Abstract
Steam-explosion is a promising technology for recovering phenolic compounds from olive mill solid waste (OMSW) due to its high impact on the structure of the fibre. Moreover, the recovery of the phenols, which are well-known microbial inhibitors, could improve the subsequent biomethanization of the dephenolized OMSW to produce energy. However, there is a considerable lack of knowledge about how the remaining phenolic compounds could affect a long-term biomethanization process of steam-exploded OMSW. This work evaluated a semi-continuous mesophilic anaerobic digestion of dephenolized steam-exploited OMSW during a long operational period (275 days), assessing different organic loading rates (OLRs). The process was stable at an OLR of 1 gVS/(L·d), with a specific production rate of 163 ± 28 mL CH4/(gVS·d). However, the increment of the OLR up to 2 gVS/(L·d) resulted in total exhaust of the methane production. The increment in the propionic acid concentration up to 1486 mg/L could be the main responsible factor for the inhibition. Regardless of the OLR, the concentration of phenolic compounds was always lower than the inhibition limits. Therefore, steam-exploited OMSW could be a suitable substrate for anaerobic digestion at a suitable OLR.
1. Introduction
The olive oil sector stands as an important economic sector for many countries; however, some current challenges could risk its economical sustainability. On one hand, the olive oil industry has to compete with other low-cost vegetable oils, such as sunflower or palm oil. On the other hand, it is required to develop sustainable and environmentally friendly management for the olive mill solid waste (OMSW) generated during extraction [1]. The OMSW results from the two-phase olive oil manufacturing process, being a high-humidity and polluting waste, where the remains of olive fruit are retained. Each ton of olive oil generates around 4 tons of OMSW each year, and due its high potential impact on the environment [2,3], it is necessary to develop an adequate OMSW management strategy to ensure environmental protection, but also economic sustainability of the olive oil sector.
One of the main processes for valorisation of OMSW is the recovery of bioactive compounds contained in its structure, which can be used in food industry, pharmacy or cosmetics. In fact, most of the bioactive compounds present in the olives remains in the OMSW [4,5]. These bioactive compounds, which are the responsible for the healthy properties of olive oil, are mainly phenols such as hydroxytyrosol, tyrosol, vanillic acid, p-coumaric acid, 3,4-dihydroxyphenylglycol, etc. [4,6]. Most of these compounds form part of complex structures, such as the cell wall, and it is therefore necessary to carry out a previous solubilisation step to recover them [7]. Thermal treatments have been shown to facilitate the solubilisation of the valuable bioactive compounds to a liquid phase, where they can be easily recovered [6].
Among the different thermal treatments, steam-explosion allows higher breakdown of the lignocellulosic structures compared to other conventional and less aggressive thermal treatments [8]. During the steam-explosion process, the material is heated with high pressure saturated steam for a short period of time followed by a fast pressure reduction, which causes an explosive decompression. During steam-explosion of OMSW, hemicellulose and cellulose structures are mainly hydrolysed, whereas the lignin is also affected to a certain degree [4]. The breakdown of the lignocellulosic structures through the steam-explosion treatment results in a high solubilisation of bioactive compounds that can be easily recovered [9].
The steam-exploded OMSW that remains after bioactive compound extraction still needs to be stabilized to avoid environmental pollution. Among the processes to stabilize OMSW, anaerobic digestion offers the possibility of obtaining methane, as an energy carrier, and the possibility to recover the remaining digestate as fertilizer [10,11]. According to Stoyanova et al. [12], stable single-stage semi-continuous OMSW anaerobic digestion proceeded at an organic loading rate (OLR) of 0.76 kgVS/(m3 d) (VS, total volatile solids). Recently, Serrano et al. [13] determined that the accumulation of VFA was the main reason for destabilization during anaerobic digestion of OMSW subjected to a thermal pre-treatment (1 h at 170 °C) at an OLR of 2 gVS/(m3·d). Inhibition of the anaerobic digestion of lignocellulosic material has been also associated with phenols and derived phenolic compounds because of their severe antimicrobial properties [14,15]. Some phenols and furans with high inhibitory properties, such as hydroxymethylfurfural or furfural, are generated during the thermal treatment of the lignocellulosic compounds in severe conditions [8,16].
Extraction of polyphenols can enable the utilization of OMSW and its valorisation with energy recovery by anaerobic digestion due to the partial removal of inhibitors [3,9]. A recent previous study showed that a steam-explosion pre-treatment of OMSW (200 °C for 5 min, prior to a rapid decompression) allowed the recovery of more than 2000 mg hydroxytyrosol/kg of steam-exploded OMSW [9]. In the subsequent anaerobic digestion step of the steam-exploded OMSW performed in the batch mode, pre-treatment and phenol extraction improved the methane yield coefficient and methane production rate of the dephenolized liquid fraction by 26.8% and 26.4%, respectively, with respect to untreated OMSW [9]. However, more information about the performance of long-term anaerobic digestion of steam-exploded OMSW in semi-continuous mode, and how the accumulation of potential toxic compounds could affect the process stability and biogas production, is still required prior to industrial implementation.
Therefore, due to the potential of the mentioned OMSW steam-explosion pre-treatment [9], the present work aimed to assess the semi-continuous mesophilic anaerobic digestion of steam-exploded OMSW during a long operational period (275 days) assessing different OLRs. Finding the adequate OLR is very important for optimizing process efficiency and, at the same time, reducing the risk of destabilization.
2. Materials and Methods
2.1. Substrate and Inoculum
The substrate used in the present study consisted of OMSW, which was subjected to a phenol recovery process. Raw OMSW was obtained from the OMSW management plant “Oleícola El Tejar” located in Marchena (Seville), Spain. The phenol recovery process entailed a steam-explosion pre-treatment (200 °C for 5 min, prior to a rapid decompression). Subsequent phenol recovery was done by a patented chromatographic system (WO 2013/007850A1) from the generated liquid phase (detailed in Serrano et al. [9]). The treated OMSW used in the present research was composed of the pre-treated solid phase and the dephenolized liquid phase. Analytical characterization of the used OMSW is shown in Table 1.

Table 1.
Characterization of the Olive Mill Solid Waste (OMSW).
A full-scale digester treating sewage sludge at the “COPERO” wastewater treatment plant (Seville, Spain) was the source of the used anaerobic inoculum (Total Solids (TS) = 21 ± 1 g/kg and VS = 19 ± 1 g/kg). The reactors were initially inoculated at a concentration of 10 gVS/L.
2.2. Anaerobic Digestion Experimental Procedure
Two-litre continuously stirred tank reactors (CSTRs) in triplicate were used to evaluate mesophilic semi-continuous anaerobic digestion of the steam-exploded OMSW (more details in [13]). The volume of methane was measured daily after the removal of the CO2 with tightly closed bubblers containing a NaOH solution (3 N). The remaining gas volume was measured by liquid displacement using Boyle-Mariotte flasks. The volume of methane was expressed at standard temperature and pressure conditions (0 °C and 1 atm).
Table 2 summarizes the experimental design, including the stages for bio-stimulation and the adaptation to the substrate. After the stimulation and adaptation periods, anaerobic digestion performance of the steam-exploded OMSW was evaluated during a long-term period of 275 days at different organic loading rates (OLR). Samples were analyzed at least twice per week.

Table 2.
Experimental design.
2.3. Chemical Analyses
TS, VS, pH and alkalinity were analysed following the recommendations of the American Public Health Association (APHA) [17]. Total chemical oxygen demand (COD; g/kg) was measured as described by Raposo et al. (2008), while the colorimetric standard method 5220D was used for the measurement of the soluble COD (sCOD; g/L or mg/L) [17]. Total phenols were quantified by spectrophotometry at 655 nm through the Folin-Ciocalteu method [5]. A gallic acid curve was used for calibration of the method. Soluble phenols were also quantified by the Folin-Ciocalteu method after filtration through a Millipore 0.45 μm filter (Albet GF 47 mm, Dassel, Germany). Individual phenols were quantified using a Hewlett–Packard 1100 liquid chromatography system (more details are described in Serrano et al. [9]). Separate volatile fatty acids (C2–C6) were determined using a Shimadzu gas chromatograph (GC-2010).
2.4. Microbial Analysis
Samples were taken at the inoculum and at 75, 175, and 250 days of the experiment in order to analyze the number of microorganisms by DAPI staining. Diluted samples of 10 L volume were fixed onto glass slides for 20 min, and then mounted with 25 L of Fluoroshield with DAPI (Abcam, Cambridge, UK) prior to visualization in a Zeiss Axio Imager M2 fluorescence microscope under UV light. Images were acquired with an AxioCam MRm digital camera. The mean number of cells was determined from 10 random images using the ImageJ software. Backgrounds were subtracted from all images to improve image clarity as described elsewhere [18].
2.5. Economic Assessment
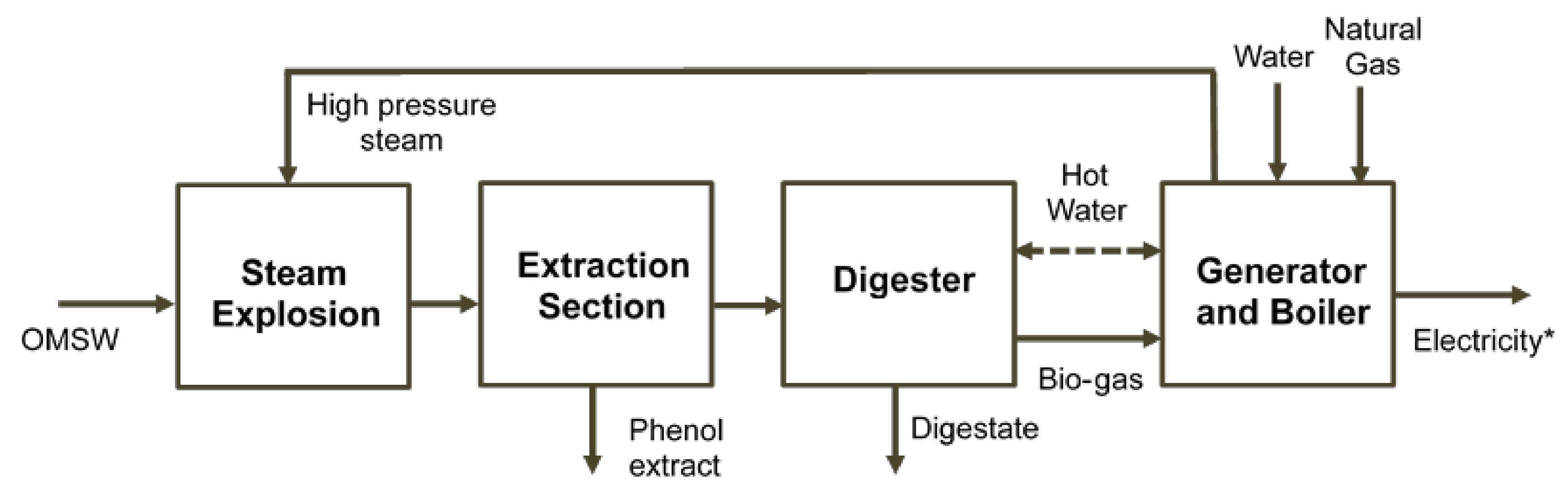
The economic profitability of a bio-refinery consisting of steam explosion pre-treatment, phenol extraction, anaerobic digestion and a cogeneration system was assessed (Figure 1). Net present value (NPV), internal rate of return (IRR) and payback were calculated according to the methodology described in Serrano et al. (2017b). An additional natural gas input was considered to cover the thermal energy requirement due to the steam explosion pre-treatment. Although a price of 520 €/kg for phenol extract [19] was initially fixed in the study, a possible drop in this price due to the implementation of the new technology could be considered in the profitability assessment. In this sense, a sensibility study was performed to calculate, making NPV equal to zero, the minimum price for which the bio-refinery project would be profitable. A full and detailed description of the economic assessment methodology is reported in Serrano et al. [3]. Due to the high initial inversion, only the financed project scenario was considered. The discount rate and prices of both sold electricity and bought natural gas were updated to 1.40%, 0.12 €/kWh [20], and 0.04 €/kWh [21], respectively.
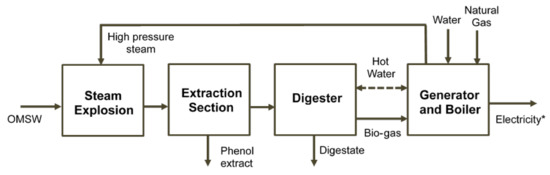
Figure 1.
Bio-refinery scheme consisting of steam explosion solubilization, phenol recovery, anaerobic digestion and a cogeneration system. * Net electricity production after subtracting the total electricity consumed from the electricity produced.
3. Results and Discussion
3.1. Methane Production at the Different Experimental Phases
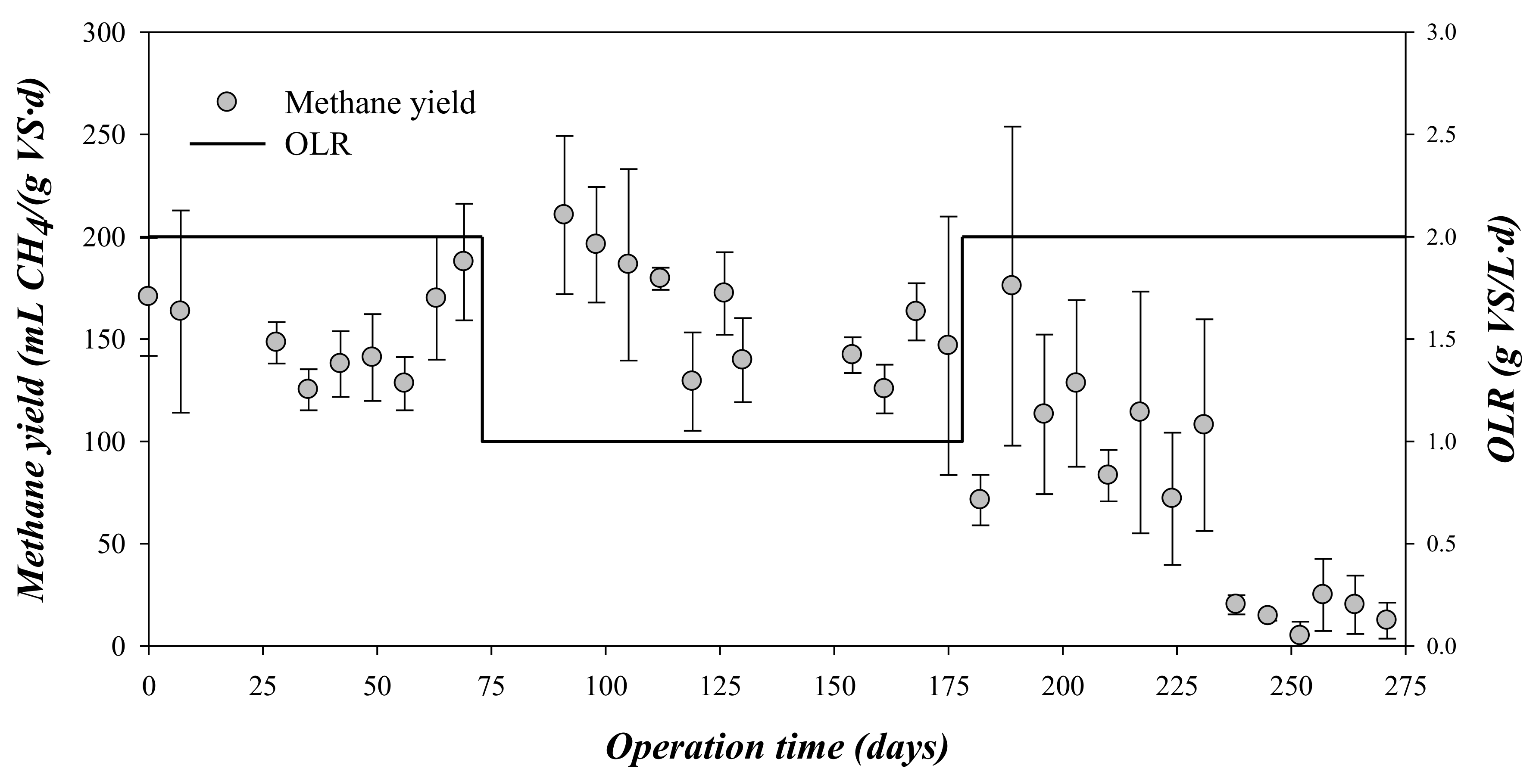
The methane production was monitored daily throughout the digestion time for the different OLRs (Figure 2). As can be observed, methane yield varied significantly when varying the OLR. In the first stage, i.e., 0–75 days and OLR = 2 gVS/(L·d), the methane production rate presented a mean value of around 0.369 ± 0.049 gCODCH4/(gCOD·d), i.e., 152 ± 21 mL CH4/(gVS·d) (Table 3).
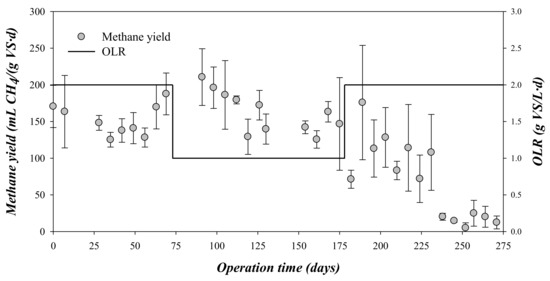
Figure 2.
Variation of the methane yield and the organic loading rate (OLR) during the experimental time.

Table 3.
Methane production rates, pH, alkalinity, COD, CODs, total phenols, soluble phenols, total VFA and individual phenolic compound concentration during the different organic loading rates (OLRs).
At day 75, the OLR was set to 1 gVS/(L·d). From days 75–175, the mean methane production rate was 0.395 ± 0.066 gCODCH4/(gCOD·d), i.e., 163 ± 28 mL CH4/(gVS·d) (Table 3, Figure 2). This value was just 7.2% higher than that obtained at the previous 2 gVS/(L·d) OLR. As the reduction of the OLR did not result in a marked increase in methane production, it could be remarked that the steam-explosion pre-treatment allowed operation at both OLRs without affecting the methane production during the assayed period. Serrano et al. [3] reported that the batch anaerobic digestion of steam-exploded OMSW produced a maximum value of 108 ± 9 mL CH4/(gVS·d), i.e., 40% and 50% lower than that obtained in the present research operating at an OLR of 2 and 1 gVS/(L·d), respectively. The higher total duration of the experiments at the semi-continuous mode, compared with the 25 day operating period of the batch experiments, allowed adaptation of the inoculum to the substrate and a higher methane production was acheived [3].
After 175 days, the OLR feed to the reactors was again augmented to 2 gVS/(L·d) to evaluate if a long operation time could result in better adaptation of the digesting microorganisms to the substrate or if the accumulation of organic compounds could affect the stability. However, the increment in the OLR entailed a complete failure of the process and a marked decrease of the methane production (Figure 2). Concretely, the values of methane production rate decreased from 0.345 to 0.030 gCODCH4/(gCOD·d), i.e., from 175 to 8 mL CH4/(gVS·d) (Table 3). The values of methane production rates in the third stage implied that the OMSW was not efficiently converted in methane. According to the obtained results, the OLR of 1 gVS/(L·d) was most recommendable for the biomethanization of steam-explosion pre-treated and dephenolized OMSW, due to the more stable operation and higher methane yield and methane production rate. Other authors have shown similar effects on the methane production by increasing the OLR. For example, Serrano et al. [13] reported that the methane production in the anaerobic digestion of OMSW subjected to a thermal treatment at 170 °C and 1 h varied from 119 ± 30 mL CH4/(gVS·d) to 172 ± 60 mL CH4/(gVS·d) by reducing the OLR from 2 to 1 gVS/(L·d). Stoyanova et al. [12] reported that the biomethanization of two-phase OMSW operated at stable conditions at an OLR of 0.76 gVS/(L·d), but the increase of the OLR up to 1.1 and 1.76 gVS/(L·d) affected the methane production and, finally, caused the total failure of the system. In the same way, Orive et al. [22] also reported low methane production rates (77 mL CH4/(gVS·d) in the semi-continuous anaerobic digestion of diluted OMSW (COD: 60 g/kg) at high OLRs (around 3 gVS/(L·d).
3.2. Chemical Oxygen Demand and Volatile Fatty Acid Concentration throughout the Experimental Time
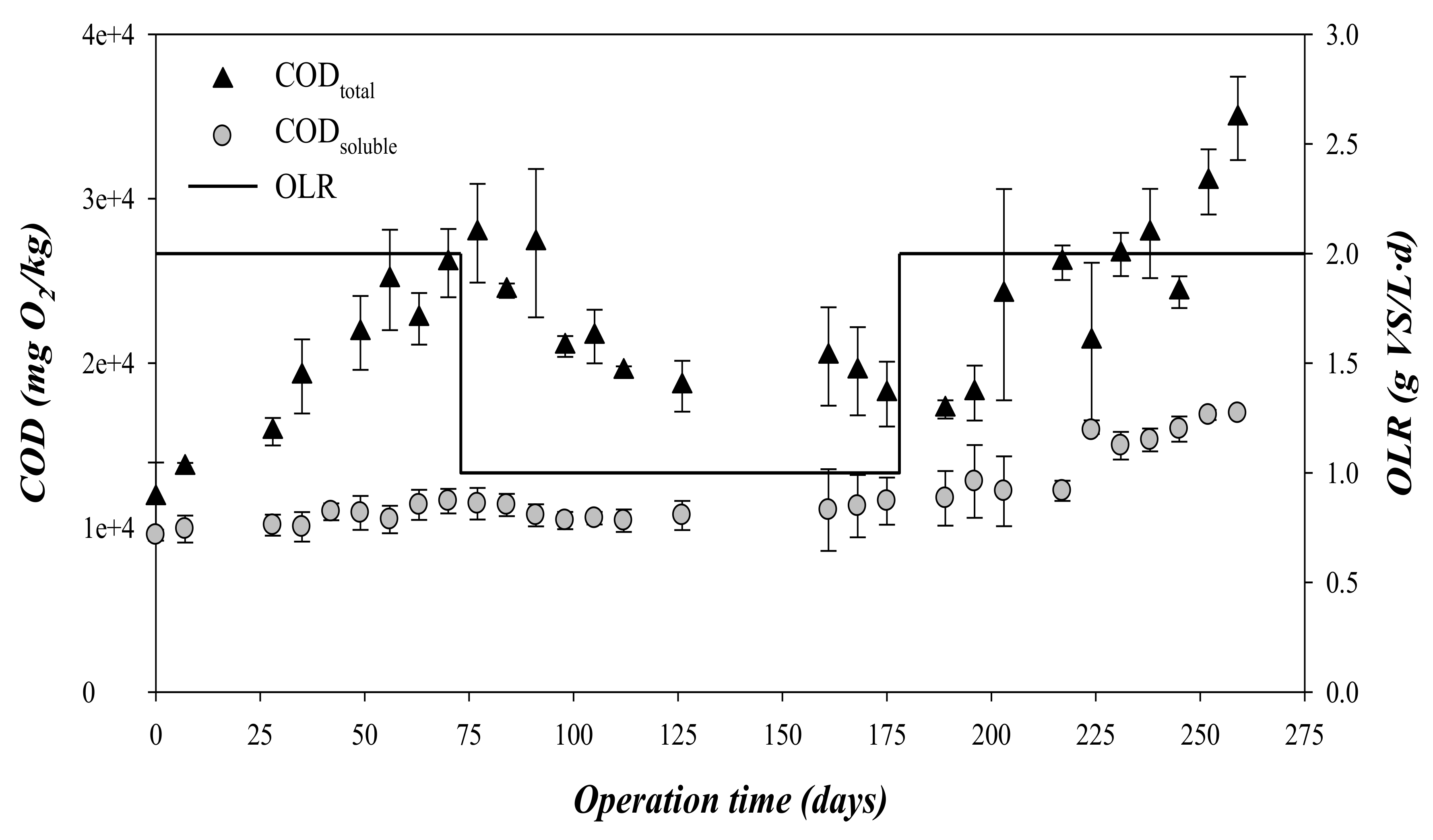
Values of CODtotal and CODsoluble, all expressed as mgO2/kg, during operational time are shown in Figure 3. During the first 175 days, i.e., stages 1 and 2, the concentration of CODsoluble remained at relatively constant values regardless of the applied OLR, with a mean value of 10,000 mgO2/kg. By contrast, the CODtotal presented a clear increment from 12.0 to 27.0 gO2/kg during the first stage, i.e., 0–75 days and OLR = 2 gVS/(L·d). The accumulation of the CODtotal, when the CODsoluble and the VFA remained at low values, indicated that the hydrolysis was probably the limiting rate-step during this stage. In fact, the hydrolysis has been widely reported to be the limiting step in the anaerobic degradation pathway for complex organic solids [23]. The decrease of the OLR to 1 gVS/(L·d), entailed a rapid decrease of the accumulated CODtotal from days 75 to 100. After this period, the concentration of CODtotal remained at a mean value of 19.6 ± 1.2 gO2/kg.
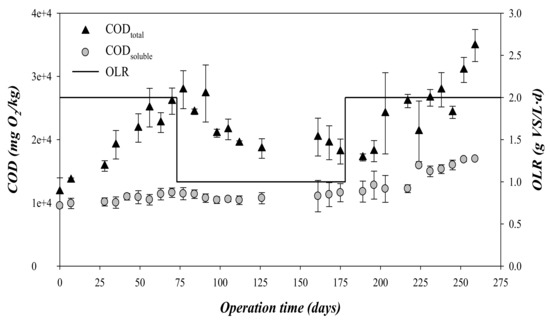
Figure 3.
Variation of the total and soluble COD and the organic loading rate (OLR) during operation time.
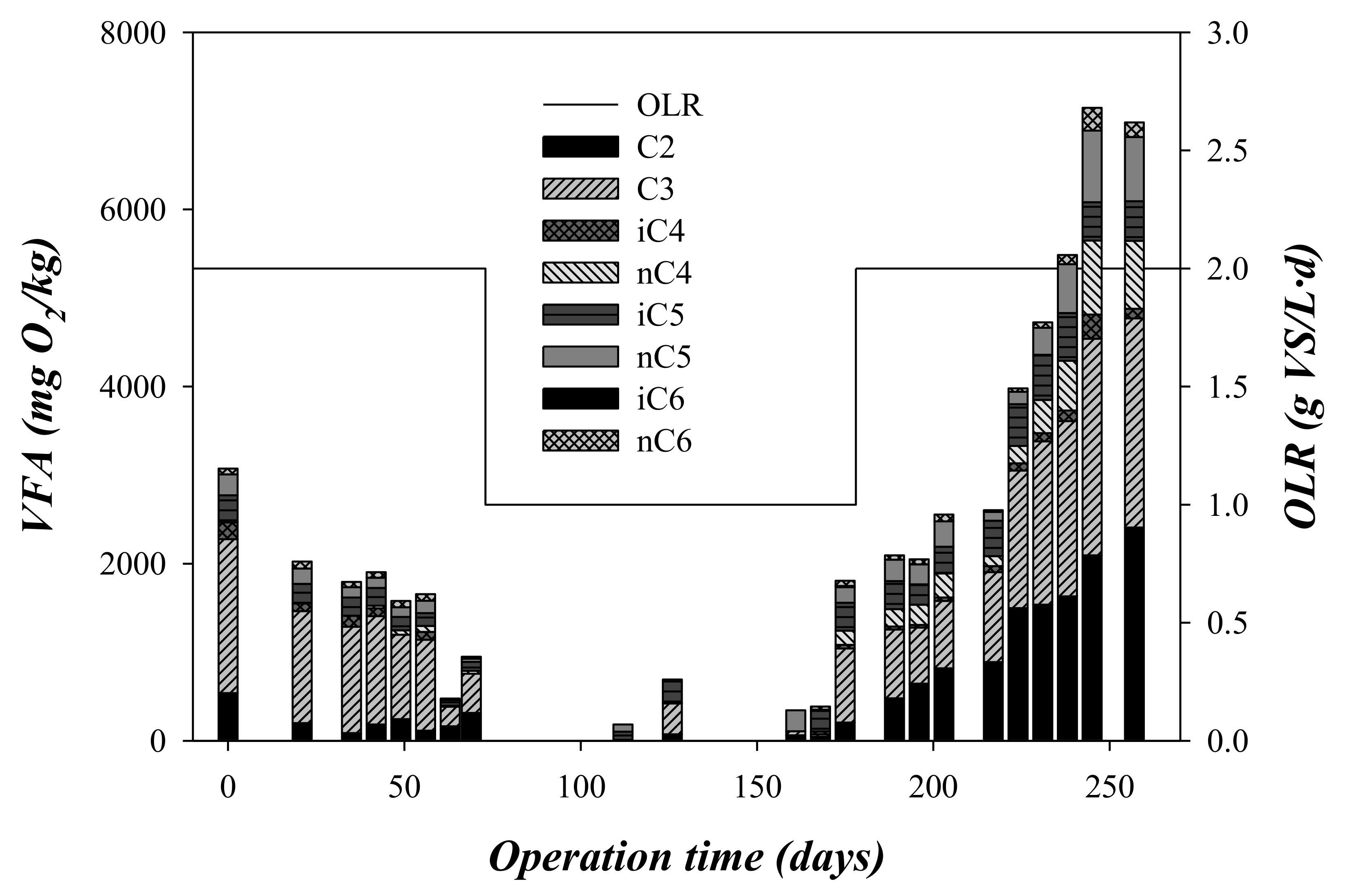
The VFA composition was also analysed and the obtained results are shown in Figure 4. VFA concentration systematically decreased during the first stage (OLR = 2 gVS/(L·d), days 0–75), from ~3000 mgO2/kg to ~900 mgO2/kg, where propionic acid was the most abundant VFA determined in these first 75 days (Figure 4). VFA concentration remained almost undetectable during stage 2 (OLR = 1 gVS/(L·d), days 75–175). The decrease of propionic acid and almost disappearance of the rest of VFA during the second stage (75–175 days) carried out at an OLR of 1 gVS/(L d) could indicate that the microorganisms required this time period to complete their adaptation to the substrate [24]. Despite the initial increase in the VFA concentration, the pH of the digesters remained at mean values of 7.81 ± 0.34 and 7.39 ± 0.41 (Table 3), during stages 1 and 2, respectively. The pH remained slightly alkaline (above 7.8) until day 80 of operation. From that day, despite having reduced the OLR of the system, the alkalinity suffers a very marked decrease, which diminished from about 3220 ± 100 to 1460 ± 400 mg CaCO3/L. It should be noted that this substrate did not provide alkalinity. This decrease in the alkalinity was also associated with a decrease in pH, from 7.9 ± 0.1 on day 75 to 7.0 ± 0.2 on day 175.
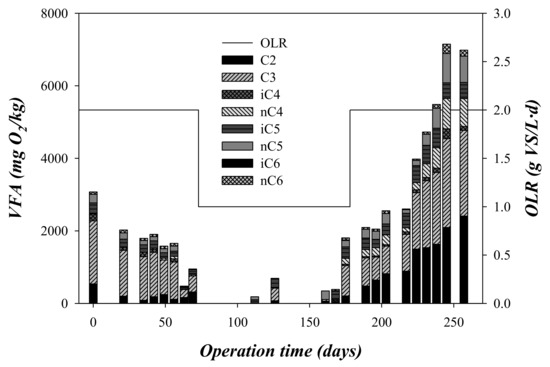
Figure 4.
Variation of the VFA and the organic loading rate (OLR) with the operation time.
After 175 days, the OLR once more increased up to 2 gVS/(L·d). In view of the previously mentioned fall in alkalinity, alkalinity was added daily from day 175 to maintain the pH of the operation within the optimum values for anaerobic digestion. As a consequence, enormous oscillations in alkalinity values were observed in the third operating period (OLR: 2 gVS/(L·d), days: 175–275). In spite of all the artificially added alkalinity, the pH during this stage falls repeatedly to values below those recommended, reaching values close to 6. Although the acidification of the digesters was not reached until the end of this third stage, the acid pH and high acetic content of the OMSW (Table 1), i.e., 4.49 ± 0.05 and 1802 ± 8 mg/L, respectively, could acidify the process if the buffer capacities of the digesters were not high enough. The alkalinity values determined in the present research were markedly lower than that reported by Rincon et al. [25] for the anaerobic digestion of steam-exploded OMSW at mesophilic conditions. These authors reported alkalinity values in a range from 7000 to 9000 mg CaCO3/L, which ensured that no acidification appeared despite the acid character of the OMSW. In the same way, Orive et al. [22] reported very high alkalinity values (10.6–21.3 g CaCO3/L) in digesters treating diluted OMSW, which allowed them to achieve pH values in the reactors within the optimal range for methanogenesis.
In this third stage, the CODtotal critically increased above 28 gO2/kg (Figure 3). CODtotal accumulation was associated with a decrease in methane production, indicating a strong inhibition of the process (Figure 2). Process failure was also detected by CODsoluble and VFA accumulation during this stage (Figure 3 and Figure 4). This accumulation indicated that the acidogenic and methanogenic microorganisms were inhibited prior to the hydrolytic microorganisms. This performance is in contrast to that observed from days 0 to 75, where the concentrations of CODsoluble and VFA presented low values (Figure 2 and Figure 3). The decrease in pH during the third phase due to the accumulation of VFA could explain the inhibition of the methanogenic activity [26]. As can be seen in Figure 4, the concentration of propionic acid increased during this third stage, being the most abundant VFA. Specifically, the mean propionic acid content increased from 251 mg/L during the second stage (OLR: 1 gVS/(L·d)) to 1486 mg/L in the third stage (OLR: 2 gVS/(L·d) (Table 3). This accumulation was probably a consequence of the inhibition of the methanogens, which are the most sensitive microorganisms to acidification in the anaerobic processes [27]. The increment in the propionic acid concentration indicated that the inhibition also affected the microorganisms involved in the acetogenesis, and not only the methanogens. Although alkalinity was added periodically during the third stage, it was not able to compensate for the VFA accumulation, dropping the pH to values below 6 at the end of the third stage. Likewise, a recent study of anaerobic digestion of untreated OMSW in CSTR reactors carried out by Stoyanova et al. [12] revealed that the increase of the OLR up to a value of 1.76 gVS/(L·d) entailed a drop in pH to values below 7. In the present study, the operation at an OLR of 2 gVS/(L·d) was not stable, resulting in a strong inhibition due to the accumulation of solids in the digester, an increase of the VFA concentration and a marked drop of the pH and the alkalinity. However, at an OLR of 1 gVS/(L·d), the effluent characterization confirmed the good performance of the digesters described for the high methane production rate obtained.
3.3. Composition and Concentration of the Phenolic Compounds
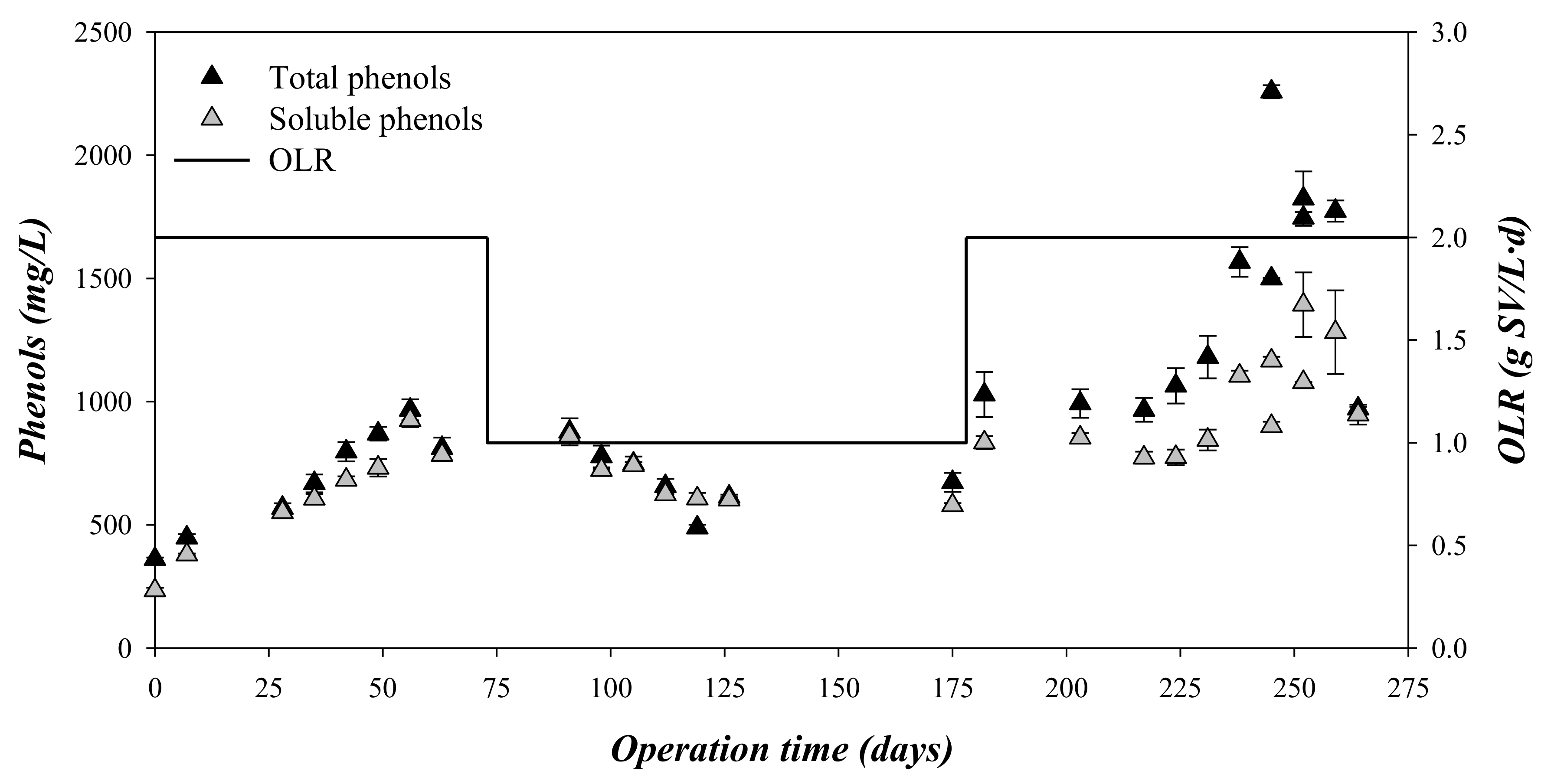
Phenolic compounds are reported to be toxic to anaerobic microorganisms, affecting the microbial growth [28]. In that sense, the presence of phenols, as total and soluble phenol concentration, and phenolic composition were monitored (Figure 5 and Table 4). The concentration of total and soluble phenolic compounds followed a similar trend to the values of CODtotal (Figure 2 and Figure 4). At an OLR = 2 gVS/(L·d), the phenolic compounds accumulated in the digesters, whereas at an OLR of 1 gVS/(L·d), the phenol concentration was kept at a lower constant concentration (Figure 5) compared to an OLR of 2 gVS/(L·d). The relation between soluble phenol and total phenol was close to 1 during stages 1 and 2. This value is much higher than the ratio of soluble phenol/total phenol in the substrate, which presented a value of 0.31 (Table 1). This difference indicated that the phenols were partially hydrolyzed during the biomethanization. However, the presence in the digesters of detectable concentrations of vanillin and 4-ethylphenol indicated that the degradation was not complete (Table 4) [3].
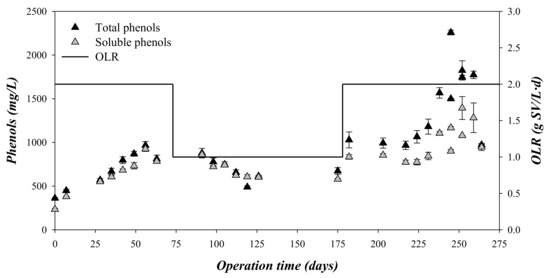
Figure 5.
Variation in the total and soluble phenols and the organic loading rate (OLR) with the operation time.

Table 4.
Individual phenolic compound concentration during the different organic loading rates (OLRs), where n.d., non detected; Traces, <0.01 mg/L.
Regardless of the OLR applied to the digester, the phenol and furanic concentration was always lower than the limits described as inhibitory for the anaerobic digestion. For example, hydroxymethylfurfural was totally biodegraded, and, therefore, was not detected, whereas vanillin concentrations were lower than 150 mg/L throughout the experimental time (Table 3). The inhibition concentrations for anaerobic processes were reported to be around 800 mg/L for hydroxymethylfurfural and 2000 mg/L for vanillin [16]. Both compounds, i.e., hydroxymethylfurfural and vanillin, have been widely defined as the most toxic phenolic and furanic compounds for biomethanization [3,16,29]. Therefore, the failure of the biomethanization process determined at an OLR of 2 gVS/(L·d) was probably a consequence of overloading of the system, with acidification of the process, instead of the toxic effect of the accumulated phenolic and furanic compounds [30].
3.4. Microbiology Assessment
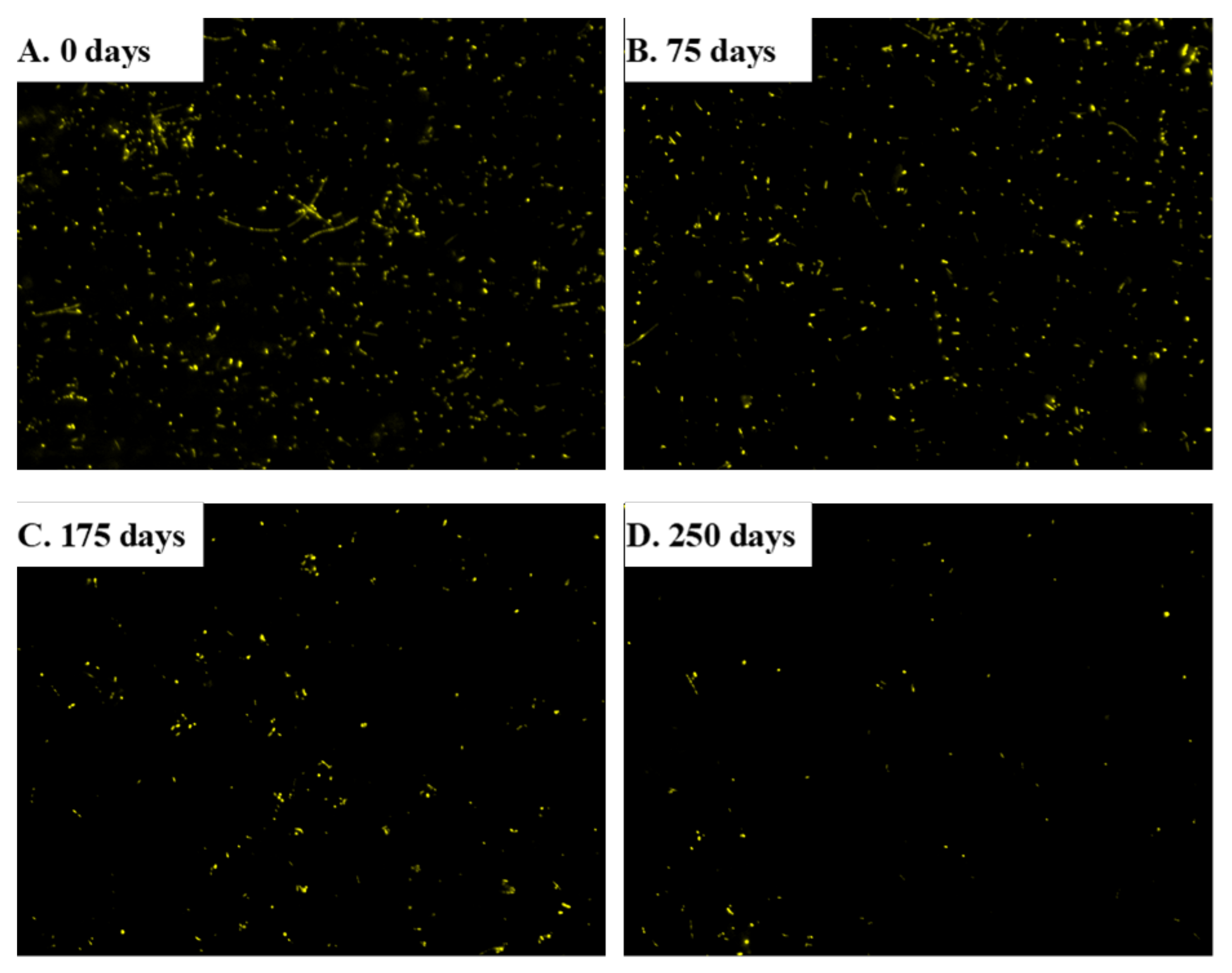
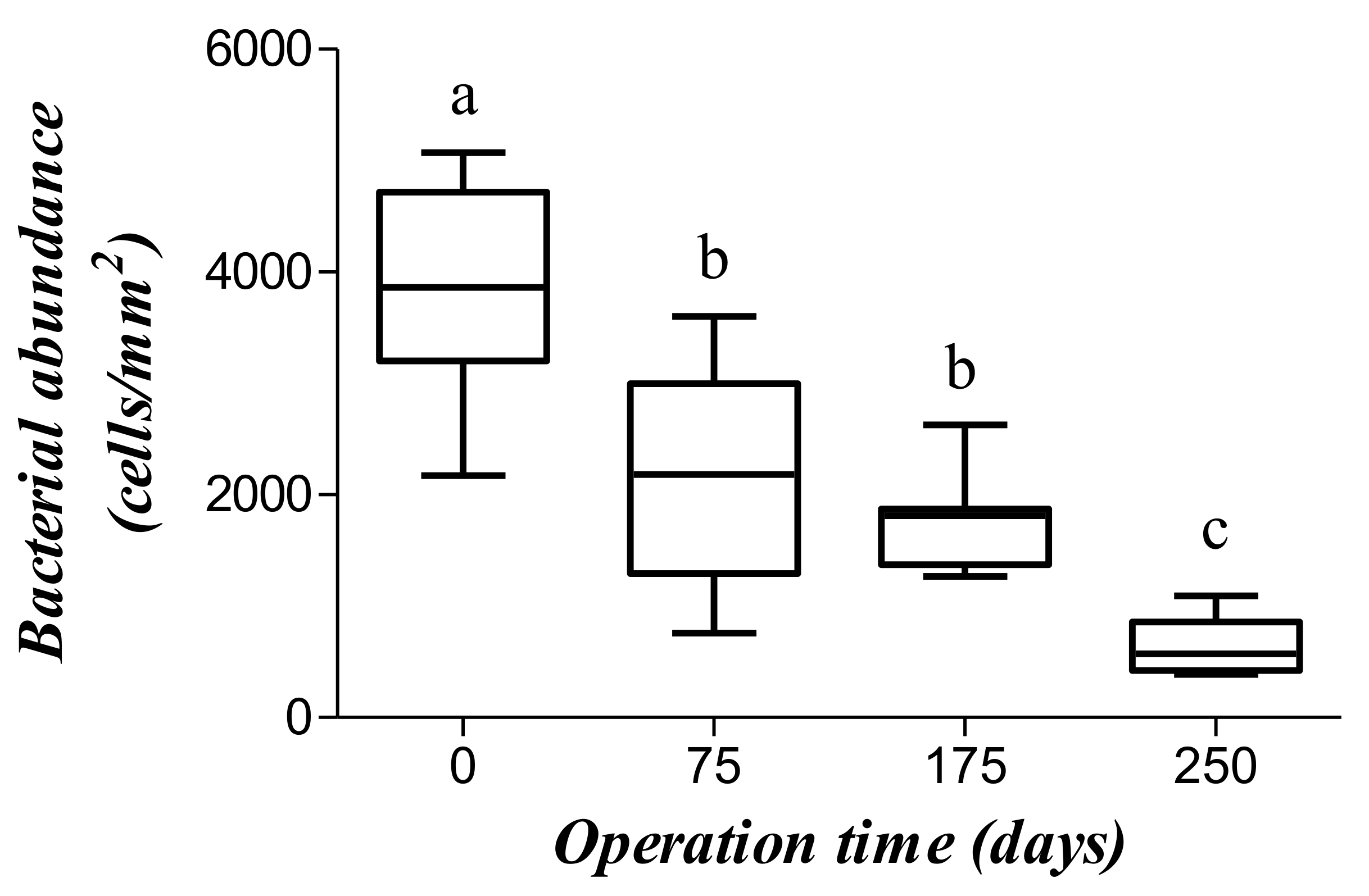
On the last day of each tested OLR, the number of microbes was determined from fluorescence images in the inoculum and at 75, 175, and 250 days after starting the experiment (Figure 6). The mean number of microorganisms decreased from 12,761 ± 3213 cells/mm2 in the inoculum to 7126 ± 3099 cells/mm2 after 75 days (Figure 7) as a result of a process of selection by the substrate [31]. The number of microbes was no different at day 175 (5739 ± 1316 cells/mm2) from day 75, which suggests that the rate of duplication met an equilibrium with the OLR. Interestingly, duplication rate was similar to the production of methane, which suggest that most cells are methanogenic bacteria. Accordingly, the number of microbes dramatically decreased at day 250 (2159 ± 801 cells/mm2) where a low production of methane was detected.
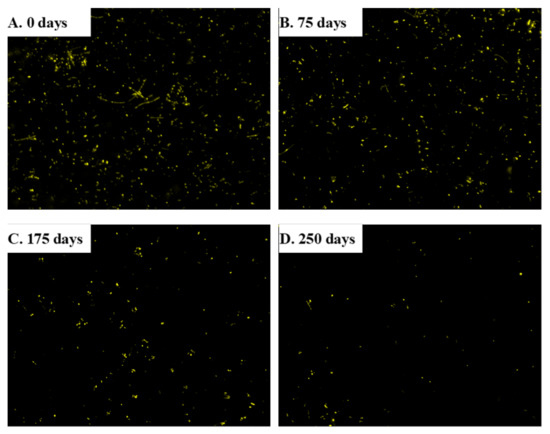
Figure 6.
DAPI stained images for the determination of bacterial abundance at distinct experimental times.
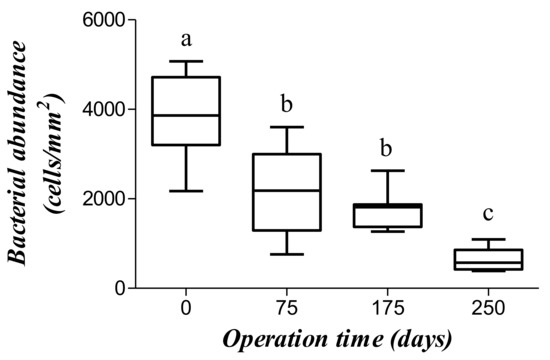
Figure 7.
Bacterial abundance at distinct operation times. Boxes with distinct letters are significantly different (p < 0.05, one-way ANOVA plus Tukey post-hoc test).
3.5. Economic Assessment
The results obtained for the profitability assessment are summarised in Table 5. The proposed bio-refinery concept (Figure 1) shows high values for both NPV (3.9·103 M€) and IRR (1011%), and a short payback period (1 year), which point to high profitability. Regarding the influence of the price of the phenol extract, the project would be profitable for prices higher than 42.2 €/kg. This minimum price is almost lower than the minimum price of 51.8 €/kg obtained for a similar bio-refinery concept in which the OMSW is pre-treated with steam at 170 °C [13].

Table 5.
Economical assessment.
4. Conclusions
The anaerobic digestion process was stable at an OLR of 1 gVS/(L·d), with a specific production rate of 163 ± 28 mL CH4/(gVS·d). However, the increment of the OLR up to 2 gVS/(L·d) resulted in total exhaust of the methane production. The increment in the total and soluble organic matter, especially the increment in the propionic acid concentration up to 1486 mg/L, described an overloaded inhibition process. Regardless of the OLR, the concentration of phenolic compounds was always lower than the inhibition limits. An economic analysis showed that the combination of the steam-explosion recovery of phenols and the anaerobic digestion, under stable conditions, could generate high profitability, which would still be profitable for a phenol extract price higher than 42.2 €/kg. Therefore, steam-exploited OMSW could be a suitable substrate for anaerobic digestion at the correct OLR.
Author Contributions
Conceptualization: A.S., F.G.F., B.A.-F., G.R.-G., S.L., J.F.-B. and R.B.; Methodology: A.S., F.G.F., B.A.-F., G.R.-G., S.L., J.F.-B. and R.B.; Validation: A.S., F.G.F., B.A.-F., G.R.-G., S.L., J.F.-B. and R.B.; Formal Analysis, A.S., F.G.F., B.A.-F., G.R.-G., S.L., J.F.-B. and R.B.; Investigation: A.S., F.G.F., B.A.-F., G.R.-G., S.L., J.F.-B. and R.B.; Resources: A.S., F.G.F., B.A.-F., G.R.-G., S.L., J.F.-B. and R.B.; Data Curation: A.S., F.G.F., B.A.-F., G.R.-G., S.L., J.F.-B. and R.B.; Writing—Original Draft Preparation: A.S., F.G.F., B.A.-F., G.R.-G., S.L., J.F.-B. and R.B.; Writing—Review & Editing: A.S., F.G.F., B.A.-F., G.R.-G., S.L., J.F.-B. and R.B.; Supervision: F.G.F. and R.B.; Project Administration: F.G.F. and R.B.; Funding Acquisition: F.G.F. and R.B.
Funding
This research was funded by the [Spanish Ministry of Economy and Competitiveness] through Project grant number CTM2014-55095-R and the Ramon y Cajal Programme (RyC 2012-10456). Sergio López acknowledges the financial support from the ‘V Own Research Plan’ of the University of Seville (VPPI-US) for his research contract. This contracts is cofunded by the European Social Fund.
Acknowledgments
The authors wish to express their gratitude to. Ainoa Botana for her assistance to this research.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mahmoud, A.E.; Fathy, S.A.; Ali, M.M.; Ezz, M.K.; Mohammed, A.T. Antioxidant and anticancer efficacy of therapeutic bioactive compounds from fermented olive waste. Grasas y Aceites 2018, 69, 266. [Google Scholar] [CrossRef]
- Tortosa, G.; Alburquerque, J.A.; Bedmar, E.J.; Ait-Baddi, G.; Cegarra, J. Strategies to produce commercial liquid organic fertilisers from “alperujo” composts. J. Clean. Prod. 2014, 82, 37–44. [Google Scholar] [CrossRef]
- Serrano, A.; Fermoso, F.G.; Alonso-Fariñas, B.; Rodríguez-Gutierrez, G.; Fernandez-Bolaños, J.; Borja, R. Olive mill solid waste biorefinery: High-temperature thermal pre-treatment for phenol recovery and biomethanization. J. Clean. Prod. 2017, 148, 314–323. [Google Scholar] [CrossRef]
- Rodríguez-Gutiérrez, G.; Lama-Muñoz, A.; Rubio-Senent, F.; Fernández-Bolaños, J.; Ruiz-Méndez, M.V. New Olive-Pomace Oil Improved by Hydrothermal Pre-Treatments; INTECH Open Access Publisher: London, UK, 2012. [Google Scholar]
- García, A.; Rodríguez-Juan, E.; Rodríguez-Gutiérrez, G.; Rios, J.J.; Fernández-Bolaños, J. Extraction of phenolic compounds from virgin olive oil by deep eutectic solvents (DESs). Food Chem. 2016, 197, 554–561. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, G.; Lama, A.; Rodríguez, R.; Jiménez, A.; Guillén, R.; Fernández-Bolaños, J. Olive stone an attractive source of bioactive and valuable compounds. Bioresour. Technol. 2008, 99, 5261–5269. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Senent, F.; Rodríguez-Gutíerrez, G.; Lama-Muñoz, A.; Fernández-Bolaños, J. New phenolic compounds hydrothermally extracted from the olive oil byproduct alperujo and their antioxidative activities. J. Agric. Food Chem. 2012, 60, 1175–1186. [Google Scholar] [CrossRef] [PubMed]
- Hendriks, A.T.W.M.; Zeeman, G. Pretreatments to enhance the digestibility of lignocellulosic biomass. Bioresour. Technol. 2009, 100, 10–18. [Google Scholar] [CrossRef]
- Serrano, A.; Fermoso, F.G.; Alonso-Fariñas, B.; Rodríguez-Gutierrez, G.; Fernandez-Bolaños, J.; Borja, R. Phenols recovery after steam explosion of Olive Mill Solid Waste and its influence on a subsequent biomethanization process. Bioresour. Technol. 2017, 243, 169–178. [Google Scholar] [CrossRef]
- Christoforou, E.; Fokaides, P.A. A review of olive mill solid wastes to energy utilization techniques. Waste Manag. 2016, 49, 346–363. [Google Scholar] [CrossRef]
- Al-Mallahi, J.; Furuichi, T.; Ishii, K. Appropriate conditions for applying NaOH-pretreated two-phase olive milling waste for codigestion with food waste to enhance biogas production. Waste Manag. 2016, 48 (Suppl. C), 430–439. [Google Scholar] [CrossRef]
- Stoyanova, E.; Lundaa, T.; Bochmann, G.; Fuchs, W. Overcoming the bottlenecks of anaerobic digestion of olive mill solid waste by two-stage fermentation. Environ. Technol. 2017, 38, 394–405. [Google Scholar] [CrossRef] [PubMed]
- Serrano, A.; Fermoso, F.G.; Alonso-Fariñas, B.; Rodríguez-Gutiérrez, G.; López, S.; Fernandez-Bolaños, J.; Borja, R. Performance evaluation of mesophilic semi-continuous anaerobic digestion of high-temperature thermally pre-treated olive mill solid waste. Waste Manag. 2019, 87, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Borja, R.; Alba, J.; Banks, C.J. Impact of the main phenolic compounds of olive mill wastewater (OMW) on the kinetics of acetoclastic methanogenesis. Process Biochem. 1997, 32, 121–133. [Google Scholar] [CrossRef]
- Kourmentza, C.; Koutra, E.; Venetsaneas, N.; Kornaros, M. Integrated Biorefinery Approach for the Valorization of Olive Mill Waste Streams towards Sustainable Biofuels and Bio-Based Products. In Microbial Applications Vol.1: Bioremediation and Bioenergy; Kalia, V.C., Kumar, P., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 211–238. [Google Scholar]
- Ghasimi, D.S.M.; Aboudi, K.; de Kreuk, M.; Zandvoort, M.H.; van Lier, J.B. Impact of lignocellulosic-waste intermediates on hydrolysis and methanogenesis under thermophilic and mesophilic conditions. Chem. Eng. J. 2016, 295, 181–191. [Google Scholar] [CrossRef]
- American Public Health Association, American Water Works Association, The Water Environment Federation. Standard Methods for the Examination of Water and Wastewater; APHA-AWWA-WEF: Washington, DC, USA, 2005. [Google Scholar]
- Siritantikorn, S.; Jintaworn, S.; Noisakran, S.; Suputtamongkol, Y.; Paris, D.H.; Blacksell, S.D. Application of ImageJ program to the enumeration of Orientia tsutsugamushi organisms cultured in vitro. Trans. R. Soc. Trop. Med. Hyg. 2012, 106, 632–635. [Google Scholar] [CrossRef] [PubMed]
- Ciriminna, R.; Meneguzzo, F.; Fidalgo, A.; Ilharco, L.M.; Pagliaro, M. Extraction, benefits and valorization of olive polyphenols. Eur. J. Lipid Sci. Technol. 2016, 118, 503–511. [Google Scholar] [CrossRef]
- EUROSTAT. Available online: https://ec.europa.eu/eurostat/web/main/home (accessed on 27 March 2019).
- endesaonline.es. Available online: www.endesaonline.es (accessed on 20 March 2019).
- Orive, M.; Cebrián, M.; Zufía, J. Techno-economic anaerobic co-digestion feasibility study for two-phase olive oil mill pomace and pig slurry. Renew. Energy 2016, 97, 532–540. [Google Scholar] [CrossRef]
- Ortega, L.; Husser, C.; Barrington, S.; Guiot, S.R. Evaluating limiting steps of anaerobic degradation of food waste based on methane production tests. Water Sci. Technol. 2008, 57, 419–422. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Wang, J.; Meng, L. Effects of volatile fatty acid concentrations on methane yield and methanogenic bacteria. Biomass Bioenergy 2009, 33, 848–853. [Google Scholar] [CrossRef]
- Rincón, B.; Rodríguez-Gutiérrez, G.; Bujalance, L.; Fernández-Bolaños, J.; Borja, R. Influence of a steam-explosion pre-treatment on the methane yield and kinetics of anaerobic digestion of two-phase olive mil solid waste or alperujo. Process Saf. Environ. 2016, 102, 361–369. [Google Scholar] [CrossRef]
- Liu, C.; Yuan, X.; Zeng, G.; Li, W.; Li, J. Prediction of methane yield at optimum pH for anaerobic digestion of organic fraction of municipal solid waste. Bioresour. Technol. 2008, 99, 882–888. [Google Scholar] [CrossRef] [PubMed]
- Borja, R. Biogas Production. In Comprehensive Biotechnology, 2nd. ed; Elsevier: Amsterdam, The Netherlands, 2011; Volume 2, pp. 785–798. [Google Scholar]
- Battista, F.; Fino, D.; Erriquens, F.; Mancini, G.; Ruggeri, B. Scaled-up experimental biogas production from two agro-food waste mixtures having high inhibitory compound concentrations. Renew. Energy 2015, 81 (Suppl. C), 71–77. [Google Scholar] [CrossRef]
- Carrere, H.; Antonopoulou, G.; Affes, R.; Passos, F.; Battimelli, A.; Lyberatos, G.; Ferrer, I. Review of feedstock pretreatment strategies for improved anaerobic digestion: From lab-scale research to full-scale application. Bioresour. Technol. 2016, 199, 386–397. [Google Scholar] [CrossRef]
- Theuerl, S.; Klang, J.; Prochnow, A. Process disturbances in agricultural biogas production—Causes, mechanisms and effects on the biogas microbiome: A review. Energies 2019, 12, 365. [Google Scholar] [CrossRef]
- Alcántara-Hernández, R.J.; Taş, N.; Carlos-Pinedo, S.; Durán-Moreno, A.; Falcón, L.I. Microbial dynamics in anaerobic digestion reactors for treating organic urban residues during the start-up process. Lett. Appl. Microbiol. 2017, 64, 438–445. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).