Safflower Biodiesel: Improvement of its Oxidative Stability by Using BHA and TBHQ
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Material
2.2. Transesterification Conditions
2.3. Antioxidant Addition
2.4. Biodiesel Characterization
2.5. FAME Characterization
2.6. Oxidative Stability Determination
3. Results and Discussion
3.1. Biodiesel Characterization
3.2. FAME Profile
3.3. Antioxidant Addition
3.3.1. BHA Addition
3.3.2. TBHQ Addition
3.3.3. BHA and TBHQ Combined Addition
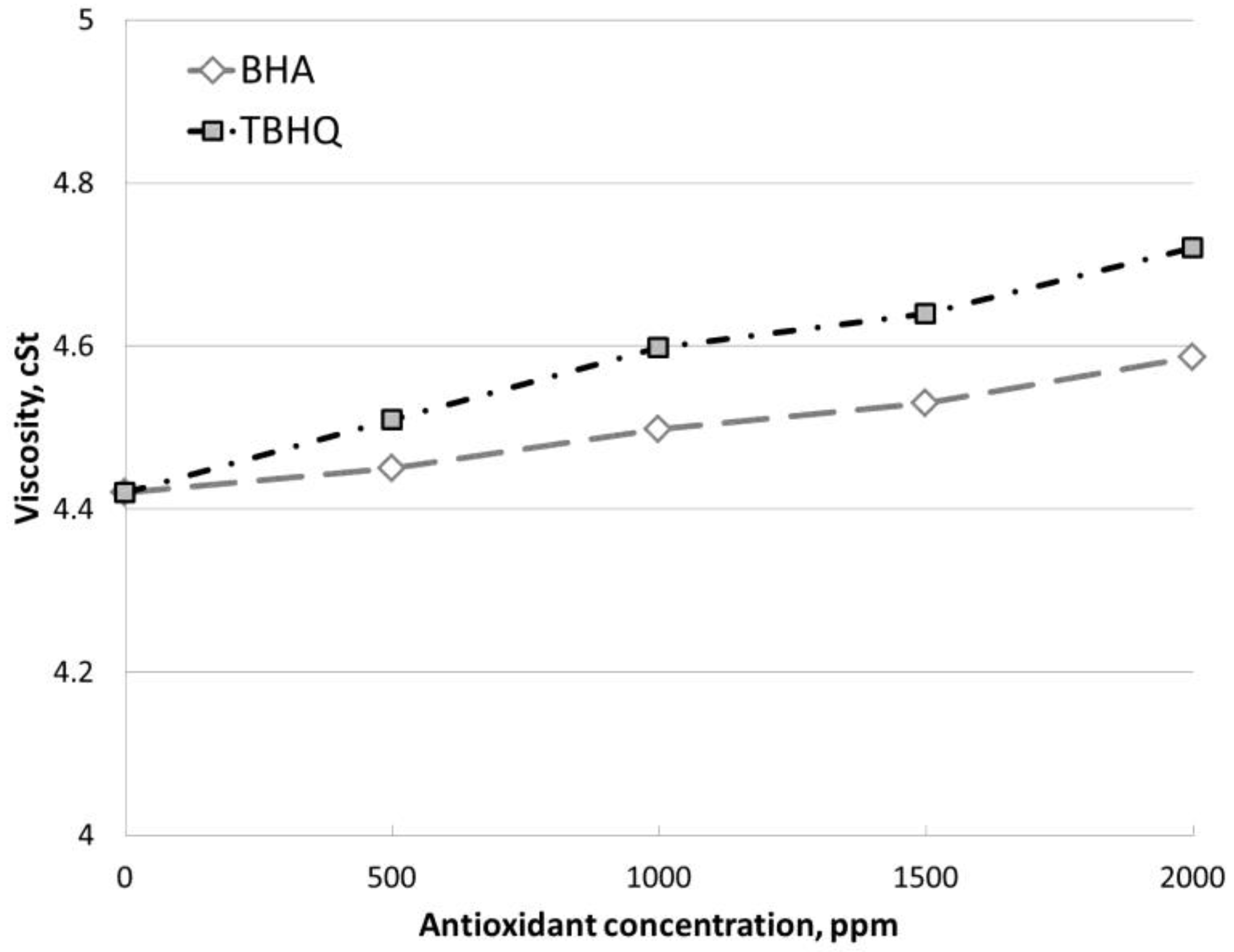
3.3.4. Effect of Antioxidants on Other Biodiesel Characteristics
4. Conclusions
- The yield of biodiesel production from safflower oil was acceptable, making this product suitable for biodiesel production from an engineering point of view.
- Most characteristics of safflower biodiesel complied with the standard, except for oxidative stability. For the latter, the use of antioxidants is mandatory, as the oxidative stability was especially short and, therefore, not suitable for storage.
- As in many other parameters, the oxidative stability was strongly influenced by the FAME profile. In the case of safflower biodiesel, methyl linoleate was the majority compound, which is more unstable than mono or saturated FAMEs, due to its conjugated double bond (susceptible to self-oxidation).
- The use of BHA and TBHQ was effective at increasing the oxidative stability of safflower biodiesel. TBHQ was, in general and at the same concentrations, more effective than BHA. In fact, BHA did not comply with the lower limit of the standard (8 h) in the range studied.
- The combined use of BHA and TBHQ did not show any synergistic effect. Indeed, the effect of their combined use was inhibitory (especially at low concentrations of BHA and TBHQ), which makes the mixture of these antioxidants not desirable in this case.
- Apart from increasing the oxidative stability, the use of BHA and TBHQ influenced on viscosity. In general, an increase in viscosity was found, which could alter the compliance with standards. Therefore, both the initial viscosity and oxidative stability (and therefore the concentration of antioxidant required) of biodiesel are important to comply with the standard range.
- Consequently, as the oxidative stability of safflower biodiesel was so low, the use of an effective antioxidant, such as TBHQ at 1000 ppm, was recommended, not mixing it with other antioxidants. The mixture with other more stable biodiesel samples could be another alternative for safflower biodiesel revaluation.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Manaf, I.S.A.; Embong, N.H.; Khazaai, S.N.M.; Rahim, M.H.A.; Yusoff, M.M.; Lee, K.T.; Maniam, G.P. A review for key challenges of the development of biodiesel industry. Energy Convers. Manag. 2019, 185, 508–517. [Google Scholar] [CrossRef]
- Knothe, G.; Razon, L.F. Biodiesel fuels. Prog. Energy Combust. Sci. 2017, 58, 36–59. [Google Scholar] [CrossRef]
- Huang, D.; Zhou, H.; Lin, L. Biodiesel: An alternative to conventional fuel. Energy Procedia 2011, 16, 1874–1885. [Google Scholar] [CrossRef]
- Rodionova, M.V.; Poudyal, R.S.; Tiwari, I.; Voloshin, R.A.; Zharmukhamedov, S.K.; Nam, H.G.; Zayadan, B.K.; Bruce, B.D.; Hou, H.J.M.; Allakhverdiev, S.I. Biofuel production: Challenges and opportunities. Int. J. Hydrog. Energy 2017, 42, 8450–8461. [Google Scholar] [CrossRef]
- Azadi, P.; Malina, R.; Barrett, S.R.H.; Kraft, M. The evolution of the biofuel science. Renew. Sustain. Energy Rev. 2017, 76, 1479–1484. [Google Scholar] [CrossRef]
- Ji, X.; Long, X. A review of the ecological and socioeconomic effects of biofuel and energy policy recommendations. Renew. Sustain. Energy Rev. 2016, 61, 41–52. [Google Scholar] [CrossRef]
- Cajamarca, F.A.; Lancheros, A.F.; Araújo, P.M.; Mizubuti, I.Y.; Simonelli, S.M.; Ida, E.I.; Guedes, C.L.B.; Guimarães, M.F. Evaluation of various species of winter oleaginous plants for the production of biodiesel in the State of Parana, Brazil. Ind. Crop. Prod. 2018, 126, 113–118. [Google Scholar] [CrossRef]
- Cardoso, B.F.; Shikida, P.F.A.; Finco, A. Development of Brazilian biodiesel sector from the perspective of stakeholders. Energies 2017, 10, 399. [Google Scholar] [CrossRef]
- Popp, J.; Harangi-Rákos, M.; Gabnai, Z.; Balogh, P.; Antal, G.; Bai, A. Biofuels and their co-products as livestock feed: Global economic and environmental implications. Molecules 2016, 21, 285. [Google Scholar] [CrossRef]
- Poudel, J.; Karki, S.; Sanjel, N.; Shah, M.; Oh, S.C. Comparison of biodiesel obtained from virgin cooking oil and waste cooking oil using supercritical and catalytic transesterification. Energies 2017, 10, 546. [Google Scholar] [CrossRef]
- Rahman, M.M.; Rasul, M.; Hassan, N.M.S. Study on the tribological characteristics of Australian native first generation and second generation biodiesel fuel. Energies 2017, 10, 55. [Google Scholar] [CrossRef]
- Costarrosa, L.; Leiva-Candia, D.E.; Cubero-Atienza, A.J.; Ruiz, J.J.; Dorado, M.P. Optimization of the transesterification of waste cooking oil with mg-al hydrotalcite using response surface methodology. Energies 2018, 11, 302. [Google Scholar] [CrossRef]
- Agarwal, A.K.; Gupta, J.G.; Dhar, A. Potential and challenges for large-scale application of biodiesel in automotive sector. Prog. Energy Combust. Sci. 2017. [Google Scholar] [CrossRef]
- Patel, P.D.; Lakdawala, A.; Chourasia, S.; Patel, R.N. Bio fuels for compression ignition engine: A review on engine performance, emission and life cycle analysis. Renew. Sustain. Energy Rev. 2016, 61, 113–149. [Google Scholar] [CrossRef]
- Hamdan, S.H.; Chong, W.W.F.; Ng, J.H.; Ghazali, M.J.; Wood, R.J.K. Influence of fatty acid methyl ester composition on tribological properties of vegetable oils and duck fat derived biodiesel. Tribol. Int. 2017, 113, 76–82. [Google Scholar] [CrossRef]
- Bobadilla, M.C.; Lorza, R.L.; Escribano-Garcia, R.; Gómez, F.S.; González, E.P.V. An improvement in biodiesel production from waste cooking oil by applying thought multi-response surface methodology using desirability functions. Energies 2017, 10, 130. [Google Scholar] [CrossRef]
- Rahman, Z.; Rashid, N.; Nawab, J.; Ilyas, M.; Sung, B.H.; Kim, S.C. Escherichia coli as a fatty acid and biodiesel factory: Current challenges and future directions. Environ. Sci. Pollut. Res. 2016, 23, 12007–12018. [Google Scholar] [CrossRef]
- Brigham, C. Perspectives for the biotechnological production of biofuels from CO2 and H2 using Ralstonia eutropha and other ‘Knallgas’ bacteria. Appl. Microbiol. Biotechnol. 2019, 103, 2113–2120. [Google Scholar] [CrossRef] [PubMed]
- Abdulkhani, A.; Alizadeh, P.; Hedjazi, S.; Hamzeh, Y. Potential of Soya as a raw material for a whole crop biorefinery. Renew. Sustain. Energy Rev. 2017, 75, 1269–1280. [Google Scholar] [CrossRef]
- Martínez, G.; Sánchez, N.; Encinar, J.M.; González, J.F. Fuel properties of biodiesel from vegetable oils and oil mixtures. Influence of methyl esters distribution. Biomass Bioenergy 2014, 63, 22–32. [Google Scholar] [CrossRef]
- Encinar, J.M.; Pardal, A.; Sánchez, N. An improvement to the transesterification process by the use of co-solvents to produce biodiesel. Fuel 2016, 166, 51–58. [Google Scholar] [CrossRef]
- Reeves, C.J.; Menezes, P.L.; Jen, T.C.; Lovell, M.R. The influence of fatty acids on tribological and thermal properties of natural oils as sustainable biolubricants. Tribol. Int. 2015, 90, 123–134. [Google Scholar] [CrossRef]
- Chavoushi, M.; Najafi, F.; Salimi, A.; Angaji, S.A. Improvement in drought stress tolerance of safflower during vegetative growth by exogenous application of salicylic acid and sodium nitroprusside. Ind. Crop. Prod. 2019, 134, 168–176. [Google Scholar] [CrossRef]
- Sabzalian, M.R.; Saeidi, G.; Mirlohi, A.; Hatami, B. Wild safflower species (Carthamus oxyacanthus): A possible source of resistance to the safflower fly (Acanthiophilus helianthi). Crop Prot. 2010, 29, 550–555. [Google Scholar] [CrossRef]
- Landry, E.J.; Fuchs, S.J.; Bradley, V.L.; Johnson, R.C. The effect of cold acclimation on the low molecular weight carbohydrate composition of safflower. Heliyon 2017, 3, e00402. [Google Scholar] [CrossRef]
- Mihaela, P.; Josef, R.; Monica, N.; Rudolf, Z. Perspectives of safflower oil as biodiesel source for South Eastern Europe (comparative study: Safflower, soybean and rapeseed). Fuel 2013, 111, 114–119. [Google Scholar] [CrossRef]
- Khalid, N.; Khan, R.S.; Hussain, M.I.; Farooq, M.; Ahmad, A.; Ahmed, I. A comprehensive characterisation of safflower oil for its potential applications as a bioactive food ingredient—A review. Trends Food Sci. Technol. 2017, 66, 176–186. [Google Scholar] [CrossRef]
- Sampaio, M.C.; Santos, R.F.; Bassegio, D.; de Vasconselos, E.S.; de Almeida Silva, M.; Secco, D.; da Silva, T.R. Fertilizer improves seed and oil yield of safflower under tropical conditions. Ind. Crop. Prod. 2016, 94, 589–595. [Google Scholar] [CrossRef]
- Khalid, A.; Tamaldin, N.; Jaat, M.; Ali, M.F.M.; Manshoor, B.; Zaman, I. Impacts of biodiesel storage duration on fuel properties and emissions. Procedia Eng. 2013, 68, 225–230. [Google Scholar] [CrossRef]
- Ministerio de Agricultura, Pesca y Alimentación. Avances Mensuales de Superficies y Producciones Agrícolas; Ministerio de Agricultura, Pesca y Alimentación: Madrid, Spain, 2018.
- Çelebi, Y.; Aydın, H. Investigation of the effects of butanol addition on safflower biodiesel usage as fuel in a generator diesel engine. Fuel 2018, 222, 385–393. [Google Scholar] [CrossRef]
- Işik, M.Z.; Aydin, H. Investigation on the effects of gasoline reactivity controlled compression ignition application in a diesel generator in high loads using safflower biodiesel blends. Renew. Energy 2019, 133, 177–189. [Google Scholar] [CrossRef]
- Yang, R.; Zhang, L.; Li, P.; Yu, L.; Mao, J.; Wang, X.; Zhang, Q. A review of chemical composition and nutritional properties of minor vegetable oils in China. Trends Food Sci. Technol. 2018, 74, 26–32. [Google Scholar] [CrossRef]
- Liu, W.; Lu, G.; Yang, G.; Bi, Y. Improving oxidative stability of biodiesel by cis-trans isomerization of carbon-carbon double bonds in unsaturated fatty acid methyl esters. Fuel 2019, 242, 133–139. [Google Scholar] [CrossRef]
- Focke, W.W.; Van Der Westhuizen, I.; Oosthuysen, X. Biodiesel oxidative stability from Rancimat data. Thermochim. Acta 2016, 633, 116–121. [Google Scholar] [CrossRef] [Green Version]
- Chan, C.H.; Tang, S.W.; Mohd, N.K.; Lim, W.H.; Yeong, S.K.; Idris, Z.; Kotwal, M.; Kumar, A.; Darbha, S.; Sharma, R.V.; et al. Tribological behavior of biolubricant base stocks and additives. Renew. Sustain. Energy Rev. 2018, 93, 145–157. [Google Scholar] [CrossRef]
- Saluja, R.K.; Kumar, V.; Sham, R. Stability of biodiesel—A review. Renew. Sustain. Energy Rev. 2016, 62, 866–881. [Google Scholar] [CrossRef]
- Kumar, N. Oxidative stability of biodiesel: Causes, effects and prevention. Fuel 2017, 190, 328–350. [Google Scholar] [CrossRef]
- UNE-EN 14214:2013 V2+A1:2018. Liquid Petroleum Products—Fatty Acid Methyl Esters (FAME) for Use in Diesel Engines and Heating Application—Requirements and Test Methods; The National Standards Authority of Ireland (NSAI): Dublin, Ireland, 2018. [Google Scholar]
- Canha, N.; Felizardo, P.; Correia, M.J.N. Controlling the oxidative stability of biodiesel using oils or biodiesel blending or antioxidants addition. Environ. Prog. Sustain. Energy 2018, 37, 1031–1040. [Google Scholar] [CrossRef]
- França, F.R.M.; dos Santos Freitas, L.; Ramos, A.L.D.; da Silva, G.F.; Brandão, S.T. Storage and oxidation stability of commercial biodiesel using Moringa oleifera Lam as an antioxidant additive. Fuel 2017, 203, 627–632. [Google Scholar] [CrossRef]
- Van der Westhuizen, I.; Focke, W.W. Stabilizing sunflower biodiesel with synthetic antioxidant blends. Fuel 2018, 219, 126–131. [Google Scholar] [CrossRef]
- Varatharajan, K.; Pushparani, D.S. Screening of antioxidant additives for biodiesel fuels. Renew. Sustain. Energy Rev. 2018, 82, 2017–2028. [Google Scholar] [CrossRef]
- Schober, S.; Mittelbach, M. The impact of antioxidants on biodiesel oxidation stability. Eur. J. Lipid Sci. Technol. 2004, 106, 382–389. [Google Scholar] [CrossRef]
- Squissato, A.L.; Richter, E.M.; Munoz, R.A.A. Voltammetric determination of copper and tert-butylhydroquinone in biodiesel: A rapid quality control protocol. Talanta 2019, 201, 433–440. [Google Scholar] [CrossRef]
- Yang, J.; He, Q.S.; Corscadden, K.; Caldwell, C. Improvement on oxidation and storage stability of biodiesel derived from an emerging feedstock camelina. Fuel Process. Technol. 2017, 157, 90–98. [Google Scholar] [CrossRef]
- Dunn, R.O. Effect of antioxidants on the oxidative stability of methyl soyate (biodiesel). Fuel Process. Technol. 2005, 86, 1071–1085. [Google Scholar] [CrossRef]
- Zhou, J.; Xiong, Y.; Liu, X. Evaluation of the oxidation stability of biodiesel stabilized with antioxidants using the Rancimat and PDSC methods. Fuel 2017, 188, 61–68. [Google Scholar] [CrossRef]
- Kivevele, T.T.; Huan, Z. Effects of Antioxidants on the Cetane number, Viscosity, Oxidation Stability, and Thermal Properties of Biodiesel Produced from Nonedible Oils. Energy Technol. 2013, 1, 537–543. [Google Scholar] [CrossRef]
- Serrano, M.; Martínez, M.; Aracil, J. Long term storage stability of biodiesel: Influence of feedstock, commercial additives and purification step. Fuel Process. Technol. 2013, 116, 135–141. [Google Scholar] [CrossRef]
- Yahagi, S.S.; Roveda, A.C.; Sobral, A.T.; Oliveira, I.P.; Caires, A.R.L.; Gomes, R.S.; Trindade, M.A.G. An Analytical Evaluation of the Synergistic Effect on Biodiesel Oxidation Stability Promoted by Binary and Ternary Blends Containing Multifunctional Additives. Int. J. Anal. Chem. 2019, 2019, 6467183. [Google Scholar] [CrossRef]
- Encinar, J.M.; González, J.F.; Pardal, A.; Martínez, G. Rape oil transesterification over heterogeneous catalysts. Fuel Process. Technol. 2010, 91, 1530–1536. [Google Scholar] [CrossRef]
- UNE-EN ISO 3104/AC:1999. Petroleum Products. Transparent and Opaque Liquids. Determination of Kinematic Viscosity and Calculation of Dynamic Viscosity (ISO 3104:1994); International Organization for Standarization (ISO): Geneva, Switzerland, 1999. [Google Scholar]
- UNE-EN 116:2015. Diesel and Domestic Heating Fuels—Determination of Cold Filter Plugging Point—Stepwise Cooling Bath Method; British Standards Institution (BSI): London, UK, 2015. [Google Scholar]
- UNE-EN 51023:1990. Petroleum Products. Determination of Flash and Fire Points. Cleveland Open cup Method; Asociacion Espanola de Normalizacion: Madrid, Spain, 1990. [Google Scholar]
- UNE-EN-ISO-12937:2000. Productos Petrolíferos. Determinación de Agua. Método de Karl Fischer por Valoración Culombimétrica; The Spanish Association for Standardization and Certification (AENOR): Madrid, Spain, 2001. [Google Scholar]
- UNE-EN 14111:2003. Fat and Oil Derivatives. Fatty Acid Methyl Esters (FAME). Determination of Iodine Value; International Organization for Standarization (ISO): Geneva, Switzerland, 2003. [Google Scholar]
- Eryilmaz, T.; Yesilyurt, M.K. Influence of blending ratio on the physicochemical properties of safflower oil methyl ester-safflower oil, safflower oil methyl ester-diesel and safflower oil-diesel. Renew. Energy 2016, 95, 233–247. [Google Scholar] [CrossRef]
- Jose, T.K.; Anand, K. Effects of biodiesel composition on its long term storage stability. Fuel 2016, 177, 190–196. [Google Scholar] [CrossRef]
- García, M.; Botella, L.; Gil-Lalaguna, N.; Arauzo, J.; Gonzalo, A.; Sánchez, J.L. Antioxidants for biodiesel: Additives prepared from extracted fractions of bio-oil. Fuel Process. Technol. 2017, 156, 407–414. [Google Scholar] [CrossRef]
- Dunn, R.O. Antioxidants for improving storage stability of biodiesel. Biofuels Bioprod. Biorefining 2008, 2, 304–318. [Google Scholar] [CrossRef]
- Borsato, D.; de Moraes Cini, J.R.; Da Silva, H.C.; Coppo, R.L.; Angilelli, K.G.; Moreira, I.; Maia, E.C.R. Oxidation kinetics of biodiesel from soybean mixed with synthetic antioxidants BHA, BHT and TBHQ: Determination of activation energy. Fuel Process. Technol. 2014, 127, 111–116. [Google Scholar] [CrossRef]

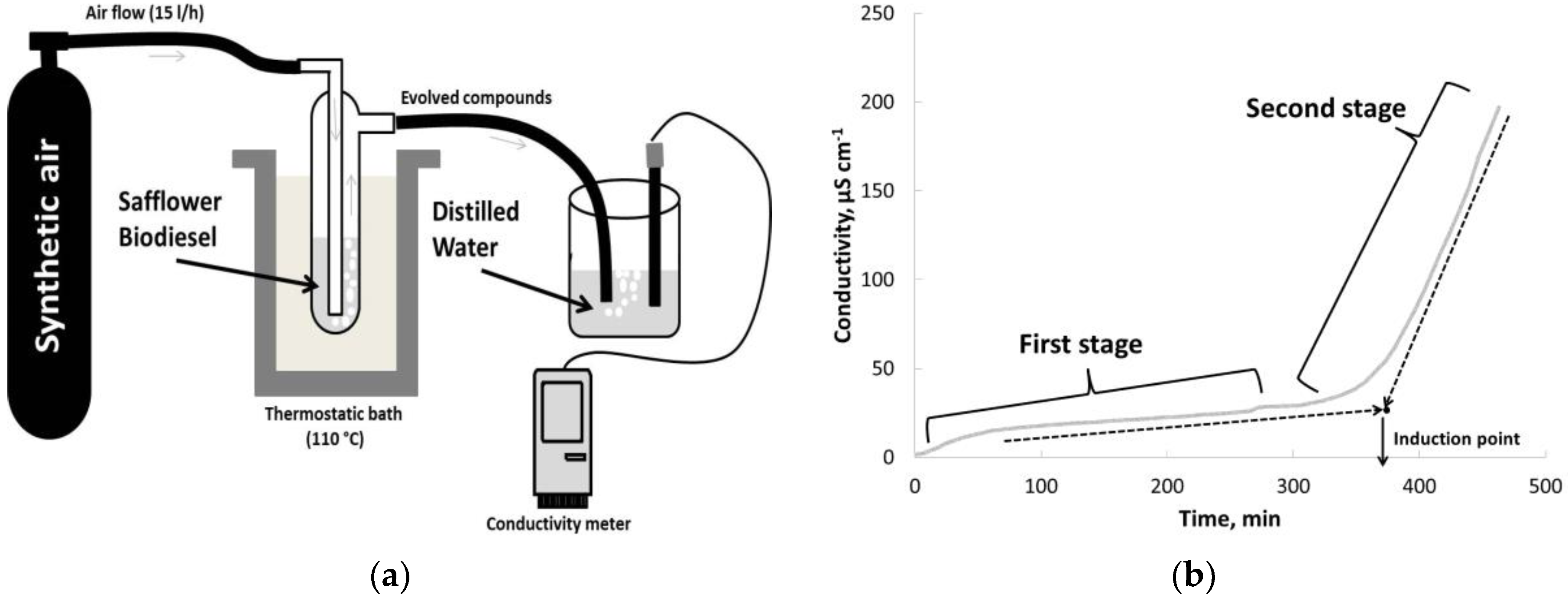
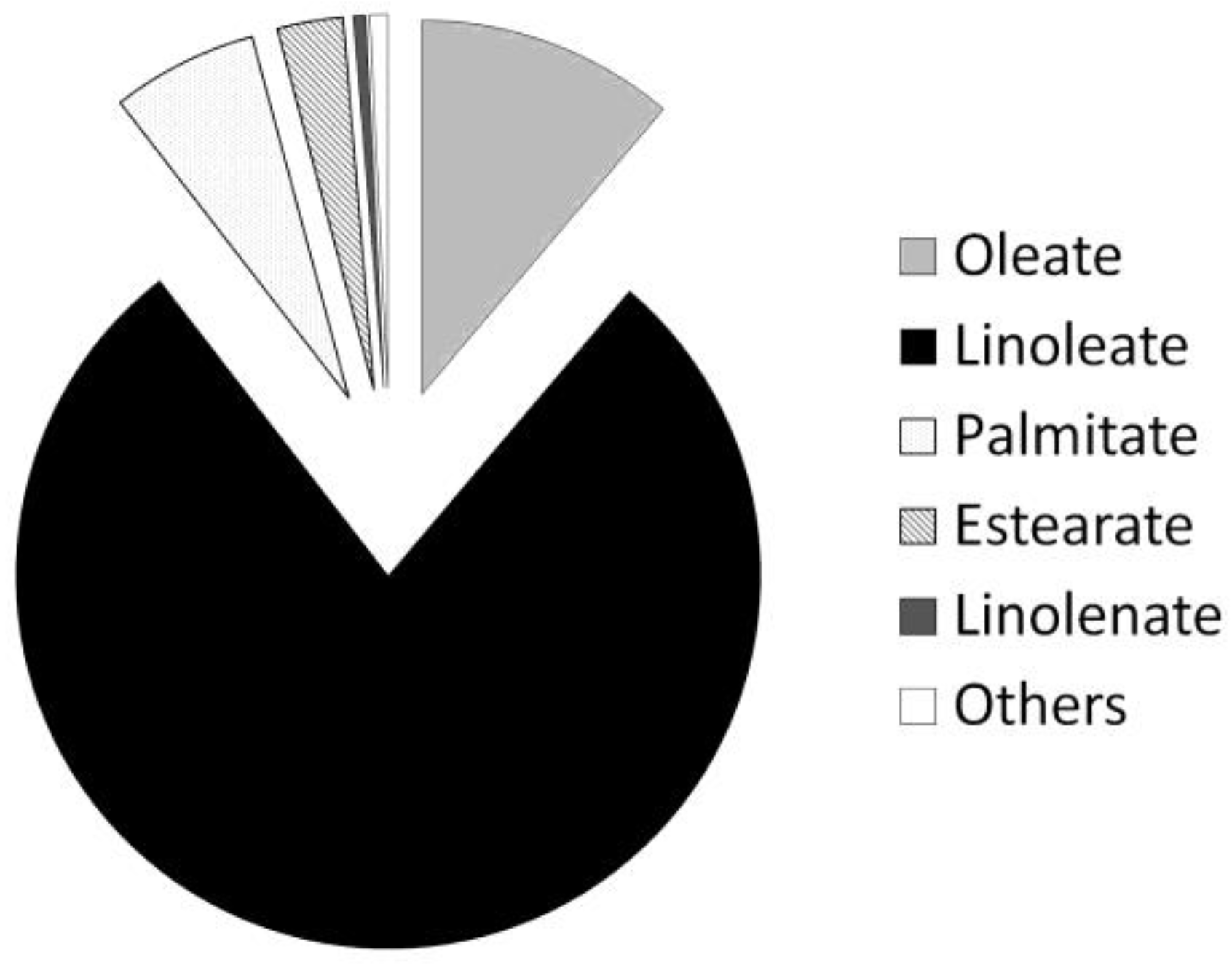

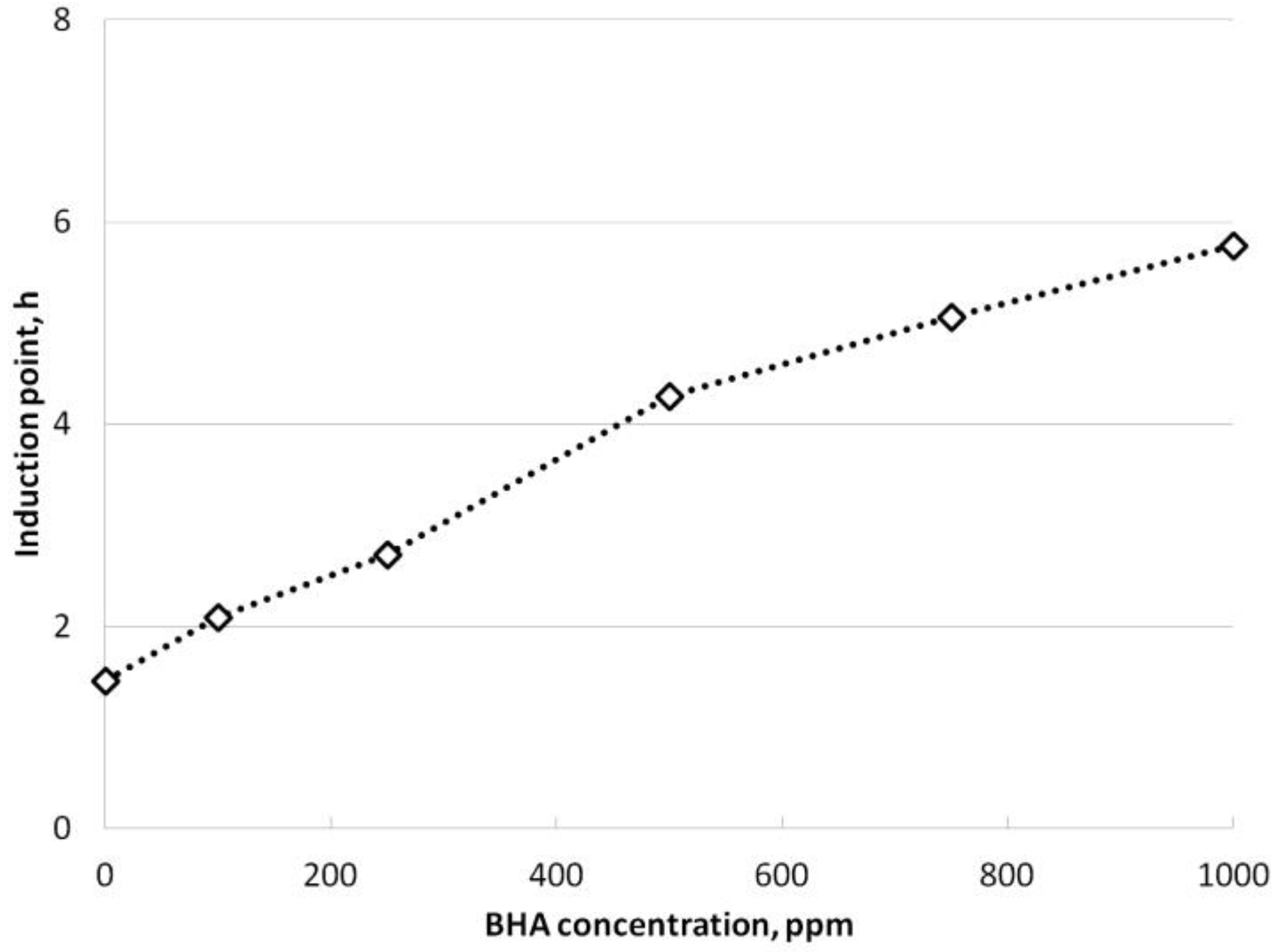

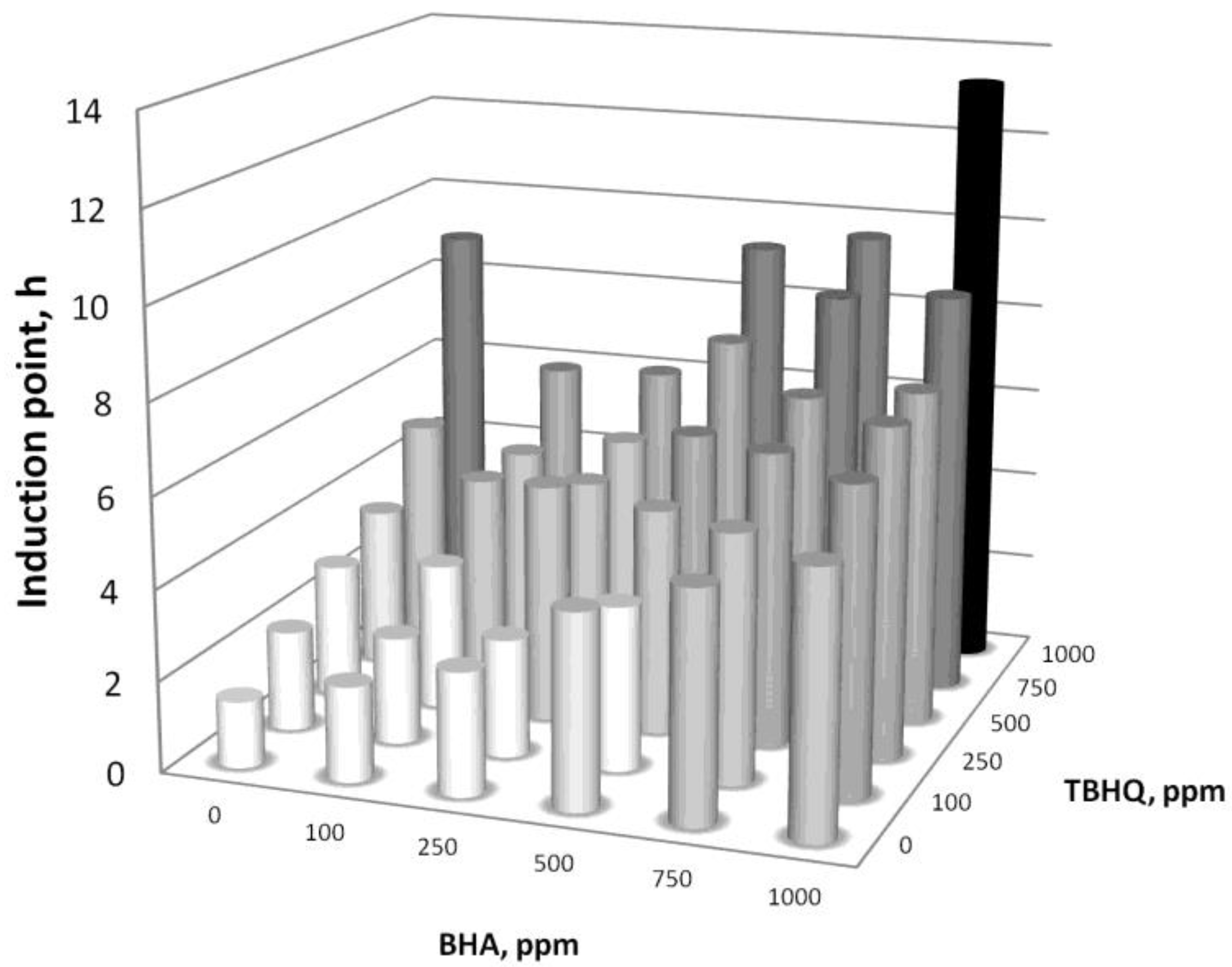
| Reaction temperature (°C) | 65 |
| Reaction time (min) | 60 |
| Methanol/oil ratio | 6:1 |
| Catalyst concentration 1 (%) | 1.5 |
| Experiment | BHA Concentration (ppm) | TBHQ Concentration (ppm) |
|---|---|---|
| Effect of BHA addition | 0, 100, 250, 500, 750 and 1000 | 0 |
| Effect of TBHQ addition | 0 | 0, 100, 250, 500, 750 and 1000 |
| Effect of BHA and TBHQ addition | 0, 100, 250, 500, 750 and 1000 | 0, 100, 250, 500, 750 and 1000 |
| Oven temperature (°C) | 220 for 23.5 min, 240 for 14 min |
| Injector temperature (°C) | 270 |
| Detector temperature (°C) | 300 |
| Column flow (cm3min−1) | 28 |
| Carrier gas | Helium |
| Auxiliary gas | Nitrogen |
| Combustible gas (cm3min−1) | Synthetic air (300) |
| Oxidizing gas (cm3min−1) | Hydrogen (30) |
| Parameter | Value | EN-14214 |
|---|---|---|
| Viscosity at 40 °C (cSt) | 4.42 | 3.50–5.00 |
| Density at 15 °C (g·dm−3) | 880 | 860–900 |
| Oxidative stability (h) | 1.46 | 8 1 |
| FAME content (%) | 96.78 | 96.5 1 |
| Flash point (°C) | 180 | 120 1 |
| Combustion point (°C) | 190 | Not included |
| CFPP (°C) 2 | −2 | −20–+5 |
| Water content (mg·Kg−1) | 400 | 500 3 |
| Acidity number (mg KOH·g−1) | 0.35 | 0.5 3 |
| Iodine number (g I2·100 g−1) | 115 | 120 3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nogales-Delgado, S.; Encinar, J.M.; González, J.F. Safflower Biodiesel: Improvement of its Oxidative Stability by Using BHA and TBHQ. Energies 2019, 12, 1940. https://doi.org/10.3390/en12101940
Nogales-Delgado S, Encinar JM, González JF. Safflower Biodiesel: Improvement of its Oxidative Stability by Using BHA and TBHQ. Energies. 2019; 12(10):1940. https://doi.org/10.3390/en12101940
Chicago/Turabian StyleNogales-Delgado, Sergio, José María Encinar, and Juan Félix González. 2019. "Safflower Biodiesel: Improvement of its Oxidative Stability by Using BHA and TBHQ" Energies 12, no. 10: 1940. https://doi.org/10.3390/en12101940
APA StyleNogales-Delgado, S., Encinar, J. M., & González, J. F. (2019). Safflower Biodiesel: Improvement of its Oxidative Stability by Using BHA and TBHQ. Energies, 12(10), 1940. https://doi.org/10.3390/en12101940







