Surface Properties and Pore Structure of Anthracite, Bituminous Coal and Lignite
Abstract
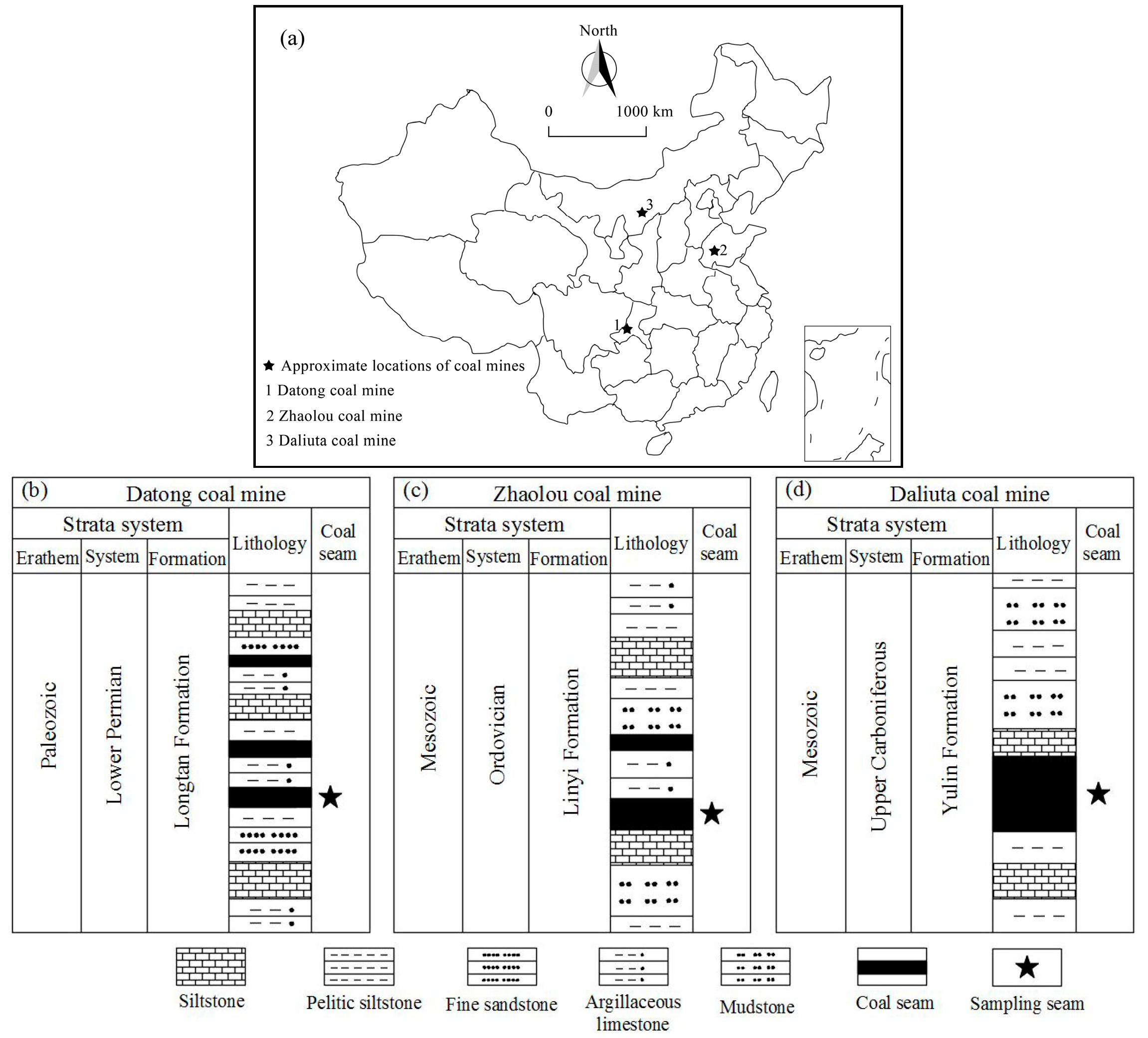
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Ultimate and Proximate Analyzes
2.3. Scanning Electron Microscopy
2.4. FT-IR Analysis
2.5. Low-Pressure N2 and CO2 Isotherm Measurements
2.6. Mercury Porosimetry Measurement
2.7. Water Vapor Isotherm Measurements
3. Results and Discussion
3.1. Ultimate and Proximate Results
3.2. SEM Results
3.3. FT-IR Results
3.4. Pore Structure for the Coal Samples
3.5. Properties Determined by Water Adsorption
3.5.1. Water Vapor Adsorption-Desorption Isotherms
3.5.2. Modeling of Water Vapor Adsorption
3.6. Comparison of Different Methods in Coal Pore Characterization
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gosiewaka, A.; Dralich, J.; Laskowski, J.S.; Pawlik, M. Mineral matter distribution on coal surface and its effect on coal wettability. J. Colloid Interface Sci. 2002, 247, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Crosdale, P.J.; Beamish, B.B.; Valix, M. Coal bed methane sorption related to coal composition. Int. J. Coal Geol. 1998, 35, 147–158. [Google Scholar] [CrossRef]
- White, C.M.; Smith, D.H.; Jones, K.L.; Goodman, A.L.; Jikich, S.A.; LaCount, R.B.; DuBose, S.B.; Ozdemir, E.; Morsi, B.I.; Schroeder, K.T. Sequestration of carbon dioxide in coal with enhanced coalbed methane recovery—A review. Energy Fuel 2005, 19, 659–724. [Google Scholar] [CrossRef]
- Dudzińska, A.; Howaniec, N.; Smoliński, A. Effect of coal grain size on sorption capacity with respect to propylene and acetylene. Energies 2017, 10, 1919–1929. [Google Scholar] [CrossRef]
- Levine, U.G.; Schlosberg, R.H.; Silbernagel, B.G. Understanding the chemistry and physics of coal structure (A Review). Proc. Natl. Acad. Sci. USA 1982, 79, 3365–3370. [Google Scholar] [CrossRef] [Green Version]
- Busch, A.; Gensterblum, Y. CBM and CO2-ECBM related sorption processes in coal: A review. Int. J. Coal Geol. 2011, 87, 49–71. [Google Scholar] [CrossRef]
- Li, Q.; Lin, B.; Wang, K.; Zhao, M.; Ruan, M. Surface properties of pulverized coal and its effects on coal mine methane adsorption behaviors under ambient conditions. Powder Technol. 2015, 270, 278–286. [Google Scholar] [CrossRef]
- Vedyagin, A.A.; Mishakov, I.V. Effect of the thermal treatment of anthracite from the Gorlovo Basin on its adsorption characteristics. Solid Fuel Chem. 2016, 50, 34–38. [Google Scholar] [CrossRef]
- Ji, X.; Song, D.; Ni, X.; Li, Y.; Zhao, H. Coal matrix deformation and pore structure change in high-pressure nitrogen replacement of methane. Energies 2018, 11, 175–193. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Mouscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Wang, F.; Cheng, Y.; Lu, S.; Jin, K.; Zhao, W. Influence of coalification on the pore characteristics of middle-high rank coal. Energy Fuel 2014, 28, 5729–5736. [Google Scholar] [CrossRef]
- Nie, B.; Liu, X.; Yuan, S.; Ge, B.; Jia, W.; Wang, C.; Chen, X. Sorption characteristics of methane among various rank coals: Impact of moisture. Adsorption 2016, 22, 315–325. [Google Scholar] [CrossRef]
- Okolo, G.N.; Everson, R.C.; Neomagus, H.W.J.P.; Roberts, M.J.; Sakurovs, R. Comparing the porosity and surface areas of coal as measured by gas adsorption, mercury intrusion and SAXS techniques. Fuel 2015, 141, 293–304. [Google Scholar] [CrossRef]
- Gürdal, G.; Yalçın, M.N. Pore volume and surface area of the carboniferous coals from the Zonguldak Basin (NW Turkey) and their variations with rank and maceral composition. Int. J. Coal Geol. 2001, 48, 133–144. [Google Scholar] [CrossRef]
- Laxminarayana, C.; Crosdale, P. Role of coal type and rank on methane sorption characteristics of Bowen Basin, Australia coals. Int. J. Coal Geol. 1999, 40, 309–325. [Google Scholar] [CrossRef]
- Perera, M.S.A.; Ranjith, P.G.; Choi, S.K.; Bouazza, A.; Kodikara, J.; Airey, D. A review of coal properties pertinent to carbon dioxide sequestration in coal seams: With special reference to Victorian Brown coals. Environ. Earth Sci. 2011, 64, 223–235. [Google Scholar] [CrossRef]
- Zhao, J.; Xu, H.; Tang, D.; Mathews, J.; Li, S.; Tao, S. A comparative evaluation of coal specific surface area by CO2 and N2 adsorption and its influence on CH4 adsorption capacity at different pore sizes. Fuel 2016, 183, 420–431. [Google Scholar] [CrossRef]
- Clarksona, C.R.; Bustin, R.M. The Effect of pore structure and gas pressure upon the transport properties of coal: A laboratory and modeling study.1: Isotherms and pore volume distributions. Fuel 1999, 78, 1333–1344. [Google Scholar] [CrossRef]
- Jian, K.; Fu, X.; Ding, Y.; Wang, H.; Li, T. Characteristics of pores and methane adsorption of low-rank coal in China. J. Nat. Gas Sci. Eng. 2015, 27, 207–218. [Google Scholar] [CrossRef]
- Gao, D.; Li, M.; Wang, B.; Hu, B.; Liu, J. Characteristics of pore structure and fractal dimension of isometamorphic anthracite. Energies 2017, 10, 1881–1892. [Google Scholar] [CrossRef]
- Hou, S.; Wang, X.; Wang, X.; Yuan, Y.; Pan, S.; Wang, X. Pore structure characterization of low volatile bituminous coals with different particle size and tectonic deformation using low pressure gas adsorption. Int. J. Coal Geol. 2017, 183, 1–13. [Google Scholar] [CrossRef]
- Amarasekera, G.; Scarlett, M.J.; Mainwaring, D.E. Micropore size distributions and specific interactions in coals. Fuel 1995, 74, 115–118. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, Z.; Lu, Y.; Choi, S.K.; Liu, X. Sorption hysteresis characterization of CH4 and CO2 on anthracite, bituminous coal, and lignite at low pressure. ASME J. Energy Resour. Technol. 2018, 140, 012203. [Google Scholar] [CrossRef]
- Mastalerz, M.; He, L.; Melnichenko, Y.B.; Rupp, J.A. Porosity of coal and shale: Insights from gas adsorption and SANS/USANS techniques. Energy Fuel 2012, 26, 5109–5120. [Google Scholar] [CrossRef]
- Cai, Y.; Liu, D.; Pan, Z.; Yao, Y.; Li, J.; Qiu, Y. Pore structure and its impact on ch4 adsorption capacity and flow capability of bituminous and sub-bituminous coals from Northeast China. Fuel 2013, 103, 258–268. [Google Scholar] [CrossRef]
- Marsh, H. Adsorption methods to study microporosity in coals and carbons—A critique. Carbon 1987, 25, 49–58. [Google Scholar] [CrossRef]
- Velasco, L.F.; Guillet–Nicolas, R.; Dobos, G.; Thommes, M.; Lodewyckx, P. Towards a better understanding of water adsorption hysteresis in activated carbons by scanning isotherm. Carbon 2015, 96, 753–758. [Google Scholar] [CrossRef]
- Clarkson, C.R.; Bustin, R.M. Variation in Micropore Capacity and size distribution with composition in bituminous coal of the Western Canadian Sedimentary Basin. Fuel 1996, 75, 1483–1498. [Google Scholar] [CrossRef]
- Emmett, P.H.; Dewitt, T.W. The low temperature adsorption of Nitrogen, Oxygen, Argon, Hydrogen, N–Butane and carbon dioxide on porous glass and on Partially Dehydrated Chabazite. J. Am. Chem. Soc. 1943, 65, 617–621. [Google Scholar] [CrossRef]
- Gan, H.; Nandi, S.P.; Jr, P.L.W. Nature of the porosity in American coals. Fuel 1972, 51, 272–277. [Google Scholar] [CrossRef]
- Liu, G.; Bai, Y.; Fan, Z.; Gu, D. Determination of Klinkenberg permeability conditioned to pore-throat structures in tight formations. Energies 2017, 10, 1575–1592. [Google Scholar] [CrossRef]
- Yao, Y.; Liu, D. Comparison of low-field NMR and mercury intrusion porosimetry in characterizing pore size distributions of coals. Fuel 2012, 95, 152–158. [Google Scholar] [CrossRef]
- Lai, J.; Wang, G. Fractal Analysis of Tight gas sandstones using high–pressure mercury intrusion techniques. J. Nat. Gas Sci. Eng. 2015, 24, 185–196. [Google Scholar] [CrossRef]
- Guo, X.; Yao, Y.; Liu, D. Characteristics of coal matrix compressibility: An investigation by mercury intrusion porosimetry. Energy Fuel 2014, 28, 3673–3678. [Google Scholar] [CrossRef]
- Nishino, J. Adsorption of water vapor and carbon dioxide at carboxylic functional groups on the surface of coal. Fuel 2001, 80, 757–764. [Google Scholar] [CrossRef]
- La’szlo’, K.; Czakkel, O.; Dobos, G.; Lodewyckx, P.; Rochas, C.; Geissler, E. Water vapour adsorption in highly porous carbons as seen by small and wide angle X-Ray scattering. Carbon 2010, 48, 1038–1048. [Google Scholar] [CrossRef]
- Charrière, D.; Behra, P. Water sorption on coals. J. Colloid Interf. Sci. 2010, 344, 460–467. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Prasetyo, L.; Nguyen, V.T.; Horikawa, T.; Do, D.D.; Nicholson, D. Characterization of oxygen functional groups on carbon surfaces with water and methanol adsorption. Carbon 2014, 81, 447–457. [Google Scholar] [CrossRef]
- Furmaniak, S.; Gauden, P.A.; Terzyk, A.P.; Rychlicki, G. Water Adsorption on carbons–critical review of the most popular analytical approaches. Adv. Colloid Interface 2008, 137, 82–143. [Google Scholar] [CrossRef] [PubMed]
- Shigehisa, T.; Inoue, T.; Kumagai, H. Water adsorption and desorption of upgraded Brown coal. Part 1: Isotherms of adsorption and desorption. Energy Fuel 2014, 28, 4986–4992. [Google Scholar] [CrossRef]
- Chen, Y.; Mastalerz, M.; Schimmelmann, A. Characterization of chemical functional groups in macerals across different coal ranks via micro-FTIR spectroscopy. Int. J. Coal Geol. 2012, 104, 22–33. [Google Scholar] [CrossRef]
- Mel’Gunov, M.S.; Ayupov, A.B. Direct method for evaluation of BET adsorbed monolayer capacity. Micropor. Mesopor. Mater. 2017, 243, 147–153. [Google Scholar] [CrossRef]
- Ozdemir, E.; Schroeder, K. Effect of moisture on adsorption isotherms and adsorption capacities of CO2 on coals. Energy Fuel 2009, 23, 2821–2831. [Google Scholar] [CrossRef]
- Monson, P.A. Understanding adsorption/desorption hysteresis for fluids in mesoporous materials using simple molecular models and classical density functional theory. Micropor. Mesopor. Mater. 2012, 160, 47–66. [Google Scholar] [CrossRef]
- Boer, J.H.D.; Lippens, B.C. Studies on pore systems in catalysts II. The shapes of pores in aluminum oxide systems. J. Catal. 1964, 3, 38–43. [Google Scholar]
- Ozdemir, E.; Morsi, B.I.; Schroeder, K. Importance of volume effects to adsorption isotherms of carbon dioxide on coals. Langmuir 2003, 19, 9764–9773. [Google Scholar] [CrossRef]
- Melnichenko, Y.B.; He, L.; Sakurovs, R.; Kholodenko, A.L.; Blach, T.; Mastalerz, M.; Radlin’ski, A.P.; Cheng, G.; Mildner, D.F.R. Accessibility of pores in coal to methane and carbon dioxide. Fuel 2012, 91, 200–208. [Google Scholar] [CrossRef] [Green Version]
- Naono, H.; Hakuman, M. Analysis of porous texture by means of water vapor adsorption isotherm with particular attention to lower limit of hysteresis loop. J. Colloid Interface Sci. 1993, 158, 19–26. [Google Scholar] [CrossRef]
- Horikawa, T.; Muguruma, T.; Do, D.D.; Sotowa, K.I.; Alcántara-Avila, J.R. Scanning curves of water adsorption on graphitized thermal carbon black and ordered mesoporous carbon. Carbon 2015, 95, 137–143. [Google Scholar] [CrossRef] [Green Version]
- Saliba, S.; Ruch, P.; Volksen, W.; Magbitang, T.P.; Dubois, G.; Michel, B. Combined influence of pore size distribution and surface hydrophilicity on the water adsorption characteristics of micro- and mesoporous silica. Micropor. Mesopor. Mat. 2016, 226, 221–228. [Google Scholar] [CrossRef]
- Rutherford, S.W. Modeling water adsorption in carbon micropores: Study of water in carbon molecular sieves. Langmuir 2006, 22, 702–728. [Google Scholar] [CrossRef] [PubMed]
- Švábová, M.; Weishauptová, Z.; Přibyl, O. Water vapour adsorption on coal. Fuel 2011, 90, 1892–1899. [Google Scholar] [CrossRef]
- McCutcheon, A.L.; Barton, W.A.; Wilson, M.A. Characterization of water adsorbed on bituminous coals. Energy Fuel 2003, 17, 107–112. [Google Scholar] [CrossRef]









| Properties | Anthracite | Bituminous Coal | Lignite |
|---|---|---|---|
| BET N2-SSA (m2/g) | 0.187 | 0.744 | 1.498 |
| BJH N2-SSA (m2/g) | 0.178 | 1.065 | 1.985 |
| D-R CO2-SSA (m2/g) | 120.38 | 54.54 | 98.71 |
| D-A CO2-SSA (m2/g) | 74.34 | 45.77 | 61.05 |
| MIP SSA | 7.422 | 16.82 | 10.69 |
| BJH N2-pore volume (×10–3 cm3/g) | 0.543 | 1.275 | 4.021 |
| MIP-pore volume (×10–3 cm3/g) | 5.5 | 20.3 | 8.2 |
| D-A CO2-micropore volume (×10–3 cm3/g) | 26.35 | 11.94 | 21.61 |
| BET N2-average pore width (Å) | 122.7 | 151.7 | 78.03 |
| BJH N2-adsorption pore width (Å) | 132.4 | 47.89 | 45.92 |
| Proximate Analysis (wt %) | Ultimate Analysis (wt % daf) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Coal Mine | Coal Rank | Cfix | Vdaf | Aad | M | C | H | N | O |
| Datong | Anthracite | 73.85 | 12.81 | 13.34 | 1.96 | 90.11 | 3.79 | 0.96 | 2.05 |
| Zhaolou | Bituminous coal | 65.1 | 28.71 | 16.24 | 1.50 | 82.54 | 4.57 | 1.08 | 11.03 |
| Daliuta | Lignite | 53.55 | 36.96 | 19.42 | 2.46 | 72.71 | 4.95 | 1.19 | 20.50 |
| Sample | Dent Model | D-W Model | |||||
|---|---|---|---|---|---|---|---|
| m0 | K1 | K2 | R-Square | s0 | s | R-Square | |
| Anthracite | 0.12 | 2.32 | 0.85 | 0.9991 | 0.85 | 0.023 | 0.9812 |
| Bituminous coal | 0.42 | 4.78 | 0.70 | 0.9825 | 3.35 | 0.026 | 0.9935 |
| Lignite | 0.90 | 2.11 | 0.63 | 0.9962 | 5.30 | 0.043 | 0.9989 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Zhang, Z.; Choi, S.K.; Lu, Y. Surface Properties and Pore Structure of Anthracite, Bituminous Coal and Lignite. Energies 2018, 11, 1502. https://doi.org/10.3390/en11061502
Liu Z, Zhang Z, Choi SK, Lu Y. Surface Properties and Pore Structure of Anthracite, Bituminous Coal and Lignite. Energies. 2018; 11(6):1502. https://doi.org/10.3390/en11061502
Chicago/Turabian StyleLiu, Zhenjian, Zhenyu Zhang, Sing Ki Choi, and Yiyu Lu. 2018. "Surface Properties and Pore Structure of Anthracite, Bituminous Coal and Lignite" Energies 11, no. 6: 1502. https://doi.org/10.3390/en11061502





