5), 1280. https://doi.org/10.3390/en11051280





