Experimental Investigation of Methane Hydrate Induction Time in the Presence of Cassava Peel as a Hydrate Inhibitor
Abstract
1. Introduction
2. Materials and Methods
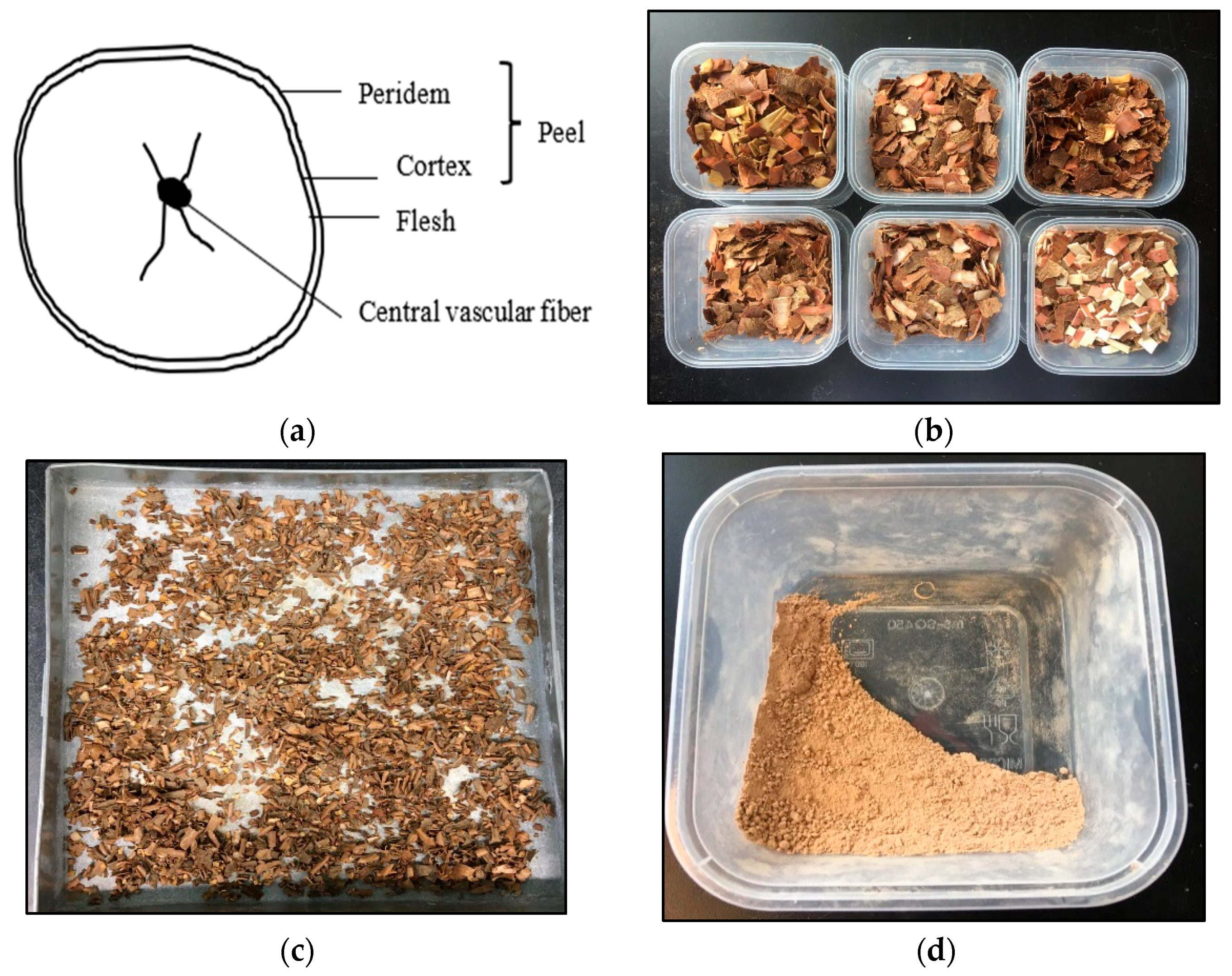
2.1. Cassava Peels Preparation
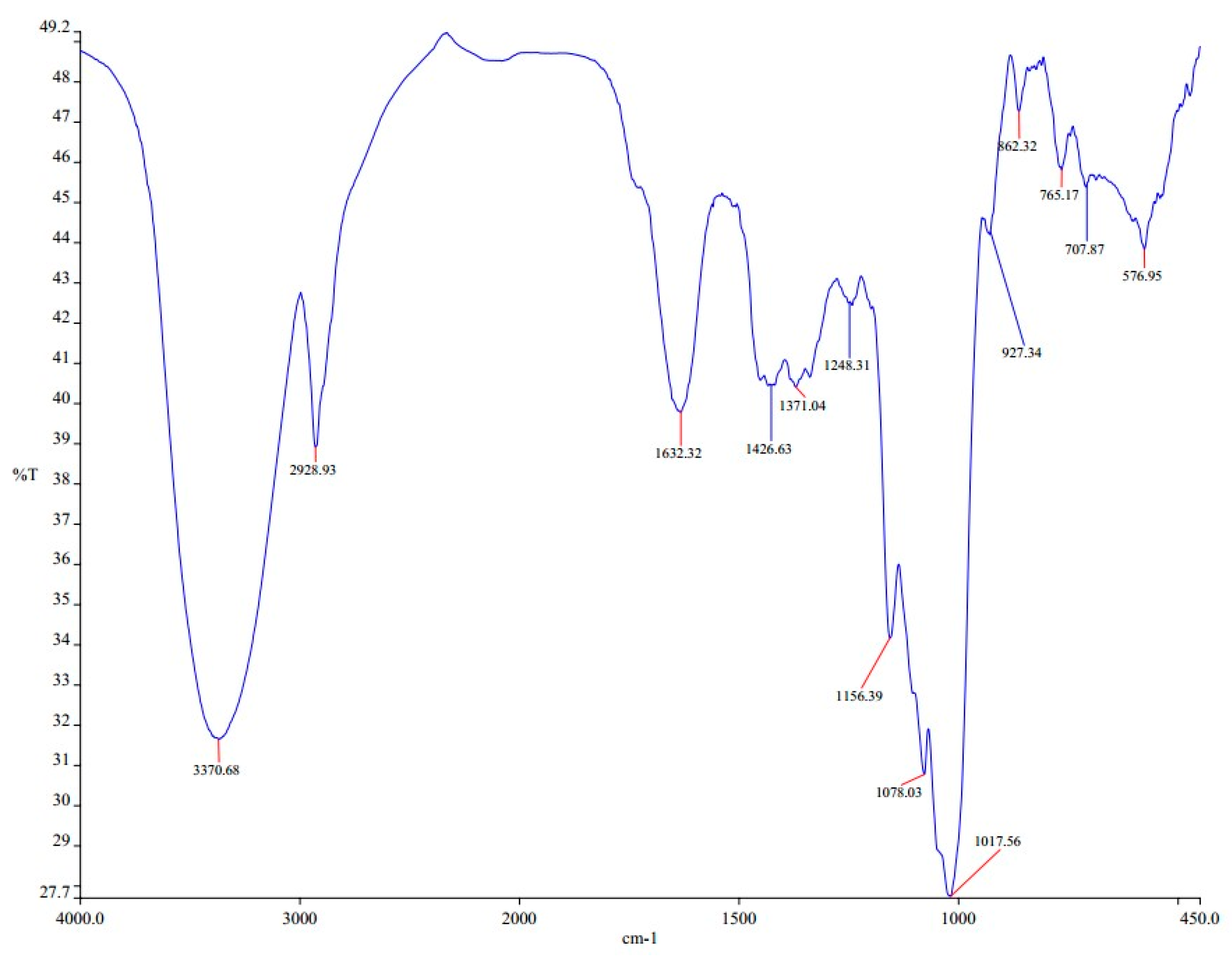
2.2. Hydroxyl Group Identification Using FTIR
2.3. Morphology and Composition Identification Using SEM
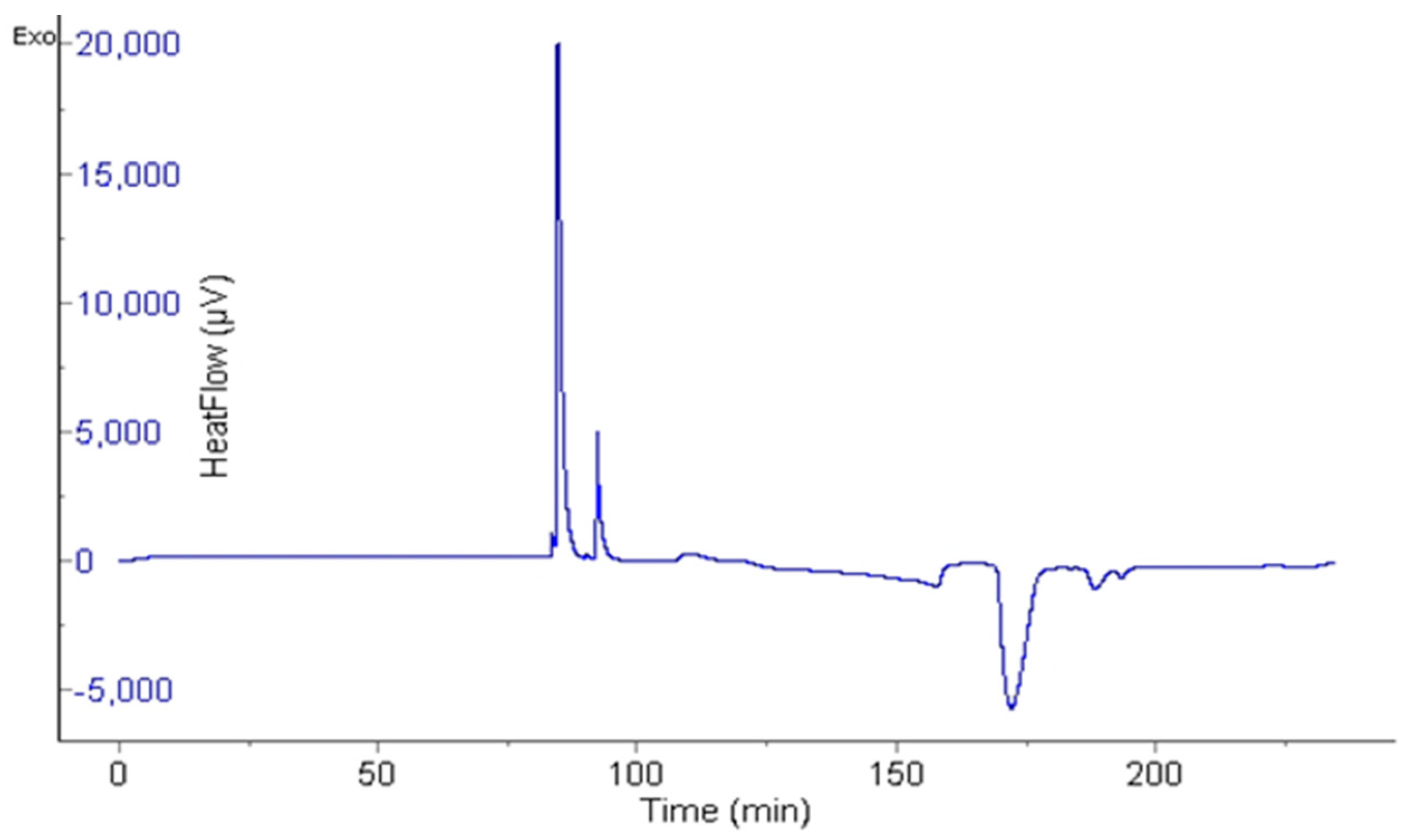
2.4. Measurement of Induction Time Using µ-DSC
3. Results and Discussion
3.1. Hydroxyl Group Identification Using FTIR
3.2. Morphology and Elemental Composition Analysis Using SEM
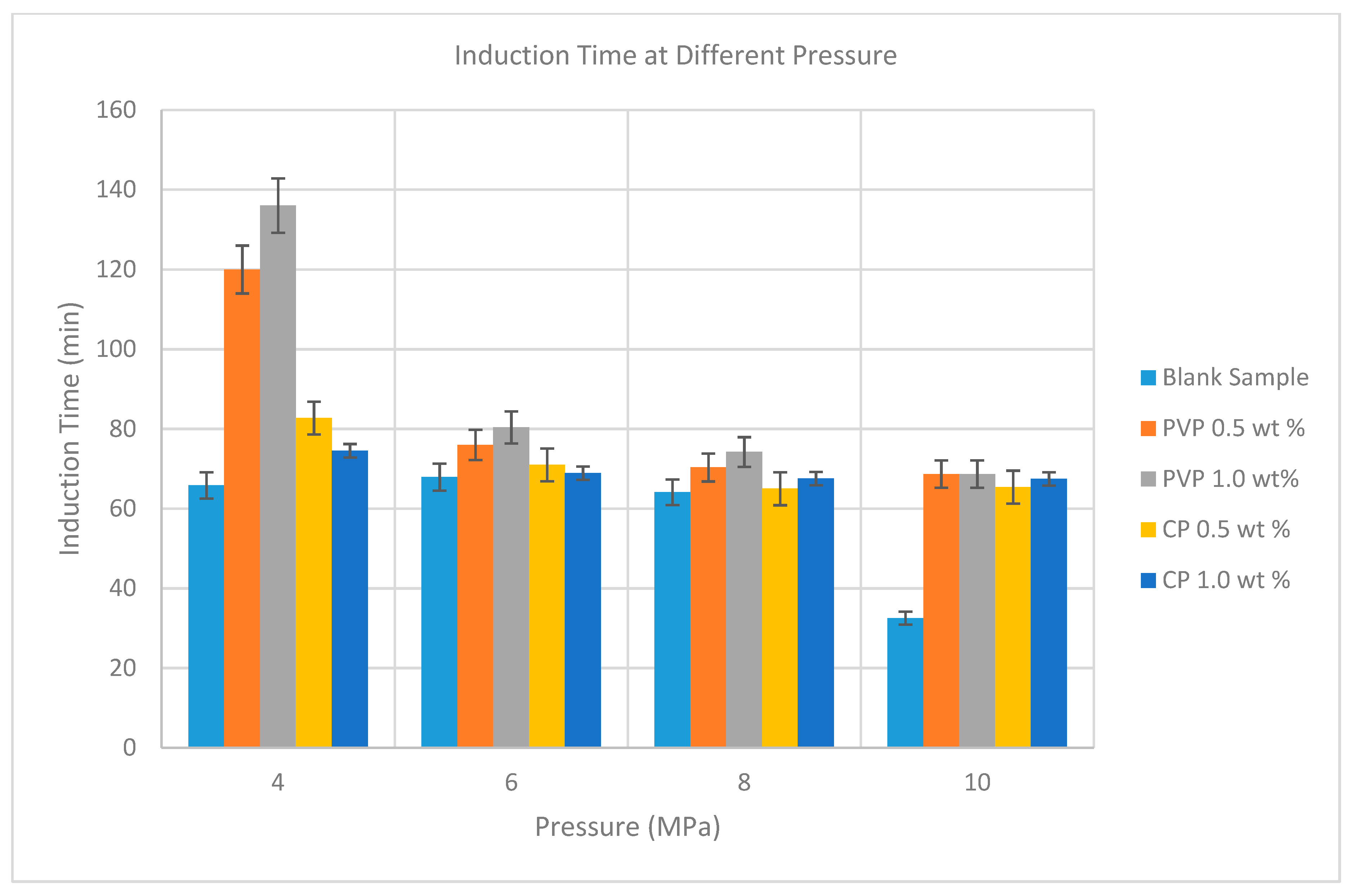
3.3. Measurement of Induction Time Using µ-DSC
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sloan, E.D., Jr. Clathrate Hydrates of Natural Gases, Revised and Expanded; CRC Press: Boca Raton, FL, USA, 1998. [Google Scholar]
- Wang, Z.; Zhao, Y.; Zhang, J.; Pan, S.; Yu, J.; Sun, B. Flow assurance during deepwater gas well testing: Hydrate blockage prediction and prevention. J. Pet. Sci. Eng. 2018, 163, 211–216. [Google Scholar] [CrossRef]
- Lee, S.Y.; Holder, G.D. Methane hydrates potential as a future energy source. Fuel Process. Technol. 2001, 71, 181–186. [Google Scholar] [CrossRef]
- Yelisetti, S.; Spence, G.D.; Riedel, M. Role of gas hydrates in slope failure on frontal ridge of northern Cascadia margin. Geophys. J. Int. 2014, 199, 441–458. [Google Scholar] [CrossRef]
- Majorowicz, J.; Grasby, S.E.; Safanda, J.; Beauchamp, B. Gas hydrate contribution to Late Permian global warming. Earth Planet. Sci. Lett. 2014, 393, 243–253. [Google Scholar] [CrossRef]
- Tang, C.; Liang, D. Inhibitory effects of novel green inhibitors on gas hydrate formation. Chin. J. Chem. Eng. 2019, in press. [Google Scholar] [CrossRef]
- Wang, Y.; Fan, S.; Lang, X. Reviews of gas hydrate inhibitors in gas dominant pipelines and application of kinetic hydrate inhibitors in China. Chin. J. Chem. Eng. 2019, in press. [Google Scholar] [CrossRef]
- Yaqub, S.; Lal, B.; Partoon, B.; Mellon, N.B. Investigation of the task oriented dual function inhibitors in gas hydrate inhibition: A review. Fluid Phase Equilib. 2018, 477, 40–57. [Google Scholar] [CrossRef]
- Creek, J.L. Efficient hydrate plug prevention. Energy Fuels 2012, 26, 4112–4116. [Google Scholar] [CrossRef]
- Aminnaji, M.; Tohidi, B.; Burgass, R.; Atilhan, M. Gas hydrate blockage removal using chemical injection in vertical pipes. J. Nat. Gas Sci. Eng. 2017, 40, 17–23. [Google Scholar] [CrossRef]
- Chatti, I.; Delahaye, A.; Fournaison, L.; Petitet, J.P. Benefits and drawbacks of clathrate hydrates: A review of their areas of interest. Energy Convers. Manag. 2005, 46, 1333–1343. [Google Scholar] [CrossRef]
- Moore, J.A.; Vers, L.V.; Conrad, P. Derstanding Kinetic Hydrate Inhibitor and Corrosion Inhibitor Interactions. In Proceedings of the Offshore Technology Conference, Houston, TX, USA, 4–7 May 2009. [Google Scholar]
- Kannan, K.; Punase, A. Low Dosage, High Efficiency and Environment Friendly Inhibitors: A New Horizon in Gas Hydrates Mitigation in Production Systems. In Proceedings of the SPE International Symposium on Oil Field Chemistry, The Woodlands, TX, USA, 20–22 April 2009. [Google Scholar]
- Kamal, M.S.; Hussein, I.A.; Sultan, A.S.; von Solms, N. Application of various water soluble polymers in gas hydrate inhibition. Renew. Sustain. Energy Rev. 2016, 60, 206–225. [Google Scholar] [CrossRef]
- Ke, W.; Svartaas, T.M.; Kvaløy, J.T.; Kosberg, B.R. Inhibition–Promotion: Dual Effects of Polyvinylpyrrolidone (PVP) on Structure-II Hydrate Nucleation. Energy Fuels 2016, 30, 7646–7655. [Google Scholar] [CrossRef]
- Kvamme, B.; Kuznetsova, T.; Aasoldsen, K. Molecular dynamics simulations for selection of kinetic hydrate inhibitors. J. Mol. Graph. Model. 2005, 23, 524–536. [Google Scholar] [CrossRef] [PubMed]
- Idress, M.; Jasamai, M.; Yuhaznel, F.N.; Peng, W.; Karimi, N. Preliminary study of natural polymer as kinetic hydrate inhibitor. Mater. Today Proc. 2018, 5, 21667–21671. [Google Scholar] [CrossRef]
- Morgan, N.K.; Choct, M. Cassava: Nutrient composition and nutritive value in poultry diets. Anim. Nutr. 2016, 2, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Ubalua, A.O. Cassava wastes: Treatment options and value addition alternatives. Afr. J. Biotechnol. 2007, 6, 2065–2073. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Sillanpää, M.; Anna, W.K. Agricultural waste peels as versatile biomass for water purification—A review. Chem. Eng. J. 2015, 270, 244–271. [Google Scholar] [CrossRef]
- Adekunle, A.; Orsat, V.; Raghavan, V. Lignocellulosic bioethanol: A review and design conceptualization study of production from cassava peels. Renew. Sustain. Energy Rev. 2016, 64, 518–530. [Google Scholar] [CrossRef]
- Xu, S.; Fan, S.; Fang, S.; Lang, X.; Wang, Y.; Chen, J. Pectin as an extraordinary natural kinetic hydrate inhibitor. Sci. Rep. 2016, 6, 23220. [Google Scholar] [CrossRef]
- Xu, Y.; Yang, M.; Yang, X. Chitosan as green kinetic inhibitors for gas hydrate formation. J. Nat. Gas Chem. 2010, 19, 431–435. [Google Scholar] [CrossRef]
- Merkel, F.S.; Schmuck, C.; Schultz, H.J. Investigation of the influence of hydroxyl groups on gas hydrate formation at pipeline-like conditions. Energy Fuels 2016, 30, 9141–9149. [Google Scholar] [CrossRef]
- Asharuddin, M.S.; Othman, N.; Zin, N.S.M.; Tajaruddin, H.A. A chemical and morphological study of cassava peel: A potential waste as coagulant aid. In Proceedings of the International Symposium on Civil and Environmental Engineering 2016 (ISCEE 2016), Melaka, Malaysia, 5 December 2016. [Google Scholar]
- Kusumayanti, H.; Handayani, N.A.; Santosa, H. Swelling power and water solubility of cassava and sweet potatoes flour. Procedia Environ. Sci. 2015, 23, 164–167. [Google Scholar] [CrossRef]
- Maeda, N.; Kelland, M.A.; Wood, C.D. Ranking of kinetic hydrate inhibitors using a high pressure differential scanning calorimeter. Chem. Eng. Sci. 2018, 183, 30–36. [Google Scholar] [CrossRef]
- Mutalib, M.A.; Rahman, M.A.; Othman, M.H.D.; Ismail, A.F.; Jaafar, J. Scanning Electron Microscopy (SEM) and Energy-Dispersive X-Ray (EDX) Spectroscopy. In Membrane Characterization; Elsevier: Amsterdam, The Netherlands, 2017; pp. 161–179. [Google Scholar]
- Gupta, A.; Lachance, J.; Sloan, E.D., Jr.; Koh, C.A. Measurements of methane hydrate heat of dissociation using high pressure differential scanning calorimetry. Chem. Eng. Sci. 2008, 63, 5848–5853. [Google Scholar] [CrossRef]
- Nashed, O.; Sabil, K.M.; Ismail, L.; Japper-Jaafar, A.; Lal, B. Mean induction time and isothermal kinetic analysis of methane hydrate formation in water and imidazolium based ionic liquid solutions. J. Chem. Thermodyn. 2018, 117, 147–154. [Google Scholar] [CrossRef]
- Bavoh, C.B.; Lal, B.; Keong, L.K.; Binti Jasamai, M.; Binti Idress, M. Synergic kinetic inhibition effect of EMIM-Cl+PVP on CO2 hydrate formation. Procedia Eng. 2016, 148, 1232–1238. [Google Scholar] [CrossRef]



| Absorption | Functional Group | Compound Class |
|---|---|---|
| 3700–3584 | O–H stretching | Alcohol |
| 3550–3200 | O–H stretching | Alcohol |
| 3500 | N–H stretching | Primary amine |
| Element | Compound | Weight (%) | Atomic (%) |
|---|---|---|---|
| C | CaCO3 | 71.50 | 77.09 |
| O | SiO2 | 28.12 | 22.76 |
| Al | Al2O3 | 0.10 | 0.05 |
| Si | SiO2 | 0.09 | 0.04 |
| K | Potassium feldspar | 0.19 | 0.06 |
| Totals | - | 100.00 | 100.00 |
| Sample | Pressure (MPa) | Induction Time (min) |
|---|---|---|
| Without inhibitor (blank sample) | 4.0 | 65.84 |
| 6.0 | 67.92 | |
| 8.0 | 64.14 | |
| 10.0 | 32.54 |
| Pressure (MPa) | Concentration (wt %) | No of Runs | Average Induction Time for PVP (min) | Average Induction Time for CP (min) |
|---|---|---|---|---|
| 4.0 | 0.5 | 3 | 120.00 | 82.73 |
| 4.0 | 1.0 | 3 | 136.00 | 74.54 |
| 6.0 | 0.5 | 3 | 76.00 | 70.97 |
| 6.0 | 1.0 | 3 | 80.40 | 68.92 |
| 8.0 | 0.5 | 3 | 70.33 | 65.00 |
| 8.0 | 1.0 | 3 | 74.22 | 67.55 |
| 10.0 | 0.5 | 3 | 68.70 | 65.40 |
| 10.0 | 1.0 | 3 | 70.52 | 67.46 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Idress, M.; Shahril, M.A.; Zuraidin, A.S.; Jasamai, M. Experimental Investigation of Methane Hydrate Induction Time in the Presence of Cassava Peel as a Hydrate Inhibitor. Energies 2019, 12, 2314. https://doi.org/10.3390/en12122314
Idress M, Shahril MA, Zuraidin AS, Jasamai M. Experimental Investigation of Methane Hydrate Induction Time in the Presence of Cassava Peel as a Hydrate Inhibitor. Energies. 2019; 12(12):2314. https://doi.org/10.3390/en12122314
Chicago/Turabian StyleIdress, Mazlin, Muhammad Afiq Shahril, Ahmad Syahir Zuraidin, and Mazuin Jasamai. 2019. "Experimental Investigation of Methane Hydrate Induction Time in the Presence of Cassava Peel as a Hydrate Inhibitor" Energies 12, no. 12: 2314. https://doi.org/10.3390/en12122314
APA StyleIdress, M., Shahril, M. A., Zuraidin, A. S., & Jasamai, M. (2019). Experimental Investigation of Methane Hydrate Induction Time in the Presence of Cassava Peel as a Hydrate Inhibitor. Energies, 12(12), 2314. https://doi.org/10.3390/en12122314





